Note: Descriptions are shown in the official language in which they were submitted.
2 ~3 '~
CONTINUOUS PRODuCTION OF POTASSIUM NITRATE
VIA ION EXCHANGE
FIELD OF THE INVENTION
This invention is directed to methods for producing
potassium nitrate generally, and more particularly to methods
for producing potassium nitrate via ion exchange, and to
apparatus capable of producing potassium nitrate via ion
exchange on a continuous basis.
BACKGROUND OF TH~ INVENTION
Potassium nitrate, otherwlse known as saltpeter or
nitrate of potash, is important ln the production of
fertllizers, explosives, glass, and numerous other industrial
chemlcals. It is one of the oldest known "industrial"
chemicals. Potassium nitrate has been used on a large scale
since around the year 1300, when the Chlnese dlscovered that
saltpeter could be combined wlth sulfur and charcoal to
produce the common exploslve known as black powder.
The ever growlng demand for potasslum nltrate for these
and other such uses has resulted ln a prolonged search for
lmproved potasslum nitrate production processes, and various
methods have been invented to produce potassium nitrate. For
example, large quantities of potassium nitrate are
commercially produced by the reaction of potassium chloride
wlth nitric acld in the presence of oxygen, yleldlng the
following overall reaction:
2KCl + 2HNO3 + 1/2 2 ---> 2KNO3 + Cl2 + H2O.
The potassium chloride and nitric acid must be reacted at
100C to produce potassium nitrate, nitrosyl chloride and
water as follows:
3KC1 ~ 4HN03 ---> 3KN03 + NOCl + C12 + 2H20.
The nitrosyl chloride is then oxidized to chlorlne and
nitrogen peroxide, N02, with nitric acid. See Ghemical
Process Industries, 4th Ed., Shreve and Brink, McGraw-Hill,
Inc., New York (1977), pp. 272-273.
Smith et al, in U.S. Patent 2,963,345, herein
incorporated by reference, disclose a process for producing
potassium nitrate, which involves agitating solid particulate
potassium chloride with liquid nitrogen peroxide under
anhydrous conditions at a temperature of 15 C; excess
nltrosyl chloride vapors produced by the reaction are
continuously withdrawn to maintaln the reaction. Potassium
nltrate and unreacted potassium chlorlde are then separated by
addltion to a brine that contains dlssolved potasslum nitrate
and potasslum chloride; the brine solution is heated to about
85 C to dissolve the potassium nitrate, but not the solid
partlcles of potassium chloride. The solid partlcles o
potassium chlorlde are then separated by flltratlon.
Large volumes of potasslum nltrate are also produced by
the reactlon of ~odlum nltrate with potassium chlorlde, the
overall reactlon belng:
XCl ~ NaN03 ---> XN03 ~ NaCl.
Thls process requlres that potasslum chlorlde be dlssolved in
2 ~
a hot solution of sodium nitrate; upon hea~ing, sodium
chloride crystals are formed. The hot potassium nitrate
solution is then run through the sodium chloride crystals
forming at the ~ottom of the reaction vessel. However, a
mixture of potassium nitrate and sodium chloride is formed, so
additional processing operations are required to separate
potassium nitrate.
Lehto, in U.S. Patent No. 3,983,222, herein incorporated
by reference, discloses a continuous process for producing
potassium nitrate, which includes the steps of extracting
nitrate from aqueous solutions with an organic amine salt
dissolved in an organic solvent, separating the organic phase
containing the extracted nitrate from the aqueous phase, and
stripping the organic base wlth a potassium salt stripping
solution having a Ph of at least 0.5. The stripping solution
also contains nitrate ions and potassium ions with the
concentration oS potassium nitrate maintalned high enough to
induce crystalllzatlon of potasslum nitrate from the stripping
solutlon contlnuously.
Dotson et al, U.S. Patent No. 4,465,568, herein
lncorporated by reference, uses an electrolytic process to
produce chloride free mlxtures of sodium nitrate and potassium
nitrate.
All of the prior art processes for producing potasslum
nltrate are expenslve or dlfflcult to perform. Processes that
utlllze nitric acld at elevated temperatures require speclally
constructed equipment to handle the highly corrosive
reactants, and further, elevated reaction temperatures require
high energy inputs. Other prior art processes suffer from low
yields of potassium nitrate or an impure product, while others
involve the use or production of nitrogen peroxide, which is
toxic, and poses a pollution problem.
Thus, there is a need for an inexpensive and continuous
process for producing large quantities of potassium nitrate at
ambient temperatures. There is also a need for a potassium
nitrate production process which does not corrode reaction
vessels, and thereby require expensive corrosion resistant
construction materials. Further, there is a need for a safe
potassium nitrate production process which produces by-
products which are easy to handle, and dispose of.
Reaction of potassium chloride with nitric acid to
produce potassium nitrate via ion exchange has not been
attempted, since a potentlal hazard exlsts in the use of
nitrlc acid ln ion exchange operatlons. There have been
several accidents lnvolvlng the use of nltrlc acld as a
regenerant or elutlon agent wlth lon exchange reslns. Nltrla
acld ls a powerful oxldlzlng agent, and the reactlon of nitrlc
acld wlth organlc ion exchange resins can result in a serious
fire or exploslon. Further, whlle the use of dllute solutlons
of nltric acld may reduce the rlsk of exploslon or flre, the
presence of metals, such as copper, and absorbed organlc
solutes ln any system contalnlng nltrlc acld can catalyze an
~ ~ ~J 7 ~
uncontrolled reaction. Even in dilute solutions, nitric acid
is believed to have a negatlve effect on the useful life of
exchange resins.
At dilute nitric acid concentratlons, larger volumes of
resin are needed, with the resulting increase in cost, without
a substantial decrease in the perceived potential for a fire
or explosion. The necessity of using large volumes of
expensive resin to achieve reasonable yields of product
further discouraged the use of ion exchange to produce
potassium nitrate.
The production of potassium nitrate by passing a neutral
nitrate salt through a catlonic exchange resin was also not
believed practical, since cationic exchange resins have an
equal affinity for potassium and other monovalent ions.
Divalent ions, such as calcium, make regeneration of such a
column difflcult, since large quantities of potassium are
necessary to displace calcium bound to the resin. Yet,
provided the aforementioned problems can be overcome,
productlon of potassium nitrate via ion exchange offers a
slmple, low cost and efficient alternative to the prior art
method8.
SU~RY OF THE INVENTION
These and other ob;ects of the present invention are
achieved by passing a solution of nitric acid through a
potassium loaded strong cationic exchange resin to produce
potassium nitrate. In a preferred embodiment, a fifteen per
cent by weight nitric acid solution (15~ wt ~NO3(~q)) is passed
through a potassium loaded strong catlonic exchange resin to
produce a solution of about fifteen per cent by weight
potassium nitrate (15~ wt KNO3(~q)) and about 0.5 per cent by
weight of nitric acid; the solution of potassium nitrate and
nltric acid is subsequently neutralized with potassium
hydroxide (KOH) to produce a substantlally pure aqueous
solution of potassium nitrate. High purity solid potassium
nitrate (KNO3(~)) can then be produced by standard
crystallization methods.
In a preferred embodiment, potassium nitrate is produced
contlnuously through the use of a modlfled advanced separation
devlce, ASD, such as that described ln U.S. Patent Nos.
4,764,276 and 4,522,726, ls8ued to Berry et al, and herein
lncorporated by reference. Preferably the ASD is modifled to
lnclude thlrty chambers, whlch are fllled with a catlonlc
exchange resln, and whlch rotate about a circular path in
perlodlc fluld communlcatlon wlth a serles of flxed feed and
dlscharge ports connected to opposlte ends of the chambers.
Preferably, the feed ports and chambers are arranged 80
that each of the chambers 18 ln fluld communlcatlon wlth no
~J7
more than one feed port at a time, and each of the feed ports
is in communication with at least one of the chambers at all
times. The discharge ports are purposely arranged so that
each of the chambers $s in fluid communication with no more
than one discharge port at a time, and each of the discharge
ports is in communication with at least one of the chambers at
all times.
Preferably, a first feed port directs a continuous supply
of a fifteen per cent by weight nitric acid solution, or first
solution, into the rotating resin filled chambers in fluid
communication therewith. Hydrogen ions are exchanged with
potassium ions bound to the resin to produce a second solution
of potassium nitrate and dilute nitrlo acid. The second
solution flows into a first discharge port, which is in fluid
communicatlon with the resin filled chambers in communication
with the first feed port; the second solution from the first
dlscharge port ls then dlrected to an adJacent second feed
port. The second solutlon ls then passed through the resln in
the chambers which are ad~acent to the chambers supplied with
the irst solution, and flows out of the ad~acent second
discharge port. The second solution flowlng from the second
dlscharge port has a hlgher concentration of potassium nltrate
and a lower concentration of nitric acid than the second
solutlon flowlng from the first discharge port.
The second solution from the second dlscharge port is
then dlrected lnto a thlrd eed port, through the resin ln the
2~2 ~3~
chambers which are adjacent to the chambers communicating with
the second feed and discharge port, and flows out of a third
discharge port. The second solution flowing from the third
discharge port is then directed to an ad~acent fourth feed
port, through the resin in the chambers filled with potassium
loaded strong cationic exchange resin, and flows continuously
out of a fourth discharge port. The solution of potassium
nitrate and dilute nitric acid leaving the fourth discharge
port is then neutralized with potassium hydroxide to convert
the remaining nitric acid to potassium nitrate.
Preferably, the direction of flow of the first and second
solutions is counter-current to the directlon of motion of the
rotating resin filled chambers. Thus, chambers, filled with
fresh potassium loaded strong cationic exchange resin, are
first contacted with the second solution which is fed through
the fourth eed port. Subsequently, the potassium loaded
resin is sequentially sub~ected to the second solution fed
from the third and second feed ports, with the second solution
being fed to the chambers progresslvely havlng a hlgher nltric
acld content and a lower potasslum nitrate concentratlon.
Thus, the potassium loaded resin will have been partially
converted to its hydrogen loaded or acid form when it is
contacted with the first solution of nitric acld provided by
the first feed port. The resin in the chambers communicating
with the irst eed port 19 substantially converted from the
potassium loaded form to the hydrogen loaded form by contact
d ~
with the first solution of nitric acid.
The chambers movlng from fluid communication with the
first feed port are subsequently moved into fluid
communication with a series of four wash water feed ports.
The rotating resin filled chambers are first moved
sequentially into fluid communication with the last, or eighth
feed port, of the four ports fed with wash water. The wash
water is initially fed to a fifth feed port, flows through the
resin filled chambers ln fluid communlcation therewith, exits
10 a fifth discharge port, is directed into a sixth feed port,
and 90 on, until the wash water containing the highest
concentration of potassium nitrate and nitric acid flows from
the elghth feed port continuously. Thus, the fifth feed port
directs substantially pure deionized water through the
15 hydrogen loaded resin to remove any resldual potassium nitrate
and nitrlc acid on the resin in the chambers rotating into and
out of fluld communication therewith. The "cleanest" wash
water is used on the "cleanest" resin last, while the
"dlrtiest" wash water 18 used on the ~dirtiest" resin first.
20 In a preferred process, the dilute wash solution of potasslum
nitrate and nitrlc acid leaving the eighth dlscharge port is
used to dilute a fifty-two per cent by weight nitric acid
solution to form a fifteen per cent by weight nitric acld
solution, which is fed to the first feed port.
The resin fllled chambers containing washed hydrogen
loaded resin are then sequentially moved into and out of fluid
7 `~ ~ ~,S~
communication with a ninth feed port, which is fed a
continuous stream of air. The air forces out any residual
wash water on the resin in the chambers in communication with
the ninth feed port. The resin filled chambers are then
sequentially moved into and out of communication with six feed
ports supplying a third solution of potassium chloride, or a
fourth solution of potassium chloride and hydrochloric acid;
the fourth solution is produced by the conversion of the
hydrogen loaded resin to the potassium loaded form.
Preferably, the third solution contains about ten to
twelve per cent by weight potassium chloride, and is fed into
a tenth and an eleventh feed port. The fourth solution
produced in the chambers communicating with the tenth and
eleventh feed ports is then directed from tenth and eleventh
discharge ports into twelfth and thirteenth feed ports, and
subsequently rom twelfth and thirteenth discharge ports to
fourteen and fifteenth feed ports. Chambers filled with
hydrogen loaded resln movlng from communlcatlon with the ninth
feed port, or alr ln~ectlon port, are flrst moved lnto and out
of communlcatlon wlth the flfteenth feed port, and are
sequentially moved lnto and out of fluid communicatlon wlth
the flfteenth, fourteenth, thlrteenth, twelfth, eleventh and
tenth feed ports.
The chambers contalnlng potasslum loaded resln are then
seguentlally moved lnto and out of fluld communlcatlon wlth
four wash water feed and dlscharge ports, referred to as the
'~2'~9~
sixteenth, seventeenth, eighteenth and nineteenth feed and
discharge ports. Wash water fed to the sixteenth feed port is
sequentially fed from the slxteenth discharge port through the
ad~acent chambers via the seventeenth, eighteenth and
nineteenth feed ports. Wash water fed to the sixteenth feed
port is deionized, and substantially pure, while wash water
enterlng the nineteenth feed port contains potassium chloride
and hydrochloric acid rinsed from resin in the preceding
chambers. The wash water leaving the nineteenth discharge
port is preferably used to dilute incoming concentrated
potassium chloride solutions, or it can be neutralized and
disposed of.
A twentieth feed port dlrects alr lnto chambers moving
from communication with the sixteenth feed port, and forces
wash water from the resin.
Thus, in the preferred apparatus, each resin filled
chamber i9 sequentially subJected to feed solutions of nitric
acld, wash water, air, potassium ahlorlde, wash water, and
alr.
BRIEF DESCRIPTION OF THE DRAWINGS
Flgure 1 ls a block dlagram lllustratlng the process of
the present lnventlon.
Flgure 2 ls a front elevatlon vlew, with parts broken
away, of the preferred apparatus for performing the method of
the present lnvention.
Figure 3 is a cross-sectional vlew taken along llne 3-3
11
of Flgure 2.
Figure 4 is a cross-sectional view taken along line 4-4
of Figure 3.
Figure 5 is a cross-sectional view taken along line 5-5
of Figure 2.
Figure 6 is an exploded perspective view of parts of the
apparatus shown in Flgure 5.
Figure 7 is a cross-sectional view with parts broken away
taken along line 7-7 of Figure 5.
10Figure 8 is a cross-sectional view taken along line 8-8
of Figure 5.
Figure 9 is a cross-sectional view taken on line 9-9 of
Figure 5.
Figure 10 illustrates a plant layout for performing the
15preferred embodiment of the present process.
Figure 11 is a schematic view illustrating a process for
producing potassium nitrate using the apparatus of Figure 2.
DETAILED DESCRIPTION OF T~E INVENTION
The advanced separation device, ASD, disclosed ln U.S.
20Patent Nos. 4,765,276 and 4,522,726, has been used in various
conflguratlons, and with numerous startlng materlals to
produce valuable products on a small scale. For example,
Berry et al, in U.S. Patent 4,704,263, disclose the production
of potasslum phosphates by lon exchange using the ASD. The
25process involves passing a phosphate salt solution through a
catlon exchange resin loaded with potasslum to form potasslum
12
- ~ ~ ?J ~
phosphate, and after washing out residual salts, regenerating
the cation exchange resin by addition of a potassium salt.
Preferably, the potassium salt is potassium chloride and the
ion exchange resin is a strong cation exchange resin.
Phosphoric acid was not directly applied to the potassium
loaded cation exchange resin to produce potassium phosphate.
In related U.S. Patent 4,704,262, ~erry d$scloses the use
of the ASD to produce dialkali metal phosphates by ion
exchange. The process involves passing an ammoniated
phosphate solution through a weak cation exchange resin and
the alkaline metal formed, so that the ammonium is exchanged
with the alkaline metal to produce an ammonium loaded resin
and a dialkali metal phosphate. In particular, the ammoniated
phosphate solution is prepared by reacting ammonium with a
water soluble phosphorus source, such as monocalcium phosphate
or phosphoric acid, and the alkali metal salt is potassium
8ul fate, potassium chloride, sodium sulfate, sodium chloride,
or sodlum carbonate.
It was desired to use the ASD in a similar fashion to
Berry et al. to produce potassium nitrate via ion exchange.
Slnce additlon of nitric acid to a cation exchange resin was
known to be dangerous, initlal experiments involved the
conversion of neutral nitrate salts to potassium nitrate via
ion exchange.
25Experiments were performed to determine if calcium
nitrate could be converted to potassium nitrate by passage of
13
3~s~
a calcium nitrate solution through a potassium loaded strong
cation exchange resin. The overall reaction is:
2Ca( NO3 ) 2 + 2RK - - - > 2KNO3 + Ca( NO3 ) 2 + R2Ca .
About ninety-five per cent of the calcium bound to the
resin, but about five per cent calcium nitrate remained
combined with the potassium nitrate produced. The calcium
nitrate is difficult to separate, requiring substantial
additional effort and expense to produce pure potassium
nitrate. Further, upon attempting to regenerate the column
from its calcium loaded form to its potassium loaded form, the
exchange capacity of the resin was substantially reduced.
Thus, the following reaction proceeded to the right only about
sixty per cent:
R2Ca + 2KCl - - - > 2RK + CaCl2 .
Attempts to pass sodium nitrate solutions through a
potassium loaded column were discouraged because of the
difficulty in obtaining hlgh yields of potassium nitrate.
Further, it was believed that the potasslum nitrate produced
would be mixed wlth large amounts of sodlum nltrate. Finally,
sodium nitrate is expensive in relation to other feed
materlals such as calclum nltrate, indicating that the process
would be expenslve as well as inefficient.
While it was believed that addltlon of nitric acid to a
strong cationi~ exchange resln might pose a fire or explosion
hazard, or could cause a substantial reduction in the useful
life of the ion exchange resin used, the failure ln the
oarller experlment~ to produce ~ vl~ble pot~e~ium nltrate lon
exchan~e productlon method left ~ need for ~ better mothod for
~roducln~ potas~lum nltrate. Thu~, s~dltlonal ex~rlments
were pQrforme~, ln whloh ~ oln~le f~xed aolumn wa9 f~lled wlth
a ~ot~081um loaded stron~ c~t~onla exch~n~e re~ln, 801~ under
the trade name DOWEX MONOSPHERE TG650C, ~nd a dilute solutlon
of nltric acld w~ pa~Qa throu~h the rosln. 6urprl~1n~1y,
th~ ~xchan~e ¢apeclty ~nd appearance of the re~n did not
a~poar to be neg~tlvely effeated by the nltrla ~cld, and hl~h
yl~ld~ of pota~slum nltrate reeulted. D~splte pr~dlatlon~
that nltrlo acld woul~ decay the ro~lns u~ed, ~urprleln~ly,
ther~ was no resln dQcay noted, and consequently resln decay
produot~ wer~ not noted ln the pot~81um nltr~te produced.
rùrthor, over the course of multlple ~xperlment~ wlth dilute
lS nlt~lo ~cld, no flr- or explo~lon occurred. ~herefore, lt was
dl-covored that pot~8slum nltrate could be produoed e~ly and
~t room t-mperature by pa~-ln~ nltrlc ~cl~ throu~h a pot~sslum
lo~d d otron~ c~tlonlc ~xohang~ re~ln.
Ur- o~ a eln~l- 1x d bed ~xchano- column r~qulred a
l~r~- ~mount o~ r~oln ln order to achleve ~r~ter th~n nlnety-
~lv ~or c-nt oonverd on of nltrla ~old to pota~lum nltrate.
In order to produce puro pot~sslum nltrate, lt 18 necessary to
neutr~llze tho excess nltrlc aold wlth pot~lum hydroxlde.
81nce ~otasslum hydroxlde le r~latlvoly expen~ive, the
com~et~tlvo a~v~ntage of producln~ pot~-~lum nltr~te vl~ ion
xch~n~e 1~ eub8tantl~11y reduced by th~ lnor~od c08t~
* Indicates a trade-mark throughout.
..
r~
involved in the purchase of large volumes of exchange resin to
increase column yields, or the purchase of large volumes of
potassium h~droxide to neutralize excess nitric acid.
Further, flxed bed exchange columns are not very
efficient, since the ion exchange process cannct be carried
out continuously. The flow of materials to the fixed bed must
be frequently interrupted so the resin can be regenerated.
Fur~her, a large amount of resln ls wasted in fixed bed
columns since the exchange zone in the fixed bed is relatively
small compared to the size of the column bed. Finally, as the
exchange zone nears the bottom of a column bed, the
concentration gradient between the potassium ions bound to the
resin and the hydrogen ions in the nitric acid feed solution
has substantially diminished, which results in a reduction in
the exchange efficiency.
The sùrprislng dlscovery that potassium nltrate could be
produced by passing a nltrlc acld solutlon through a potassium
loaded strong catlon exchange resln, wlthout causlng a flre or
exploslon, or rapldly decompo~lng the resln, suggested that
the ~uccess of Berry et al ln applylng the ASD to small scale
productlon of alkall metal phosphates, such as potasslum
phosphate, may posslbly serve as a model to produce potasslum
nitrate vla lon exchange. Early experlments wlth a laboratory
scale ASD or ion separation exchange process (ISEP) system
produced by Advanced Separation Technologies, Inc. of
Lakeland, Florida, were very successful; high yields of
16
r~
potassium nitrate were produced on a continuous basis from
continuous flows of nitric acid, potasslum chloride, and wash
water.
Therefore, an industrial scale ASD, or ISEP, was
constructed. The industrial scale ASD was essentially an
enlarged version of the laboratory scale ASD. However, the
industrial scale ISEP leaked so severely upon pressurization
of the feed solutions, that it was not possible to use the
apparatus effectLvely. Therefore, a critical valve, to be
described in more detail later hereinbelow, was redesigned,
and successfully tested; this resulted in a modified ASD
capable of producing over 5000 tons per year of potassium
nitrate from contlnuous supplles of nltrlc acld and potasslum
chloride.
With reference to Figure 1, a block diagram illustrating
the overall process of the present invention is provided. The
resin filled chambers of the ASD apparatus are represented in
Flgure 1 by different zones. In practice, each of the resin
fllled chambers is cycllcally passed through the different
zones. Resln fllled chambers passlng through the potassium
nltrate productlon zone are fllled with resln ln the potasslum
loaded form, and a dllute nltrlc acld solution 2 ls passed
through the resln. The nltrlc acld solutlon 2 is drawn from
tank 4 by pump 6. The nltrlc acid solution 2 in tank 4 ls
provided by combining a concentrated nltrlc acid source 8 with
productlon wash water. A portlon of the dllute nitric acid 2
~ ~J' ~
is combined with concentrated nltric acid solution 8 at static
mixer 10. Thls ensures that a homogeneous nltric acid
solution 2 ls provlded to the potasslum nltrate productlon
zone. Preferably, the nitrlc acid is obtained as a fifty-two
per cent by welght solutlon, and dlluted to approxlmately
fourteen to sixteen per cent by weight nitric acid before it
is pumped into the ASD.
It has been discovered that use of nltrlc acid at a lower
concentratlon reduces the risk of flre or explosion, as well
as reduces or substantially elimlnates $ast decomposition of
the resin matrix. It is preferred that the nitric acid used
be diluted below twenty-three per cent; at higher
concentrations, clogging of the columns was noted, which is
belleved to be due to preeipitation of nitrate salts in the
resin ehambers.
The potassium nitrate solutlon 12 leaving the potassium
nitrate produetion zone preferably eontalns about fifteen per
eent by welght potassium nltrate and about 0.5 per eent by
weight nltrle aeld. Preferably, thls eoneentration of
resldual nltrle aeld 18 neutrallzed wlth potasslum hydroxlde.
The amount of potasslum hydroxlde used to neutrallze the
exeess nltrle aeld 18 preferably small enough to make the
present proeess eeonomleally eompetltlve wlth the prior art
processes for produeing potasslum nltrate.
The ehambers leavlng the potasslum nltrate produetlon
zone then pass to a potasslum nltrate produetlon wash zone.
18
7 ~ ~ ~
Wash water 14 is continuously passed through the resin in the
chambers passing through the production wash zone to carry
away residual potassium nitrate and nitric acid remaining on
the resin. The effluent 16 from the production wash zone is
directed to tank 4.
Chambers passing from the production wash zone then pass
to the drain zone, where air from air source 18 forces any
residual wash water from the resin in the chamber to prevent
cross zone contamination. Preferably, the water drained from
the resln is directed to a sewer 20, after any necessary
environmental treatment steps are performed.
Preferably, the wash water 14 is deionized. Chambers
leaving the drain zone which follows the production waæh zone,
then enter a potassium chloride adsorption zone. The resin,
whlch has been converted ln the potasslum nitrate production
zone to its hydrogen loaded or acid form, is regenerated to
its potasslum loaded form ln the potasslum chlorlde adsorption
zone. A potasslum chlorlde ~olutlon 22 18 drawn from a tank
24, and fed by pump 26 lnto the chambers passlng through the
potas~lum chlorlde adsorptlon zone. Preferably, the potasslum
chlorlde eed 801utlon 22 is diluted in feed tank 24 to
approxlmately a ten to fourteen per cent by weight solution of
potasslum chloride wlth adsorptlon wash water exlting the
potassium chloride adsorption zone. The exchange of potasslum
ions for hydrogen ions bound to the resin in the potassium
chlorlde adsorptlon zone results ln the productlon of
19
hydrochloric acid. Preferably, the hydrochloric acid effluent
28 can be used in other chemical processes~ or neutralized
with lime before disposing of the solution.
Chambers leaving the potassium chloride adsorption zone
are then passed to an adsorption wash zone. Wash water 14 is
passed through the chambers in the adsorption wash zone to
remove residual potassium chloride and HCl. The effluent 30
from the adsorption wash zone is preferably combined in feed
tank 24 with the concentrated KCl feed solution 32; this
increases the efficiency of both KCl and water use. Finally,
chambers leaving the adsorption wash zone enter a drain zone
where air 18 forces any remaining water on the potassium
loaded resin out of the chambers to prevent cross zone
contamination. The air and water mixture 34 are forced from
the chambers to a drain or sewer 36.
Referring now to Figure 2, there is shown a preferred
apparatus 40 for continuously producing potassium nitrate via
lon exchange in accordance wlth the hereln dlsclosed method.
Apparatus 40 ls posltloned wlthin an access framework 42, and
comprises a dlsc-shaped rotating carousel 44, which supports
a plurality of chambers 46, some of which are at the periphery
of the disc-shaped rotating carousel 44. A plurality of
generally radlally arranged feed hoses 48 deliver liquids to
a feed distribution valve 50, and feed hoses 52 conduct
liquids from the valve 50 to the chambers 46. Hoses 54
dellver llqulds from the chambers 46 to a dlscharge
' S~ ~
distribution valve 56, and hoses 58 conduct liquids from the
discharge distrlbutlon valve 56. In a preferred embodiment,
there are thirty chambers 46, the same number of feed hoses 52
and the same number of hoses 54. There are twenty of the feed
hoses 48 and twenty of the hoses 58. As will be explained
hereinbelow, some of the hoses 58 will be the same as or are
connected to some of the feed hoses 48, to effect recycling of
discharge liquid.
A control apparatus 60 is provided, and includes
monitoring and control devlces and circuits for operating the
apparatus 40 on a continuous basis. Also shown in Figure 2
are storage tank 62 for aqueous potassium chloride and storage
tank 64 for nitric acld; tanks 62 and 64 are each connected by
hoses to one of the feed lines 48.
lS Flgure 3 shows carousel 44 with chambers 46 thereon, and
with eed hoses 48 connected to them. Note that some of
chambers 46 are located lnwardly of the peripheral chambers
46. Feed hoses 48 are also connected to these inner chambers
46. Also shown ln Flgure 3 18 a shaft 66, whlch 18 conneated
wlth carousel 44, so that rotatlon of shaft 66 rotates
carousel 44, and vlce ver~a.
Referrlng to Figure 4, there is shown a cross-section of
carousel 44 and a chamber 46, chambers 46 are preferably made
of high denslty polyethylene. Feed hoses 48 enter through the
top 68 of chambers 46 and dlscharge feed liquid into the
ahamber 46, where it passes through an upper containment
21
screen and ~upport pl~te 70. Wlthln ch4mb~r 46 thore i8
charge or body 72 of a strong c~tlonlo exchange r~sln, 8uch as
th~t herelnelbove mentlone~. Above the bottom 74 of ah4mber 46
18 ~ ~upport plste an~ a lower contalnment screen 76, upon
whioh re~ts reoln 72. Pr~forably, the ~cre~n~ ~re m~de of
polypropylene, ~nd the 8Upport plate8 are m~de of polyvinyl
ahloride, lt 18 preferred that su~ablo g~ket~ be loc~ted
b~tween each of the8~ separ~te part~ wher~ver they sre Joined.
A ~referred gasket materl~l 18 sold under the trade name
Hy~alon, and 1~ sold wlth A8Ds ~vallable from AS~ Inc. o~
Lakel~nd, Florld~. Connected to bottom 74 of each chamber 46
~re ho~eE~ 5~. Also shown in Fi57ure 4 1~ ~ drlve motor 78.
Motor 78 ~rlves ~ plnlon 80, whlch 18 ln me~h with a r1n~ ~ear
82. As wlll be ~precl~ted, rotatlon of c~rou~el 44 may b~
o~ct~d ~y other mechanl~ms than th~t shown.
~eferrlng now to Flgure 5, there 1~ shown ~ cross-sectlon
o~ dl~ah~rge dlstrlbutlon v41ve 56, lt beln~ und~r~tood that
th- aon~tru~tlon o~ the feea dletrlbutlon valve 50 is
~ubetantially th~ oame, 81thou~h v-lve 50 18 po~ltloned ln
~pp~r4tu~ 40 so ~ to be lnverte~ wlth resp~ct to valve 56.
A pedestal 90, whlch ls of hollow conf~uratlon as shown,
rests on ~ ba~e 92. At lts upper end, pedestal 90 18 provided
wtth 4 serles o~ radl~ extendln~ ~butment plate~ 94 (see
~l~o ~igure 6) ~na the~e ~upport an lnner ~nnul~r ~l~te 96 ~n~
4n outer ~nnul~r plate 98, there beln~ an annulsr sp~ce
between the ~l~tes 96 ~nd 98 Q8 shown ln Fl~ure~ 5 ~nd 6.
22
Extending downwardly ln alignment with the pedestal 90 is
a drive shaft 100 having attached to it, as by a suitable
keyway 101, a sprocket 102, located above the valve 56.
An inner valve housing ring 104 ls provided with a lower,
lnwardly extending flange 106, and an annular series of bolts
108 secure flange 106 to the inner annular plate 96. Inner
housing ring 104 is provided with an outwardly extending
flange 110 at its upper end.
An outer housing ring 112 is concentric with inner
hGusing ring 104 and has an outwardly extendlng flange 114 at
its lower end; an annular series of bolts 116 secures the
flange 114 to the outer annular plate 98. At its upper end,
the outer housing ring 112 has an inwardly dlrected flange 118
which is ln spaced, opposing relationship to the flange 110.
As shown ln Figure 6, there is provided an annular body
120 having a radial width substantially the same as the space
between the inner annular plate 96 and the outer annular plate
98. The body 120 is provlded wlth radially extending slots
122 whlch each recelves one of the gusset plates 94. An
annular serle8 of L-shaped passages 124 are provided in the
body 120, and the body 120 will be seen to have at lts top an
outwardly extendlng annular flange 126 and an lnwardly
extendlng annular flange 128. The vertlcal part 124A of the
passage 124 extends to the upper surface 130 of the body 120:
~ody 120 18, as shown Flgure 5, of T-shaped vertlcal cross-
sectlon.
23
3 ~ d~
On the upper surface 130 of body 120 there rests an
annular crown plate 132. Crown plate 132 occupies the space
above the body 120, and beneath the flanges 110 and 118 of the
inner housing ring 104 and outer housing ring 112,
respectively, and is between outer housing ring 112 and inner
housing ring 104.
As shown in Figure 6, the bottom surface of the annular
crown plate 132 has an annular series of evenly spaced ports
134. These ports 134 are each in fluid communication with a
nipple 136, which extends upwardly from the annular crown
plate 132. The number of ports 134 and their circumferential
extent are such that at any given moment, each of the ports
134 is in fluid communication with at least one of the L-
shaped passages 124. In a preferred embodiment, the centers
of thirty ports 134 are separated by 12 lncrements, so that
twenty of the L-shaped passages 124, which are distributed at
18 intervals about the annular body 120, will always be in
fluid communiaatlon with at least one of the ports 134.
As shown in Flgure 7, the sprocket 102 has indentations
138 in it8 outer end which engage the nlpples 136, in spaaed
groups o three, 80 as to drive the nipples 136 and the
annular crown plate 13Z inside o valve 56.
The lower, horlzontal portions 124B of the L-shaped
passages 124 are each in fluld communication with a hose 58.
Hence, fluid may pa88 into or out of nipples 136 to or from
hose 54, into or out of the port 134, thence into or out of
24
passage 124 in the body 120, and thence outwardly or lnwardly
through hose 58.
To avold leakage, as shown in Figure 6, an annular outer
bladder 140 underlies the flange 126, and an annular inner
bladder 142 underlies the flange 128. The bladders 140 and
142 will be seen in Figure 5 to substantially occupy the
spaces between the inner annular plate 96, the outer annular
plate 98, the inner housing ring 104, the outer housing ring
112, the vertical portion of body 120 and the flanges 126 and
128 of body 120. Pneumatic pressure is provided in bladders
140 and 142 to urge the upper surface 130 of body 120 against
the lower surface of the annular crown plate 132. Should any
lea~age occur, it will be collected by a series of bores 144
extending through the inner housing ring 104, the bores being
in fluid communication with nipples 146, which enable the
bores 144 to be connected with a collection conduit 148, a
drain conduit 150 being connected thereto.
Pneumatlc pressure can be adJusted in bladders 140 and
142 to mlnlmize wear of the valve component; suitable gauges
and controls are preferably provlded to monltor and adJust
bladder pressure. If bladder pressure is too low, leakage
rom valves 50 and 56 wlll occur, and llqu:Ld will drain from
conduits 150. Preerably, the inner bladders are malntalned
at 75 PSI pressure, and the outer bladders are malntained at
70 PSI, with maximum recommended pressures belng 105 PSI and
100 PSI, respectlvely. Overinflation of the bladders will
cause excessive torque to be required to rotate plate 132 in
the valves 50 and 56. This could result ln rupture of the
bladders, faster wear of the valves, or damage to the motor
and drive mechanism.
In a preferred embodiment, the drive mechanisms are
protected by a high torque lnterlock, which will turn off the
drive motor and nitric acid and potassium chloride feed
solutions when excessive torque is encountered. Preferably,
the speed controller for motor 78 ls located inside the
control apparatus 60. A preferred speed controller is sold
under the name Speedstar JR; lt ls a varlable frequency drive,
avallable from Electrical South Inc. of Greensboro, North
Carolina, and requlres a 230 volt single phase power supply,
converting lnput voltage to a 460 volt 3 phase output with a
controlled frequency of 0-60 Hz.
In Figures 5 and 8, there will be seen a base plate 152
for pedestal 90, whl~h rests on the base 92. An ear 154
e$tends from pedestal 90, and 18 conneated to a hydraulia pump
and motor 156 through piston 157. A seaond ear 158, having a
810t 160 thereln, extends from the base plate 152, and a bolt
162 passes through the slot 160. Bolt 162 may be loosened to
permlt rotational movement of the pedestal 90 by the motor
156. This 18 effeated in order to obtaln ad~ustment of the
valve 56, 80 that vertiaal parts 124A of passages 124 ln valve
56 are vertiaally allgned with vertlaal parts 124A of passages
124 ln valve 50. Thus, llqulds travelllng from a vertlaal
26
part 124A in valve 50, through a chamber 46, and lnto the
vertical part 124A in valve 56 which is in vertical alignment.
The effect of this ad~ustment is to annularly displace the
pedestal 90, body 120, inner housing ring 104 and outer
housing ring 112.
In Figure 7, there is shown the annular series of hoses
58, each pair of which is held by a support plate 164. There
may be seen, also, the annular flange 118, the annular row of
nipples 136, and inwardly thereof, the annular flange 110.
There is also seen the sprocket 102 with indentations 138
engaging spaced groups of three nipples 136. Also shown is
the collection conduit 148, and shaft 100.
Flgure 9 dlscloses the plates 164, hoses 58, and the
radially extending gusset plates 94 extending outwardly from
the pedestal 90. Also shown is the shaft 100.
As noted above, valves 50 and 56 are substantially
modlfied from previous ASD valves due to severe leakage
problems encountered when using the prior art valves. Valves,
such as 50 and 56 whlch lnclude body 120 have superior leak
reslstance: thls ls due to the unitary constructlon of body
120, whlch is less likely to have its shape distorted by the
rotation of crown plate 132. Further, slots 122 snugly fit
over gusset plates 94 to prevent rotational slippage of body
120 in valves 50 and 56.
Hoses 58 are connected to the portlon of body 120 whlch
pro~ects from between inner and outer annular plates 96 and
98 In order to re~uoe the pos~lble ~lexln~ or bendln~ of the
proJect~n~ port~on of body 120 upon connectlon of hoses 58 to
the horl~ont~ rt 12~B o~ ~-shaped p~ssa~es 12~, ho~es 58
~re prefer~bly permanently attaohed to passa~es 124, and
reln~orced wlth ~lates 164 Oulck rele~se connectore 166
enable r~p~d connection and dl~conn~otlon of extension~ of
hose~ 58 w~thout ~tre~elng body 120, thereby resultin~ ln B
sur~rlslngly ~mproved le~k reslst~nt ~alve aonstructlon
~his v~lve constructlon allows ~or lar~e sa~le lndu~trlal
~roductlon of the pot~lu~ nltrats throu~h contlnuous oontact
wlth tron~ c~tlonlc exoh~nge resln wlthout l~ak~e of nlt~lc
8cid ~eed ~olutlon or hydroohlorio aold disch~r~e ~olutlon
Preferably, body 120 i8 molded ~rom ~ ~olld pl~tlc,
which 18 cap~ble of resi~tin~ corrosion by the proce~s
ro~atant~ 8n~ product~ A pr-ferre~ mat~rl-l for formin~ body
120 1- high ~n~ity poly thylene ~s~nles~ ~teel ~e known to
roelot nltrlc acid, but hydroohlorlc ~cla produced in the
~ proc--~ lo ~nown to corrod~ ~t~lnlo~ ~t-el~ Thore~or~, in a
pre~erred mbod~mont, rot~tln~ arown pl-te 132 i8 formed of an
alloy ~old under ~he trade n~me Haetolloy "C22", sold wlth
A8D~ ~vall~ble from A8T Inc of Lakelan~, Florid~ Oth~r
m~ter~al~ may b~ u~ed, but may wear out f~ster The be~rln~s
and other part~ of valve~ 50 ~nd 56 ~re ~ref~rably formed of
~olypropyl-ne, ~nd chlorlnated polyv~nyl chlorlde
Prefe~ably, DOWEX MONOsPHERE TG650C* stron~ aatlon
~xch~n~e re~ln 1~ ~80~, whlah h~s a p~rtlale ~ of 20-40
;,.. .
~ ,~
~ '~ ,f,;J ~
U.S. Standard Mesh. Preferably, chambers 46 are sufficiently
large to hold a charge of 4.55 cubic meters of resin, and have
enough space to allow for resin expanslon. The perforated
resin support plate preferably has a 60 U.S. Standard Mesh
screen thereon to contain the resin in the chambers. Note
that, while the DOWEX MONOSPHERE TG650C resln ls preferred,
any other strong cation exchange resin capable of producing
potassium nitrate upon contact wlth nitric acid solution, is
contemplated as being equivalent. Although individual
chambers are used in a preferred embodiment, a large single
chamber, divided into compartments, may be used in place of
the separate chambers. Further, the number of compartments
and feed ports may be changed. The preferred chambers are 61
cm ln diameter, having a resin bed depth of 61 cm, and allow
for resin expansion of 15 cm.
As one of skill in the art will readily appreciate, a
variety of procedures can be followed to optlmize operational
parameters for apparatus 40. Further, a variety of
modlflcations can be made to the apparatus to help ensure that
the apparatus ls set up for and malntalned at peak efflciency.
In a preferred embodlment, valves 50 and 56 are kept in
the same relative rotational positions with each other, in
order to keep the zones of valve 50 synchronlzed wlth the
zones of valve 56; mlsallgnment of the valves may cause cross-
leakage ln the system. Therefore, lt ls preferred that anallgnment device (not shown) be utillzed to asslst ln the
allgnment of the valves 50 and 56. For example, ln order to
keep the fixed vertical passages 124A of valves 50 and 56 in
vertical alignment, alignment indicator lights are preferably
provided to assist in monitorlng valve alignment. Attached to
valves 50 and 56, on crown plate 132, are fixed two magnetic
pick-ups spaced at 180, which activate a sensor located on
the fixed component of the valve. The sensors transmit a
slgnal to the indicator lights when they are ln alignment with
the magnetic pick-ups. When the valves 50 and 56 are in
perfect alignment, the indicator lights for the upper and
lower valves will light simultaneously. If the valves are out
of allgnment, the lights wlll not be activated simultaneously.
In order to adJust allgnment of the valves, the necessary
connections, such as bolt 162, are loosened, and pedestal 90,
along with the components of valve 56 attached thereto, is
rotated to align the vertical parts 124A of passages 124 in
valve 56 with their corresponding parts in valve 50. Close
vertlcal alignment o valves 50 and 56 is generally preferred
for a carousel rotation rate of approximately fifty minutes to
one hour per rotation; faster carousel rotation rates may
requlre that vertlcal parts 124 in lower valve 56 lead the
correspondlng parts ln valve 50. As one of skill ln the art
can appreclate, the rotatlon rate of the carousel can be
greatly increased or reduced depending upon solution flow
rates and other process requlrements ln order to optlmlze the
performance of apparatus 40.
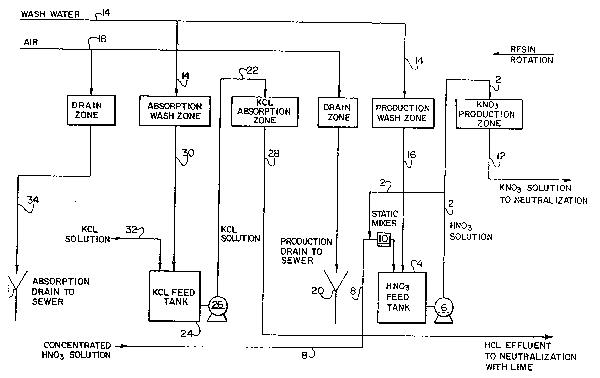
With reference to Figure 10, a preferred plant layou~ Q
illustrated, which uses an apparatus, such as apparatus 40, to
continuously produce potassium nitrate via contact of nitric
acid with a strong cationic exchange resin. Solid potassium
chloride is fed to hopper 200 where conveyor 202 directs them
to a dissolution vessel 204. Solid potassium chloride is
combined with water from line 206 in vessel 204, and stirred
by an agitator 208, which is driven by motor 210. Pump 212
directs the concentrated potassium chloride solution to filter
214.
Preferably, the concentrated KCl solution in line 213
contalns about twenty per cent potassium chloride by weight.
The filtered potassium chlorlde solutlon in line 216 is then
transferred by pump 218 to surge tank 220. Potassium chloride
solution in tank 220 ls then directed to a potassium chloride
eed tank 222 by pump 224. A sample point 226 enables samples
to be drawn from potassium chloride solution line 216, so that
its concentration and purity can be monitored. A control
valve 228 regulates the amount o potassium chloride solution
ln llne 216 belng ed to tank 222. A control loop 230 is
provlded, and preferably lncludes a 1OW lndlcator quantity
totallzer, a transducer to convert pneumatlc slgnals to
electronlc signals, and a separate 1OW control whlch uses
pneumatlc pressure to regulate control valve 228.
Potasslum chlorlde solutlon 216 is diluted in tank 222
through combination with adsorption wash effluent in line 232.
J ~
Preferably, potassium chloride feed solution in line 234 is
directed from tank 222 to ports 6 and 7 of the potassium
adsorption zone in Figure 11. Note that adsorption wash
effluent 232 contains dilute potassium chloride and very
dilute hydrochloric acid. Preferably, the potassium chloride
feed solution in line 234 contains approximately twelve per
cent potassium chloride by weight. As will be appreciated by
one of skill in the art, potassium chloride solutions of
greater and lesser concentration may be used.
A sample point 236 is provided to withdraw samples, and
a separate pneumatic control loop 238, having similar parts
and configuration to loop 230, is provided to control a
solutlon recirculation loop 240; this ensures that a
homogenous potassium chloride solution, having a stable
concentration, is directed to apparatus 40.
Fresh deionized water is fed through line 242, and is
stored ln a wash water tank 244. Preferably, tank 244 holds
approxlmately 1300 gallons of water for a plant which produces
approximately 5000 tons or more of potasslum nitrate per year,
and a suficlent quantlty of water 18 malntained ln the tank
through use o a float valve 246. Wash water ls then dlrected
by pump 248 to lines 250 and 252. Line 250 dlrects water to
the potasslum adsorptlon wash zone whlch lnltiates at port 2
in Flgure 11. Line 252 dlrects water to a potasslum nltrate
productlon wash zone lnitiated at port 13 in Figure 11.
~ine 254 carries a solutlon of potasslum nltrate produced
32
~ ~ut~ J'i$1 ~ ia-~
in apparatus 40 from port 20 in Figure 11 where it i8 directed
to a surge tank 2~6. Potasslum hydroxlde ls stored in tank
258 and pumped through line 260 to tank 256, where it is used
to neutralize residual nitric acid in the potassium nitrate
solution. Pump 262 directs neutralized potassium nitrate
solution (the reaction of potassium hydroxide and nitric acid
yielding a solution of potassium nitrate only), to a storage
or surge tank 264. Preferably, pump 266 then directs the
potassium nitra~e solution to a subsequent crystallization
procedure. A pneumatic control loop 268 regulates valve 270
and valve 272 to ensure that the proper amount of potassium
hydroxide solution from tank 258 is added to tank 256.
Line 274 carries production wash effluent from port 16,
and directs it to tank 276 where it is combined with
concentrated nitric acid from line 278. Flow of nitric acid
in line 278 i8 regulated by control valve 280 which interacts
with control loop 282. A density meter 284 interacts with
control loop 282 for a purpose to be described below.
Nitrlc acid in line 278 is combined wlth a mixture of
nltrlc acld and productlon wash water from tank 276, which is
provided by llne 286 in static mixer 288. Pump 290 circulates
solutlon from tank 276 through line 286, through static mixer
288, and lnto line 292 to ensure that a homogenous nitric acid
solution is directed into line 294. Density meter 284
measures the density of the nitric acid solutlon passing
through llne 286, and interacts with control loop 282 to
~ ~ 7~ J ~
thereby control the amount of solution flowing through lines
278, 286, 292 and 294.
Control loop 298 monitors and ad~usts for the volume of
nltric acid solution in tank 276. Excess production wash
effluent in line 274 is directed to a drain 300 by llne 302.
The production wash effluent in line 274 contains very dilute
nitric acid and potassium nitrate. Preferably, the
concentrated nitric acid is provided as a fifty-two per cent
by weight solution and is diluted in tank 276 to an
approximately twelve per cent by weight solution. Nitric acid
in llne 294 is then directed to port 17 of the potassium
nitrate productlon zone ln Figure 11.
The reactlon is generally carrled out at ambient
temperatures, although some increase in temperature ls noted
ln static mlxer 288 as a result of diluting the nitric acid.
Preferably, the temperature does not lncrease beyond about
110 F in statlc mlxer 288.
In a preferred embodiment, tanks 222 and 276 are formed
of a plastlc materlal su¢h as those sold under the name
Nalgene. Preferably, feed llnes are formed of two inch
d~ameter polyvinyl chloride plplng, although any other
materlal or slze tublng or plplng may be used, provlded lt
does not interfer~ substantlally wlth the reaction process.
The electropneumatlc control loops can be replaced wlth other
mechanlsms capable of automatlcally monltorlng and adJustlng
solutlon concentratlons and flow, or the system can be run
34
$
manually, although the later alternative is inefficient in
comparison with automatic systems. A wash system (not shown)
is preferably provided to perlodically rinse off apparatus 40.
With particular reference to Figure 11, note that line
304 provides compressed alr to ports 1 and 12 via lines 306
and 308, respectively (Please note that the numbers given to
the ports is arbitrary). Nitric acid entering fixed feed port
17 passes into one or two chambers which are moving slowly
into and out of periodic fluid communication with port 17.
The solution of nitric acid contacts potassium loaded strong
cation exchange resin in the chambers to produce a solution of
potasslum nitrate, while reducing the concentration of nitric
acid. The solutlon of potassium nitrate and nitrlc acid flows
out of the chambers in fluid communication with input port 17
lnto discharge port 17, and is sequentially dlrected to feed
and dlscharge ports 18, 19 and 20.
As the nltrlc acld solutlon passes countercurrently to
the chamber movement through ports 17 through 20, the
concentration of nltrlc acld 18 reduced, whlle the
concentratlon of potasslum nltrate ln the solutlon ls
lncreased. Thus, freshly regenerated potasslum loaded strong
catlon exchange resln ln the chambers movlng lnto and out of
fluld communlcatlon wlth ports 20 ls contacted wlth a solutlon
havlng a relatlvely hlgh concentratlon of potasslum nltrate
and a low concentratlon of nltrlc acld.
Chambers passlng from the potasslum nltrate productlon
2~
zone pass to a production wash zone. Deionized water in line
252 is passed countercurrent to the chamber movement
sequentially through ports 13 through 16. The effluent from
discharge port 16 ~n llne 274 contains dilute potassium
nitrate and very dilute nitric acid which is then directed to
tank 276 where it is combined with concentrated nitric acid
from line 278. Thus, nitric acid in line 294 will generally
contain small quantities of potassium nitrate. Chambers
passing from the production wash zone pass into fluid
communication with air from line 308 through port 12 in a
production drain zone. Air from line 308 forces residual
potassium nitrate and nltric acid into discharge port 12 where
it is subsequently disposed of from drain line 310.
Chambers passing from fluid communication with port 12
pass into a potassium adsorption zone, in which the hydrogen
loaded resin formed in the potassium nitrate production zone
i8 regenerated to its potassium loaded form. A potassium
chloride solution in line 234 is directed into ports 6 and 7,
and dlscharges through discharge ports 6 and 7. The solution
discharglng from dlscharge ports 6 and 7 i8 preferably
comblned, and is then directed to feed ports 8 and 9.
Preferably, the solution discharglng from ports 8 and 9 is
combined, and fed to input ports 10 and 11. The concentration
of potassium chloride in the solution decreases as the
potassium chloride solution passes through the chambers in the
potasslum adsorption zone towards the productlon draln zone.
36
2~2 ~
The adsorption of potasslum on the hydrogen loaded resin
results in the formation of hydrochloric acid which ultimately
discharges from ports 10 and 11 into discharge line 312.
Preferably, the hydrochlorlc acid is utillzed ln other
chemlcal processes, or ls neutralized before dlsposal.
Chambers passlng from the potasslum adsorptlon zone then
move to the adsorption wash zone where excess potassium
chlorlde and hydrochloric acid are rinsed from the potassium
loaded cation exchange resin. Note that wash water from llne
250 passes countercurrently to the dlrectlon of chamber
movement through ports 2 to ports 5. Generally, feed
solutlons are passed downwardly through the resin fllled
chambers. However, lt is preferred that at least one feed
solutlon be dlrected upward through the chamber ln order to
redlstrlbute the resln ln the chamber. Thls avolds
channelllng and other negatlve chromatographlc separatlon
effects.
Note that the adsorptlon wash feed is preferably fed
upwardly throùgh dlscharge port 5 and feed port S. The
ad80rption wa8h zone effluent enters llne 232, where lt ls
subsequently used to dllute potassium chlorlde feed solutlon
ln tank 222. Chambers movlng from the adsorptlon wash zone
then pass lnto fluld communlcatlon wlth feed port 1, where alr
from llne 306 forces the resldual solutlon of dllute potasslum
chlorlde and hydrochlorlc acld lnto dlscharge port 1; the
dllute solutlon 18 then neutrallzed and sent to a sewer
37
through line 314.
While twenty input ports and twenty dischar~e ports have
been utilized in con~unction with thirty rotating chambers, it
is contemplated that the various production zones represented
in Figure 11 can be formed with as few as one input and
discharge port for each zone, although this wlll require some
modification of the valves. It follows that the number of
chambers may be increased or decreased, or that multiple
chambers may be replaced with a single chamber d$vided into a
plurality of compartments.
EXAMPLE 1
The following non-limiting example provides an actual
material balance for a potaQsium nitrate production plant
uslng an apparatus such as described above. Other methods,
materlals, and reactlons parameters than those descrlbed above
or below can be used in the practlce or testlng of the present
lnventlon. Table 1 below presents materlal lnput and output
data for an ASD, such as descrlbed above, havlng thirty
chambers fllled wlth a strong catlon exchange resln (DOWEX
MONOSPHERE ~G650C), which periodically rotate lnto and out of
fluld communlcatlon wlth twenty vertically aligned feed and
discharge ports; the solution flow pattern represented in
Flgure 11 was followed.
r r~ r~ _ ~ .a _ _ o ~c r' r' ~ 1
N _ a N N r N _ ~ ~ ~1
~1 ~ ~ ~ ~
__ _ ___ a '~^ ~ _ _
, , . _ . . . a __ ~ .~ .
~ii ;~ N ~5 ~ O~ O~ ~_ ~a ~ O rl ~ l
L~ N N _ ~S N N _ rl o- r o o
L~ ~ a ~ . 8 o ~ a a ~ ' ~ L~
Of particular significance ln Table 1 ls the row labled
"KNO3 PRODUCT," showing that 15.9 metric tons per day of
potassium nitrate were produced, with only 0.0068 metric tons
per day being lost from the production drain zone. This
5quantity of potassium nitrate was produced from 15.12 metric
tons per day of potassium chloride and 10.63 metric tons per
day of nitric acid. This represents approximately a ninety-
three per cent conversion of the nitric acid to potassium
nitrate, with only a 0.1 per cent loss of nitric acid through
10the production drain zone. The remaining seven per cent
nitric acid was subsequently neutralized with potassium
hydroxide.
Although the resin utilized in Example 1 lost some of its
color, no decrease in resin loading capacity was noted.
15Further, close examination of the resin beads continuously
used for six months showed the beads to have maintained good
sphericlty and strength. Thus, it appears that the resin may
be used for long perlods of tlme wlthout any substantlal loss
ln lts ablllty to reverslbly exchange potasslum and hydrogen.
20Please note that by ad~ustlng the concentration and flow
rates of the varlous feed solutlons, alterlng the amount of
resln ln the chambers, and adJusting the rotation rate of the
carousel, that higher percentage converslon of nitric acid to
potassium nitrate may be obtained.
25It i8 contemplated that the process descrlbed hereinabove
18 equlvalent to processes ln whlch the potasslum nltrate
~ 27`~
production zones, production wash zone, and production drain
zones are not stationary, and the resin filled chambers are
stationary, such that the feed and discharge ports are moved
into and out of fluid communication with the stationary resin
filled chambers. It is also contemplated that the continuous
ion exchange process of the present invention can be performed
by other apparatus, in which a plurality of stationary
chambers or columns, filled with a strong cation exchange
resin, are sequentially fed solutions of potassium chloride,
wash water, nitric acid solution, and wash water solution,
with air being in~ected into the columns following the
adsorption wash and production wash solutions. There can be
provided sufficient columns and control apparatus so that
there are at least six columns, with each of the six columns
undergoing a different step of the process than the other
columns simultaneously. In this way, continuous production of
potasslum nitrate from potassium chloride and nitric acid
could also be produced.
Thus, it has been discovered that potassium nitrate can
be easily and safely produced by contactlng a solutlon of
nltrlc acld wlth the potasslum loaded strong catlon exchange
resin, It is further posslble to achleved hlgh efflclency of
this reactlon wlth minimal resln volume through the use of a
contlnuous solid liquld contactlng apparatus, such as, but not
limited to, that described above.
From the above teachings, it ls apparent that many
41
~J ;) ~J 7 1~
modifications and variations of the present invention are
possible. It is therefore to be understood that the invention
may be practiced otherwise than as specifically described.
42