Note: Descriptions are shown in the official language in which they were submitted.
FIRE EXTINGUISHANT COMPOSITIONS,
METHODS AND SYSTEMS UTILIZING BROMODIFLUOROMETHANE
.:
REFERENCE TO RELATED APPLICATIONS
This application is a continuation-in-part of the -
5 following United States Patent Applications: Serial No.
419,132, filed on October 10, 1989 and entitled FLUOROCARBON
COMPOSITIONS FOR USE AS FIRE EXTINGUISHANTS; Serial No.
488,295, filed on March 2, 1990 and 0ntitled FIRE
EXTINGUISHING METHODS AND COMPOSITIONS UTILIZING
10 2-CHLORO-1,1,1,2-TETRAFLUOROETHANE; and Serial No. 439,738,
filed on November 21, 1989, and entitled FIRE EXTINGUISHING
METHODS AND BLENDS UTILIZING HYDROFLUOROCARBONS, the last
mentioned Application being a continuation-in-part of United ;
States Patent Application Serial No. 396,841, filed on August
15 21, 1989 and entitled FIRE EXTINGUISHING METHODS AND BLENDS
UTILIZING HYDROFLUOROCARBONS. ~ -
, . ..
BACKGROUND OF THE IMVENTION
Field of the Invention:
The present invention relates to the field of fire
20 extinguishant compositions, and particularly to extinguishant
mixtures including bromodifluoromethane. These compositions h
display a surprising efficacy in fighting fires, and also are ~-
characterized by low ozone depletion potential (ODP), low
toxicity and minimal decomposition during extinguishment. ~-
Description of the Prior Art:
Several properties are desirable for fire fighting ~ -
chemicals. In addition to efficacy for fire extinguishing,
' "' i 3 r~ ~
the compositions should be relatively inexpensive, readily
discharged onto the fire, and low in both toxicity and ozone
depletion potential. Various materials have been considered
in the past for use as fire extinguishants, and some are in
5 widespread commercial use. However, these chemicals generally
have drawbacks in respect to one or more of these desired
properties.
Numerous halocarbons have been reviewed for possible fire
extinguishant use. A comprehensive study was conducted at
lO Purdue University in the late 1940's, in which numerous
halocarbons were examined. Purdue Research Foundation and
Department of Chemistry, "Fire Extinguishing Agents, Final
Report Sept. 1, 1947 to June 30, 1950", discussed in Larsen,
E.R., "Mechanism of Flame Inhibition I: The Role of Halogen",
15 JFF/Fire Retardant Chemistry, Vol. 1 (February 1974). In a
first phase of the Purdue experiments, forty-six halocarbons
were tested for their capacity to render a heptane/air mixture
non-flammable. However, these tests were indicated in the -;~
report as not correlating with fire extinguishing efficacy. ; ~ -
20 Also, after this preliminary review, several compounds
. . ~ . . .; .-
including bromodifluoromethane performed poorly. Following
this initial test, certain compounds were selected to be -~
evaluated for suitability as fire extinguishants. The i~`
selected compounds were tested as extinguishants in terms of
25 efficacy at different temperatures and pressures and with .`;
dif~ferent flammable materials, use in binary mixtures,
stability, resistivity and toxicity. Materials not performing
well in the init~ial tests, including bromodifluoromethane,
were not among the several chosen to be tested for fire
30 extinguishing utility.
The efficacy of bromodifluoromethane is surprising from a
consideration of the prior art. The prior art teaches that
the order of effectiveness of the halogens for fire
extinguishing is ~r > Cl F, as stated for example in Ford,
in Haloaenated Fire Suppressants, ACS Symposium Series 16,
J! j
--3--
Washington, DC, 1976. Ford and others further teach that
adding a second atom of halogen produces a marginal additional
increase in effectiveness. Thf~se trends are apparent in the
fluoromethane series where the flame inhibiting properties
5 have been shown to be in the order CF3Br > CFC13 > CF2C12 >
CF3Cl > CF3H > CF4, as discussed by da Cruz, et al., in Bull.
Soc. Chim Bela. 97 1011 (1988). Hence, one would predict
that the replacement of Cl with H in Halon 1211 (CF2BrCl)
would produce a less effective agent, bromodifluoromethane,
lO CF2HBr. We have surprisingly found however that
bromodifluoromethane is a very effective extinguishing agent,
and in fact on a weight basis is superior to Halon 1211
(CF2BrCl) for the extinguishment of a variety of fire types.
d Also surprising is the low level of decomposition products i- -
15 HX formed during the extinguishment of fires by
bromodifluoromethane. As noted in the Fire Protection ~
Handbook (NFPA, 1981), decomposition of halogenated agents can ~- -
take place on exposure to flame or to surface temperatures,
and in the presence of H (from water vapor or the combustion
20 process itself), the main decomposition products including HF
and HBr. It is well known that bromodifluoromethane can
undergo alpha elimination of HBr at elevated temperatures, as -
described for example in US 3,210,430 to Knight, and one might
expect that the use of bromodifluoromethane for the
25 extinguishment of flames would be characterized by the ~ ~.
;~ production of high levels of HBr. In contrast we have found
that very low levels of HBr are formed during extinguishment.
For example, in the extinguishment of n-heptane pool fires
with Halon 1301 (CF3Br) or bromodifluoromethane, smaller
; 30 amounts of both HF and HBr are formed from the
bromodifluoromethane. ~ -~
The low toxicity of bromodifluoromethane is also somewhat -~
surprising. As noted by Clayton in Fluorine Chemistrr
Reviews, v. 1, 1967, the substitution of H for Cl in general
35 leads to increased inhalation toxicity. Hence one might
.
--4--
expect the toxicity of bromodifluoromethane to be much higher
than that of Halon 1211. Bromodifluoromethane is also known
to undergo hydrolysis to form HBr and difluorocarbene, CF2, as ~-
described in Hine and Langford, J. Amer. Chem. Soc.. 79, 5~97
5 (1957), and it might be expected that such behavior would also -
render bromodifluoromethane toxic. However, we have found
that the four hour LC50 values (the concentration of agent - -~-
required to cause death in half of the sample population) in
rats for bromodifluoromethane (CHF2Br) and Halon 1211 -
10 (CF2BrCl) are 108,000 and 131,000 ppm, respectively. Thus,
bromodifluoromethane is of approximately the same toxicity as
Halon 1211, and the concentrations required for extinguishment ;
with bromodifluoromethane are at levels safe to living
organisms.
The result of past studies such as the one at Purdue
University has been to direct the art to certain compounds,
; primarily bromochlorodifluoromethane, bromotrifluoromethane
and dibromotetrafluoroethane. The effectiveness of these
three compounds in extinguishing fires has been described in
20 United States Patent No. 4,014,799 issued to Owens.
Bromotrifluoromethane and bromochlorodifluoromethane, and
a few other compounds, have been extensively utilized as fire
extinguishing agents because of their cleanliness, relatively
low toxicity, moderate cost and effectiveness. There is
25 considerable concern, however, about the apparently high ozone
depletion potential of certain totally halogenated compounds `~
in commercial use, including bromotrifluoromethane and
,~ ; blromochlorodifluoromethane. These compounds are asserted by
some to be capable of destroying the earth's ozone layer,
30 which forms a protective shield against harmful ultraviolet
radiation. Clearly a need exists for an effective agent that "-
presents a reduced threat to the earth's protective ozone
layer.
The use of emulsified sludges of halogenated methane
35 hydrates as fire extinguishing agents is described in United
.'" ,~ ~ .
. . ':
` : `; : ; ~`l `
States Patent No. 3,106,530, issued to Glew on October 8,
1963. The hydrates are dispersed, with an emulsifier, in
either water or liquid halogenated methanes. Flame
extinguishing compositions are also discussed in United States
5 Patent No. 3,479,286, issued to Gambaretto. This patent
describes a two component system combining a completely ---
halogenated alkane and a chlorofluorohydrocarbon. Japanese
Patent No. 58078677 describes fire extinguishing compositions
which comprise three component systems including
10 bromotrifluoromethane, dibromotrifluoroethane and a cyanamide
derivative.
Other prior art references also fail to teach the use of
bromodifluoromethane as a fire extinguishant, and instead
teach away from this use. For example, numerous
15 bromomethanes, typically perhalogenated compounds, are
mentioned as fire extinguishants in each of United Kingdom
Patent No. 1,297,919, Poland Patent 60068, West German Patent
1,913,405 and United States Patent Nos. 2,837,891 and
2,885,450, but bromodifluoromethane is not included in any of -
20 these references. Instead, bromodifluoromethane is identified -~
in Japanese Patent No. 59221375 as useful in an aerosol
composition, and in United States Patent Nos. 2,639,301 and
4,810,403 as a refrigerant for air conditioning units. -
- There remains a need and demand for fire extinguishing -
25 agents which are highly effective and which have minimum ozone --
depletion potential and low toxicity. Preferably, such a
composition could be utilized interchangeably with current
flire extinguishing agents in existing equipment. Contrary to
the teachings of the prior art, we have discovered that
bromodifluoromethane and mixtures including
bromodifluoromethane are effective fire extinguishing agents
which satisfy the foregoing criteria.
~ '--
, r j ~ j
-6-
,.~, ,
SUMMARY OF THE INVENTION
Briefly describing one aspect of the present invention,
there is provided an effective and low ozone depleting method
for extinguishing a fire which includes introducing to the
5 fire a fire extinguishing concentration of
bromodifluoromethane or mixtures of bromodifluoromethane and a -~
compatible propellant. Representative propellants include
nitrogen, carbon dioxide, trifluoromethane, carbon
tetrafluoride, argon and mixtures thereof. The invention also -
lO includes fire extinguishant compositions comprising ~ `
bromodifluoromethane and a compatible propellant, which are
characterized by desirable fire fighting efficacy and other :~
advantageous physical properties, such as low ozone depletion .
potential.
,
In a further aspect of the present invention, there is
provided an effective and low ozone depleting method for
extinguishing a fire which includes introducing to the fire a
fire extinguishing concentration of a composition including a
mixture of bromodifluoromethane and at least one other
`20 fluorocarbon fire extinguishant. Representative fluorocarbon
: extinguishants include bromotrifluoromethane, `~
bromochlorodifluoromethane, chlorodifluoromethane, .
: chlorotrifluoromethane, heptafluoropropane,
1,1,1,2,3,3-hexafluoropropane, 1,1,1,3,3,3-hexafluoropropane,
25 pentafluoroethane, 2-chloro-1,1,1,2-tetrafluoroethane,
dibromodifluoromethane, dibromotetrafluoroethane, :~
chloropentafluoroethane, 2-bromo-1,1,1,2-tetrafluoroethane,
C2F6, C3F8 ~ C4F10 ~
; l-bromo-1,1,2,2-tetrafluoroethane,
1-chloro-1,1,2,2-tetrafluoroethane and mixtures thereof. The
invention also provides fire extinguishant compositions,
characterized by high fire extinguishing efficacy and low . .
ozone depletion potential, and comprising mixtures of
::~ bromodifluoromethane and at least one other fluorocarbon fire
''`' ~'`
'; .'~, ' ' '
. ~ ; ! ,. ;. . j
j: ~ ,,, , : 1 ,
extinguishant. Such mixtures may further include a compatible
propellant.
In particular embodiments the compositions of the present
invention have a phase equilibrium pressure of between about
5 45 psia and about 600 psig at 70F. The compositions in
certain embodiments comprise ozone depletion potentials of
less than about 3.0, and even below about lØ Other
embodiments of the inventive compositions consist essentially
of bromodifluoromethane and propellant, or of
10 bromodifluoromethane, propellant and at least one other
fluorocarbon fire extinguishant. The foregoing fire
extinguishants have low ozone depletion potential and good
fire extinguishing efficacy. -
The present invention is also directed to fire --
15 extinguishing systems charged with the foregoing
compositions. Also covered are methods for extinguishing
fires comprising discharging from a pressurized container into
the combustion zone of a fire, a fire extinguishing amount of
a fire extinguishant composition comprising any of the
20 compositions defined hereinabove.
It is an object of the present invention to provide
methods and compositions for extinguishing fires rapidly and
effectively, and with lessened depletion of the atmospheric
ozone layer.
Another object of the present invention is to provide fire
extinguishant compositions which include bromodifluoromethane,
alone or in mixture with one or more other fluorocarbon
clomponents! the bromodifluoromethane itself and the resulting
mixtures being surprisingly effective as fire extinguishants -
30 and having relatively low ozone depletion potential and
toxicity.
Further objects and advantages of the present invention
will be apparent from the description which follows.
-8-
~RIEF DESCRIPTION OF DRAWINGS
:
FIG. 1 is a perspective view of a room enclosure having a
total flooding system in accordance with one aspect of the
present invention. ~:
5 FIG. 2 is a schematic, cross-sectional view of a portable
fire extinguisher useful in accordance with the present
invention.
~ :,
,, . ,-, ,,~,.
:' -'- '` "~.
- .; . , -
..: .
,,, i I , , - ~ . ;~- "
" .~ '"
: .- .:
''""'"'' ~,
'~' `-"~' ','
.
_~Q
DESCRIPTION OF THE PREFERRED EM~ODIMENT
For the purposes of promoting an understanding of the
principles of the invention, reference will now be made to the
preferred embodiment of the invention and specific language
5 will be used to describe the same. It will nevertheless be -
understood that no limitation of the scope of the invention is
thereby intended, such alterations, modifications and further
applications of the principles of the invention being
contemplated as would normally occur to one skilled in the art
10 to which the invention relates.
The present invention provides surprisingly effective
methods and compositions for fire extinguishing. The fire
extinguishant compositions comprise bromodifluoromethane as
w211 as mixtures of bromodifluoromethane and other
fluorocarbon fire extinguishants. Prior to this invention,
the prior art has taught away from the use of
bromodifluoromethane for fire extinguishing applications.
However, it has been discovered that bromodifluoromethane is
an effective fire extinguishant, and moreover that it has a
20 desirably low ozone depletion potential (ODP).
In addition, blends of bromodifluoromethane with other
extinguishants also exhibit superior fire extinguishing
qualities and low ozone depletion potentials. It has further
been discovered that certain of the extinguishant mixtures of
the present invention generally have even greater efficacy
than would be predicted based upon the combination of the
separate compounds. This synergistic result makes the ~-
inventive mixtures unexpectedly useful as fire extinguishants.
;~ The various inventive compositions containing
bromodifluoromethane have also been demonstrated to have low
toxicity and minimum temperature dependency, and to be capable
of effective use in a wide variety of fire extinguishing
applications. Importantly, it has been demonstrated that such
~: compositions containing bromodifluoromethane can replace high
~ ~ .
'
z-10-- .
ozone depletion potential fire extinguishing agents in
existing equipment.
Bromodifluoromethane has the molecular fo~mula CHF2Br
and is understood to have the following structural formula:
H
~r - C - F
F
Bromodifluoromethane may be prepared by a number of methods,
including fluorination of bromoform with HF in the presence of
l0 chromium-type catalysts, as described in United States Patent
No. 3,210,430 issued to Knight.
One of the notable advantages of bromodifluoromethane is
the combination of efficacy, low ODP and acceptable toxicity.
Prior art fire extinguishants typically are less desirable as
15 to one or mo~e of these properties. Bromodifluoromethane is
therefore a superior fire extinguishant in appropriate ~
settings. By comparison, known extinguishants such as ;
bromotrifluoromethane (Halon 1301) are slightly more effective
on a weight basis, but their ODP's are substantially higher, -
20 rendering them environmentally unacceptable. The ~-
bromodifluoromethane compositions of the present invention
have reduced ODP ratings, but are surprisingly effective at -~
levels safe to humans, i.e. particularly at concentrations
less than about 10% (v/v).
In view of the concern with ozone depletion in the earth's ~ :
atmosphere, the present invention provides a distinct -~
a~dvantage resulting from the use of bromodifluoromethane.
While not being bound to a particular theory, it is believed
that the hydrogen substituent of bromodifluoromethane makes it
30 less stable than fully halogenated methanes such as
bromotrifluoromethane and bromochlorodifluoromethane, thereby
making it less likely to penetrate into the stratosphere, or
to persist in that region. Accordingly, its potential for
ozone depletion is dramatically lower than more stable
r
--11--
compounds, such as CHF2Br, or CF3Br. For example,
bromodifluoromethane has been determined to have an ozone
depletion potential less than one tenth of that of
bromotrifluoromethane.
The fire extinguishant compositions of this invention
generally exhibit an ozone depletion potential of less than
about 3.0, and preferably less than about 1Ø For purposes
herein, the ODP of a pure compound may be calculated using the
following algorithm developed by G. Dana Babson of the
10 University of Virginia:
ODP - A E P [(#Cl)B ~ C(#Br)] D(#C 1)
In this expression, P is the photolysis factor. P - 1.0 if
there are no special structural features which make the
molecule subject to tropospheric photolysis. Otherwise,
15 P - F, G or H, as indicated in the following Table of
Con tants: - -
TABLE OF CONSTANTS
CONSTANT NAME VALUE
A Normalizing constant 0.446
20 B Exponent for chlorine term 0.740 -
C Multiplier for bromine term 32.000
D Constant for carbon term 1.120
E Hydrogen factor [-1.0 for no H's] 0.0625
F Photolysis factor for geminal Br-C-Cl 0.180
25 G Photolysis factor for geminal Br-C-Br 0.015
H Photolysis factor for adjacent Br-C-C-Br 0.370
~: ' ,
~; The Babson method provides a reasonable approximation of the
ozone depletion potential (ODP) as calculated by the more
complex method-described in "The Relative Efficiency of a ~ -
Number of Halocarbons for Destroying Stratospheric Ozone", by
~; D. J. Wuebbles, Lawrence Livermore Laboratory Report
UCID-18924, issued January, 1981. --
The various compositions of this invention have been found
to exhibit excellent fire extinguishment properties, a good
~ .
J ,~
-12-
combination of physical properties, such as boiling point and
vapor pressure, a good combination of handling and use
properties, such as cleanliness and superior discharge pattern
configurations, reasonable toxicity characteristics, and low
5 ozone depletion potential. Appropriate selection of the
components of the composition permit utilization of existing ~-
equipment without making expensive hardware changes, and may ;~ -~
also minimize the temperature dependency of the discharge
agent, thus broadening the range of the composition's
10 potential fire fighting applications. Also, the mixtures of
the present invention may be formulated to have a desirably
low ODP or other optimized physical characteristics by
selected proportioning of the bromodifluoromethane and the ~-
other component(s).
lS Comparative tests have indicated that the ~
bromodifluoromethane compositions of the present invention - -
provide a uniform discharge over a slightly longer discharge
period than comparable fire extinguishant compositions based -
on bromochlorodifluoromethane (Halon 1211), despite the fact
20 that bromodifluoromethane has a higher vapor pressure than
Halon 1211 at a given temperature. The bromodifluoromethane
compositions also exhibit good throw characteristics, thus
providing a significant advantage over bromotrifluoromethane
(Halon 1301), which tends to volatilize too quickly. Because
25 of the lower equilibrium pressure of bromodifluoromethane
compositions relative to the comparable Halon 1301
compositions at the same temperature, the compositions of the
plresent invention may be contained and discharged using
containers and fittings of lower pressure rating than those
30 required for comparable compositions based on Halon 1301. -;
Comparative tests have also indicated that the
bromodifluoromethane compositions provide a discharge stream
more cohesive than that provided by either Halon 1211 or Halon
1301 compositions. This affords a particular advantage in -
35 efficiency. A good cohesive discharge pattern can achieve ~ -
quick "knock down" of fire by physically displacing high
temperature combustion products and oxygen supply from the
fuel. This allows the fire to be extinguished more quickly
and/or with a lesser amount of fire extinguishant.
In a first aspect of the present invention, there are - r-~;
provided fire extinguishant compositions comprising
bromodifluoromethane, alone or in combination with a
propellant. In a related embodiment the fire extinguishants
consist essentially of bromodifluoromethane, or of
10 bromodifluoromethane and propellant.
To produce an effective composition for conventional use
as a fire extinguishing agent, bromodifluoromethane is -
preferably blended with a propellant gas to produce a gas and
liquid phase mixture of fire extinguishant component and
compatible propellant. Compatibility contemplates that the
propellant be non-reactive, stable and non-interfering with
bromodifluoromethane and that it provide suitable pressure, at
moderate weight percents, to propel the bromodifluoromethane
adequately into the fire for the given fire extinguishing
systems and conditions. Suitable gases for use in such a
blend include nitrogen, carbon dioxide, trifluoromethane,
carbon tetrafluoride, argon and mixtures thereof, or other ~
gases including air. `---
Depending on the application for which the fire -
25 extinguishant composîtion is desired, a wide range of blends -~
of bromodifIuoromethane and propellant gases may be suitable.
-:
In order to provide a composition that is effective for -
delivery under autogenous pressure from conventional fire
extinguishing apparatus the relative proportions of ~`
30 bromodifluoromethane and propellant are preferably such that ~ ~
tne equilibrium pressure at 70F of the two phase mixture is ~ ;
between about 45 psig and about 600 psig, and more preferably
between about lO0 psig and about 400 psig. For many
~ applications, this translates for binary mixtures of
;~ ; 35 bromodifluoromethane and propellant into a `~
: ,~- .:.::
' '.,, - .
~ ,fp~ `"~"~
-14-
bromodifluoromethane weight fraction of at least about 70% by -
weight. Proportions in these ranges provide highly effective ~ -- --
fire extinguishment qualities, including effective function -
over a range of ambient temperature, yet provide an ozone
5 depletion potential that is substantially lower than common
prior art compounds, including either bromotrifluoromethane or ~ ~
bromochlorodifluoromethane. -
In accordance with another aspect of the present
invention, the fire extinguishing colnpositions comprise a
o mixture of bromodifluoromethane and a fluorocarbon fire
extinguishant. The fluorocarbon fixe extinguishant may
comprise any of a wide variety of known, fluorine-containing ~ ~ -
compounds which form stable, efficacious mixtures with
bromodifluoromethane. For example, known fluorocarbon fire ~ -15 extinguishants typically are those effective at less than
about 12% (v/v). Such fluorocarbons include
bromotrifluoromethane, bromochlorodifluoromethane,
chlorodifluoromethane, chlorotrifluoromethane,
heptafluoropropane ~1,1,1,2,3,3,3-heptafluoropropane and -~-
20 1,1,1,2,2,3,3-heptafluoropropane), ~-
1,1,1,2,3,3-hexafluoropropane, 1,1,1,3,3,3-hexafluoropropane,
pentafluoroethane, 2-chloro-1,1,1,2-tetrafluoroethane,
dibromodifluoromethane, dibromotetrafluoroethane,
chloropentafluoroethane, 2-bromo-1,1,1,2-tetrafluoroethane,
25 C2F6~ C3F8~ C4F10'
l-bromo-1,1,2,2-tetrafluoroethane, -
l-chloro-1,1,2,2-tetrafluoroethane and mixtures thereof.
More generally, bromodifluoromethane may be used as a
partial replacement for halogenated hydrocarbons otherwise
30 used in fire extinguishing compositions. In other words, for
a single component fire extinguishant of the prior art,
bromodifluoromethane may be added thereto to form a fire
extinguishant mixture. For a composition including two or
more of the prior art extinguishants, bromodifluoromethane may
35 be used to partially or fully substitute for one of those
components. This has the advantage of modifying the overall
characteristics of the prior art fire extinguishant
composition, such as by increasing efficacy and/or reducing
ODP or toxicity. sromodifluoromethane is therefore seen to be
5 extremely flexible in its manner of use in preparing mixtures
for use as fire extinguishants.
For the bromodifluoromethane mixtures, the relative
amounts of the bromodifluoromethane and fluorocarbons mixed
therewith are not critical, but rather are dictated by the
10 characteristics desired for the overall composition. Thus,
certain applications may require lower toxicity, while other
instances may call for maximized efficacy. Therefore, no
particular ratios of compounds are required.
Importantly, it has also been discovered that
15 bromodifluoromethane has the characteristic of forming
synergistic mixtures with other fluorocarbons. Specifically, -
-~ the efficacies of various extinguishant compositions utilizing
. .
bromodifluoromethane have been found to exceed the predicted
~-~ efficacies based simply on a weighted average of the
20 components. Specific results are described in the Examples
hereafter.
By way of example, substantial synergism has been found
for the combination of bromodifluoromethane and
heptafluoropropane. As determined by tests (see Table llA), ~;~
25 the percentage (v/v) of bromodifluoromethane in air necessary
to extinguish a cup burner flame was about 3.9%, compared to
the 6.0% necessary for heptafluoropropane alone. The `~
combination of these two components was significantly more -~ ; -
effective than the simple weighted average for the two. For ~-
30 example, at a mixture of 50% (by mole) bromodifluoromethane ~ -
and 50% heptafluoropropane, the volume percent of the mixture
in air needed to extinguish the flame was only 4.2%,
contrasted to the p~edicted level of 5.0%, representing an ~ ~ `
increase in efficiency of 16%. Particularly desirable
35 mixtures according to the present invention are those which
,.~ ~ ,
~ , , :
~ 3
., "~
'~ ',~'
have at least about a 10% increase in fire extinguishing -
efficiency over the predicted efficiency for the weighted
average of the two components.
Effective mixtures for fire extinguishment may be prepared
5 by mixing bromodifluoromethane with a variety of known fire
extinguishing agents, such as Halon 1301 (CF3Br), Halon 1211
(CF2BrCl), Halon 1202 (CF2Br2) or Halon 2402
(~rCF2CF2Br). Such mixtures optionally include a
compatible propellant such as nitrogen, carbon dioxide, -
10 trifluoromethane (CF3H), tetrafluoromethane (CF4) or argon.
Compositions which include bromochlorodifluoromethane
(Halon 1211) preferably have an ozone depletion potential of
less than about 1.5 and contain between about S% and about 35%
by weight bromochlorodifluoromethane. Compositions which
15 include bromotrifluoromethane (Halon 1301) preferably have an
ozone depletion potential (calculated by the Babson method) of ~
- less than about 3.0 and contain between about 0.9% and about ~ ~-15% by weight bromotrifluoromethane. For some flooding 7' '~ "'''
systems or other applications, it may be preferable for the -
20 compositions to contain between about 5% and about 15% by -
weight bromotrifluoromethane.
Effective compositions for fire extinguishment are also
prepared by mixing bromodifluoromethane with ~ -~
hydrofluorocarbons such as CF3CHFCF3, CF3CF2CF2H,
25 CF3CHFCF2H, CF3CH2CF3 and CF3CF~H, optionally in
the presence of a compatible propellant such as nitrogen,
argon, carbon dioxide, CF3H or CF4. Mixtures prepared by
t,he combination of bromodifluoromethane and these saturated,
higher-fluorinated Cl, C2 and C3 hydrofluorocarbons have -
30 been found to be particularly effective fire extinguishants.
Because these hydrofluorocarbons contain no bromine or
chlorine, they have an ozone depletion potential of zero.
Furthermore, since the compounds contain hydrogen atoms, they -
are susceptible to breakdown in the lower atmosphere and hence
do not pose a threat as greenhouse warming gasses. Mixtures
. ,
of CHF2Br and heptafluoropropane are especially preferred
because the compounds have similar vapor pressures over a wide
range of temperatures and therefore the composition of the
mixture remains relatively constant during discharge or other
5 application.
Specific hydrofluorocarbons of this type are compounds of
the formula CXHyFz, where x is 2 or 3; y is l or 2; and
z is 5, 6 or 7; where y is 1 and z is 5 when x is 2; and where
z is 6 or 7 when x is 3. Hydrofluorocarbons in this class
10 include heptafluoropropane (CF3CHFCF3),
1,1,l,3,3,3-hexafluoropropane (CF3CH2CF3),
1,l,l,2,3,3-hexafluoropropane (CF3CHFCHF2), and ~-
pentafluoroethane (CF3CHF2). ~-
These hydrofluorocarbon compounds are non-toxic and are
15 economical to manufacture. For example, heptafluoropropane
may be conveniently produced via the reaction of commercially
available hexafluoropropene (CF3CF=CF2) with anhydrous HF
as described in U.K. Patent 902,590. Similarly,
l,1,l,3,3,3-hexafluoropropane may be synthesized by reacting ~-
.
20 anhydrous HF with pentafluoropropene (CF3CH-CF2).
~ l,l,l,2,3,3-hexafluoropropane may be obtained by hydrogenation
;~ of hexafluoropene (CF3CF~CF2). Pentafluoroethane may be
obtained by the addition of hydrofluoric acid to
tetrafluoroethylene (CF2=CF2).
~lends of bromodifluoromethane with these
hydrofluorocarbons desirably include the hydrofluorocarbon at
a level of at least about l0 percent by weight of the blend. --
The use of hydrofluorocarbons at higher levels in su!ch blends
further minimizes the ODP and resultant adverse environmental
30 effects. Compositions containing hydrofluorocarbons
preferably ha~e an ozone depletion potential of less than - ~ -
about 0.9 and contain between about 5% and about 90% by weight
hydrofluorocarbon.
Heptafluoropropane, having a boiling point of about -17C, ~ ;~
is highly miscible with bromodifluoromethane. Consequently,
mixtures may contain substantially any proportion of -
.
! ~ ,
"
;~) ~, ,", j
-18-
heptafluoropropane to bromodifluoromethane. Such compositions -
preferably include between about 1% and about 99% by weight
heptafluoropropane. The ODP for compositions including ~-
heptafluoropropane is preferably below about 0.9.
Effective extinguishing compositions are also prepared by
mixing bromodifluoromethane with a hydrochlorofluorocarbon
such as CF3CHFCl, CF2HCF2Cl, CF3CHC12 or CHF2Cl,
optionally in the presence of a compatible propellant such as
nitrogen, trifluoromethane, carbon tetrafluoride, argon or
10 carbon dioxide. Compositions containing
hydrochlorofluorocarbons preferably have an ODP of less than
about 0.9, and contain about 5% to 90% by weight
hydrochlorofluorocarbon. ; -
Similarly efficacious blends are achieved by the
15 combination of bromodifluoromethane and
2-Chloro-1,1,1,2-tetrafluoroethane (CF3CHFCl), a halogenated
hydrocarbon also known as CFC 124. CFC 124 has a molecular
weight of 136.48 and a boiling point of -12C. Methods for
the preparation of CFC 124 are known in the prior art. For
20 example, 2-Chloro-1,1,1,2-tetrafluoroethane may be prepared by
fluorination of CC12~CC12 with HF, as described in
European Patent Application No. 313,061 (1989). An
alternative preparation is by reaction of CF2-CFCl with
KF/formamide, as reported in the Journal of the American
25 Chemical Society, vol. 82, p. 3091 (1960).
Blends of bromodifluoromethane and
2-Chloro-1,1,1,2-tetrafluoroethane (CFC 124) are effective in
llow concentrations, and of course at high concentrations as
well. The concentration employed may depend to some extent on
30 the nature of the fire, the combusting material and the
circumstances of application. The similarity of boiling
points for the two compounds allows the composition discharged
or otherwise applied to remain essentially constant. Blends
having from about 5% to about 99% by weight CFC 124 and from
35 about 95% to about 1% by weight bromodifluoromethane are
particularly preferred.
Effective fire extinguishant mixtures are also obtained by
the combination of bromodifluoromethane and one or more of
C2F6 ~ C3F8 ' C4F10 '
5 1-bromo-1,1,2,2-tetrafluoroethane,
l-chloro-1,1,2,2-tetrafluoroethane. Ratios of the components
and the concentrations of use are similar to the previously
described blends.
A further desirable aspect of the blends of
10 bromodifluoromethane and 2-Chloro-1,1,1,2-tetrafluoroethane is ~- ~
that the mixtures are especia~ly attractive due to their low ~-
ODP. 2-Chloro-1,1,1,2-tetrafluoroethane has an ODP of 0.03 as
calculated by the Babson model. It is believed that the
presence of the hydrogen in 2-Chloro-1,1,1,2-tetrafluoroethane
15 makes the compound less stable and contributes to the lower
ODP, since the molecules are susceptible to breakdown in the
lower atmosphere.
A consideration in selecting a concentration for the
compositions of this invention is the maintenance of the area
20 in a non-toxic and non-anesthetic condition. A 50% lethal ~ `
concentration (LC50) for a compound is that concentration of ~-'the compound (volume of compound per volume of air) at which
50% of~a test population is killed; a 50% anesthetic dose (AD '~
50? is that concentration at which 50% of a test population is
25 anesthetized. For example, 2-Chloro-1,1,1,2-tetrafluoroethane ~ ~- has an LC50 of 44.7% v!v, and an AD50 of 15.5% v/v, as
reported by Davies, et al., Int. J. Quantum Chem: Quantum ~-
iology Symp No.~ 3, 171 (1976). Selection of the appropriate
usage rate of bromodifluoromethane/CFC 124 mixtures will
30 therefore be affected by these properties. For example, a
usage rate where humans may be present is preferably below-~
about 15~ v/v, and more preferably below about 10% v/v.
Mix:tures are also provided in the present invention which
comprise bromodifluoromethane and a selected fluorocarbon
component, all as previously described, and a third component
-2~-
comprising a propellant. Such propellant may be any which is
compatible and useful in combination with the other
ingredients, also as previously described. Such propellants
include nitrogen, carbon dioxide, trifluoromethane, carbon ~
5 tetrafluoride and argon. Other compatible propellants or -
other compounds may also be mixed with the fire extinguishants
of the present invention.
For minimum ozone depletion allowance, compositions
containing a non-fluorocarbon propellant such as nitrogen,
10 argon or carbon dioxide are preferred. Among such
propellants, nitrogen is particularly advantageous because it
provides a favorable balance of reasonable cost and low
dielectric constant. Carbon dioxide is also advantageous from `
a cost standpoint, but is less preferred in applications where
15 conductivity of the ~ire extinguishing agent is or may be a
problem. Compositions containing such non-fluorocarbon :
propellants preferably have a low proportion of propellant,
typically in the range of 0.1% to 5% by weight. Such
compositions typically exhibit an ozone depletion potential of
20 less than 1Ø ~ -
The bromodifluoromethane compositions blended with a `
propellant gas produce a gas and liquid phase mixture of fire ~-
extinguishant component and propellant. Depending on the
application, a wide range of blends of bromodifluoromethane
compositions and propellant gases may be suitable. In order
to provide a composition that is effective for delivery under
autogenous pressure from present fire extinguishing apparatus,
the relative proportions of bromodifluoromethane composition
and propellant are such that the equilibrium pressure of the
~- 30 two phase mixture at 70F is preferably between about 45 psig
and about 600 psig, and more preferably between about 100 psig
and about 400 psig. Higher pressures can be used, although
there will be an increase in equipment costs. For most
applications, this translates into a bromodifluoromethane
weight fraction of at least about 70% by weight. Proportions
.
-21-
in these ranges provide highly effective fire extinguishment
qualities, including effective function over a range of -
ambient temperature. At the same time, the ozone depletion ~;
potential is substantially lower than many prior art
5 extinguishants such as bromotrifluoromethane or
bromochlorodifluoromethane.
Among the preferred compositions of the invention are
mixtures containing bromodifluoromethane, nitrogen, and one of
the following: Halon 1211, Halon 1301, heptafluoropropane and
10 CFC 124. In such compositions, the ratios of the components
are as already described. For mixtures containing Halon 1211,
the nitrogen content is typically between about 0.3~ and about
10% by weight. For mixtures containing Halon 1301, the ;
nitrogen content is typically between about 0.1% and about 10%
15 by weight.
Particularly preferred compositions of the invention
include those formulated for replacement of Halon 1211 and
Halon 1301 in existing systems. Compositions adapted to -~
replace Halon 1211, e.g., in hand held fire extinguisher
applications, preferably are formulated to have a phase
equilibrium pressure of between about 100 psig and about - -~
200 psig at 70F. Compositions adapted to replace Halon 1301,
typically in large stationary flooding systems, preferably are ~`
formulated to have a phase equilibrium pressure of between
about 300 psig and about 400 psig at 70F.
The bromodifluoromethane compositions may be applied in ~ -
the variety of ways employed for other halogenated hydrocarbon
qxt~inguishants, including application in flooding systems, `
specialized systems and portable systems, described hereafter
in more detail. The methods for application of the described
~ fire extinguishing compositions include those known to be
;~ useful for other halogenated hydrocarbons, such as Halon 1211 ~
~; and Halon 1301. In broad terms, these methods utilize ~ -
application systems which typically include a supply of agent,
a means for releasing or propelling the agent from its
. , ~,
, ~ ,. ..
:~ ,-, .
-22-
container, and one or more discharge nozzles to apply the ---
agent into the area of the hazard or directly onto the burning
object. A system may also contain other elements, such as one
or more detectors, remote and local alarms, a piping network,
5 mechanical and electrical interlocks to shut down ventilation, ~-
etc., directional control valves, etc. Such systems may be
stationary or portable, and typically the fire extinguishant
may be pressurized with propellant gas at up to about 600 psig
at ambient temperature. For example, referring to Figure l
10 there is shown a typical system for a room ll having a raised
floor 12 and ceiling 13. Automatic fire detectors 14 are
installed in the ceiling and floor and activate the fire
extinguishing system when needed. The extinguishing system
comprises storage tanks 15, piping 16 and discharge nozzles
15 17. A control panel 18 operatively connects the detectors
with the fire extinguishing system to activate it.
Thus, the compositions of the present invention may be
used in a total flooding fire extinguishing system in which
the agent is introduced to an enclosed region (e.g., a room or
20 other enclosure) to surround a fire at a concentration
sufficient to e~tinguish the fire. Total flooding systems are
used, for example, for computer rooms, control rooms, special ~
storage areas, machinery spaces and the like. In a total ~ -
flooding system apparatus, equipment or even rooms or
25 enclosures may be provided with a source of agent and
appropriate piping, valves, and controls so as automatically
and/or manually to be introduced at appropriate concentrations
in the event that fire should break out. Local application
systems discharge fire extinguishing agent in such a manner
30 that the burning object is surrounded locally by a high
concentration of agent to extinguish the fire. Local systems
are often employed because the enclosure may not be suitable
to provide for total flooding. Examples include use for
presses, tanks, spray booths, and electric transformers.
Specialized systems are frequently used for specific
,' ~ ,~,, / , - J
applications or hazards, such as for aircraft, military
vehicles, eimergency generators, etc.
By way of further example, the compositions of the present
invention can be conveniently employed in local application
5 systems through the use of conventional portable fire
extinguishing equipment, such as those described in the Fire
Protection Handbook. For such systems, the supply of fire
extinguishing agent is typically directed by hand at the fire,
or larger mobile units with hoses and nozzles are employed for
10 directional spraying. It is usual to increase the pressure in
portable fire extinguishers with suitable propellant gases in
order to insure that the agent is completely expelled from the
extinguisher. Systems in accordance with this invention may
A be conveniently pressurized at any desirable pressure up to
15 about 600 psig at ambient conditions. -~
In accordance with the fire extinguishing method of the
present invention, a combustion suppressing amount of ;~
extinguishant composition is discharged into the combustion
zone of a fire for a time sufficient to suppress the fire. It -~
20 will be appreciated that the amount of extinguishant applied ~ -
may desirably be enough to fully extinguish or simply to ~r~
;~ suppress the fire. In the case of suppression of a fire, the
fire may be limited to a controllable volume, and thereafter
be fully extinguished with additional use of the
25 bromodifluoromethane compositions or with other extinguishant
;;~ materials or methods. In either circumstance, the fire
fighting efficacy of the bromodifluoromethane will be usefully
qmployed, and as used herein the term "extinguishingi amount" -~-
encompasses both suppression and extinguishment.
Minimum concentrations employed may be substantially any ~ -
at which a given fire may be suppressed or extinguished, the
exact minimum level being dependent on the nature of the fire,
the combusting material, the particular extinguishant
composition, the combustion conditions, and the circumstances
and manner of application. In general, however, best results
~ '`' '
.i:,': .'~ , `
, . ~ '.
-24-
are achieved where the compositions are present at a level of
at least about 2% (v/v), and more preferably at least about 4%
(v/v). The maximum amount to be employed will be governed by
matters such as economics and potential toxicity to living ~ ~-
5 things. About 15% (v/v) provides a convenient maximum
concentration for the compositions in occupied areas. Higher
concentrations including those up to about 25% (v/v) may be
employed in unoccupied areas, with the exact level again being
determined by the particular combustible material, the
lO extinguishant employed and the conditions of combustion. The
preferred fire extinguishing concentrations of the
compositions in accordance with this invention are in the
range of about 4% to about 10% (v/v).
; Initially, i.e., when discharge of extinguishant is
15 commenced, the extinguishant composition within the container
is a liquid and gas phase mixture of the fire extinguishant
components and propellant having an equilibrium pressure
typically of about 45 psig to 600 psig, preferably about
100 psig to 400 psig, at the ambient temperature that may ~ --
prevail at a fire scene. As the extinguishant is expelled,
the pressure falls. However, the initial composition within ~ -
the container is preferably such that a substantial pressure
is maintained as long as there is any significant residue of
liquid phase within the container. A substantial pressure
25 should be maintained until an effective amount of
extinguishant has been applied to the fire.
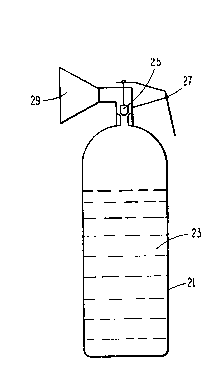
Illustrated schematically in Figure 2 is a portable fire ~
!' extinguisher which includes a cylinder 21 containing a ~-
charge 23 of the composition of the invention. The
30 bromodifluoromethane composition is maintained at a pressure
of 45 to 600 psig, preferably 100 to 200 psig, at ambient -~
temperatures contemplated for use in fighting fires. An
amount of extinguishant is provided as will be sufficient for
blanketing a fire, and thereby extinguishing it or suppressing
it to a controllable level. The charge is a gas and liquid
.
~ .
phase mixture which is substantially at equilibrium at the
aforesaid pressure and ambient temperature.
A discharge valve 25 operated by a trigger mechanism 27
provides for release of the fire extinguishant composition
5 from the container, and a discharge horn 29 is adapted to
direct the extinguishant to the combustion zone of the fire.
Alternatively, the extinguisher discharge valve may be fitted
with a hose and spray nozzle for direction of the
extinguishant. Arrangements similar to that of the drawing,
10 but having relatively large containers for the extinguisher
charge-and typically containing a charge having a phase
equilibrium pressure of 200-500 psig, are provided for mobile
or stationary systems designed for fighting large fires.
There are four basic types of extinguisher system triggering
15 devices: manual (such as a pull pin), thermatic ~similar to a
heat activated sprinkler head), electronic (activating a
solenoid), and rupture disc. Discharge temperatures typically
range from -40 to 120F (50C). Large stationary or mobile ~ -
systems typically also include means such as a filling valve ~-~
20 for charging the system with fire extinguishant. ~ ~
The mixtures of the invention may be delivered from a ~ :
single cylinder or ~ther suitable container containing the
bromodifluoromethane and the adjuvant, and optionally also
containing a propellant. Alternatively, the components may be
25 stored in separate containers and premixed through common
piping or mixture devices prior to delivery to the fire.
Hence, any desired blend may be effectively delivered to the
!~: . fire.
The invention will be further described with reference to ~ `
30 the following specific Examples. However, it will be - .;
understood that these Examples are illustrative and not
restrictive in nature. In the following Examples, percents
indicated are percents by weight unless indicated otherwise,
and all ODP's are calculated by the Babson method. ~ ~
' ';.:;
' :'.
: . ' ; I 1' j:
- z ~-
Example 1
Fire-extinguishant compositions of the present invention
were prepared by combining bromodifluoromethane and nitrogen
in the proportions shown in nos. B-l, B-2 and B- in Table 1.
5 These compositions have low ODP, suitable phase equilibrim
pressure for use in conventional systems, and are effective
fire extinguishants. Similar mixtures of bromodifluoromethane
with carbon dioxide, argon and carbon tetrafluoride yield
similarly good compositions.
As also shown in Table l, compositions were prepared by
combination of bromodifluoromethane, nitrogen and either of
Halon 1211 (H-1211) or Halon 1301 (H-1301). These
compositions, listed as T-l through T-6, also provide
advantageous fire extinguishants, although the ODP's are
15 slightly higher.
Table 1
.-~
Comp.
No. Wt.% CHF2Br Wt.% Other Components Q~ -
B-l 99.1 0.9% N2 0.88
20 B-2 99-5 0.5% N2 0.88
B-3 99.5 0.1% N2 0.88
T-l 70.0 29.5% H-1211 1.40
0.5% N2
20 0% H-1211 1.24
T-3 94 5.6% H-1211 0.98
0.4% N2
T-4 98.6 0.9% H-1301 1.00
0.5% N2
30 T-5 85.0 14.8~ H-1301 2.87
0.2% N2
T-6 76.6 23.0% H-1301 3.96
0.4% N2
Example 2
Test systems were prepared by filling containers with the
compositions set forth in Table 2. In each case the test
container was filled about half way on the basis that this
would be the minimum fill for a commercial system, maximizing
the propellant content. Table 2 also indicates the phase
equilibrium pressure exhibited by each composition at 70F.
These compositions perform well as extinguishing materials in
terms of discharge and efficacy.
:
Table 2 -
Comp. CHF2Br
No. Press. Extinauishant Other Components ~ -~
B-4 100 psig 29.3g (0.22 0.1g (0.0036 moles)
moles) N2 (1-6% N2) ~ -~
15 B-5 200 psig 29.3g (0.22 0.3g (0.01 moles) ~ ~
moles) N2 (4.8% N2) , ;~,-
B-6 200 psig 32.7g (0.26 0.4g (0.014 moles)
moles) N2 (6.5% N2) -;
B-7 400 psig 32.7g (0.26 0.8g (0.028 moles)
moles) N2 (12.3% N2)
B-8 100 psig 32.5g (0.25 0.5g (0.0071 moles) ~
moles) F-23 (2.8~ F-23) ~-
B-9 400 psig 36.3 (0.25 0.4g (0.0057 moles)
moles) F-23 (2.5% F-23) -~
Example 3 - -~
Concentrations of bromodifluoromethane (CHF2Br)
required to extinguish diffusion flames of n-heptane, n-butane
and methanol were determined using the cup burner method ~;
described by Ford in Halo~enated Fire Su~ressants, ACS
Symposium Series 16, ACS, Washington, DC, 1975, p. 16.
Bromodifluoromethane vapor was mixed with air and introduced to
-2 ~
a flame produced in a glass cup burner, with the concentration
of bromodifluoromethane being slowly increased until the flow
was just sufficient to cause extinction of the flame. The data
are reported in Tables 3-5, which also show the amount of
5 bromodifluoromethane required on a weight basis. Extinguishing
concentrations, % v/v were calculated from the relationship
flow agent (cc/min)
% v/v ~ x 100
flow agent ~ flow air (cc/min)
10 To convert volume data to mg/L, the following relationship was
employed:
(extinguishing conc, % vol) x (10) x MW -~
mg/L =
24.5
.
15 where MW , the molecular weight of the agent. The results for
Halon 1301 (CF3Br) and Halon 1211 (CF2BrCl) are also shown in
Tables 3-5 for comparison purposes.
Table 3
Extinguishment of n-Heptane Diffusion Flames
20 Agent Air flow Agent required Extinguishing Conc.
cc/min cc/min % vJv mg/L
CHF2Br 16,200 649 3.9 208
CF2BrC1 16,200 546 3.3 222
CF3Br 16,200 510 3.1 188
:
r ,~
-2 !9-
Table 4
Extinguishment of n-Butane Diffusion Flames -
'
Agent Air flow Agent required Extinguishing Conc.
cc/min cc/min % v/vmg/L
~,~
5 CHF2Br 16,200 489 2.9 155
CF2BrC1 16,200 420 2.5 168
CF3Br 16,200 396 2.4 146
Table 5 ~ ~
Extinguishment of Methanol Diffusion Flames ~-
,: "
10 Agent Air flow Agent required Extinguishing Conc.
cc/min cc/min % v~vmg/L ~
., ~ .
CHF2Br 16,200 1297 7.4 396
CF2BrC1 16,200 1184 6.8 458 -~
CF3Br 16,200 1030 6.0 365 ;~
15 As evidenced by Tables 3-5, bromodifluoromethane is seen to be ~-
more efficient on a weight basis than Halon 1211 for the
, éxtinguishment of typical Class B type fuels.
Example 4
Bromodifluoromethane (9 pounds) was charged into an Ansul
20 Model SY 0941 portable extinguisher and sufficient nitrogen
added to bring the total cylinder pressure to 125 psig. For
purposes of comparison, Halon 1211 (CF2BrCl, 9 pounds) was
charged into an identical extinguisher and pressurized with
r~
.,J 'j /~ ',' ~'" / '~
-30-
nitrogen to a total of 125 psig. Discharge testing wascondu~ted in accordance with Section 26 of UL Standard 1093,
and results are shown in Table 6.
Table 6 -
Discharge Duration Test
AgentCharge % AgentDischarge time Range
lbs Discharged seconds feet
: , .
CHF2Br 9 100 11.0 14
CHF2Br 9 100 12.0 14.5
10 CHF2Br 9 100 12.1 ~ 14.5
CF2srC1 9 100 11.0 13
, CF2BrC1 9 100 11.2 15
CF2BrCl 9 100 11.2 15
As indicated in Table 6, the average discharge durations ~ -
15 of CHF2Br and CF2BrCl were 11.7 and 11.1 seconds,
respectively, and comparable stream ranges were also
obt~ined. The results indicate comparable discharge times and
ranges for the two agents. By comparison, 9 lbs. of
bromodifluoromethane was charged at 195 psig (with nitrogen)
20 to an Amerex 1211 extinguisher having an orifice smaller than
the Ansul unit, and provided with a short discharge horn. -~
100% of the agent discharged in 11.3 seconds, yielding a
stream range of 16.5 feet.
The present discharge requirement for the Ansul SY 0941
25 extinguisher filled with 9 pounds of Halon 1211 at a charging ~-
pressure of 125 psig is 12.0 + 1.0 seconds, and it is seen
that CHF2Br also meets these requirements. The stream from
CHF2Br was observed to be more cohesive than that from Halon
1211.
:
-31- -
Example 5
Fire tests for Class A fires were conducted in accordance i
with ANSI/UL 711, Standard for Rating and Fire Testing of Fire
Extinguishers. The Class A fire tests were conducted in
5 accordance with the procedures and requirements of paragraphs -~ `
4.6 through 4.16 inclusive of that standard. Crib'
construction and ignition arrangements were conducted in - -
accordance with Table 4.2 and 4.3 of that standard. All tests
were conducted in a fire test house constructed of prestressed
10 concrete measuring 30x40 ft. on the base and 50 ft. in ;
height. A cupola with adjustable louvers to control drafting
conditions was mounted on the top of the roof.
Bromodifluoromethane was charged into an Ansul SY 0941
extinguisher and the extinguisher pressurized with nitrogen to
t ' . '' .,
15 125 psig. Results of these tests are shown in Table 7. ~
' ,: .:
Table 7 - ~ ~
Extinguishment of Class A Fires~ ;
Agent Charge~ Agent Discharge Time --`
lbsDischarged seconds ~ ~
, ,, - ".
20 CHF2Br 9 100 9.5
CHF2Br 9 100 8.4
CHF2Br 9 100 9.4
CHF2Br 7# 8 oz 100 8.5 -
CHF2Br 7# 8 oz 100 9.0
: ~-
Analysis of video tapes shows that CHF2Br extinguished the
Class A fire almost instantaneously and had no difficulty in
preventing reignition during the required 10 minute wait
period. In addition, the stream from CHF2Br was observed to be
more cohesive than that from Halon 1211, which results in an
'
-3~-
advantage in efficiency. The increased cohesiveness of the
discharge pattern results in rapid "knockdown" of the fire, in
which the high temperature combustion products and oxygen are
physically separated from the fuel, allowing the fire to be
5 extinguished with a relatively small amount of fire
extinguishant. This is seen in the last examples of Table 7,
where a reduced charge of CHF2Br is seen to be capable of
extinguishing the fire as rapidly as the larger charge. This
increased knockdown renders CHF2Br more effective than Halon
lO 1211 for a Class A fire. In another test using the Amerex 1211 --
extinguisher on a Class B fire, a discharge of 6 lb. 13 oz. ~
(75.6~) of the bromodifluoromethane agent successfully -
extinguished the fire. This Class B fire test was conducted in
accordance with Section 5 of the ANSI/VL 711 standard.
' ~
Example 6
Tests were conducted comparing the performance of
bromodifluoromethane to that of Halon 1211 in an Argus type
total flooding fire extinguishment system, used primarily for
fire protection of machinery in textile mills. Tests were
20 conducted in accordance with established Factory Mutual
criteria for approval of Halon 1211 for this application. The
system was charged with CHF2Br or Halon 1211 and pressurized
with nitrogen to 150 psig. All trials were conducted outdoors -~
by discharging the fire extinguishant agent through varying
25 piping configurations into enclosed boxes of varying size. -~
Discharge time and total elapsed time until extinguishment were -~
recorded. The two products were tested using equal amounts by
weight. A summary of the results is set forth in Table 8.
Letter prefixes in the test numbers of this table designate
particular box sizes and piping configurations utilized in the
test.
: . : ,~.';
,:
. :-
-3~
a~ ~ . .. . ...
o ~ v ~ J~ a a a
0 ~ tn ~q O O O
V J- , :
O O O
,-
ol ;.~ ~: '' '
o
Q~ .,
~o o o o o ~ o ~ -l o u~
~ 4 g~ h ~ 4 ~ , L
¢ 1 ¢ ~ ~ ~ ¢ ¢ ¢ P~ Q Q ~ ~ m ~ m '' ~ !~ :
O U~
.: ' .
:
;~ J
--3 4--
No residues of extinguishant were detected in the fire --
extinguishant containers after discharge of the product in
accordance with the test. There were no discernible
differences in cylinder filling other than some differences in
5 the equilibrium pressure of the charge. Flow of the
bromodifluoromethane composition was judged to be as good as
that of the bromochlorodifluoromethane composition, both in
balanced and unbalanced systems. No trials were made to
determine the effect of nozzle design on discharge of the
lO CHF2Br composition, but nozzle coverage was judged better
than that afforded by Halon 1211. Both products mixed well in
the test box enclosure. No equipment or valve problems were --~
noted.
As indicated in Table 8, for the various configurations ~ -~
15 tested, CHF2Br was found to be equal to or more efficient
- than Halon 1211 on a weight basis. For configuration A, Halon
1211 was seen to be only slightly more efficient on a weight ~
basis. For equipment configurations E and H, equal weights of --
Halon 1211 failed to bring about extinguishment. Tests I2 and -~
I3 indicate that the bromodifluoromethane charge achieved
results superior to that of the bromochlorodifluoromethane
~-~ charge despite the fact that the weight of the former was only ~ ~
85% that of the latter. - --
: ,- - . ~ .: :
~` ~ E~ample 7 ~
This example demonstrates the use of CHF2Br in a total i`~`
flooding system and demonstrates the low level of
decomposition products, HX, produced during the extinguishment
of fires with bromodifluoromethane.
~'~ Decomposition products were determined by a procedure ~`
similar to that described by Sheinson, et al., in Fire &
FlammabilitY, vol. 12, p. 229 (1981). Pool fires of n-heptane
were extinguished by CHF2Br total flooding at 5% in a 116
cubic foot enclosure. A distribution system allowed delivery
of 5 to 7 volume percent agent concentration within 10 to 20 -~
;: -. : :
0~ Y!IJ
-3~-
seconds following manual initiation of the discharge. A
nozzle located at the top of the chamber distributed the agent
throughout the enclosure. The fuel was ignited, allowed a ~--
preburn and then sufficient CHF2Br discharged to achieve a 5%
5 calculated concentration at 21 C. HF levels were monitored
with Sensidyne real-time continuous HF analyzers located at
different heights in the enclosure. Gas samples were
withdrawn via a 6 mm O.D. metal tube from the center of the
enclosure through a porthole. Samples were collected in a
10 Teflon trap containing a pH 5 buffer, and HBr determined via
an Orion bromide specific electrode. The results of these
tests are shown in Table 9. For purposes of comparison, Halon ~ -
1301 was examined employing the same system.
Table 9
HF and HBr Production During Total Flooding
-''-.
Agent Fire Size*DischargeHF, peakHBr, peak
Time(s) ppm ppm
CHF2Br 0.077 10 5.9 < 4
CF3Br 0.077 10 19.6 trace
20 CHF2Br 0.077 20 5.9 < 4
CF3Br 0.077 20 12.7 25.6
CHF2Br 0.077 20 103 8.6
CF3Br 0.077 20 > 120 44
:
* sq ft of fire per 1000 cu ft of enclosure
i ,
z5 As indicated in Table 9, for a given fire size and
discharge time, less decomposition products HF and HBr are
observed with CHF2Br than with Halon 1301.
Examvle 8
This example demonstrates the efficacy of blends of
-3
bromodifluoromethane with hydrofluorocarbons. Various
mixtures of bromodifluoromethane and a hydrofluorocarbon were -
tested with n-heptane fuel in a cup burner apparatus as -~-
described in Example 3, and the results are shown in Tables
5 10-13. In Tables 10A, llA and 12A, the amount of agent -~
required for extinguishment is expressed as the volume ~ in
air, i.e., ext % - [cc of agent/(cc of agent + cc of air)] x
100. In Tables 10B, llB and 12B, the amount of agent required
for extinguishment is expressed as the % added to air, i.e.,
l0 % added to air = (cc of agent/cc of air) x 100. In addition ~
to the molar composition of the blends in percent, we report ~ ~-
in Tables 10-13 the volume percents of each agent and the
total volume percent of the mixture at extinguishment, and the
concentrations of each agent and the total concentration on a ;---
15 weight basis (mg/L) at extinguishment. The Tables also
illustrate the range of ODP values obtainable with the various
mixtures. The ODP of a given mixture is calculated as the sum
of the weight percent of each agent multiplied by its ODP, as
calculated by the Babson method. For example a 50:50 (by
20 weight) blend of CHF2Br and CF3Br has an ODP of
(0.5x0.89)+~0.5x14.26) = 7.6.
The data demonstrate that effective flame extinguishment
may be obtained with mixtures of bromodifluoromethane and
various hydrofluorocarbons, and that the already low ODP of
25 CHF2Br can be further materially reduced without significant -
reduction of the extinguishing capability of the mixture. For
example, as shown in Table llB, a 43:57 by weight mixture of
CHF2Br and CF3CHFCF3 extinguishes an n-heptane flamq at a ~ -~
concentration of 4.4 % by volume, compared to 4.0 % by volume
: - :
30 for pure bromodifluoromethane. This represents a 10 % ~ ~
increase in the total volume (a 25 % increase in the total ~ -
mg/L delivered) required for extinguishment with a 56 %
reduction in ODP.
' J ~
Table 10A -
CHFZBr ~ CF3CF2H Blends
CHF2Br CHF2BrC2HF5 Total CHF2Br C2HF5 Total - -
mol % ext ~ext % ext % mg/L mg/L mg/L ODP
:,
5100 3.85 0.00 3.85 205.96 0.00 205.96 0.89
73 3.11 1.16 4.27 166.21 56.73 222.94 0.66
2.77 1.85 4.62 147.97 90.55 238.52 0.56
2.48 2.47 4.95 132.70 120.98 253.69 0.46
1.97 3.71 5.68 105.23 181.66 286.89 0.33 ~ :
9 0.63 5.99 6.62 33.60 293.35 326.95 0.09 ~-
0 0.00 8.52 8.52 0.00 417.31 417.31 0.00 -
; TABLE 10B
Extinguishment of n-Heptane Diffusion Flames
CF3CF2H / CHF2Br Mixtures
~ ~ ,- .-
H~ 15 Flow at
Extinguishment
cc/min % Added to Air
Weight %
CF3CF2H CHF2BrCF3CF2HCHF2Br TotalCF3CF2H ODP :~
~:: .
20 0 1380 0 4.0 4.0 0 0.89
196 526 1.2 3.2 4.4 25.6 0.66
314 470 1.9 2.9 4.8 37.5 0.56
421 423 2.6 2.6 5.2 47.7 0.46
~ 637 ~338 3.9 2.1 6.0 63.0 0.33
-!~, 251039 109 6.4 0.7 7.1 89.4 0.09
~ 1509 0 9.3 0 9.3 100.00 0.00
, ~
` s~ ;
-3
_able 11A
C31F2Br / CF3CIIFCF3 Blends
C}lF233r H-37 CI3F2BrTotal1-1-37CllF2~r Total
mol % ext ~O ext %ext ~Omg/Lmg/L mg/L ODP
100 0.00 3.a63.8fi 0.00206.26 206.26 0.89
0.97 2.903.87 67.52155.15 222.67 0.62
2.09 2.114.20 144.85112.88 257.74 0.39
29 3.14 1.274.42 218.2168.19 286.36 0.21 ;~
4.14 0.724.BG 287.3138.31 325.62 0.11 -;
q 5.08 0.235.31 352.4712.19 364.66 0.02
0 6.09 0.006.04 419.350.00 419.35 0.0
TA33LE 11B
Extinguishment o n-Heptane Diffusion Flames
CF3C31FCF3 / CHF2Br Mixtures
Flow at
Extinguishment
cc/min % Added to Air
Weight S ~ -~
CF3CHFCF3CHFzBrCF3C}IFCF3 C33F2Br TotalCF3CHFCF3 ODP
-~ 20 0 1380 0 q.0 4.0 0 0.89
~ 169 4B9 1.0 3.0 9.0 30.1 0.62
;~ 353 357 2.2 2.2 4.9 56.5 0.39
533 216 3.3 1.3 4.6 76.6 0.~
705 122 4.3 0.8 5.1 87.4 0.11
I869 39 5.4 0.2 5.6 97.2 0.02 -~
1042 0 6.4 0 6.q 100.00 0.00
.. -"':.,::~`.
;' .~:
. ~ :
: ~
:
-39-
TABLE 12A
CHF2Br / CF3CHFCF2H Blends
CHF2Br CHF2BrC3H2F6 Total CHF2Br C3H2F6 Total
mol %ext % ext %ext % mg/L mgfL mg/L ODP
..
1003.85 0.00 3.85 205.96 0.00 205.96 0.89
723.01 1.16 4.16 160.69 71.93 232.62 0.62
502.48 2.47 4.95 132.70 153.24 285.95 0.41
372.13 3.70 5.84 114.06 229.71 343.77 0.30 - -
201.20 4.89 6.09 64.16 303.19 367.35 0.16
TABLE 12B
Extinguishment of n-Heptane Diffusion Flames
CF3CHFCF2H / CHF2~r Mixtures
Flow at -
Extingui~hment ;-
15cc/min % Added to Air
Weight %
CF3CHFCF2H CHF2BrCF3CHFCF2HCHF2BrTotal CF3CHFCF2H ODP ~-~
0 1380 0 4.0 4.0 0 0.89
196 508 1.2 3.1 4.3 30.8 0.62
20 421 423 2.6 2.6 5.2 53.7 0.41
637 367 3.9 2.3 6.2 66.3 0.30
843 207 5.2 1.3 6.5 82.1 0.16
1' 1 i ; I , '~
.
.
~: :
. ~ ~
- ~
t'J ~ t'J ~ ~'J '; '.~ ~
-:''
_~4~
... . . ..
TABLE 13 -
CHF2sr / F23 Blends
CHF2Br CHF2BrF23 Total CHF2Br F23 Total ~
mol % ext %ext % ext % mg/Lmg/L mg/L ODP ~ ;
. ,,
5100 3.910.00 3.91 209.31 0.0~ Z09.31 0.89 - -~
88 3.410.47 3.89 182.41 13.56 195.97 0.83
77 3.250.98 4.23 173.86 27.87 201.73 0.77
63 2.941.74 4.68 157.31 49.59 206.90 0.68
2.781.88 4.66 148.53 53.80 202.33 0.65
1052 2.642.46 5.10 140.96 70.30 211.25 0.59 :
38 2.213.58 5.79 118.17 102.18 220.35 0.48 -~
33 2.044.17 6.21 108.97 119.10 228.07 0.43
8 0.768.41 9.17 40.77 240.27 281.04 0.13
0.479.45 9.92 24.97 270.07 295.04 0.08
15o 0.0012.53 12.53 0.00 357.90 357.90 0.00
Blends of CHF2Br with CF3H are particularly useful, and a
large synergistic effect is observed. For example, a 52:48 by
mole mixture of CHF2Br and CF3H affords a 34 % reduction in
~ ~ ODP, with only a 1 % increase in the total weight of agents
i~ ~ 20 required for extinguishment. In fact, for compositions
containing between ca. 60 to 95 ~ by weight CHF2Br, the total
weight of agents required is actually less than that required
for either pure agent.
For most of the hydro1uorocarbons, the volume percent of
25 the mixture required for extinguishment is significantly less
~;: ~ than that calculated on the basis of the known extinguishing ~ -
capacity of the single components. Table 14 shows the - --
percentagejdifference between the quantity of composition
theoretically necessary, and the quantity found to be actually
30 necessary for a selection of mixtures. -~ -
. - .. , ~:-,
~ . ..' ' '~'
-41-
Table 14
Mixture Composition Extinguishing Concentrati~n, Vol %
mole ~ Theoretical Actual % Difference -
CHF2Br 52
5 CF3H 48 8.0 5.1 36
CHF2Br 50
CF3CF2H 50 6.2 5.0 21
CHF2Br 50
CF3CHFCF3 50 5.0 4.2 16 ~ : ~
10 cHF2Br 71 ~ :
CF3CHFC1 29 4.2 3.8 9 ~;
' :;',.
.
Example 9
This example demonstrates the efficacy of mixtures of
bromodifluoromethane with the extinguishing agents Halons 1301
15 and 1211. Mixtures were examined according to the procedure
of Example 8, and the results are shown in Tables 15 and 16. --
~ The data demonstrate that effective flame extinguishment -
: may be obtained with mixtures of bromodifluoromethane and the
: fire extinguishment agents Halons 1301 and 1211, and that the ~ -20 ODP can be significantly reduced materially without loss of
efficiency. For example, employing a 76:24 by mole mixture of
; CHF2Br and CF3Br affords a 69% reduction in ODP compared to
:~ pure CF3Br, and the total weight of agents required is only 5%
higher compared to CF3Br by itself.
~ -
;, ~ r~
_42_ :
TABLE 15
CHF2Br / H1211 slends
CHF2BrCHF2BrH1211TotalCHF2Br H1211 Total
mol % ext %ext %ext % mg~L mg/L mg/L ODP -
5100 ~.32 0.00 3.32177.73 0.00 177.73 0.g0
sa ~.98 0.41 3.39159.43 27.71 187.14 1.16
71 2.39 0.98 3.36127.58 65.89 193.47 1.49
56 1.79 l.g0 3.20 95.85 94.58 190.43 1.77
36 1.19 2.14 3.33 63.81 143.88 207.69 2.11
1018 0.60 2.73 3.33 31.91 184.07 215.98 2.39
0 0.00 2.99 2.99 0.00 201.65 201.65 2.65
~ :,`,,
TABLE 16 -
CHF2Br / H1301 Blends -
CHF2Br CHF2BrH1301TotalCHF2Br H1301 Total
15 mol % ext % ext % ext % mg/L mg/L mg/L ODP
100 3.46 0.00 3.46184.82 0.00 184.82 0.89 ~-~
88 2.98 0.39 3.37159.47 23.58 183.05 2.61
82 2.79 0.60 3.38148.93 36.27 185.20 3.51
76 2.37 0.75 3.13126.94 45.83 17Z.76 4.44
2074 2.22 0.78 2.99118.47 47.34 165.81 4.71
61 1.80 1.17 2.96 96.08 71.04 167.12 6.57 :~
1.20 1.83 3.02 64.02 111.05 175.06 9.37 ~ ~
21 0.60 2.25 2.85 32.07 136.78 168.84 11.72 '!.
0 0.00 2.70 2.70 0.00 164.38 164.38 14.26 -~
~: . '.~ ~':.'
Example 10 ;;;;
This example demonstrates the efficacy of mixtures of
CHF2Br with hydrochlorofluorocarbons, for example CFC 124
(CF3CHFCl) or CHF2Cl. Mixtures were tested according to the
procedure of E~ample 8, and the results are shown in Table 17
and 18.
The data demonstrate that effective flame extinguishment `~
may be accomplished by employing mixtures of ~;
`, ! ~ J
-43-
bromodifluoromethane with hydrochlorofluorocarbons, while also -;
providing significantly reduced threat to depletion of ozone. -
For example, as shown in Table 17A a 71:29 by mole mixture of
CHF2Br and CF3CHFCl provides a 28% reduction in ODP compared
5 to pure CHF2Br, and is capable of extinguishment of n-heptane
diffusion flames at a concentration of 3.8~ by volume, well
within the limit for occupied areas of ca. 10%.
TABLE 17A
CHF2Br / CF3CHFCl Blends
10 CHF2Br CHF2Br CFC 124 Total CHF2Br CFC 124 Total ~-
mol % ext % ext %ext % mg/Lmg/L mg/L ODP
100 3.14 0.003.14 168.15 0.00 168.15 0.89
71 2.68 1.093.77 143.24 60.67 203.91 0.64
43 1.78 2.344.12 95.25 130.09 225.34 0.39
24 1.11 3.524.63 59.18 195.43 254.61 0.23
16 0.88 4.615.49 46.79 256.16 302.95 0.16 -~
6 0.34 5.665.99 18.00 314.06 332.06 0.07
0 0.00 6.736.73 0.00 373.31 438.65 0.018
Table 17B
20Extingui61Dnent of n-Heptane Diffusion Flames
; ~ CF3CHFCl/CHF2Br Mixture6
CF3CHFCl CF3CHFCl CHF2Br TotalCF3CHFClCHF2Br Total
mol% wt% % added % added % added mg/L mg~ ~gLL ODP ~ -
~ l~, O O 0 3.2 3.2 0 174 174 0.89
- 2529.Q29.7 1.1 2.8 3.9 63 149 212 0.64
56.857.7 2.4 1.9 4.3 136 99 235 0.39
75.176.8 3.7 1.2 4.9 205 62 267 0.23
84.184.6 4.9 0.9 5.8 271 50 321 0.16
;~ 94.494.6 6.0 0.4 6.4 334 19 353 0.07
~; 30100 100 7.2 0.0 7.2 400 0 400 0.018
,
~:: :
; ~ :
-4 ~
TABLE 18
CHF2Br / F22 Blends - -
CHF2Br CHF2sr F22TotalCHF2sr F22 Total
mol % ext % ext %ext %mg/Lmg/L mg/L ODP
5100 3.61 0.003.61193.11 0.00 193.11 0.89
3.19 1.764.94170.32 61.73 232.05 0.67
48 2.92 3.176.09156.05 111.20 267.2S 0.54
33 2.11 4.146.26113.08 145.39 258.47 0.41
21 1.82 6.558.3797.22 229.83 327.05 0.29
1010 1.12 8.849.9559.73 310.13 369.86 0.18
o o.oo 12.9012.900.00 452.93 g52.93 0.05 ~
'.~ ' .;
In view of the above, it will be seen that the s~everal ,
objects of the invention are achieved and other advantageous ~: -
results attained. As various changes could be made in the
15 above process, compositions and systems without departing from
the scope of the invention, it is intended that all matter -
contained in the above description and shown in the
accompanying drawings shall be interpreted as illustrative and - ~
not in a limiting sense. ` `
'"''''.--". ' '
-''` . ''
: ,. -
; -
:' ~ '
~ ~;
: ' :,.
`' , ;, ~
".`";'