Note: Descriptions are shown in the official language in which they were submitted.
2027~3~ -
5-{2-[(2-AMINo-2-OXOETHYL)(METHYL)AMINO]ETHYL}-
2,3-DIHyDRo-3-HyDRoxy-2-(4-METHoxypHENyL)-l~5(sH)- ~:
BENZOTHIAZEPIN-4-ONE DERIVATIVES, THEIR PREPARATION AND
THEIR APPLICATION IN THERAPY
The present invention relates to 5-{2-[(2-amino-2-
oxoethyl)(methyl)amino]ethyl}-2,3-dihydro-3-hydroxy-2-(4-
methoxyphenyl)-1,5(5H)-benzothiazepin-4-one derivatives, to
their preparation and to their application in therapy.
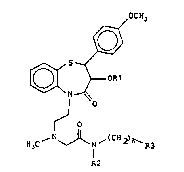
The present invention provides a compound, in the ;-
form of a pure diastereoisomer or a mixture thereof, of
- formula (I): ; .
~OC~)3
~OR1 .
~ O ~"~"',
~N ~ ~( C~ 2) ~` R3 (I)
R2
in which:
Rl represents a hydrogen atom or a C2-C4 alkanoyl
group; ; ~ .
.~
R2 represents a hydrogen atom or a C1-C4 alkyl
group; -
n represents an integer o~ from O to 3; and
R3 represents either a phenyl group bearing from 1
to 3 substituents selected from halogen atoms and
~ 202~738
trifluoromethyl, methyl, Cl-c4 alkoxy or alkylthio, nitro,
cyano, carbamoyl, acetamido, methanesulphonamido,
methoxycarbonyl or phenylcarbonyl groups, or an aromatic
heterocyclic group;
or a pharmacologically acceptable acid addition
salt thereof.
R1 is preferably a -COCH3 group. R2 is preferably a
hydrogen atom or a -CH3 or -CH(CH3)2 group. R3 i8, for
example, a pyridyl or imidazolyl group. R3 is preferably a
2-pyridyl, 1-imidazolyl or -C6H3-3,4-(OCH3)2, -C6H4-2- -~
COC6H5, -C6H3-3,4-(0cH3)2, -C6H4-3-OCH3, -C6H3-3-C1, -C6H3-
3~4 C12~ -C6H3-3~4-(CH3)2~ -c6H2-3~4~5-(ocH3)3~ -C6H4-4-
CH C H -4-CF3 -C6H4-4-F, -C6H4-4-OnC4Hg, C6H4 3
C6H4 4 OCH3~ C6H4-4-CO2CH3, -C6H4-4-Cl~ -C6H4-3-Br-4-CH3,
-C6H4-4-CN or -C6H4-4-NO2 group. The substituents on the
phenyl group represented by R3 are generally in the 3-
and/or 4-positions. When the compound of formula (I) is in
the form of a ealt, it may, for example, be in the form of
an oxalate or fumarate salt.
Since the carbon atoms at positions 2 and 3 are
asymmetric, the compounds of formula (I) can exist in
various diastereoisomeric, racemic or optically pure forms.
These various forms form part of the invention.
According to the invention, the compounds of
formula (I) may be prepared according to the scheme given
below.
2~7738
-- 3 --
SCHEME
9(II)
~(CH2)n~R3
R2
OH
~1 ( I I I J
H3C~ ~J~iN~(cH2)n~R3
R2
~OCi!l3
~VJ ~OCh3
O (O
H3C J~N~(CH2)~R3 ~-
OCH3 R2 - ~:
1~ ~ r~ ~(CN~
(VI)
~NH
H3C
; : , ,.
:;` ' '~ ''
-` 2~2773~
- 4 -
A first method consists in reacting a
benzothiazepinone of formula (V) (in which R1 is as defined
above) with a halogenated amine of general formula (IV) (in
which R2 and R3 are as defined above and Y is a halogen
S atom such as chlorine or bromine).
The second method consists in reacting a
benzothiazepinone of formula (VI) (in which R1 is as
defined above) with a halogenated derivative of general
formula (II) (in which R2 and R3 are as defined above and Y
is a halogen atom such as chlorine or bromine).
These two reactions, between either a
benzothiazepinone (V) or an amine (VI) (in the form of a ~ -
free base or of an addition salt) and a halogenated
derivative, are conventional, and hence proceed under
15 conditions well known to those skilled in the art, that is -
to say, for example, in an aprotic solvent such as acetone, ~-
2-butanone or ethyl acetate, at the refluxing temperature,
in the presence of a base to bind liberated acid (and, :
where appropriate, to liberate the base from the starting
compound), for example potassium carbonate, and optionally
in the presence of a phase transfer catalyst such as tetra-
n-butylammonium iodide or benzyltriethylammonium chloride.
It is self-evident that a compound of formula (I)
in which R1 represents a hydrogen atom may be converted to
a compound of formula (I) in which Rl represents an
alkanoyl group by an acylation of known type, and that the
^` 202~73~
- 5 -
opposite conversion is possible by hydrolysis.
The benzothiazepinones of formula (V) are described
in US Patent No. 3,562,257; those of formula (VI) are ~-
described in European Patent Applications Nos. 158,339 and
158,340.
The halogenated amine of formula (IV) may be
prepared, for example, from the alcohol of formula (III) by
the action of a halogenating agent such as thionyl
chloride. The amino alcohol of formula (III) can itself be
10 prepared according to any known method, for example from a ~ -~
halogenated derivative of formula (II) and
2-methylaminoethanol. The compounds of formula (II) are ;
accessible from chloroacetyl chloride and amines of general
formula RZ-HN-(CH2)n-R3 (in which R2, n and R3 are as ~-
15 defined above), which are described in the literature. ~ -
The Examples which follow illustrate in detail the ;-
preparation of a few compounds according to the invention.
The elemental microanalyses and the IR and NMR spectra ~`~
confirm ths structures of the products obtained. The
numbers shown in brackets for each example correspond to
those in the table which follows, and which illustrates the
structures and physical properties of a few compounds -~
according to the invention. ~ ;
:. .. '' ::.
~`''
.'
' . ' ' ': :~ ',
,~ 2 ~ 2 7 ~ 3 8
Example 1 (Compound No. lL
(+)-cis-(2S,3S)-3-Acetyloxy-2,3-dihydro-5-t2-{[2-(3,4-
dimethoxyphenyl)amino-2-oxoethyl](methyl)amino}ethyl]-2-(4-
methoxyphenyl)-1,5(5H)-benzothiazepin-4-one.
A mixture of 4 g (9.15 mmol) of (+)-cis-(2S,3S)-3-
acetyloxy-2,3-dihydro-2-(4-methoxyphenyl)-5-(2-
methylaminoethyl)-1,5(5H)-benzothiazepin-4-one
hydrochloride, 2.4 g (10 mmol) of 1-chloro-N-(3,4-
dimethoxyphenyl)acetamide and 9.9 g of potassium carbonate
in 50 ml of 2-butanone is heated to reflux for 9 h.
The mixture is filtered, the filtrate is evaporated
- and the residue is purified by chromatography on a silica
column. 5.3 g of oil are thereby obtained, which oil is
taken up with 60 ml of ethanol and treated with 0.81 g of
oxalic acid. The precipitate obtained on addition of ether
is filtered off and recrystallized in ethanol. 4 g of
oxalate are isolated.
M~lting point: 144C t~]20=+59.4 (c=0.5; MeOH).
Example 2 (Compound No. 11)
(+)-cis-(2S,3S)-3-Acetyloxy-2,3-dihydro-5-{2-t{2-t(4-
methylphenyl)methyl](methyl)amino-2- ;
oxoethyl}(methyl)amino]ethyl}-2-(4-methoxyphenyl)-1,5(5H)-
benzothiazepin-4-one.
A mixture of 4 g (9.15 mmol) of (+)-cis-(2S,3S)-3-
acetyloxy-2,3-dihydro-2-(4-methoxyphenyl)-5-(2-
methylaminoethyl)-1,5(5H)-benzothiazepin-4-one
2~277~
hydrochloride, 1.85 g (8.73 mmol) of 1-chloro-N-methyl-N-
t(4-methylphenyl)m~thyl]acetamide and 6.05 g of potassium
carbonate in 50 ml of 2-butanone is heated to reflux for
9 h. The mixture is filtered, the filtrate is evaporated
and the residue is purified by chromatography sn a silica
column. 3.5 g of oil are thereby obtained, which oil is
taken up in ethanol and treated with 1 equivalent of oxalic
acid. The precipitate obtained on addition of ether is
filtered off and recrystallized in ethanol. 2.5 g of
oxalate are isolat4d.
Melting point: 108.3-109.2C [~]20=~71.4 (c=0.3; MeOH).
Exam~le 3 (Com~ound No. 18).
(+)-cis-(2S,3S)-3-Acetyloxy-2,3-dihydro-5-{2-[{2-[2-(3,4-
,: :.. ,, ...,~
dimethoxyphenyl)ethyl](methyl)amino-2-
oxoethyl}(methyl)amino]ethyl}-2-(4-methoxyphenyl)-1,5(5H)-
benzothiazepin-4-one.
A mixture of 3 g (6.7 mmol) of (+)-cis-(2S,3S)-3- --
acetyloxy-2,3-dihydro-2-(4-methoxyphenyl)-5-(2-
20 methylaminoethyl)-1,5(5H)-benzothiazepin-4-one ---
hydrochloride, 2.4 g (8.9 mmol) of 1-chloro-N-methyl-N-[2-
(3,4-dimethoxyphenyl)ethyl]acetamide and 7 g of potassium
carbonate in 50 ml of 2-butanone is heated to reflux for ;
8 h. The mixture is filtered, the filtrate is evaporated ~
25 and the residue is purified by chromatography on a silica ; -
column. An oil is obtained, which oil is taken up in
ethanol and treated with 1 equivalent of oxalic acid. The
`- 2027738
- 8 -
precipitate obtained on addition of ether is filtered off
and recrystallized in ethanol. 2 g of oxalate are isolated.
Melting point: 90C [~]20=+52.8 (c=0.5; MeOH).
Exam~le 4 (Compound No. 20).
(+)-cis-(2S,3S)-3-Acetyloxy-2,3-dihydro-5-~2-[{2-t3-(1-
imidazolyl)propyl]amino-2-oxoethyl}(methyl)amino~ethyl}-2-
(4-methoxyphenyl)-1,5(5H)-benzothiazepin-4-one.
A mixture of 4 g (9.15 mmol) of (+)-cis-(2S,3S)-3-
acetyloxy-2,3-dihydro-2-(4-methoxyphenyl)-5-{2-
methylaminoethyl)-1,5(5H)-benzothiazepin-4-one
hydrochloride, 4 g (19.8 mmol) of 1-chloro-N-t3-(1-
imidazolyl)propyl]acetamide and 3.6 g of potassium
carbonate in 60 ml of 2-butanone is heated to reflux for
10 h.
The mixture is filtered, the filtrate is evaporated
and the residue is purified by chromatography on a silica
column. 2 g of oil are thereby obtained, which oil is taken
up with 12 ml of ethanol and treated with 0.32 g of oxalic
acid. The precipitate obtained on addition of ether is
filtered off and recrystallized in ethanol. 1.1 g of
oxalate are isolated.
! Melting point: 55C [~]20=+51.3 (c=0.5; MeOH).
D
.
` 2027~3~
g
Table
OCH3
~( ~OR1
O ( I )
H3C ' ~ Z R3
N R 1 F~2 n R3 ¦Salt I ~ ~ D2 0 M . P . (' C )
c(%),MeOH . ..
_ ._ _ _ _ '.'''' '' ''" ' ''' '
1 1 ~COCH IH ¦D ~ C6H3 4- (OCH3)2 ~03 1 03 ~1 4 ~
~ ~
COCH3 H 0 C6H4- 2 -COc6H5 ox . ~ 9 0 ,1 2 8 5
¦ 3¦COCH ICH 1 ¦ bH3-3l4-(OC 3)2 lo 1 04 ~1 0
I ~ ¦COC8 ¦N l~j C 3 3 4-(OCH3 2 13 1~34
5 COCH3 CH3 1 C6H4-3-OCH3 ox . ( 0, 3 ) 106
¦ 6 ¦COCN3¦CN3 ¦~ CbN3-3-cl lox 1 73 ¦ S
7 COCN3 CN~ i C6H3-3 4-(OCH3~2 OX 69 ~ 115
202 ~73~
1 o --
Table ( continued )
N 31 R2 n R3 ~ t I ~ 2D M P SOC)
8 COCH3 CH3 1 C6H3-3 4-C12 OX (0 5) 125
9 COCH3 CH3 1 C6H3- 3, 4-(CH3)2 OX (0695)8 92-95 5
11 COCN3 CN3 1 C6N7-3 4-5-lOCN3 3~0 67 5 143 4-1:q,6'
11 COCH3 ICR3 ¦ ~ C6Hq~4~CH3 ;ox. 71; lOH,3-109,2,
12 COCH3 CH3 1 C6H4-4-CF3 OX (O692) 126 2-128 3
13 COCH3 CH3 1 C6N4-4-F OX ~72 8 B6-88
14 COCH3 CH3 1 C6H4-4-OnC4H9 OX (0 2) 87 5-91 5
115 ¦COCH3¦CH3 1 I C6N -q-SCN3 OX ¦-55 l1 B,S 129,5¦
16 COCN3 CH3 2 C6H4- 3-OCH3 OX ~80 4 85 ~ ~ ;
17 COCH3 CH3 2 2-pyridyl ox (0S) B5
15 COCN3 CN3 2 C6H3-3,4-(0CH3)2 CX 52 H90 90
;
2027738
Table (con~luded) -~-
~; ~ R2 n R3 Salt [~D20 IM P tc)
_ _ c ( % ), MeOH ¦ . - . -
9~COC 3 CN3 C6N2-3,4,5-(ocN3 3~ .~65,4 ~I
20 COCH3 H 3 1-imidazolylox. ~51,3 ¦55
21 COCH3 I CH31 C6H4- 4 - OCH3 OX ~ 68 4 i 9 0 5
~ ~ ( 0 2 ) `
22 COCH3 CHICH ~2 1C6H~-4-CH3 fwn ~91,N 1 1-l23
23 COC 3 CH3 C6N~-4-CO2CH3~f ~ 72,5 129-131 :~
4 COC 3 CH3 C6H~-4-Clf 3n -35 129-130
25 COCH3 CH3 1C6H4-3-~r-4-CH3 ox. (0,2) 119-122 ~:
126lCCN31CH3 ¦~ ¦ C6N4-4-CN ¦fw3¦-73,5 19
27 COCN3 CN3 _ C6H~-4-NO2 f w3 ~76,2 110 1l2
Legends in the "salt" column, ox. denotes an oxalate
and fum denotes a fumarate. -
202~38
,~
The compounds of the invention were subjected to a
series of pharmacological tests which demonstrated their
value as therapeutic substances for the cardiovascular
system.
Their activity as calcium antagonists was shown by
means of a test on isolated rabbit aorta. The experimental
protocol used is a variant of that used by Godfraind and
Kaba (1969), (Blockade or reversal of the contraction
induced by calcium and adrenaline in depolarized arterial
smooth muscle, Br. J.Pharmac., 36, 549-560). The details of
the test are described in European Patent No. 0,103,500.
The molar concentration produGing a 50 % relaxation
of the response to calcium (EC50), or alternatively its
antilogarithm (pEC50), is calculated.
The pEC50 values of the compounds of the invention
range from 4.5 to 6.6.
The compounds of the invention were also subjected
to a test of binding of [3H]nitrendipine to whole rat ;
cortex. - ~
The details of the test are described in European ~ -
Patent Application No. 0,300,865.
.
The IC50 concentrations of the compounds of the
. . . -.
invention (concentrations which inhibit 50 % of the ~-
specific binding of ~3H]nitrendipine) lie betwesn 0.001 and
10 ~M.
The compounds of the invention were also subjected
to a test of inhibition of the specific binding of "PAF",
-- ~027~3~ ~
-
- 13 - -
blood platelet-activating factor, the protocol of which
test is described in European Patent Application No.
0,320,362.
The IC50 values (concentrations which inhibit the
specific binding by 50 %) of the compounds of the invention
in this test lie between 0.5 and 5 ~m.
Finally, the compounds of the invention were
subjected to a test of inhibition of blood platelet
aggregation induced by "PAF-acether" (1-0-(C16-C18 alkyl)- ~ -
2-acetyl-sn-glyceryl-3-phosphorylcholine), the protocol of
which test is also described in European Patent Application
No. 0,320,362.
The aggregation-inhibitory action of the compounds
is expressed by the IC50 concentration, the concentration
which inhibits by 50 % the aggregation produced by PAF-
acether.
The IC50 values of the compounds of the invention
in this test lie between 2 and 20 ~M.
The results of the tests show that the compounds of -
20 the invention are calcium antagonists and can, on these -~
grounds, be used for the treatment of various conditions
for which this type of agent is indicated.
Thus, in particular, they may be used in
cardiovascular medicine for the treatment of conditions
requiring modulators of transmembrane and intracellular
movements of calcium, most especially hypertension, angina
and cardiac arrhythmia.
2 0 2 ~ .~ 3 8
- 14 -
They are, in addition, capable of exhibiting
antiatherogenic, cardiac anti-ischaemic, cerebral anti-
ischaemic, antimigraine, antiepileptic, antiasthmatic and
antiulcerative effects.
In the cardiovascular field, they may be used alone
or in combination with other known active substances such
as diuretics, B-blockers, angiotensin-converting enzyme
inhibitors and ~1-receptor antagonists.
They may also be indicated for boosting the effect
10 or decreasing the toxicity of agents used in the treatment : -
of cancer or in organ transplants.
The compounds of the invention may be presented in
all forms suitable for oral or parenteral administration,
in combination with known excipients, for example in the
form of tablets, hard gelatin capsules, dragees, capsules,
and solutions or suspensions to be taken by mouth or :;~
injected.
The present invention therefore also provides a -
pharmaceutical composition which comprises a compound of
formula (I) or a pharmacologically acceptable acid addition
salt thereof and a pharamceutical excipient.
The present invention furthermore provides a
compound of formula (I) or a pharmacologically acceptable
acid addition salt thereof for use in a method of treatment
of the human or animal body by therapy, especially for use
in a method of treatment of hypertension, angina or cardiac
arrhythmia or for treatment of a condition requiring an - ;
'': '''~`
2~ 73~
- 15 -
antiatherogenic, cardiac anti-ischaemic, cerebral anti- .
ischaemic, antimigraine, antiepileptic, antiasthmatic or :
antiulcerative effect or for boosting the effect or ~- .
decreasing the toxicity of agents used in the treatment of
cancer or in organ transplants.
The daily dosage can range, for example, from 30 to
300 mg orally and from 25 to 100 mg parenterally.
: '
"