Note: Descriptions are shown in the official language in which they were submitted.
RAN 4104~180
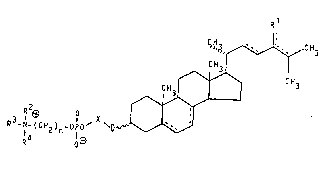
The invention i~ concerned with novel 6teroid~ of the
formula
C H ;3 f `~C H 3
C H 3 l
~ CH3
15 '~
R3 N (C!~ ) -OP0~ ~0
1 4 1~3
wherein
R is hydrogen. lower-alkyl or lower-alkylidene,
R2. R and R4 a~e hydrogen or lower-alkyl or
together with the N atom form a 5- o~ 6-membered
aromatic or ~aturated heterocyclic residue which may
be lower-alkylated,
n is the number 2, 3 OI ~,
X i~ a group of the foImula
-(CH2)p-c~Q~Q )-(Z)l. o~ O '
-CH2cH(Y)cH2-(z)l or O
-CH2cH(c~2Y)-(z~l or O
-(CH2CH~O~q-~H2cH2-(z)l or O
q i~ the number 1 or 2,
Z i5 a grou~ of the fo~mula -C(O)-~ -OC(O)-,
-OC~O)CH2-, -OCH~C(O)- or -N(T)C(O)-,
Q, Q' and T are hydrogen or lower-alkyl,
p is a whole nu~ber between 1 and 9 and, ~here Z i6
Mé/ll.9.90
s~
carbonyl, can alfio be 0,
Y i~ hyd~oxy, lower-al~oxy, lower-alkanoyloxy,
carbamoyloxy or mono- or di-lower alkyl-carbamoyl-
oxy,the dotted C-C bond6 in ~he 5(6)-, 7(8)-, 22(23)-,
24(253- and 24(2~-position are optional, whereby
the side-chain i~ either ~aturated or mono-
-un6aturated,
and ~hy~iologically compatible ~alts ~hereof.
The hydrogen atoms attached to the C a~om~ in po6ition
3 and 5 of the ~teroid part of the compound~ of formula I
can each independently have the a- or B-configuration.
The invention i8 al~o concerned with a process for ~he
manufacture of these ~teroids, medicament ba~ed on the
latter and the~e ~teroid~ a~ phar~aceutically active
substance6, as well as the usa of ~hese steroid~ in the
- manufacture of medicaments which inhibit the in~e~inal
re60rption of ~hole~terol and which lowe~ the plasma
cholesterol.
The te~m "lower" signifie that the residues denoted
thereby contain up to ~ carbon atom6 and can be ~traight-
-chain or branched. ~ethyl, ethyl, n-propyl, i~opropyl,
n-butyl ~nd t-butyl are examplefi of lower-alkyl re6idues.
Methylene and ethylidene are examples of lower-alkylidene
re6idue6. Acetoxy and propionyloxy are examples of lower-
-alkanoyloxy residue~.
PhaLmaceutically acceptable ~alt~ of the steroids of
formula I are inorgan;c 6alt8 ~uch a~ hydrochloride~ and
6ulphate6; organic ~altG ~uch a~ trifluoroaceta~e~,
mesylate~ and to~ylate6; and metal sal~ ~uch a8 sodium
salt~. The compoundfi of for~ula I can be pre~*nt in the
form of a zwitterion and tho~e in which at lea~t one of
: , ,; ~ ~ .. . .
r~
-- 3
the residues R2, R3 and R4 i~ hydrogen can be
present in the form of a hydrogen phosphate.
Among the steroids of formllla I therç are preferred
those in which Rl i~ hyd~ogen or lower-alkyl, e~pecially
ethyl, further ~hose in which E12 i~ hydrogen or
lower-alkyl and R3 and R4 are lower-alkyl. especially
methyl, or in which R2, R3 and ~4 ~ogetheL with the
N atom for~ a pyridinium or l-~ethylpyrrolidinium group.
Further, the steroids of for~ula I in which n i6 the
number 2 as well as those having a satu~ated or 5~6)-
-unsaturated or 5(6)- and 22(23)-unsaturated steroid part
are preferred.
Further preferred s~eroid6 of formula I are tho~e in
which X represents the group -(CH2)2-,
-(cH2)zo(c~2)2-~ -(CH2)20(CH2)2 ( 2 2
-CH2CHOHCH2-. -CH2CH(OCOCH3)CH2-.
-cH2cH(ocoNHcH3)cH2N[cH(cH3)2]co-~
-(CH2)2N~CO-, -(CH2)3NHCO-, -CH2CHOHCH20CO-,
-(CH(CH3)CO- or especially -(CHz)20CO-,
( 2)3 ' ( 234 ' ( H2)8
-(CH2)~0-, -(C~2~2C-- -(CH2)3C
2 ) 5CO , -CE12CH ( OCOCH3 ) CH20CO-,
-CH2C(CH3~2CO- or -CH2CH(CH20H)NHCO-.
The following are examples of pr~ferred compounds of
formula I:
O-~t2-~t(chole~t-5-en-3~-yloxy)carbonyl]oxy]çth
hydroxyphosphinyl]choline hydroxide internal 6alt,
O-[hydroxy-[3-r(3~-stigmastanyloxy)carbonyl]p~opoxy]-
pho~phinyl~choline hydroxide internal ~alt,
- -: . .
. ~ . ,. . .. - :
. :..,
~J '~ J ~ 7 ~ ~
-~12-(chole6t-5-en-3J~-yls)xy)ethoxy]hydLoxyphosphinyl]-
choline hydroxide internal 6al1: and
O-rhydro~cy-r2-~l-(5a-~eigrlafitan-3J~-yloxy)formamido]
e~hoxy]phosphinyl]choline hydroxide internal ~alt.
The following are further example~ of compound6 o
~o~ula I:
O-[r2-[t(5a-stigma~tan-3~-yloxy)carbonyl~oxy]ethoxy]
hydcoxyphosphinyl]choline hydcoxide in~ernal ~alt,
0-~hydroxy-~2- r r rE) -~ti~ma8ta-5.22-dien-3B-
yloxy]carhonyl]ethoxy]pho6phinyl]choline hydroxide
inte~nal ~alt,
0-[r3-[t(chole~t-5-en-3~-yloxyjcarbonyl]oxy]pr
hydroxyphosphinyl]choline hydroxide internal ~alt,
O-lr4-l[(cholest-5-en-3B-yloxy)carbonyl]sxy]butoxy]
hydroxyphosphinyl]choline hydroxide internal ~alt,
0-[[[8-rt(cholest-5-en-3~-yloxy~carbonyl]o~y30ctyl]-
oxy]hyd~oxypho~phinyl3choline hydroxide internal salt,
0-r~l(chole6t-5-en-3~-yloxy)carbonyl]methoxy3hydr
phosphinyl~choline hydroxide internal ~alt,
0-[[Z-~(chole6t-5-en-3~-yloxy)carbonylJethoxy]hydroxy-
pho~phinyl~choline hyd~oxide internal ~alt,
0-[hydroxy-~2-t(3~-~tigma~tanyloxy)carbonrl]ethoxy3
pho~phinyl]choline hydroxide internal salt.
thydroxy-[2-[(~tigma8ta-5~22-dien-3~-yloxy)carbonyl3
ethoxy3pho~phinyl~choline hydroxide internal salt,
- - , , ,
~, , ' ', ".,~. ,
i: , ; .
,., '~'. '` ' .' ~
, , ,
2 ~,~ r~
o-tr3-~(chole~t-5-en-3~-yloxy)carbonyl]plo~oxy]hydr
pho~phinylJcholine hyd~oxide internal salt,
0 rhydroxy-r3- r tE)-~tigmasta-5,22-dien-3~-YloxY)-
carbonyl]propoxy]phosphinyl]choline hydroxide internal
~alt,
o- rhYd~oxY- r t5-t(5a-stigmastan-3~-yloxy)carbonyl]-
pentyl~oxy]pho6phinyl~choline hydroxide internal 6alt,
0-~hydloxy-[~5-~((E)-stigmasta-5,22-dien-3~-yloxy)car-
bonyl]pentyl]oxy~phosphinyl]choline hydroxide internal
5alt,
O-thYdroxY-~[[(3B-stigmastanyl)oxy~carbonyl~methoxy]-
phosphinyl]choline hydroxide internal salt,
0-[hydroxy [[[(E)-~tigmasta-5,22-dien-3~-yloxy]-
carbonyl]methoxy]phosphinyl]choline hydroxi-de internal
~alt,
0-[hydroxy-[2-(stigmasta-5,22-dien-3fl-yloxy)ethoxy]-
phosphinyl~holine hydroxide internal ~alt,
0-t[Z-[2-~holest-5-en-3~-yloxy)ethoxy]ethoxy]hydroxy-
pho~phinyl]~holine hydroxide inte~nal sal~,
O-thyd~oxy-t2-r2-~tE)-6tig~asta-5~22-dien-3~-
yloxy]ethoxy3ethoxy]ehosphinyl]choline hydroxide internal
salt,
0-[~2-[2-t2-(chole~t-5-en 3~-yloxy)ethoxy~ethoxy]-
ethoxy~hydroxyphosphinyl]choline hydroxide internal salt,
0-[~2-1(5-chole~tan-3~-yloxy)carbonyloxy]e~hoxy]-
hydroxyphosphinyl~choline hyd~oxide internal salt,
O-rhydroxy-t2-[2-(3~-~tigma6t~nyloxy)ethoxy]ethoxy]
phosphinyl]eholine hydroxide internal 6alt,
, , ' ., ~ :; , :.
~, . . . .
:~, . : :
5.~
0-[hydroxy-~2-(3~-6ti~ma~tanyloxy)ethoxy~pho~phinyl]-
choline hydroxide internal ~alt,
l-t2-t[hydroxy-[2-[1-(5~-fitigmas~can-3~-yloxy)-
formamido]ethoxy]pho6phinyl]0xy]ethyl]pyridinium hydroxide
internal salt,
0-[hydroxy-E2 [1-t(E~-~tigmasta-5,22-dien-3B-
yloxy3forma~ido]ethoxy]phosphinyl]choline hydroxide
internal 6alt,
0-~hydroxy-r3-[l-[(E~-6tigmasta-5,22-dien-3B-
yloxy]formamido3propoxy3pho~phinyl]choline hydLoxideinternal ~alt,
O-[hydroxy-[(RS)-3-rN-i~opropyl-l-[(E~-stigma~ta-5,22-
-dien-3B-yl]oxyformamido]-2-[(methylcarbamoyl~oxy]~ropoxy3-
pho~phinyl]choline hydroxide internal salt,
-t r r2-~l-chOle~t-s-en-3~-YlOxY]~ormamido~ethoxy]-
hydroxyphosphinyl]choline hydroxide internal salt,
0-tt2-rl-(5B-chole~tan-3a-yloxy)formamido]ethoxy]-
hydroxyphosphinyl3cho~ine hydroxide internal ~alt,
1-[2-t[hydroxy-r3-[1-[(2)-stigma6ta-$,22-dien-3B-
-yloxy]formamido]p~opoxy]pho6phinyl]0xy]ethylJpyridinium
hydroxide internal 6alt,
0-[hydroxy-t2-t r t (E)-8tigma8ta-5~22-dien-3~-yloxy]-
carbonyl]oxy]ethoxy]phosphinylJcholine hydroxide internal
6alt,
5
l-t2-[[[2-[1-~chole~t-5-en-3~-yloxy3foLmamido~ethoxy~-
hydroxyphosphinyl~oxy3ethyl~ methylpyrrolidinium
hydroxide internal 6alt,
- : .
1-0-(3a,~-~tigma~tanyl~-3-0-(R5)-glyceryl-pho6pho~yl-
choline,
O-[[(RS)-Z-acetoxy-3-t5-stigmastan-3cl,~-yloxy~-
propoxy]hydro~ypho~phonyl]choline hydroxide internal ~alt,
0-[~RS)-3-[(cholest 5-en-3~-yloxy)carbonyloxy]-Z-
~hydroxypLopoxy]hydroxyphosphinyl]choline hydroxide
internal 6alt.
O-rhydroxy-(Rs)-2-hydLoxy-3-rt5a-~tigmastan-3B-
-yloxy)carbonyloxyJpropoxy]phosphinyl]choline hydroxide
int~rnal 8al~
0-[hydroxy-[(RS)-2-hydroxy-3-t~(E)-stigma~ta-5,22-
-dien-3B-yloxy]carbonyloxy]pLopoxy]phosphinyl~choline
hydroxide internal ~ialt,
O-~hydroxy-~2-~(stigmas~-5-en-3J3i-yloxy)carbonyl]oxy]-
ethoxy]pho~phinyl]choline hydroxide in~ernal ~alt,
0-[hydroxy-[3-r(~tigma~t-5-en-3~-yloxy)carbonyl]-
propoxy]phosphinrl~choline hydroxide internal salt.
O-rhydroxy-r2-(stigma~t-5-en-3~-yloxy)ethoxy]ph
phinyl]choline hydroxide inte~nal ~alt,
chole~t-5-en-3B-yl 2- r [ r2- (dimethylamino)ethoxy]-
hydroxyphosphinyl30xy]ethylcarbonate,
O-~hydroxy-r2-[1-(sti~ma~t-5-en-3B-yloxy)formamido]-
ethoxy]pho~phinyl]choline hydroxide inte~nal ~alt,
0-~(RS)-2-acetoxy-3-~(chole~t-5-en-3~-yloxy)-
carbonyl]oxy]p~opoxy]hyd~oxyphosphinyl~choline hydroxide
internal ~alt,
.
, : :. .
- 8 -
0-t[2-t~hvlest-5-en-3~-yloxy3carbonyl]-2-methylpro-
poxy]hydroxyphosphinyl]-choline hydroxide inteLnal salt and
O-[[(P~S)-2-[~-(chole~t-5-en-3~-yloxy)formamido3-~-
hydroxypropoxy]hydro~yphosphinyl]choline hydroxide
internal salt.
The s~eLoid6 of formula I and the ~alt6 the~eof can be
manufactured by
a) treating an alcohol of the formula
Rl
CH3 ~ 1'` / CH3
~ CH3 ll
HO~ ~O
with intermediary protection of a hydroxy Lesidue Y
pre6ent in the group X, with an agent which introduGe~ the
group of the formula
I ~ ~
R ~7-(CH2)n-01- (G),
R4 0
and
. . ~.
; .
'
' -
7 ~ ~
g
b) if de~ired, hydrogenating an unsatu~a~ed ste~oid of
formula I to the atu~ated steJ:oid,
c) if desired, functionally modifying a reactive residue
present i~ the group X of a steroid of formula I,
d) if de~ired, converting a ~teroid of formula I into a
~al~.
These reactions can be carried out in a manner known
per ~e. ;~
Thus, a carbonate of formula II in which X i~ a group
of the formula -(CH2)p-OC0- i~ reacted in a solvent
~uch as me~hylene chloride or chloroform in the presence
of a base such as quinoline or ~yridine firstly with a
halide of the formula PO(Hal)3, e.g. phosphorus oxy-
chloride, at room tempeLaSure and the compound obtained
can be treated with a ~alt o~ the formula
3 I W III'
R -N-(CHz)n-OH
R4
wherein W i~ lower-alkylsulphonyloxy or aryl~ulphonyl-
oxy, e.g. with choline to~ylate,
in the pre~ence of a base ~uch a6 pyridine in a fiolvent
~uch as methylene chloride at room temperature.
A carbamate of formula II in which X is a group of the
formula -(CH2)pN(T)C(0)- can be reacted fir~ely with a
halide of the formula
. ~ : - :.
., . . ~
~: . : ', . ,
' ~
-- 10 --
r~
'\ /
~ P`;~ 1
0 Hal
e.g. with 2-chloro-2-oxo-1,3,Z-dioxapho~pholane, in a
~olvent ~uch a~ tetrahydrofuran (THF) while cooling to
temperature to 0C in the pre~ence of a ba~e 6uch a~
triethylamine and the phosphate obtained can be reacted in
'che ~ame ~olvent ~ith an amine of ~che formula N~R ,R ,R )
while heating, e . g. to 50 ~o 100C.
An este~ oî formula II in which X i~ a g~oup of the
formula -CH(CE13)C0- can firstly be reacted with a halide
~uch as ~-bromoethylphosphoric acid dichloride (Pharm.
Acta Helv. 33, 1958, 349) in a 601vent ~uch a~ methylene
~hloride in the presence of a ba~ ~uch a~ triethylamine
and the ~ompound obtained can then be trea~ed in a ~olvent
such a~ t~luene with an amin~ of the f ormula
N(R2 R3 R43
As de~cribed in Example 11 hereinafter. a benzyl group
can be u~ed to protect a hydroxy residue Y pre6ent in the
group ~ of a compound of formula II. Thu~, a glycerol
deriva~ive of formula II in which X i8 a group of the
formuila -CH2CH(OH)CH2- can be reacted with tri~yl
chloride in pyridine ~o give the corre~ponding trityl
ether. Thi~ ether can be converted by mean~ of ~odium
hydride in THF a~d benzyl chloride in dimethylformamide
(DM~) at a tempe~ature up to 100C into the cQrresponding
trityl benzyl diether in which X stands for
-CH2C~(OCH2C6H5)CH2-. ~fter cleavage of the
trityl group from the diether, e.g. by mean~ o~ hydro-
,; :
: . ', ,; " . ~ . .
- , . . .......... .
,. .: ' ,
,~ ~ 4,~ J ~
~hloric acid in dioxan while heating, ~he benzyl ether :
obtained i~ ~reated as described above with an agent which
in~roduces the group
3 1 ~ D `
R -N-(CH2~n-0P- ~G).
l4
By cleavage of ~he benzyl g~oup, e.g. by hydrogenation in
the presence of palladium-charcoal (Pd/C) in methanol and
15 T~F or ~in order to avoid the ~imul~aneou~ hydrogenation ,
of double bond~ present in the ~eroid part of the ether)
in the pre~ence of palladium oxide (PdO~ in acetic acid,
there i~ obtained the glycine derivative of for~ula I in
which X i~ -CH2CH(OH)CHz-. which coLrespond6 to the
~tarting ~ompound of formula II.
A benzyl-protected steroid alcohol of formula II in
which X stands for -C~2CH(OCH2C6~5)CH2- can al60
be prepa~ed by leacting the corresponding steroid
3-chloroformate ~ith O-benzylglycerol in methylene
chloride.
As de~cribed in ~xample 22 hereinafter, a phenoxy-
carbonyl group can al80 be used for the prote~tion of a
hydroxy re~idue Y pre~ent in the group X of a compound of
formula II. Thu~, a compound of focmula II in which ~ i~ a
group -CH2CH(CH20H)N~CO- can be ~eacted with ~henyl
chlo~ofo~mate in methylene chloride, THF and pyridine. The
re~ulti~g compound containing a phenoxycarbonyloxy group
i~ then reacted, e~g. in toluene and triethylamine, with
an agent which introduce~ the group of formula G above,
e.g. with 2-chlo~o-Z-oxo-1.3~2-dioxapho6pholane, and then
., : . . ., ;
.. : . . . . :
; ... . .
~, . . ..
2 ~ 3 ~
~ 12 -
with trimethylamine in toluene and ace~onitrile. The
compound of formula 1 in which ~ i8 the ~roup
-CH2CH(CH20H)NHCo- i~ then ob~ained by cleaving off
the pheno~ycarbonyl group, e.g. using aqueou6 sodium
hydroxide.
The hyd~ogenation of an unsaturated ~teroid of
formula I can be ca~ried out in the pre6ence of Pd/C in a
solvent 6uch a~ methanol at a temperature up to 100C.
The alkanoylation of a hydroxy group Y can be
mentioned as a functional modification of a reactive
residue present in the gcoup X of a steroid of formula I.
Thus, a glycerol derivative of formula I in which ~ is the
group -CH2CH(OH)CH2- can be reacted at room
temperature in a ~olvent such as chloroorm in the
presence of a ba6e ~uch a6 pyridine and a catalyst such as
dimethylaminopyridine (D~AP) with ehe carboxylic acid
anhydride corre6ponding ~o the alkanoyl group to be
introduced.
The starting alcohol~ of for~ula II in which X is a
group of the ~ormula -(CHz)p-C(Q,Q')-Z'.
-CH2CH(Y)CH2-Z'-, -CH2CH(CH2Y)-Z'- or
- (CH2CH20)q~CH2CH2~Z ' -, Z ' i8 a group of ~he
formula -OC(0)--. -OC(O)CH2-. -OCH2C(0)- or -N(T)C(0)-
and R , Q, Q', p, q, y and T have the 6ignificance given
above are novel and as such are an object of the invention.
The alcohol~ of formula II can be prepared in a manner
known per 6e, e.g. as de~cribed in Examples A to ~ herein-
after.
Thu~, a~ alcohol e~hec of formula II in which X i6
e.g. a group -(CH2)p- can be prepared by reacting the
corre6ponding steroid-3-tosylate with the glycol
. . . . . . .
.
.
:':
. .
.
`' ' ~ ' ' '' ` "
- 13 -
H0-(CH2)p-OH in dioxan at about 120~C.
An alcohol ester of foLmula II in which X i6 e.g. a
group -(CH2)p-C0- can b~ prepared by reacting the
corresponding ~teroid-3-ol in methylene ~hloride with the
corresponding halide o the foLmula ~al-(CH2)p-COCl at
about 5C in the pre~ence of pyl id ine and converting the
halide obtained into the desired alcohol of formula II by
mean~ of pota~sium tri~luoroa~e~ate in methylene chloride,
DMF and water at about 80C.
An alcohol ester of formula II in which X i6 e.g. a
group -CH(CH3)C0 can be prepared by reacting the
corresponding ~tecoid-3-ol with lactic acid in toluene in
the presence of catalytic amount6 of p-toluene~ulphonic
acid.
An alcohol carbonate of formula II in which X is e.g.
a group -(C~z)p-~C(O)- can be prepared by r-eacting the
corresponding ~teroid-3-ol in methylene chlo~ide at about
-lODC with a ~olution of pho~gene in toluene and reacting
the stecoid-3-~hloroformate obtained in chlorofocm oc
2~ methylene chloride with a diol of the formula
H0-(CH2~p-0~ in the pre6ence of pyridine or trie~hyl-
amine at about 5C.
An alcohol carbamate of formula II in which X i~ e.g.
a group -(CH2)p-N(T)C(0)- can be prepa~ed by reacting
the corresponding steroid-3-ol in chloroform and THF with
phenyl chloroformate in the pre~ence of pyridine and
reacting the phenyl~arbonate obtained in chloroform with
the amin2 of the fo~mula H0-(CH2)p-N(T)-H~
A compound of fo~mula II in which X is a group of the
~ocmula -CH~CHEOCONH(T )]CH2N(T )CtO)- and T
and T are hydrogen or lo~er-alkyl Gan be prepaced by
,. . ~
.: . , ,
.1. .:
, , ' , ' ', ~ . , ' ,:
37~
- 14 -
reacting the corresponding l-de!oxy-l-amino-3-0-trityl-
glycerol of the for~ula T NHCH2CHOHCHzOC(C6H5)3
with a steroid-3-chlorofolmate in ~ethylene chloride in
the presen~e of pota6sium hydroxide, reacting the product
obtained in methylene ~hloride with the i~ocyanate of the
formula 0=C=N(T ) at 80C and cleaving off the trityl
group f~om the p~oduct obtained in dioxan by mean~ of
hydrochloric acid a~ 95C.
A glycerol deri~ative of formula II in which X i8 a
group -CH2CH~OH)CH~- is obtained by reacting the
co~esponding 3-0-tosyl~teroid in dioxan wi~h glycerol at
100c,
An alcohol of formula II whi~h has a double bond i~
~he ~teroid part can be hydrogena~ed to the ~orre~ponding
~aturated alcohol. e.g. in the pre~ence of Pd/C in THF.
The pre~aration of some compounds of formula II i6
described in detail in Example6 A to M hereinafter.
Exam~le_A
A ~olution of 10 g of cholesterol tosylate and 25.3 g
of ethylene glycol in 180 ~1 of dio~an i6 hea~ed to 120C
while ~tirring. The mixture is then treated with 200 ~1 of
water and e~tracted with ether. The ethereal phase is
washed fi~tly with 10% sodium caIbonate solution and then
with wa~er. The organic pha6e is dried and evapo~ated. The
residue i~ chromatographed on 6ilica ~el while eluting
with ether/hexane. There are obtained 5.6 g of 2-(cholest-
-5-en-3~-yloxy)-1-ethanol, ~.p. 92-95C. the 6tarting
material in Example 2q.
.. , ~ ,
p~ ~ ~
- lS -
Exam~le B
Analogou61y to Example A theLe are obtained:
a) 2-[[(E)-Stigma~ta-5~2-dien-3~-yl]oxy]-1-ethanol, MS:
456 (M+H ).
10 b) 2-r2-r(E~ ~tigma~ta-5,22-dien-3B-yloxy]ethoxy~-l-
-ethanol, MS: 501 (M~H ),
c) 2-[2-(cholest-S-en-3B-yloxy~ethoxyJ-l~ethanol, MS: 474
(M~H ~,
d) 2-r2-[2-~holest-5-en-3B-yloxy)ethoxy]ethoxy]ethanol,
MS: 519 (M~H ). -
Example C
1. A solution f ? g f stigma6ta~01 in 200 ml of
methylene chloride and 4 ml of pyridine i8 added dropwise
at 5C to a solution of 12.S g of 3-bromopropionyl
chloride (obta1ned from 11.1 g of bromopropionic a~id and
9~77 ~1 of oxalyl chloride in 55 ml of methylene chloride
and 3 drop~ of DMF) in 44 ml of ~e~hylene chloride. The
mixture i8 evaporated, the re~idue i6 dis~olved in 500 ml
of methylene chloride and thi~ 601ution i~ wa~hed with
dilute hydxochloric a~id. The organic pha6e is dried and
evaporated. Th~ residue i8 chro~atogLaphed on ~ilica gel
while eluting with ether hexane. There is obtained
3~-stigma~tanyl 3-bromopropionate of melting point
150-152~C.
2. Analogou~ly there are prepa~ed:
a) 5tigmasta-5,22-dien-3~-yl 3-bromopropionate,
2 -g 7 ~' r~
b) 5a-s~ig~astan-3~-yl bromoa~etate,
c) cholest-5-en-3B-yl bromoa~etate,
d~ cholest-5-en-3B-yl b~omopropionate,
e) 5a-stigmagtan-3~-yl ~-bromobutyrate~
f) chole6t-S-en-3B-yl 4-chlorobutyrate,
g) 3B-sti~mas~anyl 4-chlorobutyrate,
h) 3~-6tigmastanyl 4-iodobutyrate,
i) (E)-stigmas~a-5,22-dien-3~-yl 4-chlorobutyrate,
j) (E)-~tigma~ta-5,22-dien-3B-yl 4-iodobutyrate,
k) ~holest-5-en-3~-yl 4-iodobutyrate,
1) (E)-stigmasta-5,22-dien-3fl-yl 6-bromohexanoate,
m) 5a-stigma~tan-3~-yl 6-bromohexanoa~e,
n) ~tigmast-5-en-3~-yl 4-bromobutyrate.
3~ A ~olution of 23.1 g of 3B-~tigma~tanyl 3-bromo-
propionate in 400 ml of methylene chloride and 200 ~1 of
DMF is treated with 63.3 g of potassium trifluoroacetate
and heated a~ 80C over 72 hours. After adding 30 ml of
water the solution i~ heated at 80~C for a further 1 hour.
The solution i8 evaporated and the residue is chromato-
graphed on ~ilica gel u6ing ether-hexane. There i~
obtained 3~-~tigmastanyl 3-hydroxypropionate of mel~ing
poin~ 152-153C, the ~ta~ting matecial of ~xample 20.
. . . . : .,. , .,
.
: :
, :: :
.- 17 -
ExamP 1_
Analogou~ly to Example C there are prepared:
a) Chole~t-5-en-3B-yl glycolat:e, MS: 369 (M-HOCH~COOH),
b) 3~-~tigma~tanyl glycolate, ~S: 399 (M-HOCH2COOH),
c) cholest-5-en-3~-yl ~-hydroxypropionate, ~S: 368
(M-HOCH2CH2COOH),
d) ~tigmasta-5,22-dien-3~-yl 3~hydroxypropionate, MS: 394
(M-HOCH2CH2cooH~,
e) chole~t-5-en-3~-yl 4-hydeoxybutyrate, m.p. 98C,
f) 3~-~tig~astanyl 4-hydroxybutylate, m.p. 149C,
g) (E)-stigmasta-5,22-dien-3B-yl 4-hydroxybutyrate, m.p.
147C,
h) 5a-~tigmastan-3~-yl 6-hydroxyhexanoate, m.p. 130C,
i) (E)-stigmasta-5,22-dien-3B-yl 6-hydroxyhexanoate, m.p.
133C,
j) 6tigmast-5-en-3R-yl 4-hydroxybuty~ate, m.p. 125-126C.
ExamPle E
A 601ution of 10 g of cholesteryl chloroforma~e in
100 ml of methylene chloride and 2.16 ml of pyridine i8
added dropwi~e at 5C to a 601u~ion of 1~.8 g of e~hylene
glycol in ZOO ~1 of methylene chloride. The 601ution i~
then ~rea~ed with 300 ml of water and extracted with
methylene chloride. The organic phase i6 washed with
,,
7 .~ ~
water, dEied and evapocated. The re~idue i6 recry~tallized
in methylene chloride-ethanol. There are obtained 6.4 g of
chole6t-5-en-3~-yl 2-hydroxyethylcarbonate of melting
point 139-140C, the alcohol 6t:arting material of
Example 1.
Exam~le F
Analogously ~o Example E there are prepared:.
a) 5a-Stigma~tan-3~-yl 2-hydroxyethylcaLbonate, m.p.
184C,
b) 2-hydroxyethyl (E)-~tigma~ta-5,22-dien-3B-ylcarbonate.
m.p. 172C,
c) cholest-5-en-3~-yl 3-hydroxypropylcarbonate, m.p. 96C,
~0
d) cholest-5-en-3B-yl 4-hydroxybutylca~bonate, m.p. lQ7C,
e) chole~t-5-en-3~-yl 8-hydroxyoctylca~bonate, m.p. 79C,
f) 2-hydroxyethyl ~tigma~t-5-en-3~-ylcarbona~e, m.p.
160C.
ExamPle G
1. A solu~ion of 1.7I g of phenyl chlorsf OEmate in 5 ml
of C~C13 i6 added dropwi6e to a fiu~een6ion~ cooled to
-50C, of 4.16 g of ~tigma~tanol in 25 ml of CHC13,
12.5 ml of THF and 0.75 ml of pyridine. A further 0.25 ml
of pyridi~e îs ~hen added. The mix~ure i~ reacted at a low
temperature for 30 minutes and at ~oom temperature for a
furthec 1 hour. The reaction ~olution i6 poured into a
solution of pyridine and aqueou6 RHC03. After the
evolution of C02 has finished the ~ixture is evaporated
., ,
: ~, . ; . .
~2~
-- 19 ~
to dryne~. The re6idue is partitioned between CH2C12
and ~2 After cry~tallization from CH2C12-pentane
there are obtained ~.75 g of 3-~tigmastanyl phenyl-
carbonate. M.p. 96C.
2. 0.3 ml of ethanolamine i~ add2d to a ~olution of
1.07 g of the above phenylcarbonaSe in 3 ml of CHC13.
The mixture is r0acted at room temperature for 2 day~. The
exces~ reagent i8 di~tilled off and the phenol which
result6 in ~he reaction i~ separated a~ Na phenolate. The
compound i6 crystalli~ed~from CH2C12. There is
obtained 0.9 g of 3A-s~igmastanyl (2-hydroxyethyl)-
carbamate. ~.p. 206C, the starting ~ateLial in Example 5.
ExamPle
Analogou61y to ~xample G,
201. from ~tigma~te~ol via (E)-~tigmasta-5,2-2-dien-3~-yl
phenylcaEbonate, m.p. 156-157~C, there are obtained
a) (E)-~Sigma~ta-5,22-dien-3B-yl (2-hydroxyethyl~-
carbamate, m.p. 187C, and
b~ (E)-~tig~a6ta-S,Z2-dien-3~-yl (3-hydroxypro~yl)-
carbamate, m.p. 172-173C;
2. from chole~terol via 3~-chole6teryl ph~nylcarbonate
there i8 obtained 3~-chole~teryl t2-hydroxyethyl)
carbamate, ~.p. 168-170C;
;
3. from epicop~ostanol via epicoprostanyl phenylcarbamate
there is obtained epicopro6tanyl (2-hydroxy~thyl)-
carbamate, m.p. 98C;
~. from B-~ito~terol via ~tigma~S 5-en-3-yl phe~yl-
, ~ , , .
,. ~J~?.,~i3~
- 20 -
carbonate, m.p. 108-109C, there i6 obtained 6tigma6t-5-
-en-3~-yl (2-hydroxyethyl3carbamate, m.p. 198-199C, the
starting ma~erial in E~ample 19.
Examl~le I
1. 10 ml of a 20% phosgene ~olution in toluene are added
to a 801ution, cooled to -10C, of 4.12 ~ of fitigma6ter
in 40 ml of methylene chloride. The mixture i8 reacted
overnight and then cooled to -10C. After adding 0.7 ml of
triethylamine in 10 ml of methylene chloride the reaction
i6 continued for 24 hours. The mixture i6 neutralized with
0-7 ml of triethylamine in 10 ml of methylene chloride and
the (E~-stigma~ta-5,22-dien-3~-yl chloroformate i8
isolated.
2. A solution of 2.3 g of the above chloroformate in
10 ml of chlorofocm i~ added dropwi~e to a solution,
cooled in an ice bath, of 1.6 g of ethylene glycol and
0.5 ml of pyridine in 10 ml of chloroform. The reaction
mixture ifi poured on to ice and a ~odium bicarbonate
solution. The mixture is e~tracted with ~ethylene
chloride, chromatographed on silica gel with toluene~
chloroform/ethyl acetate (4/2~1 volO). Af~er cry~tal-
: lization from methylene chloride-~ethanol there are
obtained 1.86 g of t~)-s~igmasta-5DZ2-dien-3~-yl
l2-hydroxyethyl)carbamate, m.p. 172-173C, the alcohol
~tartin~ ~aterial of E~ample 9.
ExamPle J
1. A ~olutio~ o~ 1.45 g of 3-0-to6yl~tigma~t~rol in 20 ml
of dioxan i reacted with 2.5 g of glycerol for ~ hou~ at
100C while ~irring. After di6tillation of the ~ol~ent,
dilution of the residue wi~h water, extraction with
diethyl ether and cry~alliza~ion from methylene chloride-
. ... ..
'~.: . .
': .
~ 21 ~
-methanol there i~ obtained 1 g of 0-3,B-stiqmasteryl-
-glycerol .
2. A solution of 1.7 g of the above product in 15 ml of
THF is hydrogenated over 0.5 g of 10% Pd/C under normal
pressure. After removal of the cataly~t, dis~illation of
the solvent and crystallization from THF-methanol there is
obtained in quantita~ive yield 0-3a,~-stigmastanyl-(RS)-
-glycerol, the starting material of Example 11.
~xample_K
1. 0.74 g of (RS)-glycidol and Z.R g of tcityl chloride
in 5 ml of pyridine are reacted overnight. A ~olution of
2 g of potassium bica~bonate i8 then added while sti~riny.
After evaporation, partition of ~he cesidue between water
and methylene ~hloride and chcomatography on 6ilica gel
2~ with toluene there are obtained 2.25 g of (RS)-epoxy-l-0-
-tcitylglycerol.
2. 3 g of this product are reacted with 5 ml of
isopropylamine in a bomb tube at 100C fo~ 2 hours. Af~er
distilling off the excess ifiopropylamine 4.4 g of
(RS)-l deoxy-l-isopropylamino-3-0-tritylglyceeol, m.p. ,
128-130C, are ccystallized from methylene chloride-hexane.
3. 1.04 g of the above amine in 20 ml of me~hylene
chloride and 0.2 g of potassium hydroxide in 2 ml of water
are added dropwise while stirring to a solution, cooled to
-10C, of 1.1 g of stigmasteryl chloroformate in 4 ml of
methylene chloride. After reaction at room ~emperature for
Z hours, pha~e sepaLation in a ~eparating funnel,
chromatography on silica gel with methylene chloride-
-diethyl ether (2/1) there ace obtained 1.63 g af
(E)-stigmasta-5,22-dien-3~-yl isopropyl-[(RS)-~-hydroxy-
-3-trityloxypropyl]-carbamate, m.p. 75-75vc.
.. .~ ., .
, ,' '' , . : ~ ,
,
''' .. '' '
~ ,73
.... - ~2 -
4. A solution of 2 g of the above product in 10 ml of
methylene chloride i6 ~eacted with 2 ml o~ methyl
iRocyanate in a bomb tube at 80C for 40 hour6 and there
i~ obtained in quantita~ive yield (E)-~tigma~ta-5,22-dien-
-3~-yl i60pcopyl-[(RS)-2-methylcarba~oyl-3-trityloxy-
propyl]-carbamate, m.p. 92-93C.
lo 5- By cleaving off the tLityl group analogously to
Example llc there are obtained from 2.5 g of the above
trityl ether 1.7 g of (E)-stigmata-5,22-dien-3~-yl
[~RS)-3-hydroxy-2-[(methylcarbamoyl)oxy]pLopyl]-isopropyl-
carbamate, m.p. 240C, the 6tarting material in Example 7c.
~m~ . .
A solution of 10.2 g of chole~teryl chloroforma~e in
130 ml of methylene chloride i8 treated under argon while
~tirring with 4.6 g of 2-0-benzylglycerol in 120 ml of
methylene chloride and 2 ml of pyriidine, ~a-ken up in
500 ml of water and ~0 ml of lN HCl after 1 hour and
extracted with methylene chloride. The organic pha6e is
dried and concentrated at 50C. ~fter chromatography over
SiO2 with n-hexanie:ether (1:1) there are obtained 6.17 g
of (RS)-2-(benzyloxy)-3-hydroxypropyl chloride 5-en-3B-
-ylcarbona~e, MS: 595 (M~H ), the ~tartin~ material of
Exampls 13.
~a~
A ~olution of 3.~6 g of cholesterol and 0.~5 ~1 of
L-(~)-lactic acid (90% in water) in ao ml of toluene i6
boiled ~or 1 hour. The water i~ separated, the cooled
reaction mixture i~ treated with 0.3 g of p-toluene-
iulphonic acid ~nd boiled for a Purther 4 hour6. The
reaction mixture i~ treated with 30 ml of water and
extracted with ether. The organic pha6e i~ dried over
.
2~2$7~9
- 23 -
~odium ~ulphate and concen~rated. The residue i~ purified
on silica gel (elution agent petroleum ether/ether 4~
The~e are ob~ained 1.5 g of choles~-5-en-3~-yl (S)-2-
-hydroxyeropionate, m.p. 1~0-122~C.
The ~teroid~ of formula I and the 6alt~ thereof
inhibit ~he intestinal re~orption of cholesterol.
The inhibition of the intestinal reso~ption of
cholesterol can be demonstrated a~ follow~ in an animal
experiment:
Squirrel monkey~ are orally administered the
substances to be investiga~ed together with a te6t feed
containing a protein, stacch, triolein and ~26_14C]_
-cholesterol. Thereafter, the faece~ i~ collected for
2.5 days. The difference between the administered and the
collected radioactive chole6terol determined ln the faece~
i~ taken as the measurement of resorbed cholesterol. The
cholesterol resorption (CHORES~ i~ expres~ed in
eercentages of the control ~alue~ determined prior to the
medication.
The resul~s which have been ob~ained with ~ome
representative eroduct~ in accordance with the invention
are reproduced in ~he Table hereinafter. There are given
for each of the compounds indicated the~ein the
chole~tecol ce~orption, determined at a dosage of
100 ~mol/kg p.o., in percentage~ of that in the
pre-period. Moreove~, the Table contains data concerning
the acute toxicity of the compound~ investigated ~LD50
in mg/kg in the ca~e of ~ingle oral or intravenou~
admini~tration to mice).
,. "
,
- 24 -
Tabl~
.. _ __ _ _ _ _
Compound of formula I
of Example No. 2a 2k 2q 2r 4a
10 CHORES in % of the pre-period: 38 39 16 37 30
._ _ .. __ _____ _ __ T--------
LD~o in mg/kg p.o. 4000 4000 4000 4000
--- __
i.v. 250 25~ 250 500
Compound of formula I
of Example No. 5 6 7a 16
CHORES in % of the pre-period:40 31 3~ 40
-
LD50 in mg/kg p.o. 4000
_ _ . _ _ . _ .
The products in accordance with the invention ean be
u6ed a6 medicamen~s. e.g. in the form of pharmaceutical
preparations. The pharmaceutical ereParation~ are
administered orally, e.g. in the form of tablet6, coated
- tablet6, dragee~, hard and soft gelatine cap6ules,
35 601utions, emul6ion~ or 6uspen6ion~.
For the manufacture of pharmaceutical preparation6,
the products in accordance with the invention can be mixed
' t ~ . ;"1:, ",, , ~ " ,~ ." .
, ~
; : :
- , ~. . . ~.. . :.,
-:. . : .. ,.i.:
~ 3;i) ~J
- 25 -
with pharmaceutically inert, inorganic or organic
carriers. Lactose, maize starch, talc, ~tearic acid or it6
~alts can be u~ed, for example, as carrier~ for tablet6,
coated tablets, dragee~ and hacd gelatine cap~ule~.
Vegetable oil~, waxe~, fats or semi-solia and liquid
polyols are, for example, ~uitable carriers for soft
gelatine capsules; depending on the nature of the active
8ub6tance no carrier i8, however, generally required in
the ca~e of soft gelatine cap~ules. Water, polyol~,
~accharo~e, i~vert ~ugar and gluco6e are, for exam~le,
~uitable cacrier~ for ~he manufacture of solution~ and
~yrup6.
The pharmaceutical erepara~ions can, moreover, con~ain
pre6erving agen~ olubilizer6, ~tabilizing agen~s,
wetting agent~, emulsifying agents, 6weetening agent~
colouring agents, flavouring agent~, ~alts for varying the
osmotic pressure, buffer6, coating agents or antioxidant~.
They can also con~ain still other therapeutically valuable
substances.
As mentioned eaLlier, medicaments containing a steroid
o foLmula I or a pharmaceutically acceptable ~alt thereof
are al~o an object of the pre6ent invention, furthermore
also a proce6~ for the manufacture of ~uch medicaments
which compri~es bringing one or more product6 in
accordance with the invention and, if de6ired~ one or more
o~her therapeutically valuable sub~tance into a galenical
admini~tration form. As mentioned earlier, the product~ in
accordance with ~he invention can be used in the control
or prevention o illnes6e~.
5They can be used e~pecially in the control or
prevention of hypelchole6terolemia and of athero6clero6i~.
The dosage can vary within wide limit6 and will, of
course, be fitted to the individual requirements in each
.
' ~ .
- 26 -
particular case. ln general, in the ca6e of oral
admini~tration a daily do~age of about 50 mg to about 3 g,
pref~Lably of about 20Q mg to about 1 ~, ~hould be
appropriate.
The manufacture of compound~ of formula I i~ described
in the Examples hereinafter.
Example 1
A fiolution of 48.7 g of chole~t-5-en-3~-yl 2-hydroxy-
ethylcarbonate and 10 ml of quinoline in 500 ml of
methylene chloride i~ added dropwi6e at room t~mpera~ure
to a 601ution of lZ.5 ml of phosphoru6 oxychloride. ~he
solution i~ treated at room tempe~a~ure while stirring
with 60 ml of pyridine and 77 g of choline to~ylate in
500 ml of methylene chloride, whereupon the reaction
mixtuce is stirred at roo~ temperatuce overnigh~. The
mixture i~ treated with lZ5 ml of water and 34 g of ~odium
bicarbonate and then with 3000 ml of acetone. The
precipi~a~ed product i~ filtered off under ~uction,
dissolved in 1000 ml of chloroform~methanol 1:1 and
~tirred with 500 g of ion excha~ger (Amberlite ~B-3~. The
latter is filtered off under suction and the solution is
evaporated. The re~ultinq re6idue i~ recrystallized in a
mixture of ~ethylene chlorid~-methanol 1:1 and dioxan.
There are obtained 39 g of 0-t~2-trtcholest-5-en-3~-
-yloxy)carbonyl]oxy]ethoxy~hydroxypho~phinyl]choline
hydroxide internal ~alt of melting poin~ 224C, MS: 640
(M~H) .
Exam~le 2
Analogou~ly to Example 1,
a) starting from 5a-stig~a~tan-3~-yl 2-hydro~yethyl-
.... ~ .,.. . .. .
.. . . , . ~ , ~ .
,
. . : ,~.. ;~: :::
.: . ~
~ ~ ~ 7 ~
carbonate there i6 obtained 0-l[~2-[[(5a-~tigmastan-3~-
-yloxy)carbonyl~oxy~ethoxy]hydroxypho6phinyl]choline
hydroxide internal ~alt, m.p. ;220C
b) 6tarting from Z-hydroxyethyl (E)-~tigmasta-5,2Z-dien-
-3~-ylcarbonate there is obtained 0-thydroxy-[2-[[tE3-
-~tigma~ta-5,22-dien-3~-yloxy]carbonyl~ethoxy~phosphinyl]-
choline hydroxide internal ~alt, m.p. 220~C
c) ~tarting fro~ cholest-5-en-3~-yl 3-hydroxypropyl-
carbonate there is obtained 0-rr3-~[(cholest-5-en-3B-
-yloxy)ca~bonyl]oxy]propoxy~hydroxypho~phinyl]choline
hydroxide internal ~alt, m.p. 230C
d) 6tarting from cholest-5-en-3A-yl 4-hydroxybutyl-
ca~bonate there i~ obtained 0-rr4-~[(cholest-5-en-3~-
-yloxy)carbonyl]oxy]butoxy~hyd~oxypho6phinyl~choline
hydroxide internal 6alt, ~.p. 232C
e~ starting from cholest-5-en-3~-yl 8-hydroxyoctyl-
carbonate there i~ obtained 0-[[[B-[~(cholest-5-en-3B- ~ -
-yloxy)carbonyl~oxy~octyl]oxy]hydroxypho~phinyl~choline
hydroxide internal 6alt, m.p. 235C
f) starting from chole~t-5-en-3~-yl glycolate there i6
obtained 0-[[[~holest-5-en-3~-yloxy)carbonylJmethoxy]-
hydroxypho~phinyl~choline hydroxide internal salt, m.p.
226C
g) starting from cholest-5-en-3~-yl 3-hydroxypLopionate
there i~ obtained O-rr2-ttcholest-5-en-3~-yloxy)carbonyl~-
e~hoxy]hydloxyphosphinyllcholine hydroxide inte~nal ~alt,
m.p. 180C
h) staLting from 3~-~tigmastanyl hydroxypropionate the~e
is obtained O-thydroxy-[Z-t(3~-sti~ma~tanyloxy)carbonyl]-
'` ~' '
` .
,
, .. . . . .
7~
- Z8 -
éthoxy]phoEphînyl]cholin2 hydroxide inteLnal 6alt, m.p.
187C
i3 starting from stigmasta-5,;22-dien-3~-yl 3-hydroxy-
propionate there i~ obtained O-[hydroxy-[2-[(~tigmasta-
-5,22-dien-3~-yloxy)carbonyl~ethoxy]pho6phinyl~choline
hydroxide internal ~alt, m.p. 199C
j) 6tarting from chole~t-5-en-3~-yl 4-hydcoxybutyrate
there i~ obtained O-rt3-rtcholest-5-en-3~-yloxy)carbonyl]-
propoxy]hydloxypho6phinyl~choline hydroxide internal 6alt,
m.p. 255C
k) 6tarting from 3~-stigma~tanyl 4-hydroxybutyrate there
i~ obtained 0-thydroxy-~3-t(3~-6tigma6tanyloxy)ca~bonyl]-
propoxy]phosphinyl]choline hydroxide internal salt, m.p.
250C
1) ~tar~ing from (E)-6tigmasta-5,Z2-dien-3~-yl
4-hydroxidebutyrate there i& obtained 0-~hydroxy-[3-r(E)-
-~tigma~ta-5,22-dien-3~-yloxy)carbonyl]propoxy]pho6phinyl~-
choline hydroxide internal salt, m.p. 2Z5C
~5
m) starting ~rom 5a-~tigma~an-3~-yl 6-hydroxyhexanoate
there is obtained 0-~hydroxy-[[5-~(5a-6tigma~an-3~-
-yloxy)carbonyl~pentyl]oxy]phosphinyl]choline hydroxide
internal 6al~, m.p. 254C
n~ ~tarting from ~E)-~tigma~ta-5,22-dien-3~-yl 6-hydroxy-
hexanoate there i6 obtained 0-rhydroxy-~5-[((E)-
-~tigma6~a-5,2Z-dien-3~-yloxy)carbonyl~pentyl]oxy]-
phosphinyl]choline hydroxide internal 6alt, m.p. 215C
o) s~arting rom 3~-~tigma~tanyl glycolate ~he~e is
obtained 0-[hydroxy-r[~3B-6tigma~anyl)oxy]carbonyl3-
methoxy]phosphinyl]choline hydroxide internal ~alt, m.p.
,.,. ~,,, :
. . ::.. :.
- ,~. ~ .
"~;.' :,, :
2~2~7~
Zg
260C .
p) starting from ~)-stigmasta-5,22-dien-3~yl-yl
glycolate (not i~olated) there is obtained 0-~hydroxy-
-r tt(E)-stigma6ta-5,Z2-dien-3~-yloxy]carbonyl]me~hoxyJ-
phosphinyl]~holine hydcoxide im~ernal salt, m.p. 215C
9) star~ing f~om Z-(cholest-5-en-3~-yloxy)-1-ethanol
there is obtained 0-rr2-(cholest-5-en-3~-yloxy)ethoxy]-
hydroxyphosphinyl]choline hydroxide internal salt, MS: 595
(M~H )
r) startin~ from 2-[t(E)-stigmasta-5,22-dien-3~-yl]oxy]-
-l-ethanol there is obtained 0-~hydroxy-~2-(stigmasta-
-5,22-dien-3~-yloxy)ethoxy]phosphinyl~choline hydroxide
internal sal~, ~.p. 230C
5) starting ~rom 2-r2-(chole6t-5-en-3~-yloxy)ethoxy~
-ethanol there is obtained 0-rr2-[2-(cholest-5-en-3~-
-yloxy)ethoxy]ethoxy]hyd~oxyphosphinyl]choline hydroxide
intecnal salt, MS: 640 (M~H )
t) starting from 2-[2-[(E)-stigmasta-5,22-dien-3~-yloxy]-
ethoxy]-l-e~hanol there i8 obtained 0-thydroxy-[2-r2-r~E)-
-stigmasta-5,22-dien-3~-yloxy]ethoxy]ethoxy~phosphinyl]-
choline hyd~oxide internal ~alt, MS: 666 (M+H j
u~ sta~ting from 2-t2-rZ-(chole t-5-en-3~-yloxy~e~hoxy]-
ethoxy]-ethanol thece i8 obtained 0-~[2-~2-r2-(cholest-5-
-en-3~-yloxy)ethoxy]ethoxy]ethoxy]hydroxyphosphinyl]choline
hydroxide int~rnal salt, ~S: ~84 (~+H ).
_a~
1 g of 0-[~2-[~cholest-5-en-3~-yloxy)carbonyloxy]-
ethoxy~hydcoxyphosphinyl]choline hydroxide internal salt
:
,,
~ '3~5'J~
-
-- 30 --
is hydrogenated in 100 ml of methanol with 1 g of Pd/C 5%
under 30 bar of H2 at 80C. After filtration the
solution i8 evaporated and the residue is dis601ved in
chloroform-methanol-dioxan. By aclding ether there i6
obtained 0.6 g of 0-r~Z-[(5-chol,estan-3~-yloxy)car-
bonyloxy]ethoxy]hydroxyphosphinyl]choline hydroxide
internal salt as a hygroscopic powder, m.p. 2250C.
Example,~
In an analDgous manner to Example 3,
a) using 0-[hydroxy-[2-[2-r(E)-stigmasta-5,22-dien-3~-
-yloxy]ethoxy]ethoxy]phosphinyl]choline hydroxide internal
salt there is obtained 0-[hydroxy-[Z-[2-(3~-~tigmastanyl-
oxy)ethoxy]ethoxy]phosphinyl]choline hydroxide internal
salt, MS: 670 (M+H )
b) using 0-~hydroxy-r2-(stigmasta-5,22-dien-3~-yloxy)-
ethoxy]phosphinyl]choline hydroxide internal salt there is
obtained 0-[hydroxy-~2-(3~-stigmastanyloxy)ethoxy]-
phosphinyl]choline hydroxide internal salt, m.p. 200C.
Example_5
1 g of 3~-stigma~tanyl (2-hydroxyethyl)carbama~e and
0.35 ml of Et3N are dissolved in 5 ml of THF. A solution
of 315 mg of 2-chloro-2-oxo-1,3,2-dioxaphoseholane in 3 ml
of THF is added dropwise to the solution, which is cooled
30 in an ice bath. The suspen6ion obtained i6 stirred at room
temperature for 5 hours. The Et3N~HCl precipitate is
then filtered off and the solution remaining behind i6
evaporated.
The phosphate obtained i6 dissolved in 10 ml of THF,
the ~olution is treated in a pressure flask with 1 g of
,:
, ..
.
,: .. ~ ,
:,
. : .
.. . . ..
... .
(CH3)3N in 10 ml of THF. The mixture i~ reacted at
70C for 2~ hour~. After di6ti~Llation o~ the exce~6
reagent and of the ~olven~ the residue is taken up in
20 ml of ~eOH-CHC13 and the ~olu~ion i6 percolated
through an ion exchangee (Ambe~lite MB3). The ~roduct i~
chromatographed on æilica gel with CHC13-MeOH-H20
(S0/35/5 in vol.). Ther~ i8 obtained 0.6 g of 0-[hydroxy-
10 -r2-[l-(5a-~tigmastan-3B-yloxy)formamido]ethoxy]-
phosphinyl]choline hydroxide internal sal~, MS: 669
(M~H ).
Example 6
The preparation of the phosphate i8 repeated as
de~cribed in Example 5. The material obtained from 2 mmol
of ~tarting carbamate i~ di~solved in 5 ml of dry pyridine
and reacted at B0C for 24 hour~. The produ~t is i~olated
and purified analogously to Example 5. There i8 obtained
0.95 g of 1-[2-~[hydroxy-~2~ 5-s~igma~tan-3~-yloxy)-
formamido]ethoxy]pho~phinyl~oxy]ethyl]pyridinium hydroxide
internal salt, ~S: 689 (~+H ).
Example 7
Analogou61y to Example 5,
a) ~tarting from (E)-~ti~ma~ta-5,22-dien-3~-yl
52-hydroxyethyl)carbamate there i6 obtained 0-rhydroxy-~2-
-rl-[(E)-~tigmasta-5,22 dien-3~-yloxy]~ormamido]ethoxy~_
pho~phinyl]~ho].ine hydroxide internal 6alt, ~S: 665
(M+H )
b) ~ta~ting ~rom (~)-stigmasta-5,22-dien-3~-yl
~3-hydroxypl~pyl)carbamate there i6 obtained C-~hydroxy-
-t3- rl-r (E~-8tigma~ta-s~22-dien-3A-yloxy]foLmamido]-
propoxy]pho~phinyl~choline hydroxide internal saltJ MS:
'` : . : .
: ;
3 l ~ ~
- 32 -
679 (~H~)
c~ ~tar~ing from (E~-stigmasta-~,Z2-dien-3~-yl ~(RS)-3-
-hydroxy-2-r(methylcarbamoyl~oxy~propyl]-i~opropylcarbamate
there i~ obtained 0-rhydroxy-rRS)-3-~N-i~opropyl-l-~(E)-
-~tigma6ta-5,22-dien-3B-rl]oxyformamido]-2-r(methyl-
carbamoyl)oxy]propoxy]phophinylJcholine hydroxide
internal 6alt, MS: 794 (M~H )
d) star~ing from 3~-chole~teryl (2-hydroxyethyl)carbamate
there i~ obtained 0-[[[Z-rl-chole~t-5-en-3~-yloxy3-
formamido]ethoxy]hydLoxypho~phinyl]choline hydroxide
internal ~alt, MS: 63g (M~H )
e) starting from epicopro6tanyl (2-hydroxyethyl)carbamate
there i~ ob~ained 0-t~2-tl-(5~-chole~an-3-yloxy)-
formamido]ethoxy~hydroxyphosphinyl]choline hydroxide
internal salt, ~S: ~41 (M+H ~.
Example 8
Analogou~ly to Example S, ~tarting from (E)-stigmas~a-
-5,22-dien-3~-yl (3-hydroxypropyl)carbamate there i~
obtained 1-~2-~thydroxy- r 3-tl-t(E)-~tigma6ta-5,22-dien-3B-
-yloxy]formamido]propoxy]phogphinyl]oxy]ethyl]pyridinium
hydroxide internal ~alt, ~S: 699 (M+H ).
Example 9
A solution of 2.22 g of (E)-stigma6ta-5,22-dien-3B-yl
(2-hydroxyethyl)carbonate and 1.1 ml of pyridine in 10 ml
of chloroform i~ added dropwise to a solutio~0 cooled to
0C, of 0.75 g of pho~phoru~ oxychloride in 3 ml of
chlorofoem. The mixture i6 reacted at room ~emperature for
1 hour. Then, 1.6 g of choline tosylate in 15 ml of
pyridine are added. The mixture is stirred o~ernigh~, then
.
~ ~t f S'.~
a ~olution of 2 g of pota~sium bicarbonate i6 added. The
mixture i~ evaporated to dryne~s6 and ehe re~idue i6 taken
up in 100 ml of THF/methanol/chloro~orm (1/1/1). The
solids are filtered oEf. The ~olution remaining behind i8
percolated over an ion exchang,er ~Ambe~lite MB 3). After
chromatography on silica gel with chloroform/methanol/
water ~60/35/5 vol) and cry~tallization from methylene
chloride/acetone there are obtained 1.6 g of 0- rhydroxy-
-[2-~[~(E)-~tigma~ta-5,22-dien-3B-yloxy]carbonyl]oxy]-
ethoxy~phosphinyl]choline hydroxide internal ~alt, MS: 666
(M~H ).
Example 10
4.75 g of 3~-chol~stelyl (2-hydroxyethyl)carbamate are
reacted with 2-chloro-2-oxo-1,3,2-dioxaphospholane. The
phosphate obtained i6 treated wi~h N-methylpyrrolidine at
70C for 24 hour~. After chromatography on ~ilica gel with
~hlorofoLm/me~hanol/water t30/62.5f7.5 vol.) there are
obtained 1.2 g of 1-r2-~tr2-~l-rcholest-5-en-3~-yloxy]-
formamido]ethoxy]hydroxypho~phinyl]oxy~ethyl]-l-methyl-
pyrrolidinium hydroxide internal ~alt, MS: 665 ~M+H ).
ExamPle 11
a) 1.41 g of trityl chloride are added to a 601ution of
2.49 ~ of 0-3a,~-~itigmaRtanyl-(RS)-glycerol in 10 ml of
pyridine. After reaction for 48 hours a potafi~ium
bicarbona~e ~olution is added while stirring. The mixture
i8 evaporated to drynes~. The residue i~ partitioned
between ~ethylenie chloride and water. After chLomato~raphy
on ~ilica gel with methylene chloride there i6 obtairLed
l-0-trityl-3-0-(3a,B-~tigmastanyl)-~RS)-glycerol.
b) Z.4 g of the above trityl ether in 10 ml of THF and
then 0.46 ml of benzyl chloride in 5 ml of DMF are added
:
rJ
-- 34 --
dropwi6e to a 6u~pen~ion of 96 ~g of ~odium hydride in
5 ml of THF. After reaction at 80C for 2 hours, distil-
lation of the solvent and partition of the residue between
methylene chloride and water ~he~e is obtained l-0-trityl-
-2-0-benzyl-3-0-~3a,~-stigma6tanyl)-(RS)-glycerol.
c) 2 ml of lN hydrochloric acid are added to a 601ution,
heated to 95~C, of 2.4 g of the above benzyl ethe~ in
20 ml of dioxan. After reaction a~ 95~C for Z hourfi the
solvent is distilled off, the residue i~ taken up in
hexane and the precipitated triehenylme~hanol i&
~eparated. Afte~ ch~omatog~aphy on ~ilica ~el with
toluene/ethyl acetate (9/1) there are obtained 1.5 g of
1-0-(3a,B-6~ig~astanyl)-Z-0-benzyl-(RS)-glycerol.
d) Analogously to Example 5, from 1.4 g of the above
glycerol de~ivative fir6tly by reaction with 2-chloro-2-
-oxo-1,3,2-diox2pho pholane and then reaction of the
re~ulting phosphate with trimeehylamine there is obtained :~
0.8 g of 1-0-(3,~-stigmastanyl)-2-0-benzyl-3-0-(RS)-
-glyceryl-pho6phorylcholine, MS: 7~6 (M~H ).
e) A solution of 0.77 g of the above benzyl ether in
10 ml of methanol and 5 ml of THF i~ hydro~enated under
normal pressure in the presence of 10% Pd/C. There is
obtained in quantitative yield 1-0-(3a,B-stigmastanyl)-
-3-0-(RS)-glyceryl-phosphorylcholine, MS: ~56 (M+H ).
Exam~le 12
50 mg of DM~P and 0.5 ~1 of acetic anhydride are added
to a solution of 0.5 g of the glycecol derivative of
Example 11 in 3 ~1 of chloroform and 1 ml of ~yridine.
AfteL reaction for 1 hour the mixture is concentrated to
dryness. The ~olution of the residue in Z0 ml of methanol
i6 percolated over ion ~xchanger ~Ambellite ~B-3). There
-, ,. - ; ~ . ~
- ~ ' : , :: ::
, ~,
~$3~
- 35 -
is obtained 0.51 g of O-~r(RS)-2-acetoxy-3-[5a-
-~tigmastan-3a,~-yloxy]propoxy]hydroxypho~phonyl]choline
hydroxide internal salt, MS: 698 (~H ).
Examp]e 13
a) 1.15 ml of phosphoru~ oxychloride are treated under
argon and while stirring with 6.1 g of (RS)-2-(benzyloxy)-
-3-hydroxypropylchole~t-5-en-3~-yl carbonate and 1.5 ml of
quinoline in 80 ~1 of methylene chloride. There ace added
thereto after 4 hours 7.4 g of choline to~ylate and 6 ml
of py~idine and after 24 hours 15 ml of water and 4 g of
NaHCO3. Afte~ 30 minutes the mixture i~ poured into
500 ml of water and 50 ml of lN HCl and extracted wi~h
chlorofo~m. The organic pha~e is concentrated. The product
i~ dis~olved in 150 ~1 of MeOH-CHC13 (2:1) and tceated
with 100 g of ion exchanger (A~be~lit MB3). Af~er stirring
for 16 hou~ the ion exchanger iB filtered off under
~uction. The solution obtained is concentrated at 60C,
distilled with toluene and d~ied. The residue i6 purified
over ~ilica gel with CHC13-MeOH (1:1) and CHC13-MeOH-H2O
(60:35-5). After drying there are obtained ~.9 g of
O-r[(RS)-2-benzyloxy-3-[(cholest-5-en-3B-yloxy~carbonyl_
oxy]propoxy]hydroxypho~phinyl]choline hydroxide internal
~al~, yield: 63~. MS: 760 (M~H ).
b) 4.65 g of the product of a) and 1.~ g of PdO in 200 ml
of CH3COOH are stiLr~d under H2 undec normal pre~sure
for 20 minute6. After f;ltLation of the cataly~t the
solution i~ concentrated at 60C. The residue i6 washed in
200 ml of ether, filtered and dried. The re~idue is
dissolved i~ 40 ml of CHC13-MeOH (1:1) and treated with
25 ml of dio~can and 200 ml of ether. The separated
cly~tal~ a~e filtered off under ~uction, washed wi~h ether
and dried at 50C. There are obtained 3.25 g o~ o-r ~ (RS)-
-3-t(chole~t-5-en-3~-yloxy~arbonyloxy]-2-hyd~oxyproeoxy]-
.
.
2~7~ ~3
- 36 -
hydroxyphosphinyl]choli~e hydroxide internal salt
(strongly hygroscopic), yield: 793, MS: 670 (M~H ~.
xamP:Le 14
Analogou~ly to Example 13 there can be manufactured:
a) 0-~Hydroxy-~RS)-2-hydroxy-3-~r5-~tigmastan-3~-
-yloxy)carbunyloxy]p~opoxy]pho phinyl]choline hydroxide
internal ~alt ~epimer6 1~ S: 700 (N~H )
b~ 0-rhydroxy-[(RS~-2-hydroxy-3-[t(E)-stigma~ta-5,22- -
15 -dien-3~-yloxy]carbon~loxy]plopoxy~pho~phinrl]choline ~,
hydroxide internal salt (epimel~ 1:1), MS: 696 (~H )0
xam~e~l5
a~ A Bolution of 1 g of chole~t-5-en-3~yl (S)-2-hydroxy-
propionate and 0.37 ~1 of triethylamine i~-5 ml of
methylene chloride i~ added dro~wise to a solution of 1 g
of ~-bromoethyl-phosphoric acid d7chloride in 10 ml of
methylene chloride. ~he mixture i~ stirred firstly at room
temperature for 4 hours. then under ~eflux for 1 hour.
Aftel adding 5 ml of water the mi~tule i~ boiled at reflux
for 2 hours. The mixture is diluted with methylene
chloride, the aqueous phase is ~eparated, the organic
phase i~ wahed with water, d ied over odium sulphate and
concen~rated. The ~e~idue i~ dis~olved in ether and
treated with a ~olution of 5 g o~ barium a~etate in 20 ml
of water. The ~ixture iB ~tir~ed at ~oom ~emperature for
20 hour~, the re~ulting barium ~alt i~ fil~ered off and i~
treated with a mixSure of Z0 ml o~ 3N hydro~hloric acid
and 20 ml of methylene chloride. The mixture i~ stirred at
room temperature for 1 hour, the organic phaQe i~
~eparated, dried over ~odium sulphate and ~o~centrated.
There is obtained 0.7 g of chole~-5-en-3~-yl ~S)-2-~[(2-
" ~ ~
:, ~
-~ 7
- 37 -
-bromoe~hoxy)hydroxyphosphinyl]oxy]propionate, MS: 643
(M-H) .
b) A ~olution of 0.7 g of cholest-5-en-3~-yl SS)-2-t~(2-
-bromoethoxy)hydroxyphosphinyl]oxy]propionate in 15 ml of
toluene i6 treated with 15 ml o~ trimethylamine in a
dry-ice bath. The mixture i8 heated to 60C ~or 30 hour6.
After cooling the reac~ion ~ix~ure i~ taken up in toluene
and concentrated. The residue i~ taken up in 30 ml of
me~hanol, treated with 2 g o~ silver carbonate and stirred
at 50C for 0.5 hour. ~he precipita~e is filter~d off,
concentrated and there i~ obtained 0.6 g o~ 0-~[(S)-l-
-~(choles~-5-en-3~-yloxy)carbonyl]ethb~y]hydroxy-
phosphinyl]choline hydroxide internal sal~, MS: 624
(M+H ).
E~ample 16
Analogously to Example 1, starting from 6tigmast-5-
-en-3~-yl 2-hydroxyethylcarbonate there i8 obtained 0-
-~hydroxy-t2~[(~tigmast-5-en-3B-yloxy~carbonyl]oxy]ethoxy]-
pho~phinyl]choline hydroxide internal ~alt, m.p. 207C
~decompo6ition~.
Example 17
Analogously to Example 2k) and Example 2q), there are
Obtained
a3 0-rhydroxy-t3-t~stigmast-5-en-3~-yloxy)carbonyl]-
propoxy]phozphinyl]choline h~droxide internal salt, m.p.
220C (decompo6ition), and, respectively,
b) 0-~hydroxy-[2-(6tigmast-5-en-3~-yloxy)ethoxy]phofi-
phinyl]choline hydroxide internal salt, m.p~ 197C
~decomposition).
.
. :
:
7J~Jf37~
- 3~ -
~3~ ]e 18
Analogously to Example 5, but using dimethylamine in
place of triethylamine, there i6 obtained cholest-5-en-3~-
-yl 2-[~2-(dimethylamino)ethoxy]hydrsxyphosphinyl]oxy]-
ethylcarbonate, m.p. 150C (decomposition).
Example 19
Analogou~ly to Example 5, there i6 obtained
O-thYdLoxy-[2-rl-~stig~ast-5-en-3~-yloxy)formamido]-
ethoxy]pho phinyl]choline hydroxide internal 6alt, m.p.
lS 225C (de~omposition).
Exam~le 20
A~alogously to Example 12, there is obtained O-[t~RS~-
-2-acetoxy-3-[t(cholest-5-en-3~-yloxy)carbonyl~oxy]propoxy]-
hydroxyphosphinyl3choline hydLoxide interna-l ~alt.
130C [decompo6ition).
Example 21
a~ A solution of 11.6 g of chole~terol and 3.5 g of
hydroxypivalic acid i~ R0 ml of toluene i8 ~reated with
1 g of p-toluenesulphonic acid and boiled for 4 hour~. The
reaction mixture is treated with water and extracted with
ether. The organic pha6e is dried and concentrated and the
re6idue i6 purified ove~ 6ilica gel ~ith petroleum ether/
ether $4:1). There are obtained 1.9 g of chole6t 5-en-3~-
-yl 3-hydroxy-2,2-dimethyl-propionate. ~.p. 174-176C.
b) To a ~olution of 1.9 ~ of the produ~t of a) in 60 ml
of methylene chloride are added dropwise fir~tly 1.1 ml of
triethylamine and then a 601ution of 1.42 g of ~-bromo-
ethyl-pho~phoric acid di~hloride in 30 ml of methylene
.
,
..
.:
3 ~ j ~
- 39 -
chloride. The mixture i6 boiled under reflux for 18 hour~,
and, after the addition sf watler, boiled under reflux for
1 hour. The aqueou~ phase i~ ~eparated and the organic
phase is wa~hea wi~h water, dried and concent~ated. The
residue i8 di~olved in ether and trea~ed with a ~olution
of 15 g of barium acetate in 30 ml of water. The mixture
is ~tirred for 20 hours, the resul~ing barium ~alt i~
filtered off and treated wi~h a mixture of ~0 ml of ~N
hydrochlo~ic acid and 50 ml of me~hylene chloride. The
mixture i8 ~irred at ~oom temperature for 1 hour and the
organic phase i8 separa~ed, dried and concent~ated. There
are obtained 1.6 g of chole~t-S-en-3~-yl 3-t[(2-bromo-
ethoxy)hydLoxy-phosphinyl]oxy]-2,2~dime~hylpropionate,
m.p. 98C.
c) A ~olution of 1.6 g of the product of b) in 15 ~1 of
~oluene is trea~ed with 15 ml of trimethylamine while
cooling i~ a dry-ice bath. The mixture i6 heated to 60C
for 2 day~. After cooling in a dry-ice bath the reaction
mixture i6 taken up in toluene and concentrated. The
residue is taken up in methanol, treated with 3 g of
silver carbonate and stirred at 50C for 0.5 hour. The
precipitate i8 filtered off and the filtrate i~ con-
centlated. By recry~allization of the refiidue from ethee
there is obtained 0.8 g (5Z~) of 0-rtZ-~chole6t~5-en-3B-
-yloxy~carbonyl]-2-methylp~opoxy~hydro~yphosphinyl~choline
hydroxide inte~nal ~alt, m.p. 210C.
Example 2?
a) A ~olution of 5.00 g of cholesteryl chloroformate i~
20 ml of methylene Ghloride i8 added d~opwi~e to a mixture
of 1.4 g of DL-~erinol~HCl and 3.1 ml of triethylamine
in 30 ml o~ methylene choride. After 6tirring for 45 hour~
the mixture is taken up in ayueous methanol and extrac~ed
with chloroform. The organic phase i6 dried and con-
,
,:
- ~J~2~37
- 40 -
centrated. ~fte~ chromatography over SiO2 with chloro-
form and then with ether ~here are obtained 4.3 g of
cholest-5-en-3B-yl [2-hydroxy-:L-(hydroxymethyl)ethyl]car-
bamate. m.p. 158C ~decomposition~.
b) A solution of 3.7 g of the product of a) in 30 ml of
methylene chloride, 10 ml of ~HF and Ou6 ml of pyridine i8
treated under argon with 1 ml of phenyl chlo~oformate in
15 ml of methylene chloride. A~ter stirring for 1 hour the
solution is taken up in 200 ml of water and 50 ml of O.lN
HCl and extracted with lSO ml of methylene chloride. The
organi~ pha~e i~ dried and concentrated. After chroma-
tography over SiOz with n-hexane/ether (1:1) and ether
there are obtained 2.1 g of chole~t-S-en-3B-yl [(RS)-2-
-hydroxy-l-~(phenoxycarbonyl)oxymethyl]ethyl~carbamate.
c) 0.05 ml of 2-chloco-2-oxo-1,3,Z-dioxapho~pholane is
added to ~ mixture of 320 mg of the product of b) in 20 ml
of toluene and 0008 ml of ~riethylamine under argon while
~tirring at room temperaeure. The (Et)3N:HCl is filtered
off under 6uc~ion afte~ 16 hour~O The solution obtained i~
concentrated and the residue i6 di~olved in a solution of
2 g of ~rimethyla~ine in 20 ml of toluene and 5 ml o~
acetonitrile and held at 80C. Ths ~olution is concen-
trated after 6 hours. The product, O-[r(RS)-2-rl-
-(cholest-5-en-~-yloxy)focmamido~-3-(phenoxycarbonyloxy)-
propoxy]hydroxyphosphinyl]choline hydroxide internal ~alt,
is u6ed in the next step without purification.
d) The product of c) i~ dissolved in 20 ml of methanol-
-chloroform ~1:1) and t~eated with 2 ml of lN NaOH at room
temperature ~while ~Sirring. AfteL adding 10 g o~ ion
exchanyer (Ambellite MB-3) and tirring for 4 hours the
~olution i8 concentrated, azeotropically distilled with
toluene and then dried. The re~idue is chro~atoqraphed on
~ilica gel with CHC13-MeOH-H20 (60/35/5 by vol.).
.. : : ....................... . . . :
.
- - 41 -
Aftel drying there are obtained 200 mg Of O-t~RS)-2-[1-
-(cholest-S-en-3~-yloxy)formamido]-3-hydroxypropoxy]-
hydroxyphosphinyl]choline hydLoxide internal salt, m.p.
220C (decomposition).
Tablets and capsule~ of the following composition are
manufactuced in a mann2l known pe~ se:
ExamPle a
Tablets 1 tablet contains
15 Compound of formula I200 mg
Mic~ocrystalline cellulose 155 my
Maize starch 25 mg
Talc 25 mg
Hydroxypropylmethylcellulose 20 m~
425 mg -
Example b
Capsules 1 capsule contains
Compound of formula I100.0 mg
Maize starch 20.0 mg '`
Lactose 95.0 mg
Talc 4.5 mg
30 Magnesium steara~e 0.5 m~
200.0 mg
..
. ~ ' ' :
. : .. : .
, , ' : ' ' : ' :
,: :
. :
,............................................ . : ..