Note: Descriptions are shown in the official language in which they were submitted.
2029868
SPECIFICATION
Title of the Invention
Insulated Wire
Field of the Invention
The present invention relates to an insulated wire,
- and more particularly, it relates to an insulated wire
such as a distribution wire, a wire for winding or the
like which is employed under high-vacuum environment or
high-temperature environment such as a high-vacuum
apparatus or a high-temperature service apparatus.
Background of the Invention
An insulated wire may be applied to equipment such as
heating equipment or a fire alarm, for which safety under
- a high temperature is required. Further, the insulated
wire is also used under environment in an automobile,
which is heated to a high temperature. An insulated wire
formed by a conductor which is coated with heat resistant
organic resin such as polyimide, fluorocarbon resin or the
like has generally been used as such an insulated wire.
As to application for which high heat resistance is
required, or employment under environment for which a high
degree of vacuum is required, organic coating is
insufficient in view of heat resistance, no gas emission
property and the like. Thus, an insulated wire of such a
form that a conductor is inserted in an insulator tube of
- 1- ~
-- 2029868
ceramics, an MI cable (Mineral Insulated cable) of such a
form that a conductor is inserted in a heat resistant
alloy tube of a stainless steel alloy etc. which is filled
with metal oxide powder of magnesium oxide etc., or the
like has been employed for such application.
A fiber-glass braided insulated wire employing
textile glass fiber as an insulating member etc. is listed
as an insulated wire for which flexibility is required
with heat resistance.
~ 10 In the aforementioned insulated wire coated with
organic resin having heat resistance, the highest
temperature at which insulability can be maintained is
about 200C at the most. Therefore, it has been
impossible to employ such an organic insulated coated wire
for application for which guarantee for insulability is
required under a high temperature of at least 200C.
Further, the insulated wire which is improved in heat
resistance through an insulator tube of ceramics has
disadvantages such as inferior flexibility. The MI cable
-20 is formed by a heat resistant alloy tube and a conductor,
and hence the outer diameter of the cable is increased
- with respect to the conductor radius. Thus, the MI cable
has a relatively large section with respect to electric
energy allowed by the conductor which is passed through
the heat resistant alloy tube. In order to use the MI
20~9868
cable as a wire for winding which is wound on a bobbin
etc. in the form of a coil, however, it is necessary to
bend the heat resistant alloy tube in prescribed
curvature. In this case, bending performed on the heat
resistant alloy tube involves difficulty. When the MI
cable is wound in the form of a coil, further, it is
difficult to improve space factor since the tube of its
outer layer is thick as compared with the conductor.
Further, when the fiber-glass braided insulated wire
., .
having heat resistance is employed and worked into a
prescribed configuration in response to its application,
the network of the braid is disturbed to cause a
-:- breakdown. In addition, dust of glass is generated from
-~ the glass fiber. This glass dust may serve as a gas
adsorption source. Therefore, when the fiber-glass
- braided insulated wire is used under environment for which
a high degree of vacuum is required, it has been
impossible to maintain a high degree of vacuum due to the
gas adsorption source provided by the glass dust.
On the other hand, there has generally been the so-
called alumite wire prepared by performing anodic
oxidation treatment on a wire of aluminum or an aluminum
~- - alloy, as an insulated wire which is excellent in heat
resistance, insulability and heat dissipativity. In this
alumite wire, its base material is restricted to aluminum.
2029868
~urther, an inorganic insulating layer formed on the base
material is also restricted to aluminum oxide. Thus,
there has been such a problem that it is impossible to
select combinations of the base material and the inorganic
insulating layer which are suitable for various uses.
Disclosure of the Invention
Accordingly, the present invention has been proposed
in order to solve the aforementioned problems, and its
object is to provide an insulated wire comprising the
~ 10 following items:
(a) It has high insulability under environment of a
high temperature.
(b) It is excellent in flexibility.
(c) It comprises no gas adsorption source.
(d) Combinations of a base material and an inorganic
insulating layer suitable for various uses can be
selected.
An insulated wire according to the present invention
comprises a base material, a chromium oxide contA;ning
layer, and an oxide insulating layer. The base material
has an outer surface, and includes a conductor. The
~ chromium oxide cont~;n;ng layer is formed on the outer
-`! surface of the base material. The oxide insulating layer
is formed by applying a precursor solution of a metallic
oxide onto the chromium oxide containing layer by a sol-
~_ 2029868
gel method or an organic acid salt pyrolytic method.
The chromium oxide cont~ining layer is preferablyformed by an electrochemical technique. The
electrochemical technique includes electrolytic plating or
S electroless plating. The underlayer to be provided with
the oxide insulating layer may be a CrO3x (1.5 < X < 2.5)
layer, in order to preferably serve as an adhesion layer.
Namely, the layer formed by the electrochemical technique
has a chromium oxide layer as its outermost layer. The
.
oxide insulating layer preferably contains silicon oxide,
aluminum oxide or zirconium oxide. As to the base
material, copper or a copper alloy is ~referably employed
in view of high conductivity and the cost. In
consideration of a use at a higher temperature or the
like, nickel chromium, silver, iron or a ferroalloy, a
stainless steel alloy, or titanium or a titanium alloy is
preferably contained in the surface layer of the base
material.
It is known that a chrome plated layer is formed on a
conductor of copper or a copper alloy etc. as an excellent
adhesion layer. However, insulating oxide ceramics such
as silicon oxide obtained by heat treatment of a precursor
solution of a metallic oxide hardly exhibits adhesion with
respect to the chrome plated layer. This is based on
recognition of the inventors.
2029868
In an insulated wire obtained by directly forming a
thin film of ceramics on a surface of a conductor made of
copper, further, the ceramics thin film serving as an
insulating layer has insufficient adhesion with respect to
a base material.
According to the present invention, therefore, a
layer having a chromium oxide layer as its outermost layer
is formed on an outer surface of a base material.
Insulating oxide ceramics adheres onto the chromium oxide
layer as a layer having excellent adhesion.
The aforementioned chromium oxide layer is formed by
an electrochemical technique. When the chromium oxide
layer is formed by electrolytic plating, a substance
- obtained by adding a small amount of organic acid to an
aqueous solution of chromic anhydride is used as an
electrolyte. Although a sergeant bath mainly composed of
chromic anhydride or sulfuric acid is known as an
electrolytic bath employed for chrome plating, it is
different from this bath in the following point: Namely,
mineral acid mixed into the electrolytic bath has a
function of dissolving chromic anhydride which is
generated on the surface of a plated layer in electrolytic
plating. Therefore, a glossy metallic chrome layer is
plated when a sergeant bath is employed. In the present
invention, it is necessary to preferentially plate
2029~68
chromium oxide. Therefore, a small amount of organic acid
is added to an electrolytic bath employed in the present
invention. In a case of using mineral acid such as
sulfuric acid, further, it is necessary to employ a
particularly diluté electrolytic bath. Namely, chromic
anhydride concentration is not more than 50 g/Q and
sulfuric acid concentration is not more than 1 g/Q.
Further, while a thin film of insulating ceramics is
formed on the outer surface of a layer mainly composed of
chromium oxide by heat treatment of a precursor solution
of a metallic oxide, the layer mainly composed of chromium
oxide preferably has a roughened surface, in order to
further increase adhesion of the thin film.
The chromium oxide containing layer may be formed by
electrolytic plating employing an electrolyte which is
prepared by adding sodium citrate, sodium carbonate or the
like, for example, to an aqueous solution of sodium
chromate. In this case, the as-formed layer is mainly
composed of chromium oxide, which is generated by
trivalent reduction of hexavalent chromium contained in
the electrolyte. If copper is used as a base material in
this electrolytic plating treatment, the base material
surface is oxidized and the chromium oxide contA;~;ng
layer is formed in the exterior thereof. However,
adhesion of the chromium oxide cont~; n; ng layer with
2029868
respect to the base material is not reduced by such
oxidation of the base material surface.
Conditions for electrolytic plating for forming the
inventive chromium oxide containing layer are different
from those for general bright plating in treatment current
d`ensity etc. Although the current density is set at 10 to
60 A/dm2 in the bright plating, depending on the treatment
temperature, the current density is set at 100 to 200
A/dm2 in the present invention. A chromium oxide
con~;n;~g layer having a roughened surface can be formed
by this condition of the current density.
On the chromium oxide containing layer, an insulating
oxide layer is formed by application of a precursor
solution of a metallic oxide. The precursor solution of a
metallic oxide mentioned in this specification is a
~~ solution prepared from a metal organic compound, which isbroadly classified in correspondence to a sol-gel method
or an organic acid salt pyrolytic method, and those of the
following two types are included:
The first type of precursor solution is a solution
which is generated by making hydrolytic reaction and
dehydration/condensation reaction of a compound cont~;n;~g
hydrolyzable metal-oxygen-organic group bonds such as
metal alkoxide or acetate of a metal. This solution may
contain an organic solvent such as alcoholj a raw material
.
-- 8 --
2029868
compound such as metal alkoxide, and water and a catalyst
required for hydrolytic reaction. Further, it generally
contains an organic residual group such as alkoxide,
dissimilarly to hydroxide sol that is generated from
inorganic salt.
The second type of precursor solution is a solution
prepared by dissolving a metal organic compound such as
organic acid salt of a metal in an appropriate organic
solvent. In a method employing this type of precursor
solution, a metallic oxide is generated by pyrolyzation
through heating after application. Therefore, a
decomposition temperature of the employed metal organic
- compound must be lower than its boiling point or
~ sublimation point.
The metal organic compound mentioned in this
specification is a concept similar to "metal-organic
compounds" described in Journal of Materials Science 12
(1977) pp. 1203 to 1208, for example.
Further, the applied layer must be left at a
temperature higher than the room temperature, for
volatilization of the organic solvent and removal of a
residual organic substance. However, the temperature of
the atmosphere for such leaving must not be higher than
the melting point of the metal forming the base material.
It is possible to form almost all metallic oxide-
-
2029868
based ceramics covering by application of a precursor
solution of a metallic oxide. SiO2, Al2O3, ZrO4, TiO2, MgO
or the like can be listed as an example of a metallic
oxide formed by this method. Further, ethoxide,
propoxide, butoxide or the like can be listed as metal
alkoxide employed for the first type of precursor
solution. Metallic salt such as naphtanic acid, caprylic
acid, stearic acid, octylic acid or the like is preferable
as organic salt employed for the second type of precursor
solution.
The oxide insulating layer formed from the precursor
solution of the metallic oxide by the sol-gel method or
the organic acid salt pyrolytic method is an oxide which
is completely converted to a metallic oxide. This oxide
- 15 is preferably formed by heat treatment under an atmosphere
in an oxygen current. In general, decomposition of the
compound contained in the solution which is applied onto
the chromium oxide cont~ini ng layer is completely
terminated at a temperature of about 500C. If the same
is heat treated at a higher temperature, however, reaction
- between elements forming the chromium oxide cont~i n i ng
layer and a metal or semimetal contained in the applied
,~
solution is facilitated, whereby adhesion between the
chromium oxide cont~i n i ng layer and the oxide layer is
improved.
-- 10 --
2029868
Thus, the oxide insulating layer converted to
ceramics exhibits excellent heat resistance/insulability
also under a high temperature of at least 500C. Further,
the chromium oxide containing layer is excellent in
adhesion to the conductor forming the base material.
Therefore, adhesion between the oxide insulating layer and
the outer surface of the base material is improved as
compared with the case of directly forming the oxide
insulating layer on the outer surface of the conductor by
heat treatment of the precursor solution of the metallic
oxide. Thus, the insulated wire provided according to the
present invention has heat resistance/Lnsulability, as
- well as excellent flexibility.
Further, the oxide insulating layer formed on the
chromium oxide contAin;ng layer has a smooth outer
surface. Therefore, a high breakdown voltage
proportionate to the film thickness can be obtained, while
it is possible to reduce a gas adsorption source.
According to the present invention, in addition, the
chromium oxide contA;ning layer is formed between the base
material and the oxide insulating layer. Therefore,
combinations with the inorganic insulating layer suitable
for various uses can be selected through the chromium
oxide containing layer.
Brief Description of the Drawings
-- 11 --
20298fi8
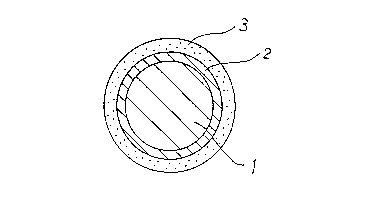
Fig. 1 is a sectional view showing a cross section of
an insulated wire according to the present invention in
correspondence to Example 1.
Fig. 2 is a sectional view showing a cross section of
an insulated wire according to the present invention in
correspondence to Example 2.
: Fig. 3 is a sectional view showing a cross section of
an insulated wire according to the present invention in
correspondence to Example 3.
.,
Fig. 4 is a sectional view showing a cross section of
an insulated wire according to the present invention in
correspondence to Example 4.
Fig. 5 is a graph showing the result of measurement
of surface roughness of a chromium oxide cont~in;ng layer
formed in accordance with Example 3 or Example 4.
Fig. 6 is a graph showing the result of measurement
of surface roughness of a chrome plated layer formed in
accordance with Reference Example.
Best Modes of Carrying Out the Invention
ExamPle 1
- (a) Formation of Chromium Oxide Cont~;ning Layer
Electrolytic plating treatment was performed on an
outer surface of a copper wire of 2 mm~ in wire diameter.
At this time, an electrolyte was prepared from that having
concentration of 40 g/Q of chromic anhydride and 0.45 g/Q
- 12 -
2029868
of sulfuric acid. As to plating conditions, the bath
temperature was 50C, the current density was 140 A/dm2,
and the treatment time was two minutes. Thus~ a chromium
oxide cont~;n;ng layer was formed on the outer surface of
the copper wire with a film thickness of about 1 ~m.
(b) Preparation of Coating Solution used for Sol-Gel
- Method
Nitric acid was added to a solution mixed in mole
ratios of tetrabutyl orthosilicate:water:isopropyl alcohol
= 8:32:60 in a ratio of 3/100 mole with respect to
tetrabutyl orthosilicate. Thereafter this solution was
heated/stirred at a temperature of 80C for two hours.
Thus, a coating solution used for a sol-gel method was
synthesized.
(c) Coating
' The wire obtained by (a) was dipped in the coating
solution of (b). A step of heating at a temperature of
400C for 10 minutes was performed ten times on the wire
whose outer surface was thus coated with the coating
solution. Finally, this wire was heated in an oxygen
current of 500C in temperature for 10 minutes.
An insulated covered wire obtained in the
aforementioned manner is shown in Fig. 1. Fig. 1 is a
sectional view showing a cross section of the insulated
wire obtained according to Example 1. Referring to Fig.
2029868
1, a chromium oxide containing layer 2 is formed on an
outer surface of a copper wire 1. On this chromium oxide
.
contAining layer 2, a silicon oxide layer 3 is formed by
the sol-gel method as an oxide insulating layer. The film
- thickness of an insulating layer formed by the chromium
oxide contAining layer 2 and the silicon oxide layer 3 was
about 4.0 ~m.
A breakdown voltage was measured in order to evaluate
insulability of the obtained insulated wire. Its
breakdown voltage was 800 V under the room temperature,
and was 600 V under a temperature of 800C. Even if this
insulated wire was wound on an outer pe~ipheral surface of
a cylinder having a diameter of 10 cm, no cracking was
caused in the insulating layer.
ExamPle 2
(a) Formation of Chromium Oxide ContAining Layer
A copper wire of 2 mm~ in wire diameter was vapor-
degreased through use of perchloroethylene. Thereafter
the copper wire was dipped in a solution mixed in volume
ratios of 85 % phosphoric acid:70 % nitric acid:water =
15:2:3, thereby roughening its surface.
Then, the copper wire was used as a cathode and a
stainless steel plate was used as an anode to perform
electrolytic plating treatment by feeding a direct current
of 0.05 A/dm2. At this time, a solution of about 1 Q
- 14 -
- 2029868
prepared by dissolving 30 g of sodium chromate, 30 g of
sodium citrate and 30 g of sodium carbonate in water
respectively was used as an electrolyte.
Thus, a copper oxide layer having a film thickness of
about 1 ~m was formed on the outer surface of the copper
- wire, and a chromium oxide containing layer was formed in
its exterior with a film thickness of about 0.1 ~m.
(b) Preparation of Coating Solution used for Sol-Gel
Method
A solution mixed in mole ratios of tetrabutyl
orthozirconate [(C4H9O)4Zr]:water:n-butyl alcohol = 5:15:80
was heated/stirred at a temperature of~120C for two
hours. Thus, a coating solution used for a sol-gel method
was synthesized.
(c) Coating
The wire obtained by (a) was dipped in the coating
solution of (b). A step of heating at a temperature of
400C for 10 minutes was performed ten times on the wire
whose outer surface was thus coated with the coating
solution.
An insulated covered wire obtained in the
aforementioned manner is shown in Fig. 2. Fig. 2 is a
sectional view showing a cross section of the insulated
wire obtained according to Example 2. Referring to Eig.
2, a copper oxide layer 12 is formed on the outer surface
- 15 -
2029868
of a copper wire 11. Further, a chromium oxide containing
layer 13 is formed in the exterior of this copper oxide
layer 12. On this chromium oxide cont~;n;ng layer 13, a
-zirconium oxide layer 14 is formed by the sol-gel method
as an oxide insulating layer. The film thickness of an
insulating layer formed by the copper oxide layer 12, the
chromium oxide cont~ining layer 13 and the zirconium oxide
layer 14 was about 3.0 ~m.
A breakdown voltage was measured in order to evaluate
insulability of the obtained insulated wire. Its
breakdown voltage was 700 V under the room temperature,
and was 500 V under a temperature of 7~0C. Even if this
insulated wire was wound on an outer peripheral surface of
a cylinder having a diameter of 10 cm, no cracking was
caused in the insulating layer.
Example 3
(a) Formation of Chromium Oxide Cont~;ning Layer
Electrolytic plating treatment was performed on an
outer surface of a nickel-plated copper wire of 1.8 mm~ in
wire diameter. At this time, an electrolyte was prepared
from that having concentration of 200 g/Q of chromic
anhydride, 20 g/~ of ammonium methavanadate and 6.5 g/l of
acetic acid. As to plating conditions, the base material
was used as a cathode, while the bath temperature was
50C, the current density was 150 A/dm2, and the treatment
- 16 -
2029868
time was two minutes. Thus, a chromium oxide cont~in;ng
layer was formed on the outer surface of the nickel-plated
copper wire with a film thickness of about 1 ~m.
As to the surface state of the chromium oxide
cont~in;ng layer, the center line average roughness Ra was
0.15 ~m and the maximum height Ry was 0.87 ~m in
accordance with Surface Roughness of IS0468-1982. The
surface roughness was measured by using a surface contour
measurer DERTAR3030 made by Sloan Inc., U.S.A., under
conditions of a tracer diameter of 0.5 ~m, a stylus
pressure of 10 mg, a reference length of 50 ~m, and no use
of a cutoff filter. The result of measurement is shown in
Fig. 5.
(b) Preparation of Coating Solution used for Organic
Acid Salt Pyrolytic Method
A coating solution was prepared by dissolving 20 g of
2-ethyl-hexanoic silicate in 100 mQ of dibutyl ether.
(c) The wire obtained by (a) was dipped in the
coating solution of (b). A step of heating at a
temperature of 500C for 10 minutes was performed ten
times on the wire whose outer surface was thus coated with
the coating solution.
An insulated covered wire obtained in the
aforementioned manner is shown in Fig. 3. Fig. 3 is a
sectional view showing a cross section of the insulated
2029868
wire obtained according to Example 3. Referring to Fig.
3, a nickel-plated copper wire comprising a nickel-plated
layer 22 formed on an outer surface of a copper wire 21 is
used as a base material. A chromium oxide cont~ining
layer 23 is formed on the outer surface of this nickel-
plated copper wire. On the chromium oxide cont~i n i ng
layer 23, a silicon oxide layer 24 is formed by an organic
acid salt pyrolytic method as an oxide insulating layer.
The film thickness of an insulating layer formed by the
chromium oxide cont~ining layer 23 and the silicon oxide
layer 24 was about 5 ~m.
A breakdown voltage was measured i~n order to evaluate
insulability of the obtained insulated wire. The
breakdown voltage was 500 V under the room temperature,
- 15 and was 300 V under a temperature of 800C. Even if this
insulated wire was wound on an outer peripheral surface of
a cylinder having a diameter of 5 cm, no cracking was
caused in the insulating layer.
ExamPle 4
(a) Formation of Chromium Oxide Containing Layer
- The so-called stainless steel clad copper wire of 1.8
mm~ in wire diameter, in which a stainless steel alloy
(SUS304) was engaged on an outer surface of a copper wire,
was used as a base material. Electrolytic plating
treatment was performed on the outer surface of this
- - 18 -
2029BC8
stainless steel clad copper wire. At this time, an
electrolyte was prepared from that having concentration of
200 g/Q of chromic anhydride, 20 g/Q of ammonium
methavanadate and 6.5 giQ of acetic acid. As to plating
conditions, the base material was used as a cathode, while
the bath temperature was 50C, the current density was 150
A/dm2 and the treatment temperature was two minutes.
Thus, a chromium oxide contAining layer was formed on the
outer surface of the stainless steel clad copper wire with
a film thickness of about 1 ~m.
As to its surface state, the center line average
roughness Ra was 0.15 ~m, and the mAxi~l-m height Ry was
0.87 ~m in accordance with Surface Roughness of IS0468-
1982. The measurement was performed by using a surface
contour measurer DEKTAK3030 made by Sloan Inc., U.S.A.,
- under conditions of a tracer diameter of 0.5 ~m, a stylus
pressure of 10 mg, a reference length of 50 ~m, and no use
of a cutoff filter. As the result of this measurement,
that shown in Fig. 5 was obtained similarly to Example 3.
(b) Preparation of Coating Solution used for Organic
Acid Salt Pyrolytic Method
25 g of aluminum tetra-i-butoxide was dissolved in
100 mQ of diethylene glycol monomethyl ether, and
thereafter heated/stirred at 150C for one hour. This
solution was stood to be cooled to the room temperature,
-- 19 --
2029868
and thereafter mixed with 3 g of alumina particles of 0.03
~m in nominal particle size, thereby preparing a coating
solution.
(c) Coating
The wire obtained by (a) was dipped in the coating
solution of (b). A step of heating at a temperature of
500C for 10 minutes was performed ten times on the wire
whose outer surface was thus coated with the coating
solution.
An insulated covered wire obtained in the
aforementioned manner is shown in Fig. 4. Fig. 4 is a
sectional view showing a cross section of the insulated
wire obtained according to Example 4. Referring to Fig.
4, a stainless steel clad copper wire having a stainless
steel alloy layer 32 on an outer surface of a copper wire
31 is used as a base material. A chromium oxide
contA;ning layer 33 is formed on the outer surface of the
stainless steel clad copper wire. On this chromium oxide
contAining layer 33j an aluminum oxide layer 34 is formed
by an organic acid salt pyrolytic method as an oxide
insulating layer. This aluminum oxide layer 34 consists
of an aluminum oxide mixed layer containing aluminum
- particulates which have been mixed in the coating solution
from the start. The film thickness of an insulating layer
formed by the chromium oxide contAining layer 33 and the
- 20 -
202~868
aluminum oxide layer 34 was about 12 ~m.
A breakdown voltage was measured in order to evaluate
insulability of the obtained insulated wire. Its
- breakdown voltage was 900 V under the room temperature,
and was 700 V under a temperature of 800C. Even if this
insulated wire was wound on an outer peripheral surface of
a cylinder having a diameter of 15 cm, no cracking was
caused in the insulating layer.
Reference Example
(a) Formation of Metallic Chrome Plated Layer
Electrolytic plating treatment was performed on an
- outer surface of a nickel-plated coppe~ wire of 1.8 mm~ in
wire diameter. At this time, an electrolyte to be used
was prepared from that having concentration of 250 g/Q of
chromic anhydride and 2.5 g/Q of sulfuric acid. As to
plating conditions, the base material was used as a
cathode, while the bath temperature was 50C, the current
density was 40 A/dm2, and the treatment time was two
minutes. Thus, a chrome contAining layer was formed on
the outer surface of the nickel-plated copper wire with a
film thickness of about 1 ~m.
As to its surface state, the center line average
roughness Ra was 0.06 ~m and the m~ximllr height Ry was
0.51 ~m in accordance with Surface Roughness of ISO468-
1982. The measurement was performed by using a surface
2029868
contour measurer DEXTAK3030 made by Sloan Inc., U.S.A.,
under conditions of a tracer diameter of 0.5 ~m, a stylus
~ pressure of 10 mg, a reference length of 50 ~m, and no use
of a cutoff filter. The result of this measurement is
shown in Fig. 6. A glossy metallic chrome layer was
formed on the outer surface of the nickel-plated copper
wire.
(b) Preparation of Coating Solution used for Organic
Acid Salt Pyrolytic Method
A coating solution was prepared by dissolving 20 g of
2-ethyl-hexanoic silicate in 100 mQ of dibutyl ether.
(c) Coating
The wire obtained by (a) was dipped in the coating
solution of (b). A step of heating at a temperature of
500C for 10 minutes was performed on the wire whose outer
surface was thus coated with the coating solution, whereby
the as-formed insulating layer was separated like a film
after heating, and exhibited no adhesion.
Industrial Availability
- 20 As hereinabove described, the insulated wire
according to the present invention is suitable for a
distribution wire, a wire for winding or the like, which
is employed under high-vacuum environment or high-
temperature environment such as a high vacuum apparatus or
a high temperature service apparatus.
- 22 -