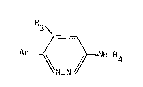Note: Descriptions are shown in the official language in which they were submitted.
203~ 3~
P~RIDAZINE DERIVATIVES, PROCESS FOR_THEIB PREPARATION
A~D PHARMACEUTICAL COMPOSITIONS CONTAINING T~EM
~or many years pyridazine derivatives have been sug~ested
as ~edicines, in particular medicines active on the cardiovascular
system or on the central nervous system.
In particular, French patent 2 510 99~ and European
patent 72 726 disclose pyridazine derivatives variously substituted
on the pyridazine ring and all bearing at position 3 an amine
substituent of the type ~ -alkylene-,`~l in which X and Y independently
represent hydrogen, alkyl or form together with the nitrogen atom to
which they are attached a heterocycle such as morpholine.
.~11 of these co~pounds exilibit an activity on the central
nervous system as antidepressants.
-~ccording to the present invention, novel derivatives o~
pyridazine have now been discovered which have lost their antidepressant
activity and acquired a useful activity as li~ands of cholinergic
receptors, in particular receptors of the Ml type.
In accordance with a first feature, the object of the prescnt
invention is novel derivatives of pyridazine corresponding to the
formula:
R3
Ar ~ ~ NH-~4 (I)
in which
- Ar represents a phenyl group substituted by Rl and R2 or a hetero-
cyclic radical such as a pyridyl group, unsubstituted or substituted
by methyl or methoxy, or a thienyl group, unsubstituted or substituted
by chlorine, methyl or methoxy;
~ Rl and ~2 each independently denotes hydrogen, halogen, trifluoro-
methyl, hydroxy, C1-C4 alkoxy or Cl-C4 alkyl;
- R3 represents a C1-C4 linear or branched allcyl, C3-C7 cycloalkyl,
benzyl, phenethyl or the ~r' radical, I~r' being phenyl substituted
by P~l and g2;
..
.- . - - . - :
- . . :.
.. ,, . . , ~ .
:,.. - ~ , : . . . . :
': . . - ~
203~3~
- R4 re?resents:
Cl~2Xl R5
a ~ CH2 - C - (Cl~2)n ~ N \ group9 with n = 0 or 1
CH2X1 ~6
in ~/hich ~1 represents hydrogen or methyl;
- R5 represents a Cl-C6 linear alkyl group;
- !?~ represents a Cl-C, linear al'.cyl group, or R5 and R6 also constitute
together with the nitrogell atom to which they are attached a hetero-
cycle selected from morpholine, pyrroli(line or piperidine;
as well as their salts with organic or mineral acids.
Preferentially, .~r represents phenyl, unsubstituted or mono-
substituted at position 2, more particularly ~r represents a group
selected from phenyl, 2-'nalo~phenyl, in particular 2-chloro-pll~llyl,
2-metho~y-phenyl or 2-hydro~y-phenyl, ?~3 represents phenyl or n-propyl,
T-5 and R~ each represents ethyl, n = 0 and ~1 = H.
The followin~ co;npounds ~re particularly preferred:
3-(2-diethylamino-2-methyl-propyl)amino-6-phenyl-5-propyl-
pyridazine and its salts;
3-(2-cliethylamino-2-methyl-propyl)amino-5,6-diphenyl-
pyridazine and its salts.
The salts of the compounds of formula I accorcling to the
present inventiotl includes those with both mineral and organic acids
which enable the compounds of formula I to be separated or suitably
crystallized, such as picric acid or oxalic acid, those which form
pharmaceutically acceptable salts such as the hydrochloride,
hydrobromide, sulfate, hydrogen sulfate, dihydrogen phosphate, methane
sulfonate, methyl sulfate, maleate, fumarate, 2-napht~alene sulfonate.
In accordance with a second feature, the present invention
relates to a process for the preparation of the compounds of formula
(I).
.~ccording to the present invention, the process for the
preparation of the compounds of Eormula (I) is characterized in that
an amine ~4N7~2 is reacted with a 6-chloro pyridazine of formula:
: ' :
203~33
R3
Ar--<~Cl (II)
N_N
in which Ar and R3 have the meanin2s indicated above Eor (I) and,
optionally, the compound thus obtained is converted into a salt with
a mineral or orOanic acid.
The substitution reaction of the 6-chloro pyrida2ine (II)
by the amille R4~H2 is carried out between 100 and 150C, optionally
in the presence of ammonium chloride. The reaction is performed without
solvent or in the presence of an inert solvent such as n-butanol. ~le
product (I) is isolated by extraction and purified, for example, by
chromato~raphy.
The product oE formula I thus obtained is isolated in the
form of the free base or a salt according to standard techniques.
'lhen the compound of formula I is obtained in the Eorm of
the free base, salt formation is carried out by treatment with the
selected acid in an organic solvent. By treatment of the free base,
dissolved for example in an alcohol such as isopropanol, with a solution
oE the selected acid in the same solvent, the correspondin~ salt is
obtained which is isolated according to standard techniques. In this
way, the hydrochloride, the hydrobromide, the sulfate, the hydrogen
sulfate, the dihydrogen phosphate, the methane sulfonate, the methyl
sulfate, the oxalate, the maleate, the Eumarate and the 2-naphthalene
sulfonate are prepared.
At the end o-E the reaction, the compound of formula I may
be isolated in the form of one of its salts, for example the
hydrochloride; in this case, if necessary, the free base may be prepared
by neutraliæation of the said salt with a mineral or organic base such
as sodium hydroxide or triethylamine or an alkali metal carbonate or
bicarbonate such as sodium or potassium carbonate or bicarbonate.
~lhen Rl andjor R2 represent a hydroxyl group, the compound
according to the invention is obtained startino from compound ~I) in
which Rl and/or R2 denote alkoxy and all of the other substituents
:
, . . , ~ - - -
- . - - : :: ~
~ : ' i - : ~: :
203~3~
have the above definitions, I)y deallcylation using 1cno~/n methods.
The 6-chloro pyridazines (II), used as startino materials,
are prepared from the correspon(ling 211-pyrldazin-3-ones (III) by
reaction ~ith an e~cess of hot phosphorus oxychloride in the absence
oE a solvent or in the presence of an inert solvent such as
acetonitrile, accordino to the :Eollot~ing reaction scheme:
R3
Ar ~\ ~ O POCl3 ~II)
N-N -->
H
(III)
15The 2EI-pyridazin-3-ones ~III) are known or prepared by known
methods.
Tl1us, when P~3 is a Ar' radical, the 211-pyridazin-3-ones are
obtainetl according to the method described by P.SCf{MIDT et al. in llelv .
Chim. Acta, l954, 15, 134-140, starting Erom malonic acid tliethyl ester
20antl a hydrazone derivative according to the follo~ino reaction scheme:
Ar'-CO-C-Ar + CH2-CO2C2H5
N-NH2 C02C2H5
25Ar~ C02C2Hs Ar~ COOH
- > Ar ~ O - > Ar ~ O
N-N N-
H H
Ar' :
- > Ar ~ ~ O :
-N :
1 ~: :
(III) ~ -
. .- . ~
2~3~3~
11hen ~3 represents an alkyl or cycloallcyl radical, the
compoun(1s ~III) are prepared ~rom a ketone Ar-CO-C~2R3 (1):
CllO R3 OH
COOC2Hs CH-CH
5 Ar-C-C112-R3 -> Ar - C C = O
l 1~sC20 2
R3 OH R3 11
lO NH2NH2 CH-CH\ C=C
-----> Ar - C C = O > Ar - C\\ C = O ~
N - N N - ~ -
3 ~ (III) H
The hydro~y keto ester 2 is obtained from the ketone 1 by
heating it with ethyl ~lyoxylate at a temperature between 80 and 140C.
The crude reaction mixture is then taken up in an inert solvent such
as n-butanol and hydrazine hydrate is added. By heating at refluc for
24 hours, the 4,5-dihydro 4-hydro~y pyridazin-3-one 3 is obtained
which, when heated in an acidic medium, leads by dehydration to the
211-pyridazin-3-one (III).
The amines R4N112 are known or prepared by lcnown methods.
Thus, when n = O, they rnay be prepared from a cyano derivative of
formula:
XlCHz OH
C (IV)
XlCH2 CN
By reaction with an amine H~R5R6 by heating at a temperature
between 40 and 80C, optionally in the presence of a salt of a strong
acid such as sodium sulfate or magnesium sulfate, a compound of formula:
X lCH2 NR5R6
C ~V)
XlCH2 CN
:
-
-. :
,
. . - ~ . . ~ .
2~3~3~
is first prepared, then this compound is hydrated by reaction with
a strong acid such as hot sulEuric acid in order to produce the
corresponding aMide:
XlCH2 / NR5R6
C \ (VI)
XlCH2 CONH2
Finally, reduction by heating with a metal hydride such as
boron hydride or lithium aluminium hydride leads to the formation of
the amine R4Nll2.
~ lhen n = O, the amine P~4N!12 may also be prepared from a
chloronitroso derivative (VII) accordino to the procedure described
in J. Prakt. Chem., 1978, 320 (3), 433-451.
Xl,CH2 XlCH2
C1 - C - CH2NO + R5R6NH2 - ~ R6RsN - C - CH = NOH
XlCH2 XlCH2 : `
(VII) (VIII)
Li Al H4 XlCH2
> R6R5N - C - CH2 - NH2
X lCH2
The compound of formula (VII) may be used in the form o:E
a dimer (VIIa) which is obtained by reaction of nitrosyl chloride with
the appropriate olefin (IX) according to the procedure described in
J. Prakt. ChemO, 1965, 29 (4), 123.
,C,112 C1~l2Xl ~ O C~2~1
C + NOCl > Cl - C - CH2 - N = N - CH2 - C - Cl
X lCH2 CH2X 1 CH2X 1 C112X 1
(IX) (VII a )
~: . . . -
2~3~33
Finally, when n = 1, the amine R4NH2 may be prepared according
to the mcthod described in ~eilstein 4 (3)I 5~6, i.e. by reaction o
lithiuln aluminium hydride on the oxime (X):
CII2X1 LiAlH4 CI12Xl
IIo - N = CH - C - C~Iz - NR5a6 > H2N - CH2 - C - CH2- NRsR6
CH2X 1 CH2X 1
(X)
ine ~II2 ~ CX2C(CI3)2 ~ CH2-~(C~I3)2 is commercially
available.
The following exaIllples illustrate the invention without in
any way limiting it. The compounds are characterized by their melting
point (~I.p.) expressed in degrees centigrade.
E~A,IPLE 1
3-(2-diethylamino-2-methyl-propyl)amino-6-phenyl-5-propyl-
pyridazine sesquifumarate: SR 46559 ,~.
A~ 6-chloro-3-phenyL-4-propyl-pyridazine.
1. Ethyl 2-hydroxy-4-oxo-4-phenyl-3-propyl-butyrate.
A mixture of 48.c,7 g of valerophenone and 45.94 g of ethyl
~lyoxylate is heated at 120C Eor 15 hours.
The crucle reaction product is used as such in the following
step,
2. 6-phenyl-5-propyl-2rl-pyridazin-3-one.
The crude product obtained above is dissolved in 450 ml of
n-butanol, then 30 g of hydrazine hydrate are added and the mixture
is heated at reflux -Eor 24 hours.
The n-butanol is evaporated ~d2r vacuum. The residue is taken
up in a mixture of 300 ml of acetic acid and 30 ml of concentrated
hydrochloric acid. The mixture is heated at 100C for 3 hours. The
solution is poured into cold water and the product is left to
crystallize.
The solid is filtered off ancl dried.
I,~eight: 44 g M.p.: 160C.
, .: : . - - . .
- : .
2~3~3~
Yield : 69~
3. 6-chloro-3-pllenyl-4-propyl-pyridazine.
250 ml of phosph~s oxychloride are added to 44 g of
pyridazinone obtained above and the mi~ture is heated at 80~C for ~
hours. After being left to stan~ o~erni~ht at room temperature, the
reaction mixture is concentrated to 3/4 and then poured slowly onto
ice. Tl-e mixture is extracted twice with 300 ml of dichloro!nethane,
the e~tracts are tlried over sodiwn sul~ate and concentrated.
Chrolna~ography on silica is then carried out by elutin~ h an ethyl
acetate-r,lethylene chloride mixture (50/50 v/v).
After recrystallization ~rom isopropyl ether, 43.7 g of the
expected product are obtaine~d.
I.p. : 60C
Yield: 9~%.
~) Preparation of 2-diethylamino-2-methyl-propylaMine.
1. 2-diethylamino-2-methyl-propionitrile.
85.1 g of the cyanohydrin of distilled acetone and 73.1 g
of diethylamine are mixed, S5.7 g of magnesium sulfate are added and
the mixture is heated under gentle reflux for 20 hours with stirrin~.
The sulfate mass which is formed is filtered off and washed with ether.
The filtrate is concentrated and tllen distilled.
86.S g of the expected product are recovered.
Yieltl : 62%
3.p. = 68-70C at 15 mm of mercury.
2. 2-diethylamino 2-methyl propionamide.
To 95.9 g of the nitrile prel~ared in the preceding step 450
ml of sulfuric acid and 70 ml or water are added with stirring and
the mixture is heated on an oil bath at 100-110C for 2 hours. The
reaction mixture is poured slowly during one hour into 1.4 1 of a 20%
~0 ammonia solution and 400 ml of water cooled in a Dry Ice/acetone
bath. The mixture is extracted 3 times with S00 ml of methylene
chloride, the extracts are dried over sodium sulEate and concentrated. -
The expected product is obtained by distillation.
I1eight : 102.5 g
Yield : 95%
, . .:
: - -: . : ~. :: . :
:' : ~ - :: - : - ~ .:
- 2~3~;~3~
B.p. : 13~-139C at 15 mm of mercury.
3. 2-diethylamino-2-me~hyl-propylamine.
A mixture containil~, 52.4 g~ of the ami(l~ prepared in the
preceding step and ~0 ml of tetrahydroftlran are heated at 45-50C.
So ml of the borane-dimethylsulEide complex are added under an
atmosphere of nitro~en durino one hour and heating is continued for
3 hours on an oil bath at ~0-~5C.
After being left overnight at room temperature, the mixture
is cooled in an ice bath, then 315 ml of 6~ llydrochloric acid are added
slowly during 3 hours and the mixture is heated again at 135C for
3 hours. After being left overnight at room temperature, the reaction
mixture is cooled whilst 200 ml of 30~O~ sodium hydroxide are added.
The mixture is extracted 3 times with 250 ml of ether, the extracts
are dried over sodiu,n sulfate and concentrated.
The expected product is obtained by distillation.
~eight: 23
Yield : 48%
3.p. = 71-73C at 15 mm of mercury.
C) SP~ 46559 A
- t~ mixture oE 2.5 g of the chloro derivative obtained above
in step A and 4.6 g oE the ~iamine obtained in step B are heated at
120C overnight. 150 ml of ethyl acetate are added, then the mixture
is extracted twice with 50 ml of hydrochloric acid. The mixture is
then made alkaline by the addition of 50 ml of 30% sodium hydroxide
and then extracted with ethyl acetate. The extracts are washed with
dilute salt solution, dried over sodium sulfate and concentrated.
Chromatotgraphy on alumina is carried out by eluting with a methylene
chloride-ethyl acetate mixture (70/30, v/v~.
3.2 g of an oil is obtained which crystallizes.
.-I.p. = 75-77C
Yield: 87"
Sesquifumarate
3.1 g of the base obtained in the preceding step are taken
up in 50 ml of acetone and 1.6 ~ of fumaric acid in 150 ml of ace~one
are added. The mixture is filtered hot. The total volume recovered
.
.
- , : ~ ~:
- ~ :
- ~ .
.
2~3~.~33
(175 ml) is concentrated to 130 ml. The product i9 allowed to
crystallize, the crystals are filtered off and then ~ashed witll acetone.
.l g of the expec~ed product are obtained.
Overall yield of step C = 74,~
`I.p. = 151~C
rXA;~IPL~ 2
S?~ 46559 .~
Aj 6-chloro-3-?henyl-4-propyl-pyridazine, described in e~ample 1.
B) 2-diethylamino-2-methyl--propylannine.
1. Pre?aration of the compound of formula ~VII a) with ~1 = H.
47.14 g of isobutylene are dissolved in 150 ml oE n-heptane,
the mixture is cooled to a temperature bet-~een -10 and -20C and 50 g
of nitrosyl chloride are added. The temperature is allowed to rise~+5C)
during one and a half hours, then the temperature is brought to between
10C and 20C and the mixture is stirred for one and a half hours. The
precipitate formed is filteretl off, washed with heptane and then dried.
;I.p. = 102-104C
m = G4 g
2. Preparation oE the compound of forlnula VIII~ 5
= ~6 = C2~15
21.7 g of the compound prepared in the preceding step are
suspellded in 150 ml of a~solute alcohol, 39.17 g of diethylamine are
added antl the mixture is heated at 60C for 6 hours. An oil is obtained
which solidifies.
m = 19.5 g
~ I.p. ~ 50C
3. 2-diethylamino-2-methyl-propylamine
7.01 g of lithium aluminium hydride are added to 50 ml of
an ethereal solution of the compound obtained in the preceding step
during l hour. After being stirred for one and a half hours at room
temperature, the mixture is refluxed for 4 hours. r~hile the mixture
is maintained between 0C and -10C, 7.1 ml of water are added during
1 hour, 7.1 i711 of sodium hydroxide durino 30 minutes and 21.3 ml of
water during 3G minutes. After being stirred for 2 hours at room
:: , . :
:
20~3~
11
temperature, the solution is filtered, the precipitate is washed ~ith
anhydrous ether, the filtrate is dried over sodium sulfate and the
solvents are removed un~ervacuum. The pro~uct is distilled:
~.p. = 72-75C at 15 mrn of mercury.
m = 4.2 ~
C) SR 46559 A is then prepared as described in example 1.
EX~`IPLE 3
3-(2-diethylamino-2-methyl-propyl)amino-6-(2-chloro-phenyl)-
5-propyl-pyridazine sesquifumarate. SR 47863 A.
1.7 g of 3-chloro 6-(2-chloro-pilenyl) 5-propyl pyridazine
and 6 ml of 2-diethylalDino 2-methyl propylamine are heated at 110C
under nitro~en for 20 hours.
After evaporation unde~ vacuum, the mixture is talcen up in
dichloromethane and uashed with a solution of sodium bicarbonate. The
organic phase is decanted, dried over magnesium sulfate, filtered and
concentrated undsrvacuum. The residue is chromatographed on silica
nel, eluant: dichloromethane/methanol 98/2.
The conccntration of the pure fractions gives an oil ~hich
is dissolved in 10 ml of methanol. Fumaric acid is added, the methanol
is evaporated under vacuum and the sesquifumarate crystallizes from
ether.
m = l.G ~
~I.p. = 144~C.
E~ lPL~ 4
3-(2-diethylamino-2-methyl-propyl)amino-6-(2-methoxy-phenyl)-
5-methyl-pyridazine.
1.6 g of 3-chloro-o-~2-methoxy-phenyl)-5-methyl pyrida~ine,
4 g of 2-diethylamino-2-methyl-propylamine and 0.36 o of ammonium
chloride are melted together at 120C and the reaction mixture is left
at this temperature for 24 hours.
The mixture is cooled to room temperature, extracted with
ethyl acetate and washed with a saturated aqueous solution of sodium
chloride.
.
.
,- ., :
12 2~3~3
The orgaIlic phase is separated, ~ried over ~gS0~. filtered and
evaporated to Iryness in a vacuum.
The residue is chromatol7rapIled on alumina, eluent: ethyl
acetate +2~o of triethylamine.
The concentration of the pure fractions g~ives the e~pected
product. Tlle structure is confirZ~Ied by ~iR spectral analysis.
E~A`PL~ 5
3-(2-diethylamino-2-methyl-propyl)amino-5-methyl-6-(2-hydroxy-
phenyl)-pyridazine. SR 96376.
1 g oE the product obtained previously in e~ample 4 is
dissolved in 50 ml of 4~O hydrobromic acid and the mixture is heated
at reflu~ for 4~ hours. After this time, the Ieac-~.on mixture is
evaporated to dryness under vacuum, the residue is made allcaline with
an aqueous solution of potassium carbonate and the solution is extracted
with dicIlloromethane. The org7anic phase is decanted, dried over-i~IOS04.
filtered and evaporated to dryness ~der vacuum.
The residue is chromatographed on alumina, eluent: ethyl
acetate/methanol 9/1 + 2% of triethylamine.
The concentration of the pure fractions ~ives a residue which
is crystallized from isopropanol.
m = 200 mg
~ 59.2C
~'~A`IPLES 6 T0 35
A) By using the procedure indicated in e~ample lA, but by varying the
starting ketone, the 6-chloro-pyridazines assembled in the tables
1 and 2 are obtained.
.: . .~ .
.- , . :
- 13 -
2 ~
TABLE 1
6 R3
OS ~ >\ ~ Cl
___________________________________________________ ,
: R1 : R2 : R3 : Physical
: : constan~s
H H -CH2CH2CH3 M.p.: 52-53C
Cl (4) H . -CH3 M.p; 178-180C ;
lS Cl (4) H -CH2CHzCH3 ;M.p; 95C
OCH3 (4) }I ; -CH2CH2C1l3 M.p~ 68-!69C
: H : I{ : CH3 :M.p: 123-124C :
: H H phenyl M.p:. 115C
H H : cyclopropyl M,p.119C
: F (4): H isopropyl :M.p-.89-90C
-. :
: Gl (2) : H : CH2CH2CH3 : oil, NMR ~ :
:
: OCH3(2) : H : CH3 NMR
: : : : :
: H : H : benzy~ :M.p; 92C
:
- 20~13~
Cl (4~ H ; C1-4 phenyl M p... 118-119C
: H : H : Cl-4 phenyl :M.p_ 130C
:
OS : Cl (4) : H : phenyl :M.p; 125C
Cl ~2) Cl (4) CH2CH2CH3 M.p; 71-72C
: Cl (3) : H : CH2cl~2cl~3 :M.p; 48C
: CH3 (4) : H : Cl-4 phenyl :M.p'. 140C
:
: OCI13(2) : ll : C1l2CII2CH3 :oil~ NMR
: OCH3(3) : H : Cll2CH2CH3 :oil, NMR
:
: F ~4) : H : Cl-4 phenyl :M.p. 139C
____ _ ____________________________________________
NMR : NM~ spectral analysis enables the structure
of ~he above compounds to be confirmed.
.:
203~33
TABLE 2
R3
Ar_\ ~ Cl
N-N
: : : Physical
Ar : R3 : constants
: 2-thienyl : phenyl :M.p: 148C
: 2-C1 5-thienyl . phenyl . NMR
3-pyridyl : phenyl :M.F. 184C
: 2-pyridyl : phenyl : M.p. 138C
: 4-pyridyl : phényl : M.p 193~C
--------------------------------------~~~~~~~~~~~
B) Starting ~rom the chloro derivatives of table 1 and by followinn
~he procedure e~nployed in evample 1, the co~pounds according to
the invention assembled in table 3 belo~ are obtained by ~tarying
the amines `.lll2~4 use(!.
~ :
, ~ - :
.,
-
:,
- 16 -
~3~3~
TABLE 3
5 6 R3
4 ~ ~ NH-R4
05 Rl N_N
3 2
____________________________________________________________________
:SR No. : sal~ or base:
:Ex. No. : Rl R2 : R3 : R4 : M.p.
: : : : CH3 : di`nydro-
I ~ chloride
: 46729A : Cl(4) : H : CH3 : CH2C-N > ; - :
: 6 : : : : CH3 ~ : 240-242~ :
: : : : : : sesqui-
: 46732A : }I : H : nC3H7 : : fumarate
: 7 : : : : : lS9-161C
CH3/C2Hs : Fumarate
: 46733A :C1(4) ~ }I : CH3 CH2-C-N\ : 138-140C
: 8 : : : : CH3 C2Hs
: 47020A : H : H : CH3 t- ~ : sesquifuma- :
: 9 : : : : : rate 161C
- . . . . . .
: 47047A : H : H : phenyl. " " : fumarate
: 10 : : : : : 193C
:- : : : : :
: 47054A : Cl(4) : H : nC3H7 : : sesqui-
: 11 : : : : : fumarate :
: : : : : 152-154C
:
: . :. . . :
- , : . . .. : . . ..
, ~ . , , . - . ' - , :
- , : ; . : . -. : , :,
2 ~
: 47068 : oCH3~4): H : nC3H7 : " " : 65-66C base:
: 12 : : : :
:
: 47069A : OH(4) : H : nC3H7 : : hydrobromide:
05 : 13 : : : : : 179-181C
:
: 47097A : H : H : cyclo- : " " : sesqui-
: 14 : : : propyl : : fumarate
: : : : : : 158-160C
1 0
: : : : : : sesqui-
: 47098A : F(4) : H : iPr : " " : fumarate
: 15 : : : : : hemihydrate :
: : : : : : 143-145C
: : : CH3 ~ : sesqui-
: 47138A : H : H : nC3H7 : CII2-C-N O : fumarate
: 16 : : : : CH3 ~ : 149-151C
:
: : C1~l3 C~l3 : 164C
: 47153A : H H : phenyl : CH2-c-c~2-~ : sesqui-
: 17 : : : : CH3 CH3 : fumarate
:
: : : : : CH3/C2Hs : fumarate
: 47227A : H : H : benzyl : CH2-C-N~ : 163C
: -18 ~H3 C2H5
: 47297A : Cl (4) : H : Cl-4 : : dihydro-
: 19 : . : : phenyl : a~l~ide 138C
: : : : : : hydrate
:
: 47608A : H - : H : " : : dihydro-
:20 : ~id~ 147C
'' . :- . ' . ' .
,
.
-
-
.
- 18 -
2~3~3~
: 47609A : Cl (4) : H : phenyl : " " :d~hydlo
21 chl~id~470c
.
: 47655A : Cl (2) :Cl-~4): nC3H7 : " " : sesquifuma- :
05 : 22 : : : : : rate
: : : : : : 165-166C
:
: : : : : C2Hs CH3 : base
: 47673 : H : H : nC3H7 : CH2-C-N. : oil
1023 : : : : C2Hs CH3 : ~MR
:
: : : : : CH3 C2Hs : sesquifuma- :
: 47878A : Cl (3) : H : nC3H7 : CH2-C-N : rate 146C
: 24 : : : : C~13 C2l~s
: 47890A : CH3 (4) : H : Cl-4 : " " dihydro-
: 25 : : : phenyl : Chloridle O
:
: 47967 : F (4) : H : Cl-4 : : 98C
: 26 : : phenyl : : base
: 48079A : OCH3 (Z) ': H : nC3~17 : : sesquifuma- :
: 27 : : .: : : rate 95C
: 48080 : OH (2) : H : nC3H7 : : 15g,5 C
: 28 : : : : : base
: 4808lA : OCH3 (3) : H : nC3H7 : ' dihydro-
: 29 : : : : chloride
: 48082 : OH (3) : H : nC3H7 : : 166~C
30 : : : : base
________________________________________________ ___________________ :
- : . . . . : : :
;, ,. ~ ~
~ ~ 3 ~
19
.`r;lR spectrurIl of SR 47673
~D:IS0 d6; 200 `-~Tz)
0.70 (t: 3 11); O.S0 ~t; 6TI); 1~JO (q; 21l); 1.50 (m; 411);
2.30 (s; ~)ll~; 2.40 (m; 2M); 3,40 (m; 211); 6.20 (In; lll); 6.S0 (s;
lH); 7.35 (s; 5M).
~I~IR spectrum of SR 4SOSl ~
(D`IS0 d; 200 lI!lz)
0.3d (t; 3TM~; 1.40 (m; 31.1); 1.5 (s; 61T); 2.56 ~q; 211);
3.40 (b.s. ; 4Tl); 3,30 (s ; 3~1); 4.00 (d ; 21'); 7.02 (In; 3~1); 7.40
lO (t; l1l~; 7.S3 (b.s.; lM).
The following abbreviations are used for the analysis of
a ~ s~ectruIn.
s = singlet ; I).s. : broa(l sin;,let; d : doublet; t : triplet;
q : quadruplet; ;n : multiplet.
15 C) Starting from the chloro derivatives of table 2 and by l~ollowing
the pro^edure described in e:cample 1, the compounds according- to
the invention asseml)led in table 4 belo~ are obtained by varying
the amines .'~112R4 used.
,, .
~, :. . , . : '
. ~ . , .
.
'' ~ ' . ' ' . ' '
..
2~3~:~33
TA~LE 4
R3
Ar ~ NH R4
~___________________________________________________________________
: SR No. : Ar : R3 : R4 : Salt or ~ase:
: Ex. No. : : : : :
1 0
:: : : CH3 C2H5 : dihydro- : -
:47674 A : 2-thienyl : phenyl ~ : CH2-C-N \ Chlrilde3C
:31 : : : CH3 C2H5 : : .
47675 A : 5-Cl : phenyl : " " : dihydro-
:32 2-thienyl chloride 130C
:: : : : decomposi-
: tion
: 47802 A : 3-pyridyl : phcnyl : " " : trihydro- :
33 ' ' , chlnride 225C ,
: 47803 A : 2-pyridyl : ph~nyl : " " trihydro-
: 34 : : ~ oride233C
~5 : 47804 A : 4-pyridyl : phenyl : " " : ~rih~d~o-. :
: 3S : chloride
: : : : ~ 239 C
____________________________________________________________________
The compounds according to the invention were studied with .~ --
respect to their pharmacological properties and in particular with :~
respect to their affinity for the muscarinic cholinergic receptors .
of type ;~1l and ~12-
In vitro, the compounds (I) were assayed according to the
technique described by '.~1atson J.D. et al. (Life Science.s, 19S2, 31,
. .
.
;
2~3~3~
21
2019-2029) as far as their affinity for the receptors of type ~ll is
concerned and according to the technique described by ~lanmmer 1>~. et
al. (`-ature, 19S0, 2S3, 90-92) and llulme I~.C. et al. (~`~olecular
Pharmacology, 1973~, 14, 737-750! as far as their af Einity for the
receptors of the ~'~2 type is concerned.
The compounds accordino to the invention exhibit good affinity
for the receptors of type ~1l and a mar~ced specificity for the central
receptors o E type `:ll as opposed to receptors of type li2.
As an exainple, the co npound SR 46559 A showed an inhibitin~ -
concentration 50 expressed in micromoles of 0.11 and 2.2, respectively,
on the ~ll and i l2 receptors .
Similarly, the compound SR~ 47047 A showed inhibitin~
concentrations 50 o 0.04 and 0.9, respectively, on the `11 and ~t2
receptors .
In vivo, the compollnds accordin~ to the invention were assayed
for their effect on the rotations induced by intrastriatal pirenzepine
in the test tlescribed by r?orms P, et al. (Psychopharinacology, 19S7,
93, 4S9-493) modified in that the adlninistration of the compounds by
the oral route toolc place 4 hours before, instea(l o~ 30 minutes before,
the injection of pirenzepirle.
At a dose o E 3 m;-g per Icg of body wei-r,ht, the compoullds
according to the invention strongly inhibit the number of rotations
induced by pirenzepine. Thus, as an exainple, the compound SR 4G559 A
inhibits the rotations induced by pirenzepine by 73%.
Furtherlllore, the coalpounds according to the invention were
shown to be active in the passive avoidance tests in the rat described
by Jarvik ;il.E. et al. in Psychol. :led., 19S7, 21, 221-224 and by l~orms
P . et al . in Psychopharmacol ., 19S9, 9S , 2~$-2~33.
Thus, according to the results of these tests, the compounds
according to the invention counteract the amnesia induced by scopolamine
administered by the intraperitoneal route at 0.5 mg~!cg and the amnesia
induced by Ipirenzepine administered intraperitoneally at 75 mg/lcg.
For example, SP~ 46559 A exhibits an oral efficien~ dose 50 of 0.25
mg/kg and 0.027 m~,/lcg, respectively,in each of these tests.
'loreover, some compounds according to the invention were
- , ',
2~3~3~ .
studied in se~veral predictive models oE antidepressant activity such
as the forced suimming test described by Porsolt et al. (Arch. Intern.
Pharmacodyn., 1977, 229, 327-33S) and the test of antagonism of
reserpine-induced ptosis described by Gouret et al. (J. Pharmacol.
(Paris), 1977, o, 333-350). S~ 46559 A in particular was shown to be
inactive in these tests at oral doses varyillg from 0.1 to 10 mg/lcg.
linally,the compounds according to the invention did not
sho~ any sign of toxicity at the doses at which tlley are active.
Consequently, the compounds ~I) may be used as medicines.
The results indicated show that the compoun(ls according to
the invention e~hibit good affinity for the muscarinic receptors and
good activity in the tests of amnesia induced by scopolamine or
pirenzepine. They allow the use of the products accordillO to
the invention to be contemplated in all cases in which a cholinergic
deficit is indicated and particularly Eor the treatinent of cognitive
and memory disorders, and degenerative syndromes associated with
senescence and senile dementia.
In accordance with another of its features, the present
application thus relates to pharmaceutical compositions containing
at least one of the compoull(ls of formula (I) or one oE their salts
as active ingredient.
In the pharmaceutical compositions of the present invention
for oral, sublingual, transdermal or rectal administration, the active
ingredients of formula I above may be administered in specific forms
of administration, in combination with the standard pharmaceutical ~
vehicles, to humans especially for the treatment oE cognitive or memory ;-
disorders or degenerative syndromes. ~he appropriate specific forms
of administration comprise the forms used for the oral route such as
tablets, capsules, powders, granules and solutions or oral
suspensions, the forms used for sublingual and buccal administration,
the forms for subcutaneous, intramuscular or intravenous administration
and the forms for rectal administration.
In order to obtain the desired effect, the dose of the active
ingredient may vary between 0.5 and 500 mg per day.
: :
.
,
:. ~
2 ~ 3 ~
23
Each unit dose may contain from 0.1 to 100 mo of active
inoredient in combination with a pharmaceutical vehicle. This unit
dose may be administered 1 to 5 times per clay.
~Ihen a solid composition is prepared in the form of tablets
the principaL active ingredient is mixed with a pharmaceutical vehicle
such as gelatine starch lactose magnesium stearate talc num arabic,
or si;nilar substances. The tablets may be coated with sucrose or
other suitable materials or they may be treatetl so that they have
sustained or clelayed activity and so that they release continuously
a predetermined amount of active ingredient.
t~ preparation oi- capsules is obtained by nixinO the active
ingredient ~ith a diluent and ~y pourino the mixture obtained into
soft or hard capsules.
The powders or granules dispe~sible in water may contain the
active ingredient mixed with dispersing agents or wetting agents or
suspending agents such as polyvinylpyrrolidone as well as with
sweeteners or taste modifiers.
In the case of rectal administration suppositories are used
which are prepared with binders me~tino at the rectal ten~perature,
for example cocoa butter or polyethylene glycols.
~or parenteral administration aclueous suspensiolls, isotonic
saline solutions or sterile ancl injectable solutions which contain
pharmacologically compatible wetting andfor dispersing agents, for
example propylene glycol or butylene glycol are used.
The active ingredient may also be formulated in the form
of microcapsules, with or without one or more additives or supports.
As an example of a galenic preparation capsules may oe
prepared containing:
SR 46559 l~ 0.010 8
~0 Lactose 0.050 g
.~iagnesium stearate 0.005 g
by mixing the aboYe ingredients intima~ely and pouring the mixture
into gelules of hard gelatine.
.:
. ~ .
. ' ~.
,
.