Note: Descriptions are shown in the official language in which they were submitted.
i;~ ;~~ !_i ~:; ~,.: .;., n.:i
1
This invention relates to a hydrotreating
process. More particularly, this invention relates to
hydrotreating a feedstock at relatively low pressure
followed by a selective distillation to obtain an
effluent suitable for hydroprocessing or hydrocracking
over a noble metal containing catalyst.
Hydrotreating is used to improve the quality
of petroleum feedstocks by treating the same with
hydrogen in the presence of a catalyst. Depending on
the precise feed and the purpose of the operation,
hydrotreating can markedly improve the odor, color,
stability, reaction characteristics and other important
quality characteristics of the feed. Hydrotreating is
capable of removing sulfur, nitrogen and other nonhydro-
carbon components.
Severe hydrotreating is presently used to
prepare hydrocracking feedstocks. Hydrocracking is
known to significantly improve both distillate and
naphtha product quality. Hurning qualities are
improved and gasoline yield is increased.
The hydrocracking process consists primarily
of a high activity noble metal catalyst which saturates
aromatics to low levels or causes significant conver-
sion to naphtha, In order to function properly, this
catalyst must operate on a "sweetened" process stream,
that is one where sulfur and nitrogen have been removed
to very low levels. ~'or acceptable conversion activ-
ity, sulfur and nitrogen should be typically reduced to
levels of 40 ppm and 10 ppm, respectively, or lower.
Some commercial units occasionally operate to levels
slightly above this range, but must increase tempera-
ture in the conversion reactor to compensate for the
,. _, .., . .. -,.,
pJ '..l ' ~1 J ~,_
- 2 -
lower catalyst activity. For acceptable hydrogenation
activity, still lower heteroatom levels are recom-
mended, in particular, nitrogen levels of 2 ppm or
less.
The removal of these sulfur and nitrogen
contaminants requires the hydrotreating stage to be
upstream of the hydrocracking process. This upstream
hydrotreating stage is referred to as an "R-1" hydro-
treater. In the past, R-1 hydrotreaters have been
designed and built for high pressure operation, in the
league of 1500 psig total pressure. Such design was
deemed necessary due to the difficult task of removing
contaminants to the low levels required by the Hydro-
cracking Unit, particularly the second stage thereof
comprising a sweet zone. 69ith high pressure operation,
significant aromatics hydrogenation can occur, although
undesirably consuming much hydrogen and requiring large
amounts of treat gas to be circulated through the unit.
A large compressor has traditionally been needed,
further escalating the costs of the high pressure R-1
hydrotreater.
The prior art discloses various methods for
reducing hydrogen consumption in a hydrotreating or
hydrodesulfurization operation. U.S. Patent No.
4,179,355 and 4,179,354 to Frayer et al. relate to a
multistage desulfurization of a residual oil, while
avoiding deep, hydrogen inefficient hydrodesulfuriza-
tion of the heavy portion of the oil. Frayer et al.
teaches separating an interstage stream into distillate
and residual components so that the downstage hydro-
desulfurization stage is relieved of accomplishing
relatively deep desulfurization of refractory residual
components necessary to produce a low sulfur effluent
stream. U.S. Patent No. 4,592,828 to Chu et al.
discloses a hydrodesulfurization process far upgrading
r ~. ,.,
Z'
r 1 ;J >.i ~,~ '.: _.. B
- 3 -
petroleum residuum. Relatively severe conditions, for
example at 1500 to 2500 psig, are preferred. A distil-
lation unit is used for the purpose of dewaxing and
producing gasoline directly. The combination of a
hydrotreater with a distillation means in an integrated
process generally is known. U.S. Patent No. 3,806,444
to Crouch et al. discloses a hydrodesulfurization
process for converting heavy crudes or crude fractions
to liquid industrial fuels with a maximum of 1.0%
sulfur. The hydrotreating is under relatively severe
conditions and, in particular, the pressures are in the
range of 1000 to 6000 prig. A distillation unit
subsequent to the hydrotreater produces a product
stream substantially free from sulfur.
HRIEF DESCRIPTION OF THE INVENTION
It has now been discovered that sufficient
clean-up of a process stream for subsequent upgrading
in a sweet processing stage such as a hydrocracker can
be accomplished at relatively low pressure. The
present process features hydrotreating of the raw
process stream at total pressures of 500 prig or below
and elevated temperatures, followed by a distillation
or separation of the product stream into predominantly
"sweet" fractions. One or more sweet fractions are
hydroprocessed over a noble metal catalyst and the
bottoms may be passed to a catalytic cracker. A fused
iron catalyst is optionally added after the distilla-
tion step to increase the amount of product which can
be sent to the sweet processing stage.
These and other obaects are accomplished
according to our invention, which comprises:
(1) passing a first stream comprising a
petraleum distillate 'in admixture with a hydrogen
i,l 's.i ~.~ ~~..~. . .. :.y
- 4 -
containing gas through a hydrotreating zone and in
contact with a hydrotreating catalyst under a pressure
of about 500 psig or below, such that substantial
hydrodesulfurization is carried out;
(2) introducing the process stream from step
(1) into a fractionation zone, separating the stream
into a distillate overhead stream which is substan-
tially free from heteroatoms and a distillate bottoms
stream; and
(3) passing the distillate overhead stream
from step (2) into a sweet processing zone containing a
noble metal catalyst.
BRIEF DESCRIPTION OF THE DRAWINGS
The process of the present invention will be
more clearly understood upon reference to the detailed
discussion below upon reference to the drawings
wherein:
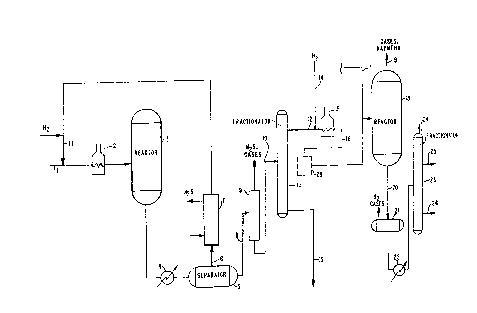
FIG. 1 shows a schematic diagram of one
process scheme according to this invention where a
relatively low pressure hydrotreater prepares a portion
of the feedstock to a reactor employing a noble metal
catalyst:
FIG. 2 contains a graph illustrating the
nitrogen distribution in the overhead of a distillation
unit following a hydrotreater, according to one embodi-
ment of the present invention: and
FIG. 3 contains a graph illustrating the
sulfur distribution in the overhead of a distillation
unit following a hydrotreater, according to one embodi-
ment of the present invention.
,,
>~..~ .i ..:~ .': ... '_~
_
DETAILED DESCF2TPTTON OF THE TNVENTTON
The present invention is directed to a
process for relatively low pressure, high temperature
hydrotreating of a petroleum distillate combined with a
selective distillation or separation to produce a
segregated product substantially free of heteroatoms.
The hydrotreating is conducted at pressure and temp-
erature conditions where heteroatom removal across the
entire liquid product would otherwise not be complete.
After an appropriate distillation or separation, the
product fraction which is substantially free from
heteroatoms can be fed to a noble metal catalyst system
for further upgrading, while the product containing
significant heteroatom levels can be fed to a catalytic
cracker for conversion to motor gasoline. A greater
ratio of sweet to dirty product can be obtained by
further increasing hydrotreating temperature, or by
cutting selectively deeper into the product and insert-
ing a fused iron cleanup bed to further decrease
nitrogen levels. Extending distillation under a vacuum
enables the removal of additional incremental amounts
of sulfur and nitrogen free light products.
Tn the following description of the inven-
tion, the term '°petroleum rlis~illate feed" is meant to
include virgin petroleum feedstock or a distillate
thereof. Materials which may be advantageously treated
according to the present process include light cataly-
tic cracker oil (LOCO), and thermally cracked distill-
ates which have a substantial amount of aromatics and
heteroatoms such as nitrogen.
The term "hydrogen containing gas" as used
herein includes by definition substantially pure
hydrogen, for example 90 to 97 percent by volume H2, or
a recycle hydrogen stream recovered subsequently in the
;a
f:~ ',; .. ,
- 6 -
process and containing at least 40 percent by volume
hydrogen, in a hydrogen rich gas as elsewhere obtained
from the processing of hydrocarbonaceous fuels and
containing at least 40 percent by volume hydrogen.
Although the term '°fractionator'° is used
herein, those skilled in the art will appreciate that
this encompasses a distillation column or alternatively
a high pressure separator, equivalent to a one plate
distillation unit.
Referring to FIG.1, a petroleum distillate
feedstream 1 comprising for example virgin naphtha,
enters hydrotreater reactor 3 designated R-2. Before
being passed to the hydrotreater reactor 3, the feed-
stream is typically mixed with a hydrogen containing
gas stream 21 and heated to a reaction temperature in a
furnace 2. The hydrogen containing feedstream prefer-
ably passes downward through the reactor 3. Depending
on the feedstock and operating conditions, all of the
oil may be vaporized or as much as 80-90~ may remain in
the liquid phase.
The hydrotreater reactor typically contains
a catalyst in the form of extrudates or pills. The
possible catalysts are well known in the art and
include molybdena on alumina, cobalt molybdate on
alumina, nickel molybdate on alumina, nickel tungstate,
or combinations thereof. Which catalyst is used may
depend on the particular application. Cobalt molybdate
catalyst is generally used when sulfur removal is the
primary interest. The nickel catalysts find applica-
tion in the treating of cracked stocks for olefin or
aromatic saturation. One preferred application for
molybdena catalyst is sweetening or the removal of
mercaptans.
i ~ ''f ~:. .._ . .. '~~
Various types of reactions occur during
hydrotreating. Tn one type of reaction, the mercap-
tans, disulfides, thiophenes, benzothiophenes and
dibenzothiophenes are desulfurized. The mercaptans and
disulfides are representative of a high percentage of
the total sulfur in lighter virgin ails, such as virgin
naghtha and heating oil. Thiophenes, benzothiophenes
and dibenzothiophenes appear as the predominant sulfur
form in heavy virgin oils and even more in cracked
stocks of all boiling ranges. Hydrotreating also
removes nitrogen from various nitrogen compounds.
Removal of nitrogen is much more difficult than sulfur
removal: 90~ desulfurization might be accompanied by
only 40~ nitrogen removal.
Total hydrogen consumption in the hydro-
treater reactor 3 is relatively low, in the range of
about 100 to 1000 SCF/B, typically 200 to 800 SCF/B,
depending on feedstock and actual processing condi-
tions. This includes hydrogen required for hydro-
desulfurization, hydrodenitrogenation and saturation of
aromatics and olefins. It is significantly less than
the 1200 SCF/B hydrogen cansumptions typically encoun-
tered in high pressure hydrotreating units. A further
reduction in hydrogen consumption may be obtained at
equivalent heteroatom removal by reducing the hydro-
treating pressure and increasing the operating tempera-
ture.
The relatively low pressure in the R-1
hydrotreater is suitably less than about 500 psia,
preferably 100 to 500 psia, and most preferably 200 to
500 psia. The temperature is suitably aver 500°F,
preferably 600 to 800°F. Temperatures over 800°F are
not recommended, since undesirable reforming or aroma-
tics production may occur in addition to excessive
catalyst deactivation. Temperatures are varied to meet
h ';a!;' ..'. ... :.1
_ 8 _
distillate specifications and satisfy unit run length
requirements. Temperature is often increased to
compensate for catalyst deactivation until end-of-run.
The hydrotreating reactor effluents are typi-
cally cooled in a heat exchanger 4 and then passed to a
gas-liquid separator 5. A gas stream 6 may be recycled
to the feed stream 1 for retreatment. The recycled gas
is usually first passed through scrubber 7 to remove
the hydrogen sulfide because of the inhibiting effect
of hydrogen sulfide on the kinetics of hydrotreating
and also to reduce corrosion in the recycle circuit.
Sometimes, when treating a light stock with a very low
sulfur content, the recycle gas is not scrubbed because
the H2S is at an acceptably low level.
Subsequent to the hydrotreating step, the
cooled and degassed stream 8 is typically passed to a
stripper 9 to remove residual H2S and other light
gases. In many cases, the liquid praducts are given a
light caustic wash to assure complete removal of H2S.
Small quantities of HAS, if left in the product, will
oxidize to elemental sulfur upon exposure to air, and
will cause the product to exceed pollution or corrosion
specifications.
Subsequent to the hydrotreating, the stripped
stream 10 is then introduced into a fractionator 11.
Fractionation is carried out to separate the inlet
stream into the following fractionation productss a
distillate (overhead) stream 12 which is relatively
depleted in sulfur and nitrogen and a bottoms stream 13
which is relatively concentrated in such heteroatoms.
Of course, additional streams may be taken for integra-
tion into other refinery operations. The distillate
stream 16 is sent for further treatment to a sweet
upgrading zone comprising a noble metal catalyst, as
-, . , ~,
,' .e ~~~.~ !_i ~J ~.
-
more fully explained below. The distillate may repre-
sent greater than 25 percent of the feed to the dis-
tillation unit, preferably greater than 75 percent and
most preferably greater than 90 percent of the inlet
feed to the fractionator 10. The bottoms stream 13 is
most preferably less than 55 percent, preferably less
than 25 percent and most preferably less than 10
percent of the inlet feed to the fractionator 11. A
split of distillate to bottoms of greater than or equal
to about 1 to 1 is possible.
The fractionator 11 may be operated at atmos-
pheric pressure or lower. Preferably, the pressure in
the fractionator may be dropped to about 25 to 100 mm
Hg to obtain additional distillate of acceptably low
heteroatom content.
The heavier fractions from the distillation
unit typically have heteroatom levels which exceed the
recommended levels for sweet upgrading. In addition,
they frequently have a high concentration of mufti-ring
aromatic structures. This is especially true for
catalytic cracker oils. Many of the aromatics have
been partially saturated, since pressures and tempera-
tures of about 500 prig and 700°F, respectively, are
similar to catalytic feed hydrotreating conditions.
The bottoms stream 13 comprising these heavier cuts may
thus make good catalytic cracker feeds for high octane
motor gasoline production. Alternatively, the bottoms
stream 13 may be cooled and sent to the heavy distill-
ate pool for further processing into light distillate.
The bottoms may also be used directly as heating oil,
fuel oil, or the like.
FIGS. 2 and 3 show a typical distribution of
sulfur and nitrogen, respectively, to be found in the
fractionator overhead stream 12. As shown in FIG. 2,
-., m; ~% r~; ' .n
S
rJ Su ~a '.% ~:. . .
...
45% or more of the entire product sample can be
distilled overhead, resulting in a clean distillate
stream with a nitrogen level of 5 ppm or less. The
material also has a sulfur content which, at less than
ppm, is well below that required for good noble
metal catalyst hydrogenation activity. Thus, greater
than 45% of the entire product may be fit for hydrogen-
ation in a sweet environment.
As shown in FIG. 3, in some cases, nearly 90%
of the total liquid product (TLP) can be distilled
overhead before distillate sulfur levels exceed 40 ppm.
At this point, nitrogen levels will have climbed to
over 50 ppm. The still relatively low sulfur content
along with the absence of the tail fraction makes this
part of the product an ideal stream to be processed
with fused iron. Fused iron is a highly active
material for hydrodenitrogenation as disclosed in U.S.
Patent No. 4,629,533, but it requires low sulfur
levels, preferably less than 50 wppm, in order to
function. Thus a small fused iron cleanup bed allows
almost 90% of the mildly hydrotreated material to be
introduced into a sweet hydrocracking or sweet hydro-
genation zone. Another way to increase the yield of
"sweet" product is to increase the temperature. For
example, by increasing the temperature from 700°F to
750°F, sweet product yield is typically increased from
about 45% to 70%. A further increase is possible by
continuing distillation under a vacuum.
The distillate stream 12 from the fraction-
ator 11 is admixed with a hydrogen stream 14 and heated
in furnace 15 to an appropriate reaction temperature.
As mentioned above, the feedstream 16 may be passed
through an optional fused iron cleanup bed 13 in order
to further remove nitrogen therefrom prior to hydro-
cracking.
i , ~)
x.
i J %.i '.J 'J ._. ..,.
_ 11 -
Also entering the hydrocracking reactor 18
can be a feed 17 treated in a relatively high pressure
hydrotreater knot shown). This hydrotreater may be used
to treat other feeds at a higher pressure than the
relatively low pressure hydrotreater 3. A pressure of
over 500 psia is currently used in the high-pressure
hydrotreaters. A typical pressure is about 1500 psia.
The effluent 17 from the high pressure hydrotreater is
typically less than 5 ppm in nitrogen and less than 40
ppm in sulfur.
The hydrocracking reactor 18 shown in FIG. 1
can be any conventionally known system employing a
noble metal catalyst. A hydrocracking reactor is pri-
marily used for gasoline conversion. The operating
conditions of the hydrocracker are flexible in response
to refinery needs. For example, higher temperatures
are usually employed for gasoline products as compared
to jet fuels. High pressures are employed, suitably in
the range of 800 to 2500 psia. Hydrogen gas at a rate
of 2000 SCF/B to 5000 SCF/B must be introduced into the
reactor. The temperature, pressure, and hydrogen feed
may be varied to adjust to the changing feeds and
intended products of the refinery.
A series of hydrocracking reactors are typi-
cally employed in a refinery. The distillate stream 12
from the fractionator may be used to back-out the cur-
rent hydrocracking recycle stream which enters the most
downstream hydrocracking reactor. This would free up
the hydrocracker recycle stream for direct disposition
into the distillate pool. Since the hydrocracker
recycle stream is high in both cetane and gravity, this
exchange would more effectively utilize refinery
streams and at the same time help de-bottleneck the
hydrocracking reactors.
i~~ rn i ~ ,~
Y E . .o
Vr, "r,,~ ':.l ~~J ,'..~~
- 12 -
The hydrocracking reactor 18 produces a
gas/naphtha stream 19 and an effluent stream 20 which
is typically passed to a second fractionator or distil-
lation unit 23 via degasser 21 and cooler 22. By
conventional operating conditions, the fractionator 23
yields a gaseous stream 24 comprising methane, ethane
and other light gases, a gasoline stream 25, and a jet
fuel stream 26. The normally gaseous components leave
the top of the fractionation tower and are sent to
light ends processing.
An advantage of the present invention is that
the low pressure hydrotreater operating at a typical
hydrogen feed rate of 750 SCF/B will be much less
costly than a high pressure hydrotreater operating at a
typical hydrogen feed rate of 1500 SCF/B.
By means of the process of the present
invention, additional gasoline or jet fuel may be
produced by a refinery at less cost and hydrogen
consumption. Instead of adding to the distillate pool
via further conversion reactions under relatively
severe conditions, virgin crude may undergo hydrode-
sulfurization under relatively mild conditions prior to
hydrocracking. Other advantages include reduced
hydrogen consumption and reduced light gas yields.
COMPARAT3VE EXAMPLE 1
A highly aromatic light catalytic cracker oil
(LOCO) feedstock having a nominal boiling range pf 350
to 700°F had the following characteristics:
b
CA 02030416 1997-11-19
- 13 -
Gravity, API 12.7
Sulfur, ppm 15500
Total Nitrogen, ppm 493
Basic Nitrogen, ppm 42
Pyrrole Nitrogen, ppm 28.0
Aniline Pt., F <30.0
Carbon, wt% 89.17
Hydrogen, wt% 9.03
Hydrogen by Nl~t, wt% 9.01
Freeze Point, F -22.2
Cloud Pt., F 0.0
Bromine Number 20.0
Refractive Index, 20C 1.5765
GCD, °F
IBP/5 321/449
10/20 458/492
30/40 503/528
50/60 548/578
70/80 608/638
90/95 669/688
99.5 735
LV% by HPLC:
Saturates 19.9
Total Aromatics 80.1
1-Ring 11.1
2-Ring 51.3
3+Ring 17.7
This oil was hydrotreated at conditions of 500 psig,
0.6 hHSV, 4000 SCF/B H2 and a somewhat elevated operat-
ing temperature of 700°F. The catalyst used in the
upgrading was a proprietary CoMo catalyst, designated
RT-3~ commercially available from American Cyanamid
(Hartford, Conn.). Feed sulfur and nitrogen levels
were 1.55 weight percent and 493 ppm, respectively.
As expected, the total liquid product (TLP)
was a murky fluorescent brown color and had sulfur and
nitrogen levels well above those required for satisfac-
tory noble metal catalyst performance. Sulfur and
nitrogen levels in the TLP were measured at 102 and 136
ppm, respectively.
Trademark
.) " ..) .s . , ;o
i.J .i.~ rj -.j
- 14 -
EXAMPLE 2
The product generated in the above Compara-
tive Example 1 was distilled via 15/5 distillation (15
theoretical plates with a 5/1 reflux ratio) into 13
narrow fractions and each fraction was analyzed for
sulfur and nitrogen content. The results of these
analyses are given in Table 1 below. Using the analy-
tical results acquired on each fraction of the TLP,
contaminant levels in the cumulative overhead were
calculated as a function of the amount of product
sample allowed in the overhead stream. The results are
shown in FIGS. 2 and 3. Total hydrogen consumption
during the hydrotreating step was low at 738 SCF/B.
This includes hydrogen required for hydrodesulfuriza-
tion, hydrodenitrogenation, and aromatics and olefins
saturation.
Surprisingly, much of the product described
in Comparative Example 1 is fit "as is" for introduc-
tion into 'the second stage of a hydrocracker which is
operating in a conversion mode. Again referring to FTC.
2, almost 60~ of the total liquid product (TLP) can be
distilled overhead before the cumulative distillate
nitrogen level exceeds 10 ppm nitrogen. Thus, nearly
60~ of the product could be introduced into the second
hydrocracking stage of today's commercial hydrocrackers
with no ill effects on hydrocracker performance.
t,~ '~. ~.~ ai 'r.' .'. !.)
- 15 -
Table 1
Fractionation Results
Sulfur level, N level,
Cut No, wt% of TLP wppm wpm
1 0 - 11.3 <10 5.0
2 11.3 - 23.2 <10 5.4
3 23.2 - 35.1 <10 4.1
4 35.1 - 45.3 <10 5.3
45.3 - 59.0 <10 23
6 59.0 - 65.0 80 144
7 65.0 - 70.9 100 166
8 70.9 - 76.9 80 155
9* 76.9 - 83.0 20 36
83.0 - 89.3 130 222
11 89.3 - 95.8 540 774
12 95.8 - 97.2 '640 1006
13 97.2 - 99.9 740 1263
(Bottoms)
*Distillation was continued under 100 mm vacuum at this point.
The heavier fractions from the above examples
having heteroatom levels which exceed the recommended
levels for sweet upgrading have a high concentration of
multi-ring aromatic structures as shown in the follow-
ing Table 2.
y ~ is 'i ~:,: .'.
- 1.6
Table 2
Aromatics Distribution in Heaw Product Cuts
Product Cut Numbers (From _Table 1)
LCGO Feed 10 11 12 13
(bottoms)
Aromatics, LV%
1-ring 11.1 17.9 11.7 12.3 12.0
2-ring 51.3 29.3 18.6 13.4 10.9
>_3-ring 17.7 23.4 35.7 36.4 37.0
Total aromatics 80.1 71.1 66.0 62.1 59.9
N level, wppm 493 222 774 1006 1263
The process of the invention has been des-
cribed generally and by way of example with reference
to particular embodiments for purposes of clarity and
illustration only. It will be apparent to those
skilled in the art from the foregoing that the various
modifications of the process and materials disclosed
herein can be made without departure from the spirit
and scope of the inventiono