Note: Descriptions are shown in the official language in which they were submitted.
) 7 ~ I
F-5324
INTEGRATED PROCESS FOR ENHANCING HIGH OCTANE ETHER
PRODUCTION AND OLEFIN CONVERSION IN GASOLINE
This invention relates to an integrated process
for the conversion of light olefins to ethers including
methyl tertiary bu~yl ether and high octana gasoline.
More particularly, the inven~ion relates to the
catalytic hydration and etherification of light olefins
to produce mixed ethers followed by unreacted olefins
aromatization or conversion to gasoline. The mixed
ether products of the integrated process are useful as
high octane blending stocks for gasoline
In recent years, a major technical challenge
presented to the petroleum refining industry has been
the requirement to establish alternate processes for
manufacturing high octane gasoline in view of the
regulated requirement to eliminate lead additives as
octane enhancers as well as the development of more
efficient, higher compression ratio gasoline engines
requiring higher octane fuel. To meet these
requirements the industry has developed non-lead octane
boosters and has reformulated high octane gasoline to
incorporate an increased fraction of aromatics. While
these and other approaches will fully meet the
technical requirements of regulations requiring
elimination of gasoline lead additives and allow the
industry to meet the burgeoning market demand for high
octane gasoline, the economic impact on the cost of
gasoline is significant. Accordingly, workers in the
field have intensified their effort to discover new
processes to manufacture the gasoline products required
by the market place. One important focus of that
research is processes to produce high octane gasolines
blended with lower aliphatic alkyl ethers as octane
boosters and supplementary fuels. C5-C7 methyl alkyl
2 0 3 ~ rJ~ ~ ~
F-5324 -2-
ethers, especially methyl tertiary butyl ether (MTBE)
and tertiary amyl methyl ether (TAME) have been found
particularly useful for enhancing gasoline octane, as
has di-isopropylether(DIpE). Therefore, improvements
to the processes related to the production of these
ethers are matters of high importance and substantial
challenge to research workers in the petroleum refining
arts.
It is well known that isobutylene may be reacted
with methanol over an acidic catalyst to provide methyl
tertiary butyl ether (MT~E) and isoamylenes may be
reacted with methanol over an acidic catalyst to
produce tertiary amyl m~thyl ether (T~ME). The reaction
is a useful preparation for these valuable gasoline
octane enhancers and is typical o~ the reaction of the
addition of lower alkanol to the more reac~ive tertiary
alkenes of the type R2C=CH2 under mild conditions to
form the corresponding tertiary alkyl ethers. The
feedstock for the etherification reac~ion may be taken
from a variety of refinery process streams such as the
unsaturated gas plant of a fluidized bed catalytic
cracking operation containing mixed light olefins,
preferably rich in isobu~ylene. Light ole~ins such as
propylene and isomers of butene other than isobutylene
in the feedstock are essentially unreactive toward
alcohols under the mild, acid ca~alyzed etherification
reaction conditions employed to produce lower alkyl
tertiary butyl ether. The further utilization, without
recycle, of these unreacted olefins and alcohols to
meet the overall product goals of the process would be
a welcomed improvement.
Lower molecular weight alcohols and ethers such a
isopropyl alcohol (IPA) and diisopropyl ether (DIPE)
are in the gasoline boiling range and are known to have
a high blending octane number. In addition, by-product
propylene from which IPA and DIPE can be made is
usually available in a fuels refinery. The
203~797
F-5324 -3-
petrochemicals industry also produces mixtures of light
olefin streams in the C2 and C7 molecular weight range
and the conversion of such stream5 or fractions thereof
to alcohols and/or ethers can also provide products
useful as solvents and blending stocks for gasoline.
The catalytic hydration of olefins to provide
alcohols and Pthers is a well-established art and is of
si~nificant commercial importance. Representative
olefin hydration processes are disclosed in U. S.
Patents Nos. 2,262,913; 2,477,380; 2,797,247;
3,798,097; 2,805r260; 2,830,090; 2,861,045; 2,891,999;
3,006,970, 3,198,752: 3,810,848; 3,989,762, among
others.
Olefin hydration employing zeolite catalysts is
known. As disclosed in U. S. Patent No. 4,214,107,
lower olefins, in particular propylene, are
catalytically hydrated over a crystalline
aluminosilicate zeolite catalyst having a silica to
alumina ratio of at least 12 and a Constraint Index of
from 1 to 12, e.g., acidic ZSM-5 type zeolite, to
provide the corresponding alcohol, essentially free of
ether and hydrocarbon by-product.
The production of ether from secondary alcohols
such as isopropanol and light olefins is known. As
disclosed in U. S. Patent No. 4,182,914 DIPE is
produced from IPA and propylene in a series of
operations employing a strongly acidic cation exchange
resin as catalyst.
The surprising discovery has been made that
conversion of the light alkenes component of a
hydrocarbon feedstream for high octane ether production
can be substantially increased by serially integrating
the initial iso-olefins etherification step with
processes capable of converting unreacted light olefins
to alcohols and ethers by hydration and etherification,
followed by aromatization or oligomerization of
remaining unreacted olefins to higher hydrocarbons
2030797
F-5324 -4-
such as gasoline and distillate. The process enhances
the production of both the desirable tertiary alkyl
ethers as well as iso-ethers.
More particularly, a process has been discovered
for the conversion of C4~ olefinic hydrocarbon
fPedstock containing C4-C5 ~ertiary olefins into high
octane gasoline boiling range oxygenates and higher
molecular weight gasoline boiling range hydrocarbons.
The process comprises the following steps:
(a) reacting a fresh mixture of excess lower
alkanol and said hydrocarbon feedstock in the presence
of acidic etherification catalyst under etherification
conditions whereby an etherification effluent stream
containing lower alkyl tertiary ~lkyl ethers, unreacted
lower alkanol and linear C4+ olefinic hydrocarbons is
produced;
(b) separating said effluent stream to
recover C5+ gasoline containing high octane lower alkyl
tertiary alkyl ethers and a stream containing said
unreacted lower alkanol and C4- hydrocarkons:
(c) introducing said unreacted alkanol and
C4-hydrocarbon stream and feedstream containing C3
hydrocarbons and water into an olefins hydration zone
in contact with acidic hydra~ion catalyst under olefins
hydration and etherification conditions whereby C3+
aliphatic oxygenates are produced;
(d) separating step (c) effluent stream and
recovering said oxygenates containing high octane
ethers and a stream containing unreacted linear C4-
olefins and alkanol by-product;
(e) contacting said unreacted linear olefins
stream with an acidic metallosilicate catalyst in a
conversion zone under olefins conversion conditions at
elevated temperature whereby higher molecular weight
gasoline boiling range hydrocarbons are produced.
- Optionally, step (d) unreacted olefins stream
containing alkanol by-product is separated and the
7 ~ 7
F-5324 -5-
olefins portion is passed ~o step (e). Also, step (c)
nydration zone may be a common hydration zone for C3
and C4 hydrocarbons or a two reactor system to upgrade
C3 and C4 hydrocarbons separately under different
conditions.
In one embodiment of the invention the C3
hydrocarbon feedstream to the hydration and
etherificaton step is omitted and only unreacted
olefins from the iso-olefin etherificaton step are
reacted in the hydration and etherification zone.
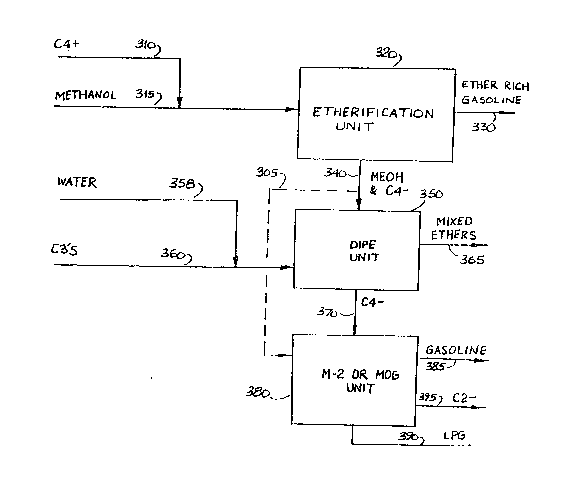
The Figure is a flow schema~ic of the process of
the present invention.
In the preferred embodiment of the instant
invention the principal components of known processes
are integrated in a manner providing a highly
advantageous and surprising advancement in refinery
technology leading to the production of high octane
gasoline blending components as well gasoline
distillate and/or aromatics. Known processes are
combined in a unique configuration that provides
enhancement of the performance of component processes,
achieving surprising advantages for the integrated
process. The processes integrated include the
etherification of tertiary olefins to produce lower
alkyl tertiary alkyl ethers such as MTBE (methyl
tertiary butyl ether) and TA~E (methyl tertiary amyl
ether), olefins hydration to produce alcohols and
ethers and olefins conversion over zeolite catalyst to
produce gasoline (MOG-Mobil Olefins to Gasoline
process), distillate (MOGD-Mobil-Olefin to Gasoline and
Distillate process) or aromatics (M-2 Forming-Mobil
Aromatization process). Olefin feedstock may be
produced, in entirety or in part, by including a
paraffins dehydrogenation step in the process.
Lower alkyl in the present invention refers to
Cl-C4alkyl derived from etherification using methanol,
ethanol, l-propanol, isopropanol, 2-butanol and
2~3~7~
F-5324 -6-
l-butanol. Tertiary alkyl refers to C4~C5 tertiary
alkyl groups derived from the etherification of
tertiary olefins such as isobutene and isoamylene. The
term oxygenates or oxygenate as used herein comprises,
individually or in combination, Cl-C8 low r aliphatic,
acyclic alcohols or alkanol and symmetrical or
unsymmetrical C2-Cg ethers.
The process of the present invention is directed
to maximizing the utilization of C3-C4 refinery streams
for the production of those gasoline range oxygenated
species, or oxygenates, known to exhi~it high octane
numbers which are useful for gasoline product blending.
Table 1 lists some of those oxygenated species of
particular interest as products of the present
invention.
Table 1
Product Blending octanes
Research Motor
Methyl Tertiary Butyl Ether (MTBE) 120 100
Di-isopropyl ether (DIPE) 109 99
Isopropyl alcohol (IPA) 116 95
Butanol (2-BuOH) 110 97
Ethyl Tertiary Butyl Ether (ETBE) 118 105
Isopropyl Tertiary Butyl Ether (IPTBE) 116
Other ethers which can be produced as products of
the present invention include methyl isopropyl ether
methyl isobutyl ether and di-isobukyl ether.
In the process of the instant invention it has
been discovered that the known greater reactivity of
tertiary olefins of the structure R2C=CH2 or R2C=CHR
compared to linear olefins of the structure RC~=CHR in
etherification reaction with lower alcohols can be
advantageously utilized to selectively etherify
2 0 3 ~ 7 ~ 7
~-5324 -7-
isobutylene and tertiary pentene in the presence of
linear olefins to produce high octane lower alkyl
tertiary butyl ether and tertiary amyl ether. Then, in
a sequential process configuration, unreacted linear
olefins are converted to high octane gasoline range
oxygenates by hydration and etherification. The
unreacted linear olefins feed to the hydration and
etherification step may be augmented with olefin
containing feedstock such as C3 hydrocarbons containing
propene. Propene can also be processed in a separate
reactor.
Isobutylene etherification conditions are known in
the art and, in the instant invention, comprise mild
conditions of low tempera~ure and high liquid hourly
space velocity (LHSV). Isobutylene etherification
temperature can range from 20C to 150C and preferably
between 60 and 125C.
In the preferred embodiments of this invention,
methanol is reacted with C3-C4 olefinic hydrocarbon
feedstock such as FCC unsaturated gas containing
olefins, particulaxly iso-olefins, to produce methyl
tertiary butyl ether. In the reaction, methanol is
generally present in a stoichiometric excess amount
between l and lO0 percent, based upon isobutylene.
Unreacted alkanol such as methanol largely will end up
in the MTBE product as a result of azeotrope formation
during fractionation. Typically, this would present
problems of an aqueous phase formation in gasoline.
However, isopropyl alcohol (IPA~ and sec-butyl alcohol
(SEC) formed in subsequent olefin hydration steps of
the overall process mitigates against two phase
formation by solubilizing methanol in the gasoline
pool.
Methanol may be readily obtained from coal by
gasification to synthesis gas and conversion of the
synthesis gas to methanol by well-established
industrial processes. As an alternative, the methanol
203~7~i
F--5324 --8--
may be obtained from natural gas by other conventional
processes, such as steam reforming or partial oxidation
to make the intermediate syngas. Crude mathanol from
such processes usually contains a significant amount of
water, usually in the range of 4 to ~0 wt%. The
etherification catalys~ employed is preferably an ion
exchange resin in the hydrogen form: however, any
suitable acidic catalyst may be employed. Varying
degrees of success are obtained with acidic solid
catalysts; such as, sulfonic acid resins, phosphoric
acid modified kieselguhr, silica alumina and acid
zeolites such as zeolite beta and ZSM-5. Typical
hydrocarbon ~eedstoc~ materials for etherification
reactions include olefinic streams, such as FCC light
naphtha and butenes rich in iso-olefins. These
aliphatic streams are produced in petroleum refineries
by catalytic cracking of gas oil or the like.
The reaction of methanol with isobutylene and
isoamylenes at moderate conditions with a resin
catalyst is known technology, as provided by R. W.
Reynolds, et al., The Oil and Gas Journal, June 16,
1975, and S. Pecci and T. Floris, Hvdrocarbon
Processina, December 1977. An article entitled "MTBE
and TAME - A Good Octane Boosting Combo," by J.D.
Chase, et al., The Oil and Gas Journal, April 9, 1979,
pages 149-152, discusses the technology. A preferred
catalyst is a bifunctional ion exchange resin which
etherifies and isomerizes the reactant streams. A
typical acid catalyst is Amberlyst 15 sulfonic acid
resin, a product of Rohm and Haas Corporation.
MTBE is known to be a high octane ether. The
article by J.D. Chase, et al., Oil and Gas Journal,
April 9, 1979, discusses the advantages one can achieve
by using these materials to enhance gasoline octane.
The octane blending number of MTBE when 10% is added to
a base fuel (R~O = 91) is 120. For a fuel with a low
motor rating (M+O = 83) oc~ane, the blending value of
2~3~97
F-5324 -9-
MTBE at the 10% level is 103. On the other hand, for
an (R+O) of 9S octane fuel, the blending value of 10
~TBE is 114.
Processes for producing and recovering MTBE and
other methyl tertiary alkyl ethers from iso-olefins are
known to those skilled in the art, such as disclosed in
U.S. Patents 4,544,776 (Osterburg, et al.) and
4,603,225 (Colaianne et al.). In tha prior art various
suitable extraction and distillation techniques are
known for recovering ether and hydrocarbon streams from
etherification effluent.
The opera~ing conditions of the olefin hydration
process herein are not especially critical and include
a temperature of from 60 to 450C, preferably from 90
to 220C and most preferably from 120 to 200C, a
pressure of from 120 to 200C, a pressure of from 690
to 16.6X103kPa ~100 to 3500 psi), preferably from
3.45X103 to 13.8X103kPa (500 to 2000 psi), a water to
olefin mole ratio of from 0.1 to 30, preferably from
0.2 to 15 and most preferably from 0.3 to 3.
The olefin hydration process of this invention can
be carried out under dense phase, liquid phase, vapor
phase or mixed vapor-liquid phase conditions in batch
or continuous manner using a stirred tank reactor or
fixed bed flow reactor, e~g., trickle-bed, liquid-up-
flow, liquid-down-flow, counter-current, co-current,
etc. Reaction times of from 20 minutes to 20 hours
when operating in batch and an LHSV o~ from 0.1 to 20,
preferably 0.1-2, when operating continuously are
suitable. A portion of unreacted olefin may be
recovered and recycled to the reactor.
The catalyst employed in the olefin hydration and
etherification operations which are connected
sequentially downstream of isobutylene etherification
operations is shape-selective acidic zeolite. In
general, the useful catalysts embrace two categories of
æeolite, namely, the intermediate pore size variety as
2 0'~
F-5324 -lO-
represented, for example, by ZSM-5, which possess a
Constraint Index of greater than 2 and the large pore
variety as represented, for example, by zeolites Y,
Beta and ZSM-12, which possess a Constraint index no
greater than 2. Preferred catalysts include Zeolite
Beta, Zeolite Y, ZSM-12, ZS~-S and ZSM-35. Both
varieties of zeolites will possess a framework
silica-to-alumina ratio of greater ~han 7. In addition,
acid resin catalysts are useful.
For purposes of this invention, the term "zeolite"
is meant to include the class of porotectosilicates,
i.e., porous crystalline silicates, which contain
silicon and oxygen atoms as the major components.
Other components can be present ~n minor amounts,
usually less than 14 mole %, and preferably less than 4
mole %. These components include aluminum, gallium,
iron, boron, and the like, with aluminum being
preferred. The minor ccmponents can be present
separately or in mixtures in the catalyst. They can
also be present intr nsically in the framework
structure of the catalyst. The frameworX
silica-to-alumina mole ratio referred to can be
determined by conventional analysis. This ratio is
meant to represent, as closely as possible, the mole
ratio of silica to alumina in the rigid anionic
framework of the zeolite crystal and to exclude any
alumina which may be present in a ~inder material
optionally associated with the zeolite or present in
cationic or other form within the channels of the
zeolite. In addition, zeolites as otherwise
characterized herein but which are substantially free
of aluminum, i.e., having silica-to-alumina mole ratios
up to and including infinity, are useful and can even
be preferable in some cases.
A convenient measure of the extent to which a
zeolite provides controlled access to molecules of
varying sizes to its internal structure is the
2~3~797
F-532~
aforementioned Constraint Index of the zeolite. A
2eolite which provides relatively restricted access to,
and egress from, its internal structure is
~haracterized by a relatively high value for the
; Constraint Index, i.e., above 2. On the other hand,
zeolites which provide relatively free access to the
internal zeolitic structure have a relatively low value
for the Constraint Index, i.e., 2 or less. The method
by which Constraint Index is determined is described
fully in U.S. Patent No. 4,016,218.
Useful zeolite catalysts of the intermediate pore
size variety, and possessing a Constraint Index of
greater than 2 up to 12, include such materials as
ZSM-5, ZSM-ll, ZSM-23, ZSM-35, and ZSM-38.
ZSM-5 is more particularly described in U.S.
Reissue Patent No. 28,341 (of original Patent No.
3,702,8~6); ZSM-ll is more particularly described in
U.S. Patent No. 3,709,979: ZSM-23 is more particularly
described in u.S. Patent No. 4,076,842; ZSM-35 is more
particularly described in U.S. Patent N~. 4,016,245;
and ZSM-38 is more particularly described in U.S.
Patent No. 4,046,859. Although ZS~-38 possesses a
Constraint Index of 2.0, it is often classified with
the intermediate pore size zeolites and will therefore
?5 be regarded as such for purposes of this invention.
The large pore zeolites which are useful as
catalysts in the process of this invention, i.e., those
zeolites having a Constraint Index of no greater than
2, are well known to the ar~. Representative of these
zeolites are zeolite Beta, zeolite X, zeolite L,
zeolite Y, ultrastable zeolite Y (USY), dealuminized Y
(Deal Y), rare earth-exchanged zeolite Y (REY3, rare
earth-exchanged dealuminized Y (RE Deal Y), mordenite,
ZSM-3, ZSM-4, ZSM-12, ZSM-20, and ZSM-50 and mixtures
of any of the foregoing. Although zeolite Beta has a
Constraint Index of 2 or less, it should be noted that
this zeolite does not behave exactly like other large
203~79r'1
F-5~24 -12~
pore zeolites. However, zeolite Beta does satisfy the
requirements for a catalyst of the presenk invention.
Zeolite Beta is described in U.S. Reissue Patent
No. 28,341 (of original U.S. Patent No. 3,308,069);
Zeolite X is described in U.S. Patent No. 2,882,244;
Zeolite L is described in U.S. Patent No. 3,216,789;
Zeolite Y is described in U.S. Patent No. 3,130,007.
Low sodium ultrastable zeolite Y (USY) is
described in U.S. Patent NosO ~,293,192; 3,354,077:
3,375,065; 3,402,996,; 3,449,070; and 3,595,611.
Dealuminized zeolite Y (Deal Y) can be prepared by
the method found in U.S. Patent No. 3,442,795.
Zeolite ZS~-3 is described in U.S.Patent No.
3,415,736; Zeolite ZSM-4 is described in U.S. Patent
No. 3,923,639; Zeolite ZSM-12 is described in
U.S.Patent No.3,832,~49, Zeolite ZSM-20 is described in
U.S.Patent No.3,972,983; and Zeolite ZSM-50 is
described in U.S. Patent No. 4,640,829.
Also, included within the definition of the useful
zeolites are crystalline porous silicoaluminophosphates
such as those disclosed in U.S. Patent No. 4,440,871,
the catalytic behavior of which is similar to that of
the aluminosilicate zeolites.
The zeolite(s) selected for use herein will
2S generally possess an alpha value of at least 1 and
preferably at least 10. For the olefins hydration
reaction, the most preferred alpha value for fresh
catalyst is at least 400. "Alpha value", or "alpha
number", is a measure of zeolite acidic functionality
and is more fully described together with details o~
its measurement in U.S. Patent No. 4,016,218, J.
Catalysis, 6, pp. 278-287 (1966) and J. Catalysis, 61,
pp. 390-396 (1980~. Zeolites of low acidity (alpha
values of less than about 200) can be achieved by a
variety of techniques including (a) synthesizing a
zeolite with a high silica/alumina ration, ~b)
steaming, (c) steaming followed by dealuminization and
21~ J ~ r~
F-5324 -13-
(d) substituting framework aluminum with other species.
For example, in the case of steaming, the zeolite(s)
can be exposed to steam at elevated temperatures
- ranging from 260 to 6500c and preferably from 400 to540C. This treatment can ~e accomplished in an
atmosphere of 10~% steam or an atmosphere consisting of
steam and a gas which is substantially inert to the
zeolite. A similar treatment c~n be accomplished at
lower temperatures employing elevated pressure, e.g.,
at from about 177 to 370C wi~h from 10 to 200
atmospheres (1013.0 to 20260 ~Pa). Specific details of
several steaming procedures may be gained from the
disclosures of U.S. Patent Nos. 4,325,994; 4,374,296;
and 4,418,235. Aside from, or in addition to any of
the foregoing procedures, the surface acidity of the
zeolite(s) can be eliminated or raduced by treatment
with bulky reagents as described in U.S. Patent No.
4,520,221.
In practicing the ole~in hydration and
~0 etherification proces~ of the present invention, it can
be advantageous to incorporate the z801ite(s) into some
other material, i.e., a matrix or binder, which is
resistant to the temperature and other conditions
employed in the process. Useful matrix materials
include both synthetic and naturally-occurring
substances, e.g., inorganic materials such as clay,
silica and/or mPtal oxides. Such materials can be
either naturally-occurring or can be obtained as
gelatinous precipitates or gels including mixtures of
silica and metal oxides. Naturally-occurring clays
which can be composited with the zeolite include those
of the montmorillonite and kaolin families, which
families include the sub-ben~onites and the kaolins
commonly known as Dixie, McNamee-Georgia and Florida
clays or others in which the main mineral constituent
is haloysite, kaolinite, dickite, nacrite or anauxi~e.
Such clays can be used in the raw state as originally
~03~7~
F-5324 -14-
mined or initially subjected to calcination, acid
treatment or chemical modification.
In the instant invention, ~fter iso-olefin
etherification and linear olefin hydration steps,
unreacted butenes and lower olefins along with or
without alcohol by-product from the hydration zone are
converted to gasoline, distillate or aromatics by the
MOG, MOGD or M-~ Forming process in contact with
metallosilicate zeolite-type catalyst such as ZSM-5. In
the case of conversion to aromatics of the hydration
zone effluent, paraffins in the effluent can also be
converted to aromatics by the M-2 Forming process.
Recent developments in zeolite catalyst and hydrocarbon
conversion processes have created interest in using
oxygenates and olefinic feedstocks for producing C5~
gasoline, diesel fuel, etc. In addition to the basic
work derived from ZSM-5 type zeolite catalyst, a number
of discoveries have contributed to the development of a
new industrial process. This process has significance
as a safe, environmentally accepta~le technique for
utilizing feedstocks that contain lower olefins,
especially C2-C5 alkenes. In U.S. Patents 3,960,978
and 4,021,502, Plank, Rosinski and Givens disclose
conversion of C2-C5 olefins, alone or in admixture with
paraffinic components into higher hydrocarbons over
crystalline zeolites having controlled acidity.
Garwood et al~ have also contributed improved
processing techniques in U.S. Patents 4,150,062,
4,211,640 and 4,227,992. The conversion of paraffins
and/or olefins to aromatics is described in U.S.
patents 3,760,024 and 3,756,942 to Cattanach, UOS.
patent 3,845,150 to Yan et al., U.S. patent 4,090,949
to Owen et al.
Operating details for the typical conversion of
olefins to gasoline or distillats as incorporated in
the preferred unique embodiments of the present
2~)30r~7
F-5324 -15-
invention are disclosed in U.S. Patents 4,456,779;
4,497,968 to Owen et al. and 4,433,185 to Tabak.
Referring now to the Figure a pre~erred embodiment
of the instant invention is illustrated. In the process
C4* hydrocarbons feedstoc~ are passed 310 to
etherification zone 320 containing an acidic catalyst
under tertiary olefin etherification conditions as
previously described herein in con~unction with
methanol feedstream 315. Typically, methanol may be in
excess of the stoichiometric amount to etherify
tertiary olefins in the hydrocarbon ~eedstock to assure
essentially complete conversion to the corresponding
lower alkyl tertiary alkyl ether. The excess can be
between 1-100%, but preferably 30%. The etherification
effluent stream is separa~ed by distillation to yield
C5+ ether rich gasoline 330 containing MTBE and a
portion of unreacted methanol. The remaining unreacted
methanol and linear C4~ olefins are passed 340 to the
hydration and etheri~ication zone 350 in contact with
catalyst and under such condition as described
previously herein. Water and C3 hydrocarbon feedstock
360 containing propylene are also introduced to the
hydration zone 350 wherein olefins are hydrated to
alcohols and alcohols etherified. While the preferred
embodiment of the invention includes C3 feedstream to
the hydration æone, this i8 no~ required and a useful
embodiment omits C3 feed, limiting hydration and
etherification to linear olefins in the iso-olefin
etherification effluent. C3 hydration can be
carried out in a separate reaction vessel as part of
hydration zone 350. The products are separated 365 as
primarily acyclic lower aliphatic oxygenates including
mixed ethers. The oxygenates comprise isopropyl
alcohol, 2 butanol, di-isopropyl ether, di-butyl ether,
methyl sec-butyl ether, methyl isopropyl ether. An
unreacted C4- hydrocarbon stream 370 is passed to
conversion zone 380 under olefins to gasoline
203~7~
F-5324 -16-
conversion conditions at elevated temperature in
contact with zeolite catalyst such as ZSM-5. C5+
gasoline 385 is produced. Other effluents from the
conversion zone may include LPG 390 and C2- hydrocarbon
395. Optionally, The C4-hydrocarbon stream 370 may be
converted to gasoline and distillate, or aromatics, in
contact with zeolite catalyst under conditions well
known in the art and described in ~he referenced
patents herein before.
In an optional variation of the present invention,
all or a portion of effluent stream 340 may be passed
to unit 380 through conduit 305.
In the present inven~ion, it is possible to use
acidic zeolite such as ZSM-5 in each of the individual
lS process steps: iso-olefin etherification, linear olefin
hydration and etherification, and olefin conversion to
higher molecular weight product. Accordingly, when the
same catalyst is used a common catalyst regeneration
system may be used. This feature of the invention
provides a significant occasion for cost savings.
. . ,