Note: Descriptions are shown in the official language in which they were submitted.
2030975
1 FIELD OF THE INVENTION
2 This invention relates to an improved catalytic
3 hydrocracking process which finds application for treating heavy
4 oil containing high asphaltene and sulfur contents. The process
incorporates use of an oil-soluble molybdenum compound which
6 reacts in situ with sulfur moieties of the asphaltenes to form
7 a colloidal catalyst for the hydrocracking reaction.
8 BACKGROUND OF THE INVENTION
9 The invention was developed in connection with a
research project undertaken to find a way to reduce coke
11 formation in the refining of heavy oil fractions and improve the
12 conversion of asphaltenes in the feedstock to useful products.
13 The feedstock which was used in the research was vacuum
14 distillation bottoms derived from bitumen. The composition of
this material included high contents of pentane-insoluble
16 asphaltenes (typically 25% by weight) and sulfur (typically 5%
17 by weight).
18 It was proposed to apply catalytic hydrocracking to the
19 feedstock, to upgrade it to refinery-treatable fractions.
However the production of solid coke and the low asphaltenes
21 conversion have heretofore made it impractical to practise
22 hydrocracking on such a feed. In practice, it is discarded as
23 waste material. So the objective of the work was to modify
~030975
1 conventional hydrocracking to develop a process characterized by
2 improved asphaltene conversion and reduced solid coke formation.
3 The present work has much in common with a process
4 described in the published literature of R. Bearden and C. L.
Aldrich of Exxon Research and Development Laboratories.
6 More particularly, in their paper entitled "Novel
7 catalyst and process to upgrade heavy oils", published December,
8 1981, in Energy Progress, Vol. 1, No. 1-4, they taught:
9 - adding a small àmount (as little as 100 ppm by
weight) of an oil-soluble compound that is a
11 catalyst precursor, specifically molybdenum
12 naphthenate, to a heavy oil fraction, and
13 - subjecting the mixture to hydrocracking
14 conditions, that is, temperature in the range 400-
454~C and hydrogen overpressure of 6.9 MPa to 17.2
16 MPa;
17 - with the result that coking is suppressed and high
18 conversion to distillable products is achieved.
19 They further disclosed:
- that by using an oil-soluble compound, good
21 distribution of the precursor is achieved;
22 - that, at hydrocracking conditions, closely spaced,
23 solid, micron-sized, catalytic particles,
24 comprising metal and coke, materialize in the oil;
and
26 - that these well dispersed, catalytically active
27 "M-coke" particles are operative to enable
28 effective hydrocracking to take place.
203Q~75
1 In their U.S. patent No. 4,226,742, Bearden and Aldridge
2 further teach adding molybdenum naphthenate to heavy oil and
3 reacting the mixture at a temperature in the range 325 - 415~C and
4 at a hydrogen overpressure of 500 - 5000 psig, to form in situ what
are asserted to be non-colloidal solid particles that are
6 catalytically active, for hydrocracking and suppression of coke
7 formation.
8 It will be noted that, in order to conduct this prior art
9 process, one must produce some solid coke, which requires
hydrocracking conditions (that is a temperature greater than about
11 300~C and a hydrogen overpressure).
12 SUMMARY OF THE INVENTION
13 In the first step of the present invention, an oil-
14 soluble, hydrocracking catalyst precursor, specifically a
molybdenum compound, preferably molybdenum naphthenate, is mixed
16 with heavy oil at an elevated temperature, typically in the order
17 of 150~C. The heavy oil contains a high asphaltene content
18 (greater than 10% by weight) and a high sulfur content (greater
19 than 2.5% by weight). Preferably, the precursor is provided in an
amount in the range 5-100 ppm weight molybdenum of the precursor in
21 heavy oil, most preferably in an amount in the range 10-30 ppm.
22 The purpose of this first step is to achieve a needed
23 extent of dispersion of the precursor through the oil and to
24 preferentially associate the molybdenum with the asphaltenes,
apparently with their sulfur moieties. At the temperature
26 involved, the viscosity of the oil is sufficiently high to enable
27 dispersion, yet it is below the temperature at which significant
28 decomposition of the precursor takes place. Such dispersion of
2030975
the precursor and its association with the sulphur moieties of
2 the asphaltenes is essential for the subsequent formation of a
3 colloidal catalyst, thought to be molybdenum sulphide, that shows
4 high selectivity for conversion of asphaltenes in a hydrocracking
process.
6 The product from the mixing step is then further heated
7 to hydrocracking temperature (preferably to 440-485~C, most
8 preferably about 455~C) and is introduced into a hydrocracking
9 reactor. Here the mixture is reacted with hydrogen supplied and
vented at a sufficient rate so as to maintain mixing of the
11 liquid and to strip light ends from the liquid, as they are
12 produced. Preferably, in the reactor the volumetric flow of
13 hydrogen should be greater than about 8 times (most preferably
14 greater than 10 times) the liquid flow, the Peclet number for the
liquid phase should be less than about 0.5, more preferably less
16 than 0.2, and the Peclet number for the gas phase should be
17 greater than about 3Ø These limitations in effect define a
18 single reactor system in which the liquid phase in the reactor
19 is well mixed and the light ends produced are continually being
stripped from the liquid.
21 The invention is based on the following discoveries:
22 - that formation of coke is associated with the
23 formation of a separate liquid phase rich in
24 asphaltenes and other coke precursors. This
separation is exacerbated by the presence of light
26 ends - therefore the light ends are stripped from
27 the liquid in the reactor using a prolific
28 hydrogen flow; and
2030975
~ that it is necessary to ensure that the catalytic
2 species (which appears to be a form of molybdenum
3 sulfide) is colloidal in form, associated with
4 the asphaltenes, and is well dispersed. This is
achieved by mixing the oil-soluble compound with
6 the partly heated oil, as a result of which the
7 molybdenum selectively becomes concentrated in
8 the asphaltenes and appears to loosely bond with
9 their sulfur moieties. When the temperature is
later increased in the hydrocracking pre-heating
11 step, the molybdenum apparently combines with the
12 sulfur moieties to provide a colloidal catalyst
13 concentrated in the asphaltenes which in turn may
14 be concentrated in separate liquid phases rich in
coke precursors such as asphaltenes.
16 The practice of the invention on an experimental basis
17 has led to conversion in the order of 99% with little or no solid
18 coke formation.
19 Broadly stated, the invention is a process for
hydrocracking heavy oil containing asphaltenes and sulfur
21 moieties, comprising: mixing the heavy oil with an oil-soluble
22 molybdenum compound hydrocracking catalyst precursor at an
23 elevated temperature that is sufficiently high to enable
24 dispersion of the precursor in the oil but sufficiently low so
that significant decomposition of the precursor is avoided, for
26 sufficient time to disperse the precursor and associate it with
27 the asphaltenes; then further heating the mixture to
28 hydrocracking temperature and reacting it with hydrogen in a
29 hydrocracking reactor at hydrocracking pressure, said hydrogen
20309 75
-
~ eing supplied and vented at a rate sufficient to ensure mixing
2 of the liquid in the reactor and stripping of light ends, so that
3 catalyst particles are formed which are colloidal in size; and
4 recovering and separating the gaseous and liquid products from
the hydrocracking step.
6 DF-~GPTPTION OF TE~E DRAWINGS
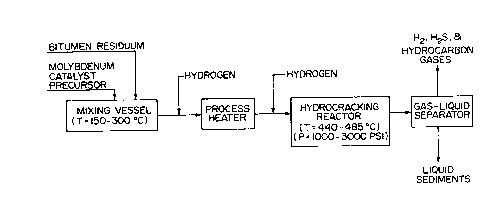
7 Figure l is a block diagram showing the steps,
8 reactants and conditions of the process;
9 Figure 2 is a schematic showing in broad outline the
laboratory circuit used to conduct the research;
11 Figure 3 is a series of IR spectra demonstrating the
12 effect of change in temperature in the mixing step; and
13 Figure 4 shows asphaltene conversion versus pitch
14 conversion for experiments providing pitch conversions between 42
and 99%.
16 ~-~G~TPTION OF TE~E ~S~"~ EMBODIMENT
17 The preferred feedstock for the present process is a
18 heavy oil fraction having an asphaltene content of at least 25%
19 by wt. and a sulfur content of at least 3% by wt. Typically the
feedstock is a high boiling fraction derived from bitumen. In a
21 particular example of such a feed, vacuum tower bottoms derived
22 from bitumen were used in developing the process. The
23 characteristics and composition of this feed were as follows:
C~
- 2030q75
-- TABLE I
2 Distillation Wt. % IBP - 430~C
3 IBP - 525~C 24.0
4 +525~C 76.0
Elemental ComPosition Wt. %
6 Carbon 83.6
7 Hydrogen 9.7
8 Nitrogen 0.8
9 Sulfur 5.9
Oxygen 0.0
11 H/C 1.4
12 TLC/FID Class Composition, Hydrocarbons 75.0
13 Asphaltene (includes Pre-asphaltene)25.0
14 The catalyst precursor used is an oil-soluble
15 molybdenum compound that decomposes in the oil when heated above
16 about 300~C. Preferably, the catalyst precursor is molybdenum
17 naphthenate. The precursor is supplied in an amount sufficient
18 to provide about 5 to l00 ppm weight of Mo in the catalyst
19 precursor per weight of bitumen. Preferably, the amount is l0 to
20 30 ppm.
21 The catalyst precursor is first mixed with the heavy
22 oil feedstock at atmospheric pressure at a temperature, typically
23 150~C. At this temperature the viscosity of the oil is reduced,
24 to assist in dispersion of the catalyst precursor, but the
25 temperature is still low enough to preclude significant
26 decomposition of the catalyst precursor or the formation of solid
27 coke in any significant amount. In this step, it is observed that
28 the catalyst precursor becomes preferentially associated with the
203097~
1asphaltenes and subsequent heating provides the catalyst,
2believed to be MoS2 in a colloidal, well distributed form that
3remains associated with the asphaltene.
4In the standard experimental embodiments the runs were
5conducted at various conditions that are now described by way of
6ranges and specific preferred values. The standard experimental
7procedure will also be described with reference to the
8experimental circuit shown in Figure 2.
9The oil feedstock previously described in Table I and
10the molybdenum naphthenate catalyst precursor were placed in a
11heated tank 1. The precursor was added in an amount in the range
1215-300 ppm. These components were mixed for 10 hours at 150~C by
13means of the mixing pump la.
14The mixing step product was pumped out of the heated
15tank 1 and passed through a line 2 and process pump 3 to the
16process heater 5. Hydrogen was added to the product in the line
174. The hydrogen was at conventional hydrocracking pressure, more
18particularly 1000 to 3500 psig. The resultant reaction mixture
19was heated to a temperature in the range 440-485~C as it passed
20through the heater 5.
21The hot reaction mixture was then introduced through
22line 6a to a conventional tubular reactor 6 where the temperature
23was maintained in the hydrocracking range 440-485~C.
24The hydrogen was added in an amount sufficient to
25ensure vigorous mixing of the liquid during hydrocracking and to
26strip off light ends. The hydrogen was supplied at an amount in
27the range 5000 - 20,000 SCF/BBL. The oil was pumped into the
28reactor at a rate in the range 0.4 - 4.0 LHSV. More particulary,
2030975
the hydrogen was preferably supplied in an amount sufficient to
2 provide the following conditions in the reactor 6:
3 Volumetric flow of H2/liquid = about 19,000 SCF/Barrel
4 Liquid Peclet No. = about 0.3
Gas Peclet No. = about 12.0
6 These Peclet numbers were determined from tracer
7 studies using Xe133 and I131.
8 The reaction products from the reactor 6 were passed
9 through line 7 to a gas-liquid separator 8. The light ends and
10 unreacted hydrogen exited the separator 8 through the line 9 and
11 were condensed to yield distillable hydrocarbon fractions and
12 hydrogen gas. The liquid products exited the separator 8 through
13 line 10. For the feedstock described in Table 1, these
14 conditions provided a pitch conversion in the range 42 - 99%.
The process is illustrated by the following examples.
16 EXAMPLE
17 This example describes in greater detail the process
18 as practised in specific runs in the laboratory and shows that
19 high conversion of asphaltenes with minimal production of solid
20 coke was achieved.
21 An asphaltene-rich feedstock of Cold Lake vacuum
22 residuum IBP greater than 430~C was charged to a O.Olm3 surge
23 tank. 300 ppm of molybdenum, as molybdenum naphthenate, was
24 added to the tank which was equipped with a stirrer and recycle
25 pump and mixed homogeneously therewith. The mixture was heated
26 under a nitrogen blanket to 200~C. The mixture was then pumped
27 through the process heater into the reactor. Hydrogen was
28 admixed with the mixture at the entrance to the process heater.
2030975
1 The process heater consisted of a 2.9 mm I.D. 6100 mm long coil
2 immersed in tin at about the hydrocracking temperature.
3 The volume of the hydrocracking reactor was 669 cc.
4 It was a stainless steel cylinder 25 mm I.D. and 1370 mm high,
manufactured by Autoclave Engineers, Erie, Pa.
6 The LHSV was 0.4 to 1.0 hl. It usually required 10-
7 12 hours for the reactor to reach steady state operating
8 conditions. The hydrocracking took place at a temperature of
9 455~C and pressure of 2000 psig. The reactor effluent comprising
a mixture of gases and liquids was fed to the hot separator where
11 gases and liquid were separated.
12 Table II provides typical results for the process.
203097~
1 TABLE II
2 Reaction Temperature, ~C 455 455
3 LHSV, h~1 0.41 1.03
4 Pressure, psig 2000 2000
H2 flow rate, scf/bbl 18,800 13,400
6 Product Yields. wt.% on feed
7 H2S 4.41 3.88
8 Cl-C3 8.00 9.01
9 C4-195~C 20.30 6.88
195-350~C 46.00 39.73
11 350-525~C 21.42 35.21
12 +525~C 0.11 5.76
13 Coke 0.00 0.86
14 C4-525~C 88.40 81.82
C4-525~C, vol. % 108.42 96.44
16 Pitch Conversion, wt.% 99.2 91.2
17 Asphaltene Conversion, wt.% 100.0 84.4
18 HDS, % 82.8 72.7
19 H2 Cons., wt.% of feed 2.5 1.9
The above hydrocracking tests were conducted on Cold
21 Lake vacuum bottoms described in Table I and the precursor
22 concentration was 300 ppm Mo on feed. After each test, all units
23 of the experimental circuit were opened, examined and found to
24 be free of coke or other fouling.
EXAMPLE II
26 This example shows the catalyst to be colloidal in
27 form.
2030975
~.~,,
1 Hydrocracking residuum was dispersed in methylene
2 chloride and the mixture was injected into a gel permeation
3 column. The molybdenum containing component was found to have
4 an apparent molecular weight range 400 to 3000 with respect to
this particular gel permeation column calibrated with respect to
6 polystyrene. This range corresponds to colloidal particles of
7 diameter greater than 0.002 micron but less than 0.01 microns.
8 EXAMPLE III
9 This example shows the effect of preferential
association of catalyst precursor with the asphaltenic fraction
11 of bitumen residue feedstock.
12 Table III shows data from two tests, one with catalyst
13 and one without catalyst. These tests demonstrated the
14 differences on asphaltene conversion and coke yield, in
particular. Although the pitch conversions for the two
16 experiments were similar, the asphaltene conversions differed by
17 a factor of 2; the catalyst selectively converted the asphaltene.
13
203097~
.., ~
1 TABLE III
2 No Catalyst 300 ppm Mo
3 Reaction Temperature; ~C 455 455
4 LHSV; h1 3.63 3.65
Pressure; psig 2500 2500
6 H2 flow rate; scf/bbl 7900 7800
7 Product Yields. wt.% on feed
8 HzS 1.94 2.40
g Cl-C3 2.59 2.22
C4-195~C 5.16 3.55
11 195~C-350~C 22.40 20.20
12 350~-525~C 31.78 35.82
13 +525~C 36.25 36.09
14 C4-525~C 59.9 60.10
Coke 6.5 0.79
16 Pitch Conversion, % 52.9 52.6
17 Asphaltene Conversion, % 23.1 58.5
18 HDS, % 31.8 39.3
19 H2 cons., wt.% of feed 0.42 0.91
Additional evidence of the effect of catalyst precursor
21 on selective asphaltene conversion and coke suppression is shown
22 in Table IV where the compositions of two +525~C hydrocracking
23 residua (pitch) are compared.
14
2030g75
TABLE IV
2 Fraction Pitch I Pitch II
3 Yield % Sulfur % Yield % Sulfur %
4 Maltenes 63.2 3.9 41.5 4.7
Asphaltenes 36.6 5.8 33.4 6.3
6 Preasphaltenes 16.3 6.2
7 Coke 0.2 -- 8.3 6.7
8 Pitch I was derived from a test containing molybdenum
9 naphthenate catalyst precursor. Pitch II was derived from a test
not containing molybdenum napthenate catalyst precursor.
11 Figure 4 shows that asphaltene conversion was favoured
12 by the presence of the catalyst for a broad range of pitch
13 conversion, 42 to 99%. In the presence of catalyst the process
14 units remained clean and free of coke. In the absence of
catalyst, the process units became fouled by coke.
16 EXAMPLE IV
17 This example shows that the process operates very
18 successfully over a broad range of concentration of precursor in
19 the bitumen residuum.
2030975
1 TABLE V
2 30 ppm Mo 300 ppm Mo
3 Reaction Temperature 455 455
4 LHSV; hl 1.03 1.03
Pressure; psig 2000 2000
6 H2 flow rate; scf/bbl 16,400 13,400
7 Product Yields wt.% on feed
8 H2S 2.86 3.88
g C1-C3 8.43 9.01
C4 - 195~C 12.13 6.88
11 195~-350~C 36.92 39.73
12 350~-525~C 34.37 35.21
13 t525~C 5.88 5.76
14 Coke 0.43 0.86
C4-525~C 83.42 81.82
16 C4-525~C; vol.% 100.52 96.44
17 Pitch Conversion, % 91.6 91.2
18 Asphaltene Conversion,% 87.4 84.4
19 HDS, % 53.6 72.7
H2 Cons., wt.% of feed 1.66 1.90
21 EXAMPLE V
22 This example shows that the catalyst precursor,
23 molybdenum naphthenate, decomposes at temperatures greater than
24 about 300~C in the absence or presence of bitumen residuum.
Figures 3a and 3b show that the catalyst precursor is
26 stable at temperatures less than 250~C. Figure 3c shows that the
27 catalyst precursor begins to decompose and polymerize slowly at
16
2030975
-
1 a temperature of 300~C. At higher temperatures the decomposition
2 was more rapid and coke was produced.
3 Figures 3d and 3e show that the catalyst precursor
4 dissolved in bitumen residuum was stable at temperatures less
than 250~C. Figure 3f shows that the catalyst precursor
6 dissolved in bitumen began to decompose slowly at a temperature
7 of 300~C. No coking was evident for the low heating rates
8 obtained.
9 Injection of the catalyst precursor into bitumen
residuum at 350~C produced coke containing molybdenum.