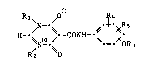Note: Descriptions are shown in the official language in which they were submitted.
,t.~f j
AP/5-17858/+
Anthelmintics
The present invention relates to novel 1,3-disubstituted 5-azolyloxy-
phenylcarbamoyl-4,6-pyrimidinedione derivatives of the formula I below
and the hydrogenated analogues thereof of the formula Ia below having
anthelmintic activity; to the said compounds for use in a method of
controlling helminths; to anthelmintic compositions that contain those
compounds as active ingredients; to the preparation of the compounds and
compositions; and to the use of the compounds or compositions for
controlling helminths, especially nematodes, cestodes and trematodes in
domestic animals and productive livestock of the class of mammals.
The compounds according to the invention have the formula I
Rl\ /O ~4
H-~ --CONH--~ ~i- (I) -
R2
or, in their hydrogenated form, formula Ia
Rl\ ~O ~4
/N--\ /H ~- ~Rs
H2_.\ ~--CONH--\ X- tIa)
R2 ~iO =- OR3
:
wherein Rl and Rz are each independently of the other Cl-C6alkyl, allyl,
Cl-C6cycloalkyl, benzyl or phenyl; and R3 is an unsubstituted or substi-
tuted heteroaromatic five-membered ring azole, bonded via carbon,
selected from the series benzimidazole, benzoxazole, benzothiazole,
imidazole, oxazole, thiazole, oxadiazole, thiadiazole and triazole; and
R4 and Rs are each independently of the other hydrogen, Cl-C6alkyl,
Cl-C6haloalkyl, Ci-C6alkoxy or C1-C6haloalkoxy, and include their
tautomeric forms and physiologically tolerable salts.
.
~ ''
., - : ' .
.
- 2 - 2 ~
An interesting group comprises compounds of formula I wherein R3, R4 and
Rs are as defined under formula I and R1 and R2 are each independently of
the oth,er C~-C6alkyl, allyl, C3-C5cycloalkyl or phenyl.
The five-membered ring azole (R3) is accordingly always bonded via a
carbon atom to the phenolic O-atom of the aminophenol moiety of the
molecule and can in turn be unsubstituted or substituted. Compounds of
formulae I and Ia wherein R3 has, for example, one of the meanings given
below form, according to the definition of R3, one of the following
preferred groups a) to f) according to the invention:
~roup R3 Type
4.~ R6 Benzimidazol-2-yl
a) 3 N ' S Benzoxazol-2-yl
2/ y \,~ Benzothiazol-2-yl
4/R8 Imidazol-2-yl
b) 3 N - Oxazol-2-yl
2 . 1! s Thiazol-2-yl
4 ~ 3 [ lH-1,2,4-Triazol]-5-yl
c) S \ /~ 2 8 [1,2,4-Oxadiazol]-5-yl
Z [1,2,4-Thiadiazol]-5-yl
[1,2,4-Oxadiazol]-3-yl
[l~2~4-Thiadiazol]-3
4 s/Ra
d) 3 ~ ~ 1 [lH-1,2,4-Triazol]-3-yl
~N R11
e) 3 ~ - N 4 [1,3,4-Oxadiazol]-2-yl
2 . . s [1,3,4-Thiadiazol]-2-yl
/ y Rg [4H-1,2,4-Triazol]-5-yl
or
R~1
f) 1~ ~ 2
~N 3 R9 [lH-1,2,4-Triazol]-5-yl
. . .
, .
- 3 - h-.;~'~
wherein
Y is oxygen, sulfur or NR1o;
Z is oxygen, sulfur or NH;
R6 and R7 are each independently of the other hydrogen, Cl-C4alkyl,
Cl-C4haloalkyl, Cl-C4alkoxy, Cl-C4haloalkoxy, Cl-C4alkylthio, Cl-C4-
alkylsulfonyl, Cl-C4alkylsulfinyl, Cl-C4haloalkylsulfonyl, Cl-C4halo-
alkylsulfinyl, (Cl-C4alkyl)2N, (C1-C4alkyl)HN, halogen, nitro or
cyano;
Rg and Rg are each indepcndently of the other hydrogen, C1-C6alkyl,
C1-C6haloalkyl, Cl-C6alkoxy, Cl-C6alkylthio, halogen or nitro; or are
each independently of the other unsubstituted or halo- or Cl-C3-
alkyl-substituted C3-C7cycloalkyl;
Rlo is hydrogen or Cl-C6alkyl; and
Rll is Cl-C6alkyl.
Within groups b) to f), interesting compounds of formulae I and Ia are
also those wherein Y, Z, Rlo and Rll are as defined above but Rg and Rg
are each independently of the other unsubstituted or halo- or Cl-C3alkyl-
substituted C3-C7cycloalkyl, preferably cyclopropyl or cyclohexyl.
The substituent R3 accordingly has, inter alia, the following
meanings:
~ R~ ~ R6 .~ R6 .~R6
a) 11 11 1 ~ lNI il 1 lNI 11 1 , 11 il 1 .
R ~ ~ R7 ~ R7 R7
: ~ 8 ~a /R8 /Rg
N I ~ N ~; .
b) ~ I~ 1, 1!
/ ~ ~9 / \~N/ \Rg / \O Rg S Rg
1!1 Rl o
/Rg /Ra ~R8
N_ . . ~-- 1 I
C) ~ \o~ ' ~ !`S~ ' R/g \~ R/
.
'
r~
/Ra
~==.
~N Rl;
e) S ; ~ \Z , 2/.\ /,\s , / \S/ \R '
Rll
~N Rs
wherain the substituents R6 to Rll are as defined above.
Preferred groups of compounds of formulae I and Ia are all those which
are formed formally by combination of the molecule fragments I' and Ia'
~N~ 4~ ~N--~ H .~4.~Rs
H- ~ ~ / -CONH \ XO_ or H2- ~ / CONH \ XO_
(I') (Ia')
wherein Rl, R2, R4 and Rs are as defined under formula I, with one of
the azoles R3 mentioned under a) to f). The present invention relates to
each of those groups.
Within all the said sub-groups, preferred compounds of formulae I and Ia
are those wherein Rl and R2 are each independently of the other methyl,
ethyl, isopropyl, allyl or cyclopropyl, and R4 and Rs are each
independently of the other hydrogen, methyl, CF3, OCH3, OCHF2 or
OCF3.
'
,,.~. ' ' :
Within the group in which the five-membered ring azole is a grouping
indicated under a), additional preferred compounds of formulae I and Ia
are those wherein R6 and R7 are each independently of the other
hydrogen, methyl, CF3, methoxy, halomethoxy, fluorine, chlorine or
bromine, and the other substituents are as defined above.
Within the groups in which the five-membered ring azole is one of the
groupings indicated under b) to f), additional preferred compounds of
formulae I and Ia are those wherein R8 and Rg are each independently of
the other hydrogen, methyl, ethyl, C3-C7cycloalkyl, CF3, C2Fs, C3F7,
CCl3, CHCl2, CHzCl, SCH3 or halogen; Rlo is hydrogen or methyl and Rll is
C~-C4alkyl, especially methyl.
Compounds of formulae I and Ia wherein the OR3 group is bonded to the
phenyl radical in the para-position are especially noteworthy.
.
Only one of the numerous possible betaine structures is given in formula
I; other isoelectronic structural formulae are, for example:
R~ CONH~ R~ CON~
H- ~ ~ ~ -CONH~ X~---~ \ -CONh--~ ~X
or, to summarise:
R ~ \ _.XoR3
R2
- 6 - ~J t~ ,~ i'.i ',
The compounds of formula Ia may exist, for example, in the following
tautomeric forms:
Rl\ ~O ~4 Rl\N /OH ~4
\ / \-/CONH ~ --CONH--~
H /N--~ \ XOR H /N--~ =- OR3
The present invention extends to all forms of the compounds of formulae I
and Ia.
Depending upon the number of carbon atoms indicated, the term alkyl by
itself or as a component of another substituent is to be understood
within the scope of the present invention as including, for example, the
following straight-chain and branched groups: methyl, ethyl, n-propyl,
isopropyl, n-butyl, sec.-butyl, tert.-butyl, isobutyl, etc.. Haloalkyl by
itself or as a component of haloalkoxy is a mono- to per-halogenated
alkyl substituent, for example CHzCl, CHCl2, CCl3, CH2F, CHF2, CF3,
CH2Br, CHBr2, CBr3, CH2I, CI3, CHClF, CHBrCl, CFBrCl, C2Fs, CH2CH2Cl,
CHClCH3, C2Cls, CHFCHClz, etc., preferably CF3. Here and hereinbelow,
halogen is to be understood as being fluorine, chlorine, bromine or
iodine, preferably fluorine, chlorine or bromine, but especially
chlorine.
Cycloalkyl by itself or as a component of a substituent is, depending
upon the number of carbon atoms indicated, cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl, etc.. Cyanoalkyl is an alkyl group in which a
hydrogen atom has been replaced by CN, preferably an alkyl group in which
the CN group is located at the terminal carbon atom.
The term "physiologically tolerable salts" of compounds of formulae I and
Ia includes the alkali metal, ammonium or amine salts, with sodium,
potassium, ammonium or alkylamine salts, especially triethylamine salts,
being preferred. The term also includes, however, the addition salts of
inorganic and organic acids formed by the addition of an equivalent
amount of a salt-forming acid to the base molecule.
r - ! ~
- 7 -
Examples of salt-forming acids are inorganic acids: hydrohalic acids,
such as hydrofluoric acid, hydrochloric acid, hydrobromic acid or
hydriodic acid, and also sulfuric acid, phosphoric acid, phosphorous
acid, nitric acid, and organic acids, for example acetic acid, trifluoro-
acetic acid, trichloroacetic acid, propionic acid, glycolic acid,
thiocyanic acid, lactic acid, succinic acid, citric acid, formic acid,
benzenesulfonic acid, p-toluenesulfonic acid, methanesulfonic acid,
salicylic acid, p-aminosalicylic acid, phthalic acid, 2-phenoxybenzoic
acid or 2-acetoxybenzoic acid.
The compounds of formulae I and la are for the most part stable solids at
room temperature that have a melting point of from approximately 100 to
approximately 300C. They have very valuable anthelmintic properties and
can be used curatively and preventively for the control of a number of
worm-related disorders in warm-blooded animals, especially in domestic
animals and productive livestock, more especially mammals.
Especially preferred individual compounds of formula I are:
1,3-dimethyl-5-[4-(6-trifluoromethoxy-benzothiazol-2-yloxy)-phenyl-
carbamoyl]-4(6)-oxo-6(4)-oxido-(lH,5H)-pyrimidinium betaine; 1,3-di-
methyl-5-[4-(6-fluoro-[5-or 7-chloro]-benzothiazol-2-yloxy)-phenyl-
carbamoyl]-4(6)-oxo-6(4)-oxido-(lH,5H)-pyrimidinium betaine; 1,3-di-
methyl-5-[4-(5-tert.-butyl-1,3,4-thiadiazol-2-yloxy)-phenylcarbamoyl]-
4(6)-oxo-6(4)-oxido-(lH,5H)-pyrimidinium betaine; 1,3-dimethyl-5-[4-(6-
chloro-benzothiazol-2-yloxy)-phenylcarbamoyl]-4(6)-oxo-6(4)-oxido-
(1H,5H)-pyrimidinium betaine.
Especially preferred compounds of formula Ia are:
1,3-dimethyl-5-[4-(6-trifluoromethoxy-benzothiazol-2-yloxy)-phenyl-
carbamoyl]-4,6-(lH,3H,5H)-pyrimidinedione; 1,3-dimethyl-5-[4-(6-fluoro-
[5- or 7-chloro]-benzothiazol-2-yloxy~-phenylcarbamoyl]-4,6-(lH,3H,5H)-
pyrimidinedione; 1,3-dimethyl-5-[4-(5-tert.-butyl-1,3,4-thiadiazol-2-
yloxy)-phenylcarbamoyl]-4,6-(1H,3H,5H)-pyrimidinedione; 1,3-dimethyl-5-
[4-(6-chloro-benzothiazol-2-yloxy)-phenylcarbamoyl]-4,6-(lH,3H,5H)-
pyrimidinedione.
S ~ r-
~ 8 ~ f~
The compounds of formulae I and Ia are prepared according to the inven-tion by desulfurizing a compound of formula II
R1\N /OH ~4
S=-\ ~--CONH--~ ~ (II)
/N--~ =- OR3
R2 0
in which R1 ? R2, R3, R4 and Rs are as defined for formulae I and Ia.
The desulfurization can be carried out, for example, by hydrogenation,
preferably by catalytic hydrogenation. In a preferred embodiment, the
compound of formula II is hydrogenated in an inert solvent or solvent
mixture at tempera~ures ranging from 20 to 180C~ preferably from 60 to
120C, especially at reflux temperature, with trialkyltin hydride,
trialkyltin halide, trialkylgermanium hydride, trialkylgermanium halide,
alkylmercury hydride or alkylmercury halide, the reaction being carried
out additionally in the presence of NaBH4 when a halide is used. In this
context, alkyl is preferably C1-C6alkyl, especially C2-C4alkyl, and
halide is especially chloride or bromide. The chlorides are especially
suitable. A further suitable hydrogenating agent that may be mentioned is
tris(trimethylsilyl)silane ([(CH3)3Si]3SiH).
The hydrides and halides are used in at least an equimolar amount, based
on the starting compound of formula II. NaBH4 can also be added in an
equimolar amount.
..
The reaction can, in addition, be carried out in the presence of a
radical initiator, which can be added in catalytic amounts. Suitable
radical initiators are, for example, azoisobutyronitrile (AiBN),
peroxides, such as benzoyl peroxide, and also UV light or heat. Examples
of suitable inert solvents, alone or in admixture, are: aliphatic and
aromatic hydrocarbons, for example pentane, hexane, petroleum ether,
ligroin, benzene? toluene, xylenes, etc.; ethers and ethereal substances,
such as tetrahydrofuran, anisole, dioxane, etc.; halogenated hydro-
carbons, such as carbon tetrachloride, tetrachloroethylene, chloro-
benzene, etc..
_ 9 _ ~t .r~ r~ 1 ~
The compounds I and Ia are generally obtained together, but by suitableselection of the solvent it is possible so to arrange the system that,
for example, the sparingly soluble betaines of formula I are precipitated
and can be removed from the reaction mixture, for example, by filtration,
whilst the more soluble compounds of formula Ia can be obtained from the
solution, for example, by concentration or precipitation. It is also
possible, however, to isolate a mixture of compounds I and Ia and then to
separate the two compounds, for example on the basis of their different
solution behaviour, for example by fractional crystallisation or by
column chromatography. The skilled choice of solvents, however, may also
result in pure products, that is to say either wholly in compounds I or
wholly in compounds Ia. The compounds of formula I can be converted into
the compounds of formula Ia according to conventional methods by hydro-
genation, for example with Raney nickel, NaBH4, tributyltin hydride,
etc..
As has already been mentioned, the compounds of formulae I and Ia can
also be in the form of adducts with bases or acids.
Whilst acid addition salts have already been described in detail at thebeginning, mention has yet to be made of the inorganic and organic bases
suitable as adduct formers. These are, for example, preferably tertiary
amines, such as trialkylamines (for example trimethylamine, triethylamine
or tripropylamine), pyridine and pyridine bases (for example 4-dimethyl-
aminopyridine or 4-pyrrolidylaminopyridine), picolines and lutidines and
also oxides, hydroxides, carbonates and hydrogen carbonates of alkali
metals and alkaline earth metals (for example CaO, BaO, NaOH, KOH,
KHCO3, NaHCO3, Ca(OH)2, K2CO3 or Na2CO3), and also acetates, for
example CH3COONa or CH3COOK. In addition, alkali metal alcoholates, for
example sodium ethanolate, sodium propanolate, potassium tert.-butanolate
or sodium methanolate, are also suitable bases. The base is advantageous-
ly added in an amount of from 10 to 100 % of the equimolar amount in
relation to the reactants.
~ ~ 3 ~
In many cases it may be advantageous to carry out the reaction under a
protective gas atmosphere. Suitable protective gases are, for example,
nitrogen, helium and argon.
The reaction of compounds II with trialkyltin hydride, especially in the
presence of a radical initiator, is especially preferred.
The thiobarbiturates of formula II are known, for example from EuropeanPublished Application EP-192,180 or the corresponding British Patent GB 2
171 099 B, or can be prepared analogously to the compounds described
therein.
The compounds of formulae I and Ia according to the invention can existin different tautomeric forms, namely in the keto or enol form or as a
mixture of the keto and enol forms. The present invention relates to the
individual tautomers and mixtures thereof, and also to the salts of any
of those forms, and to the preparation thereof.
The present invention relates to the described prepara~ion process
including all variants.
It is generally known that of the endoparasites occurring in warm-blooded
animals it is particularly the helminths that cause great damage to the
animals infested with them. Where there is chronic and especially
epidemic occurrence of worm-related disorders in herds of animals, such
damage caused by helminthiases can assume serious economic proportions.
In the animals affected, the damage manifests itself inter alia as
losses of productivity, reduced resistance to other diseases and in-
creased mortality. Especially dangerous worm-related disorders are caused
by helminths parasitising the gastro-intestinal tract and other organs
and may occur, for example, in ruminants such as cattle, sheep and goats
as well as in horses, pigs, fowl, red deer, dogs and cats.
In this description the term "helminths" is to be understood as meaningespecially parasitic worms that belong to the Platyhelminthes (cestodes,
trematodes) and Nemathelminthes (nematodes and related species), there-
fore tapeworms, sucker worms and roundworms of the gastro-intestinal
tract and other organs (for example the liver, lungs, kidneys, lymph
vessels, blood etc.).
There is therefore an urgent need to develop therapeutic agents suitable
for controlling helminths in all stages of their development and also for
guarding against attack by such parasites.
Although a number of substances having anthelmintic activity are known
that have been proposed for controlling the various species of helminth,
these have not proved completely satisfactory either because at a
tolerable dose it is not possible to make full use of their spectrum of
activity or because at therapeutically effective doses they exhibit
undesired side effects or properties. In this respect, the resistance to
certain classes of substances, which occurs more and more today, is also
increasingly significant. "Albendazol", which is described, for example,
in the literature (British Pat. No. 1464326; Am. J. Vet. Res. 38,
1425-1426 (1g77); Am. J. Vet. Res. 37, 1515-1516 (1976); Am. J. Vet.
Res. 38, 807-808 (19~7); Am. J. Vet. Res. 38, 1247-1248 (1977)), has
only a limited spectrum of anthelmintic activity in ruminants. Its action
against benzimidazole-resistant nematodes and adult liver flukes is
completely inadequate, and in particular the pathogenically important
immature migrating forms of the latter are not affected at doses
tolerated by the host animal.
It has surprisingly been found that the novel compounds of formulae I and
Ia have a broad spectrum of activity against helminths that parasitise
the animal organism, especially mammals, such as nematodes, cestodes and
trematodes, their action being directed especially against nematodes
(roundworms).
As a particular feature of the compounds of formulae I and Ia attentionis drawn to their surprisingly high tolerability by warm-blooded animals,
as a result of which they are superior to the known thiobarbituric acid
derivatives. The practical handling thereof in the treatment of worm-
infested animals is extraordinarily simplified by the fact that they aretolerated by the treated animals without symptoms even at relatively high
doses.
The novel compounds of formulae I and Ia according to the invention aresuitable, for example, for controlling parasitic nematodes of the orders
(according to K.I. Skrajabin)
Rhabditida,
Ascaridida,
Spirurida,
Trichocephalida,
or for controlling cestodes of the orders (according to Wardle & McLeod)
Cyclophyllidae,
Pseudophyllidae,
or for controlling trematodes of the order
Digenea,
in domestic animals and productive livestock, such as cattle, sheep,
goats, horses, pigs, red deer, cats, dogs and fowl. They may be
administered to the animals either as a single dose or repeatedly, the
single doses preferably being from 1 to 20 mg per kg of body weight
depending upon the species of animal. In many cases, protracted
administration may result in an improved action or may permit the use of
smaller total doses.
The compositions according to the invention are prepared by bringing the
compounds of formula I or Ia into contact with liquid and/or solid
formulation adjuvants, by mixing and/or grinding in stages, in such a
manner that an optimum display of the anthelmintlc activity of the
formulation, conformable to the application, is achieved.
The formulation stages can be supplemented by kneading, granulating
(granulates) and optionally compressing (pellets).
Suitable formulation adjuvants are, for example, solid carriers, solvents
and, where appropriate, surface-active substances (surfactants~.
' ' ' ' ' '
'
:. . ': . ' ' '
'.
:, .
- 13 - ~t ~ .~ i` ,J ' j~
The following formulation adjuvants are used to prepare the compositions
according to the invention:
solid carriers, for example kaolin, talcum, bentonite, sodium chloride,
calcium phosphate, carbohydrates, cellulose powder, cottonseed meal,
polyethylene glycol ethers, where appropriate binders such as, for
example, gelatin, soluble cellulose derivatives, if desired with the
addition of surface-active substances, such as ionic or non-ionic
dispersants; and also natural mineral fillers, such as calcite, mont-
morillonite or attapulgite. In order to improve the physical properties
it is also possible to add highly dispersed silicic acid or highly
dispersed absorbent polymers. Suitable granulated adsorptive carriers are
porous types, for example pumice, broken brick, sepiolite or bentonite;
and suitable nonsorbent carriers are, for example, calcite or sand. In
addition, a great number of pregranulated materials of inorganic or
organic nature can be used, e.g. especially dolomite or pulverised plant
material.
Suitable solvents are: aromatic hydrocarbons, preferably the fractions
containing 8 to 12 carbon atoms, e.g. xylene mixtures or substituted
naphthalenes, phthalates such as dibutyl phthalate or dioctyl phthalate,
aliphatic hydrocarbons such as cyclohexane or parafins, alcohols and
glycols and their ethers and esters, such as ethanol, ethylene glycol,
ethylene glycol monomethyl or monoethyl ether, ketones such as cyclo-
hexanone, strongly polar solvents such as N-methyl-2-pyrrolidone,
dimethyl sulfoxide or dimethylformamide, as well as vegetable oils or
epoxidised vegetable oils, such as epoxidised coconut oil or soybean oil,
and water.
Depending on the nature of the compound of formula I or Ia to be
formulated, suitable surface-active compounds are non-ionic, cationic
and/or anionic surfactants having good emulsifying, dispersing and
wetting properties. The term "surfactants" will also be understood as
comprising mixtures of s~rfactants.
Both so-called water-soluble soaps and water-soluble synthetic surface-
active compounds are suitable anionic surfactants.
1.,.. ' ' .
.
.~ :
14 ~ ~ ~
Suitable soaps are the alkali metal salts, alkaline earth metal salts or
unsubstituted or substituted ammonium salts of higher fatty acids
(ClO-C2z), e.g. the sodium or potassium salts of oleic or stearic
acid, or of natural fatty acid mixtures which can be obtained e.g. from
coconut oil or tallow oil. Mention may also be made of fatty acid
methyltaurin salts.
Frequently, so-called synthetic surfactants are used, especially fatty
sulfonates, fatty sulfates, sulfonated benzimidazole derivatives or
alkylarylsulfonates.
The fatty sulfonates or sulfates are usually in the form of alkali metal
salts, alkaline earth metal salts or unsubstituted or substituted
ammonium salts and contain a C~-C22alkyl radical which also includes
the alkyl moiety of acyl radicals, e.g. the sodium or calcium salt of
lignosulfonic acid, of dodecyl sulfate or of a mixture of fatty alcohol
sulfates obtained from natural fatty acids. These compounds also comprise
the salts of sulfated and sulfonated fatty alcohol/ethylene oxide
adducts. The sulfonated benzimidazole derivatives preferably contain 2
sulfonic acid groups and one fatty acid radical containing 8 to 22 carbon
atoms. Examples of alkylarylsulfonates are the sodium, calcium or
triethanolamine salts of dodecylbenzenesulfonic acid, dibutylnaphthalene-
sulfonic acid, or of a condensate of naphthalenesulfonic acid and
formaldehyde.
Also suitable are corresponding phosphates, e.g. salts of the phosphoric
acid ester of an adduct of p-nonylphenol with 4 to 14 moles of ethylene
oxide, or phospholipids.
Non-ionic surfactants are preferably polyglycol ether derivatives of
aliphatic or cycloaliphatic alcohols, saturated or unsaturated fatty
acids and alkylphenols, said derivatives containing 3 to 30 glycol ether
groups and 8 to 20 carbon atoms in the aliphatic hydrocarbon moiety and 6
to 18 carbon atoms in the alkyl moiety of the alkylphenols.
. ~ ' . ' " '.
- 15 - ~ ~ ~
Further suitable non-ionic surfactants are the water-soluble adducts ofpolyethylene oxide with polypropylene glycol, ethylenediaminopoly-
propylene glycol and alkylpolypropylene glycol containing 1 to 10 carbon
atoms in the alkyl chain, which adducts contain 20 to 250 ethylene glycol
ether groups and 10 to 100 propylene glycol ether groups. These compounds
usually contain 1 to 5 ethylene glycol units per propylene glycol unit.
Examples of non-ionic surfactants are nonylphenolpolyethoxyethanols,
castor oil polyglycol ethers, polypropylene/polyethylene oxide adducts,
tributylphenoxypolyethoxyethanol, polyethylene glycol and octylphenoxy-
polyethoxyethanol.
Fatty acid esters of polyoxyethylene sorbitan, e.g. polyoxyethylene
sorbitan trioleate, are also suitable non-ionic surfactants.
Cationic surfactants are preferably quaternary ammonium salts which
contain, as N-substituent, at least one C8-Czzalkyl radical and, as
further substituents, unsubstituted or halogenated lower alkyl, benzyl or
hydroxy-lower alkyl radicals. The salts are preferably in the form of
halides, methyl sulfates or ethyl sulfates, e.g. stearyltrimethylammonium
chloride or benzyldi(2-chloroethyl)ethylammonium bromide.
The surfactants customarily employed in the art of formulation are
described, inter alia, in the following publications:
"Mc Cutcheon's Detergents and Emulsifiers Annual",
MC Publishing Corp., Ridgewood New Jersey, 1980;
Sisley and Wood, "Encyclopedia of Surface Active Agents",
Chemical Publishing Co., Inc. New York, 1980.
Suitable binders for tablets and boli are chemically modified natural
polymer substances that are soluble in water or alcohol, such as starch,
cellulose or protein derivatives (e.g. methylcellulose, carboxymethyl-
cellulose, ethylhydroxyethylcellulose, proteins such as zein, gelatin and
the like) and synthetic polymers, such as, for example, polyvinyl
~ 16 ~ ~ rJ ,~
alcohol, polyvinylpyrrolidone etc.. The tablets also contain fillers
(e.g. starch, microcrystalline cellulose, sugar, lactose, etc.), glidants
and disintegrators.
If the anthelmintic compositions are in the form of feed concentrates,
then the carriers used are, for example, performance feed, feed grain or
protein concentrates. In addition to the active ingredients, such feed
concentrates or compositions may contain additives, vitamins, anti-
biotics, chemotherapeutic agents or other pesticides, especially
bacteriostatics, fungistatics or coccidiostatics, or hormone prepara-
tions, substances having an anabolic activity or substances that promote
growth, influence the meat quality of animals for slaughtering or are
useful to the organism in some other way. If the compositions or the
compounds of formula I or Ia contained therein are added directly to the
feed or to animal drinks, then the prepared feed or the prepared drink
preferably contains the active ingredients in a concentration of
approximately from 0.0005 to 0.02 percent by weight (5-200 ppm).
The compositions according to the invention can be administered to the
animals to be treated perorally, parenterally or subcutaneously, the
compositions being in the form of solutions, emulsions, suspensions
(drenches), powders, tablets, boli and capsules.
The anthelmintic compositions according to the invention generally
contain from 0.1 to 99 % by weight, preferably from 0.1 to 95 % by
weight, of the compound of formula I, formula Ia or a mixture of the two,
from 99.9 to 1 % by weight, preferably from 99.8 to 5 % by weight, of a
solid or liquid adjuvant, including from 0 to 25 % by weight, preferably
from 0.1 to 25 % by weight, of a surfactant.
Whereas commercial products will preferably be formulated as concen-
trates, the end user will normally employ dilute formulations.
The compositions may also contain further auxiliaries such as
stabilisers, antifoams, viscosity regulators, binders and tackifiers as
well as other active ingredients for obtaining special effects.
.. .
The present invention relates also to such anthelmintic compositions
employed by the end user.
In each of the methods according to the invention for controlling pestsand in each of the pesticidal compositions according to the invention,
the compounds of formulae I and Ia can be used in any of their tautomeric
forms, as mixtures thereof or in the form of their salts.
The invention also includes a method for the prophylactic protection ofanimals against parasitic helminths, which method comprises administering
the compounds of formulae I and Ia or the active ingredient formulations
to the animals as an additive to feed or to drinks or in solid or liquid
form orally, by injection or parenterally.
The following Examples serve to illustrate the invention without implying
any limitation thereof.
1. Preparation Examples
1.1. Preparation of 1,3-dimethyl-5-[4-(6-trifluoromethoxy-benzothiazol-
2-yloxy)-phenylcarbamoyl]-4(6)-oxo-6(4)-oxido-(lH,5H)-pyrimidinium
betaine
A solution of 5.3 g (10 mmol) of 1,3-dimethyl-5-[4-(6-trifluoromethoxy-benzothiazol-2-yloxy)-phenylcarbamoyl~-2-thiobarbituric acid, 126 mg (0.8
mmol) of azoisobutyronitrile and ô.ô g (30 mmol) of tributyltin hydride
is stirred under reflux in benzene for 3 hours and under a nitrogen
atmosphere. After the reaction mixture has cooled to room temperature,
the precipitated product is filtered off, washed repeatedly with diiso-
propyl ether and dried, yielding 1.2 g (24 ~0) of the title compound. M.p.
230-232C.
1H-NMR (300 MHz, DMSO d~, TMS): 11.00, s, NH; 9.16, s, H-C(3); 7.40, d,
J=9.5, 2H; 7.32, d, J=9.5, lH; 7.23, 6rs, lH; 6.92-6.81, m, lH; 6.87, d,
J=9.5, 2H; 3.16, s, 2 x CH3.
5~ f ~
- 18 - ~ t.~
1.2. Preparation of 1,3-dimethyl-5-[4-(6-trifluoromethoxy-benzothiazol-
2-yloxy)-phenylcarbamoyl]-4~6-(lH~3H~5H)-pyrimidinedione
The filtrate obtained according to Example 1.1. is concentrated and the
precipitated product is filtered off and recrystallised repeatedly from
diisopropyl ether, yielding 2.03 g (46 %) of the title compound. M.p.
109-113C.
lH-NMR (300 MHz, CDCl3, TMS): 18.25, s, OH; 11.91, s, NH; 7.73, d, J=8.0,
2H; 7.59, d, J=6.5, lH; 7.55, brs, lH; 7.32, d, J=8.0, 2H; 7.28-7.23, m,
lH; 4.51, s, H2-C(3); 3.01, s, CH3; 2.97, s, CH3.
1.3. Preparation of 1,3-dimethvl-5-[4-(5-tert.-butYl-1.3,4-thiadiazol-
2-yloxy)-phenylcarbamoyl]-4,6-(lH,3H,5H)-pyrimidinedione
A solution of 5 g (11 mmol) of 1,3-dimethyl-5-[4-(5-tert.-butyl-1,3,4-
thiadiazol-2-yloxy)-phenylcarbamoyl]-2-thiobarbituric acid, 9 ml (34
mmol) of tributyltin hydride and 90 mg (0.5 mmol) of azoisobutyronitrile
is stirred under reflux in 200 ml of toluene for 5 hours (nitrogen
atmosphere). After cooling to room temperature the mixture is concen-
trated and the crude product is washed with hexane/ether, filtered, dried
and recrystallised from ethyl acetate, yielding 2.1 g of the title
compound. M.p. 128-130C.
1H-NMR (300 MHz, CDCl3, TMS): 18.22, s, OH; 11.88, s, NH; 7.53, d, J=9.5,
2H; 7.18, d, J=9.S, 2H; 4.50, s, H2-C(3); 3.02, s, CH3; 2.96, s, CH3;
1.42, s, 9H.
1.4. Preparation of 1,3-dimethyl-5-[4-(5- or 7-chloro-6-fluoro-benzo-
thiazol-2-yloxy)-phenYlcarbamoyl]-4,6-(lH,3H,5H)-pyrimidinedione
A suspension of 461 mg (1 mmol) of 1,3-dimethyl-5-[4-(5- or 7-chloro-6-
fluoro-benzothiazol-2-yloxy)-phenylcarbamoyl]-4(6)-oxo-6(4)-oxido-
(lH,5H)-pyrimidinium betaine and 68 mg (2 mmol) of NaBH4 in 10 ml of
ethanol is stirred at room temperature under nitrogen for 1 hour. The
reaction mixture is cooled to 0C, acidified with 2N HCl, then
neutralised with saturated NaHCO3 solution and extracted with ethyl
acetate. The organic phase is washed with saturated NaHCO3 solution and
~, .
sr r~
1 9 _ f. , ~ 1 3
then with aqueous saturated NaCl solution, dried on MgSO4 and con-
centrated. After recrystallisation from ethyl acetate, 253 mg of the
title compound are obtained. M.p. 179-183C.
H-NMR (300 MHz, CDC13, TMS): 18.25, s, OH; 11.93, s, NH; 7.80-7.16, m,
6H; 4.52, s, H2-C(3); 3.03, s, CH3; 2.98, s, CH3.
The compounds of formulae I and Ia mentioned in the following Tables can
be prepared analogously to the described procedures.
,, .
'
.
~ , 'J
O N
. ~0
_~
OOOOo o
~a Z _~ t~
V ~ o U~ O
,0 ~ ~
U~ . O
_ .
.CI
~0~ ~
g O 1-1 ~ ~ ~ ~ ~ ~ ~ ~ 3
'~ .O
V ~
.~1 V ~
~ r o
V~ O O
~ ~>
r~
.=. F~O ~ t) ~ 0 ~4
1=i~
:r/ I~ r~
~ // X ~; ~ ~ ~ 2
~ il O O
\\/- I U~
o .> ~>
o\ J\//o ~2 :C 2 2 2 ~: 2 :~: O O
~A~ ~
O N tC 2 ~ ~ S N ~
e
O ~ ~ ~ ~ ~ ~ ~ r~
2 :~ X ~ 2~
~- N ~ ~~1 ~O 1~ O ~ ~ ~
~ ~i z ~
r~ ~ A r~
r~l N
X O OOOOO
V 5 V~ C1~ ) O O~ t~
E~ u~
r. O~ 0~ O~ ~ O G~ a~
~0
~ ~ 0~
O O ~1 ~ ~ ~ ~ ~ ;t 3 .$ ~ ~ ~ ~ ~t
u~ O V~ Z v~
Z Z
~ ~ N
I~ ~ X 'T- ~ X ~
OOO ~)OOOO
~o ~ ~
P~ ~r X ~ r~ X :1: X ~ ~ x ~ ~:
~ In
: ~ _ r~
~ 3~ 1 0 ~ 2 :~: 2 :~ X Sl r~ I I o
_~ N N N
l U~
~i) P~; N j~ N j~ ~ ~ ~ 5 j~j ~ j~ 3~ j~ @ ~ j~
C i~
o ,, 2 5~ C X ~
1:: . ~ ~ U~ ~D ~ CO O~ O ~ ~ ~) ~ Il~ ~D 1` CO cr~ o
~ ~ _. ~ _I ~ _I ~ _I ~ C~ ~ ~ ~ ~ ~ ~ ~ C~
C o o
.
~V~ i . ?~
~ :r:
o
X
~,
~, ~
O ~D
~' . o
~x
G~
V ~
~1
~ o o ~ ~ ~ ~ ~ ;~
~ Ll
V
U~ ~ X
O rf G
V~
~ ~.
~ l r~ r ~) ~ N
,~~ ~ ~ ~ ~ o O
O O O Z V~
~) ~4 0 0
~D
P ~~ ~ X 3~ z v~
U~
u- ~O 5
P~
..
I
r~ r~ r~
N
l P~ ~
I
O r~ ~ ~ ~ ~.> r~ r~ ~ r7 r~ r
I ~ ~
. ,_ ~ ~ ~ u~ ~ r~ oo a~ o
I
I ~
~) N
O
~_ ~
r .
r I
V~
O
O O ~ ~J ~ ~f)
.,.1 ~ ~0
0~ 0
~ ~ .
2~ 2
Z ~) Z C~ o o V) V~
Z Z
~ i ~ ~
P~ ~ ) X 2 :~ 2
.~.
~: ~
(I)o\~l\//o ~:~ :1~ 2 ~ 2 2 2 2
N U~
1~1 N ~ 2 ~ 2 N :r N
~: o fl; ~
Ei
~ ~ ~ ~> .. 7 ~ ~ ~0 r~l
X T 2
~1 E~
E-l ~ Z ~ ~
:~ , ' . ;' ,' .,,:
S`~ ' .d ~'~
_~
~ O
e ~ __
__
~0 ~ ~
3 ~ ~ ~
X
o
o o
~4 ~
~.~ ,i!
L
e
o ~ ~ ,. .0 ~ .0
~ P.
~ ~u : :
~z;
: ~: : :
~ , ' . . ~ - ' `
.,- :
~, ,
J
U~
.
~_- X
U~ o
rC
4,
o ~ P.
C o
.,1 ~ C~
V ~
o r o
:~ o vl 2 o v~ v~. o
~ 1 ~
. / ~
// X P~ n ,~ ,~ ~ 2 2
vvvvv
/ ~
Zo
~O o\//\.//o ~ :r 1 X
W ~ ~
~ :~ 2 ~ 2 5~
E3
.. 1~; ~ 2 :I: 2 3:: 2
,-1
~ Q. ~ ~ ~ ~ u~
~ .......
~7 z ~
.~ :
~,
C~l N
~1)
U~
~C ~
. O Z
~)
O O ~ O O
æ ~ ~ _, o ~ O
~ I ._ 0~ ~ ~ ~ '`
v :r _1 ~ ~ ~ a~
~ V~ I I ~
_. ~ o r~ o ~ r~
E~
~a O u~
.,1 ~ n
U)
~C~
. ~ ~ ~ oo
V ~
o
O O ~ ~ ~ -J
.,1 ~ ~0
.,~
,~ o a) x
~D ~O .C O
./ ~ _
\= / ~ V~ o o ~
~0 ~ ~ D C~ o
~1
/
:6 ~: :~ 1 X ~ X 2 3
O o
~' ao ~/!, /,o
~ N ~ ~ 2 ~ o O :1
. : ~
~ P'; i;~ j3 jS j~ N
O
.. ~ j~ jS j~ X j~ j~
~: ~
d~ o r~
O ~i u~
.
I_ ~
o
x
~o l
:~
~ v~
t~S O C~ ~I O ~
O ~ ~ ~ o~
~ l l cl l o l l l l
~ ~ CO
~ ~ c~ ~
~l
4~ ~
o ~ ~
~ ~ o
O O ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ -J
~
~n ~ X
,r o
.
P~ V~ o V~ Z V~
Z Z
_
14 r~ 1.>
O ~ X ~ S ~ O
:~ :C X S S S 2 S t~ N S S ~ S ;
O O O C~
L~
'~
S~-32SOS~SSSSS~
N N N
~ ~S~ ~
~ S ~11 11 11 ~r~~.>~.>r~ ~ ~ r~ ~o r ~ ~ 1.>
N N N S S S 2 S S S 2 S S S 2 ~S~ ~S~ S
. ~ 2 ~ S
C f
O ~ 1'7 ~ ~ ~ ~ ~ ~ ~ ~ ~ 1.> r~ ~ ~ ~ ~ ~
rl ~ ~ ~S~ ~ 2 ~ tS~ ~S cS~ ~S~ cS~ X ~ S~ ~S~
~ . ~~ ~ ~ ~00 ~ O ~ oo
J-- ~ ~ Ir~ ~~I NC~C~l ~~1~I C~J ~1 ~ ~1
~ ~ z ~ ~ U~
.
.
r~ J ' .
0~ N
X
Z
~ I
~ V~
r~
~ O
~ r--l
.C ~ ~
,C;~
~I r--I
O
~ O
rl ~ ~0
u~ a~ X
. ~rC O
. V~
N r
.) ~ ~ o~ ~ r~ X r~
~4 0 0
OOOOOOZU~ X~3 ~
~ O O
1~ ~ r X ~ Z I u~ I ,
. ~
C 5 r~
P~ t~ r~ N C
I
I r~ rq r~l r.7 ~ ~ ro r~l ~ ro r~l ro r.> ro
l N ~ X ~ X ~
I
"
rq ~ r~ ~ ~rq ro ~ ro~ ~ ro ~ ~7
. l ~1 ~ ~ S
C~ U ~
I
. l O r~ O --IC~l
V ~ ~ t~) ~ ~1 ~ ~) ~ ~ t~ ~ ~ ~ ~J
l Eo~ o Il~ Il`) Il') u~ In 1~i U') It) U'~ ) Il~
C~ ~) Z
~: . 3 ~
~ X
. ~
~,
v :r:
V~
~, ~
U ~ ..
.,, ~
0
~: E
rC ~
~ ,1
~ ~ o~
o o
.,1 ~ ~o
.,.1 ~ ~
0 ~ X
o . r o
~ Z C~ Z o o ~ V~
CD O~
~' t~ X 2
~T
J O
tl ~
~!
./
~o
o ~\~ O ~ ~ K
t~ ~ 1.7 r~
lo ~ ,e ~I C 3 1: N X N
aO
E P:;
~D
E~ ~ Z
:, ` .
:
.
V 3~
~ ~:
ra o
.
r e '~
O ) Q.
:~ ~
~ ~ O
O O
v al
OrC O
P.
~\
i / ~ o O V~
Z;=i
.~ O
// >~ 0 ~
~ / ~
: :
Z
O
~ O~1 In
~tA~ ,: ~
~I N
O ~ 11~
~Y; t~
:: ~
~ ~ .. P~ ~ X
l_
P ~ Z
N
`_ o
~ Z ~<
V ~:: 0~
V~ N
C~
~o_
.,
V~
W
O
~ ~ O
.,~
V W
-~ V X
O S O
Z ~ ~ O Ul ~ O Vl V~ O
I ~
~ i ~
U//>~ ~ VVVVV
~ / ~ a~ w ~ w
,~ 0~ 0 ~ P~ 3: q X 3:
T ~
O ~ ~ ,., ,~, ~ ~
o ~
col i x~
~ .-- N ~
E-1 ~ z Ot~ co Co CO CO CO Co
`' . `
,
' :. ` : :
-- 32 -- C? r 3 ` ~ i
2. Formulation Examples (throughout, percentages are by weight)
2.1. Emulsifiable concentrates a) b) c)
a compound of the Tables25 % 40 % 50 %
calcium dodecylbenzenesulfonate5 % 8 % 6 %
castor oil polyethylene glycol
ether (36 moles of ethylene oxide) 5 %
tributylphenol polyethylene glycol
ether (30 moles of ethylene oxide) - 12 % 4 %
cyclohexanone - 15 % 20 %
xylene mixt~re 65 % 25 % 20 %
Emulsions of any desired concentration can be produced from such concen-
trates by dilution with water.
2.2. Emulsifiable concentrates a) b) c)
a compound of the Tables10 % 8 % 60 %
octylphenol polyethylene glycol
ether (4-5 moles of ethylene oxide) 3 % 3 % 2 %
calcium dodecylbenzenesulfonate3 % 4 % 4 %
castor oil polyglycol ether
(35 moles of ethylene oxide) 4 % 5 % 4 %
cyclohexanone 30 % 40 % 15 %
xylene mixture 50 % 40 % 15 %
Emulsions of any desired concentration can be produced from such concen-
trates by dilution with water.
2.3. Suspension concentrate
a compound of the Tables 40 %
ethylene glycol 10 %
nonylphenol polyethylene glycol
ether (15 moles of ethylene oxide) 6 %
sodium lignosulfonate 10 %
carboxymethylcellulose 1 %
33 - ~ '' ` `' '
37 % aqueous formaldehyde solution 0.2 %
silicone oil in the form of a 75 %
aqueous emulsion 0.8 %
water 32 %
The finely ground active ingredient is intimately mixed with the
adjuvants, giving a suspension concentrate from which suspensions of any
desired concentration can be obtained by dilution with water.
2.4. Powder mixtures dispersible in water a) b) c)
a compound of the Tables 25 % 50 % 75 %
sodium lignosulfonate 5 % 5 %
oleic acid 3 % ~ 5 %
sodium diisobutylnaphthalene-
sulfonate - 6 % 10 %
octylphenol polyethylene glycol
ether (7-8 moles of ethylene oxide) - 2 %
highly dispersed silicic acid 5 % 10 % 10 %
kaolin 62 % 27 %
The active ingredient is thoroughly mixed with the adjuvants and the
mixture is thoroughly ground in a suitable mill, affording wettable
powders which can be diluted with water to give suspensions of the
desired concentration.
2.5. Dusts a) b)
a compound of the Tables 2 % 5 %
highly dispersed silicic acid1 % 5 %
talcum 97 %
kaolin - 90 %
Ready-for-use dusts are obtained by intimately mixing the carriers with
the active ingredient and grinding the mixture.
~ ..... . .
,: '
. ,' ' ' ~
.. :
- 34 -
2.6. Granulate a) b)
a compound of the Tables 5 % lO %
kaolin 94 %
highly dispersed silicic acid l ~0
attapu:Lgite - 90 %
The active ingredient is dissolved in methylene chloride, the solution is
sprayed onto the carrier, and the solvent is subsequently evaporated off
in vacuo. Such granulates can be admixed with the animal feed.
2.7. Granulate
a compound of the Tables 10 %
sodium lignosulfonate 2 %
carboxymethylcellulose l %
kaolin 87 %
The active ingredient is mixed and ground with the adjuvants, and the
mixture is moistened with water. The mixture is extruded and then dried
in a stream of air.
2.8. Granulate
a compound of the Tables 3 %
polyethylene glycol (mol. wt. 200) 3 %
kaolin 94 %
The finely ground active ingredient is uniformly applied, in a mixer, to
the kaolin moistened with polyethylene glycol. Non-dusty coated
granulates are obtained in this manner.
2.9. Tablets or boli
I a compound of the Tables 33.00 %
methylcellulose 0.80 %
highly dispersed silicic acid 0.80 %
cornstarch 8.40 %
,
- 35 -
II crystalline lactose 22.50 % ~ ;7 ~ f
cornstarch 17.00 %
microcrystalline cellulose 16.50 %
magnesium stearate 1.00 %
I The methylcellulose is stirred into water. Once the material has
swelled, the silicic acid is stirred in and the mixture is made into
a homogeneous suspension. The active ingredient and cornstarch are
mixed together and the aqueous suspension is incorporated into that
mixture which is then kneaded to a paste. The mass so obtained is
granulated through a 12M sieve and dried.
II All 4 adjuvants are thoroughly mixed.
III The pre-mixes obtained according to I and II are mixed together and
compressed to form tablets or boli.
2.10. In,jectable formulations
A. OilY vehicle (slow release)
a compound of the Tables0.1-1.0 g
groundnut oil ad 100 ml
a compound of the TablesO.l-l.O g
sesame oil ad 100 ml
Preparation: The active ingredient is dissolved in a portion of the oil
with stirring and optionally with gentle heating, and after cooling the
solution is made up to the desired volume and sterile-filtered through a
suitable 0.22 ~m membrane filter.
B. Water-miscible solution (medium rate of release)
a compound of the Tables0.1-1.0 g
4-hydroxymethyl-1,3-dioxolane
(glycerol formal) 40 g
1,2-propanediol ad 100 ml
.
,
.
,,
- 36 - ~ 5 ~ t.
a compound of the Tables 0.1-1.0 g
glycerol dimethylketal 40 g
1,2-propanediol ad 100 ml
Preparation: The active ingredient is dissolved in a portion of the
solvent with stirring, and the solution is made up to the desired volume
and sterile-filtered through a suitable 0.22 ,um membrane filter.
C Aqueous solubilisate (rapid release)
.
a compound of the Tables 0.1-1.0 g
polyethoxylated castor oil
(40 ethylene oxide units) 10 g
1,2-propanediol 20 g
benzyl alcohol 1 g
aqua ad inject. ad 100 ml
a compound of the Tables 0.1-1.0 g
polyethoxylated sorbitan monooleate
(20 ethylene oxide units) 8 g
4-hydroxymethyl-1,3-dioxolane
(glycerol formal) 20 g
ben~yl alcohol 1 g
aqua ad inject. ad 100 ml
Preparation: The active ingredient is dissolved in the solvents and thesurfactant, and the solution is made up to the desired volume with water.
Sterile-filtration is then carried out through a suitable membrane filter
of 0.22 ~m pore diameter.
The aqueous systems can also be used in a preferred manner for oral
and/or intraruminal administration.
3. Biological Examples
The anthelmintic activity is demonstrated by means of the following
tests:
- 37 -
f ~ r~ f ..~
3.1. Trial with sheep infested with nematodes such as Haemonch~us
contortus and Trichostrongylus colubriformis
The active ingredient is administered in the form of a suspension using a
stomach probe or by intraruminal injection to sheep that have previously
been artificially infested with nematodes such as Haemonchus contortus
and Trichostrongylus colubriformis. 1 to 3 animals are used for each dose
per trial. Each sheep is treated only once with a single dose.
A first evaluation is made by comparing the number of worm eggs excreted
in the faeces of the sheep before and after treatment.
Seven to ten days after treatment the sheep are sacrificed and dis~ected.
The evaluation is carried out by counting the worms remaining in the
intestine after the treatment. Sheep simultaneously and similarly
infested but untreated are used as a control or comparison.
In this test a marked reduction in nematode infestation is achieved with
compounds of formulae I and Ia. For example, a reduction in nematode
infestation of approximately 90 % is achieved using 20 mg of active
ingredient per kg of body weight with the following compounds: 1.1, 5.1
and 5.2. ~ith some compounds this result is obtained even with a further
reduced dosage, for example with 10 mg of active ingredient per kg of
body weight or even smaller amounts of active ingredient.
3.2. Trial with sheep infested with cestodes such as Moniezia benedeni
The active ingredient is administered in the form of a suspension using a
stomach probe or by intraruminal injection to sheep that have previously
been artificially infested with cestodes such as Moniezia benedeni. 3
animals are used for each dose per trial. Each sheep is treated only once
with a single dose.
Seven to ten days after treatment the sheep are sacrificed and dissected.
The evaluation is carried out by counting the worms remaining in the
intestine after the treatment. Sheep simultaneously and similarly
infested but untreated are used as a control or comparison. In this test,
,,,, -
- : ' '
.
.
~ .: C.r .~ . Cr
- 38 -
an approximately 90 ~0 reduction in cestode infestation is achieved with
compounds of the Tables, such as, for example, compounds Nos. 1.1, 5.1
and 5.2, at doses of less than 20 mg/kg of body weight.
3.3. Trial with sheep infested with Fasciola hepatica
The active ingredient is administered in the form of a suspension using a
stomach probe or by intraruminal injection to sheep that have previously
been artificially infested with Fasciola hepatica. 3 animals are used for
each dose per trial. Each sheep is treated only once with a single dose.
A first evaluation is made by comparing the number of worm eggs excreted
in the faeces of the sheep before and after treatment.
Three to four weeks after treatment the sheep are sacrificed and dis-
sected. The evaluation is carried out by counting the liver flukes
remaining in the gall-bladder ducts after the treatment. Sheep simultane-
ously and similarly infested but untreated are used as a control or
comparison. The difference in the number of liver flukes counted in the
two groups gives the degree of effectiveness of the test compound.
In this test, pronounced activity against Fasciola hepatica is achieved
with compounds of the Tables at doses of less than 20 mg/kg of body
weight.
. ~ .