Note: Descriptions are shown in the official language in which they were submitted.
~ 0 3 ~
BACKGROUND OF THE INVENTION
1. Field of the Invention
This invention relates to a method for con-
verting methane to higher molecular weight hydrocarbons
and hydrogen using microwave radiation.
2. Description of Related Art
Microwave energy has been used to convert
methane to other hydrocarbons. For example, U.S.
Patent 4,574,038 discloses that methane can be con-
verted to ethylene and hydrogen in a batch process at
pressures of from 0.3 to 1 atmosphere by subjecting the
methane to microwave radiation in the presence of a
metal powder catalyst. Another example of methane
conversion using microwave energy is U.S. Patent
3,663,394.
However, neither patent suggest~ the parti-
cular methane conversion process deccribed below.
SUMMARY OF ~E INVENTION
Thi~ invention concerns the synthesis of
higher molecular weight hydrocarbons and hydrogen from
a methane source. More specifically, methane can be
converted into higher molecular weight hydrocarbons
(e.~. acetylene and ethylene) and hydrogen by irradiat-
ing the methane with microwave radiation in the
presence of at least one elongated plasma initiator
that is capable of initiating an electric discharge in
an electromagnetic field. In a preferred embodiment,
molecular hydrogen will be present initially and the
~03~ 9
plasma initiator will comprise a plurality of elongated
metal wire segments arranged in close proximity to one
another.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a graph of methane conversion and
product yields versus time.
Figure 2 is a graph of methane conversion
versl~s time which shows the effect of C~/H2 mole ratio
on methane conversion.
Figure 3 is a graph of methane conversion
versus average power which shows that various plasma
initiators are effective for methane conversion.
Figure 4 is a graph of acetylene/ethylene
weight ratio versus pressure which shows the effect of
elevated pressure on product distribution.
Figure 5 is a graph of methane conversion
versus average power which shows that the proximity of
the plasma initiators affects methane conversion.
Figure 6 is a graph of methane conversion
versus average power which shows the effect of the
number of plasma initiators on methane conversion.
DETAILED DESCRIPTION OF THE INVENTION
This invention requires th~ presence of
methane, at least one elongated plasma initiator
capable of initiating an electric discharge in an
electromagnetic field, and a source of microwave
energy.
~3i.~.~9
- 3 -
The methane may be pure or mixed with other
hydrocarbons (e.~. as in natural gas). Non-hydrocar-
bon~ (e.~. C02, H2S, N2, etc.) may be present as well.
The plasma initiator may be essentially any
material capable of accumulating an electric charge
when placed in an electromagnetic field and then
dissipating the charge (or initiating an electric
discharge), for example, by ionizing a gas environment.
This includes metal initiators, non-metal initiators
(including semi-conductors), and composites of metal
and non-metal initiators. As used herein, "composite"
is meant to include mixtures (or combinations) of
metals and non-metals. Examples of suitable metal
initiators are tungsten, iron, nickel, copper, their
alloys, or mixtures thereof. Preferred metal initia-
tors are tungsten, iron, or mixtures thereof, with iron
being particularly preferred. Examples of suitable
non-metal initiators include carbon, alumina, manganese
dioxide, magnetite, nickel oxide (e.~. Nio), iron oxide
(~.y. Fe304), calcium aluminate, cobalt oxide, chromium
nitride, iron sulfide (e.g. FeS2, Fel_xS), copper
sulfide (e.g. CuS2), or mixtures thereof. Calcium
aluminate, carbon, iron oxide, or their mixtures are
preferred non-metal initiators, with carbon being
particularly preferred. Silica is not a suitable
non-metal initiator. However, silica composited with a
metal initiator or another non-metal initiator would be
a suitable plasma initiator.
Although methane conversion can be effected
using only one plasma initiator, conversion is enhanced
if more than one (e.~., 6 or more) plasma initiators
are used. Preferably, a plurality of plasma initiators
are used. Most preferably, the plasma initiator will
comprise a plurality of metal wire segments. Each
plasma initiator should be of at least a minimum length
203 ~ ~.X9
that is sufficient to initiate an electric discharge
when placed in an electromagnetic field. However, the
precise minimum length of each initiator may vary with
the frequency of the microwave source as well as the
geometry of the reaction zone and of the initiator.
If more than one plasma initiator is used, a
minimum distance should be maintained between each
initiator to facilitate dissipation of the electric
charge. However, the minimum distance will vary
depending upon the frequency of the microwave source.
As an example, the minimum distance should be at least
about 0.25 cm, preferably at least about 0.5 cm, for a
frequency of 2.45 GHz.
The plasma initiators should be elongated,
but may be formed, combined, or bent in any convenient
shape (e.~., straight, helix, spiral, and the like).
Preferably, the initiators should be formed such that
there are points or sharp edges at the ends or on the
surface of the initiators.
The plasma initiators may be stationary
within the reaction zone or they may be in motion. The
motion can result from the initiators being fluidized
by a gas (~.~. the methane feedstock) or by other means
(e.y. an external magnetic field gradient).
The frequency of the microwave source can
vary broadly. Typically, the microwave energy will
have a frequency of at least 0.3 GHz, with frequencies
centered around 0~915, 2.45, 5.80, or 22.0 GHz present-
ly being preferred in North America: particularly
frequencies centered around Q.915, 2.45, or 5.80 GHz:
especially frequencies centered around 0.915 or 2.45
GHz.
2~3~9~
- 5 -
The microwave energy used in this invention
may be continuous or pulsed, the latter allowing better
temperature control of the plasma initiators to
optimize energy use. If pulsed, the duration of
on-time pulses can vary broadly, but typically will
range from about 1 nanosecond to about 20 seconds,
preferably from about 1 millisecond to about 10
seconds, and most preferably from about 0.01 to about
O.2 seconds. The duration of off-time rests can vary
broadly as well, but typically will range from about 1
nanosecond to about 100 seconds, preferably from about
0.003 to about 60 seconds, and most preferably from
about 0.3 to about 5 seconds.
Molecular hydrogen should also be present in
the reaction zone to maintain the activity of the
plasma initiators for methane conversion. The amount
of hydrogen in the reaction zone during conversion
should be sufficient to maintain a mole ratio of
methane to hydrogen greater than 1:1, preferably at
least 1:1.5, more preferably at least 1:2, and most
preferably at least 1:4. Although some methane conver-
sion may occur at mole ratios of 1:1 or less, greater
conversion will be obtained at higher mole ratios
because hydrogen tends to reduce or inhibit the forma-
tion of carbonaceous deposits on the plasma initiators.
While not wishing to be bound by any particular theory,
it i8 believed that at lower mole ratios, greater
amounts of carbonaceous deposits accumulate on the
initiators and inhibit their ability to ionize the gas
environment.
Although extraneous molecular hydrogen need
not be added, if a sufficient amount of hydrogen is not
present initially in the reaction zone, the initiators
will deactivate until a sufficient amount of hydrogen
is present (or has accumulatad, for example, by
recycling the hydrogen formed during conversion) to
~03~
-- 6 --
retard deactivation and maintain the mole ratio at a
level that will stabilize the methane conversion at a
particular level. This so-called induction period
results in an initial loss of initiator activity and,
hence, a lower level of methane conversion than if
hydrogen had been present initially. To avoid this
undesirable loss of conversion, it is preferred to add
extraneous hydrogen to the reaction zone initially to
minimize or prevent the initial loss of initiator
activity and methane conversion. This extraneous
hydrogen may be pure or in a mixture with other gases
(e.~. as from a naphtha reformer) and may be added to
the reaction zone separately or in mixture with the
methane.
This invention can be practiced at any conve-
nient temperature and pressure, including ambient
conditions. However, the relative amounts of acetylene
and ethylene formed will vary with pressure, with a
greater amount of ethylene being formed at elevated
pressures (i.e., pressures greater than atmospheric).
In addition to acetylene and ethylene, this invention
also contemplates the formation of aromatic compounds
such as benzene, alkyl benzenes, xylenes, and the like.
This invention will be further understood by
reference to the following Examples which are not
intended to restrict the scope of the appended claims.
xample 1 - Conversion of Methane Using Pulsed Micro-
wave Radiation
A methane/hydrogen mixture (1:4 mole ratio)
flowing at 25 ml/min (milliliters/min) at atmospheric
pressure was contacted with 1.5 gm of tungsten wire
(about 0.03 inches in diameter and cut into 45 mm
lengths) in a reactor fabricated from WR430 waveguide
bounded by quartz plate glasc windows and positioned
2~3 ~.~ 19
approximately one-quarter waveguide wavelength from a
short circuit plate. The reactor was irradiated with
microwave radiation centered at a 2.45 GHz frequency
and pulsed in an on/off cycle (0.14 seconds on in a
total of 3.5 seconds) with an average power of 3.6
watts. Methane conversion was calculated according to
the following equation:
% Methane Conversion = [1 wt ~0 methane ~n the products~ x 100
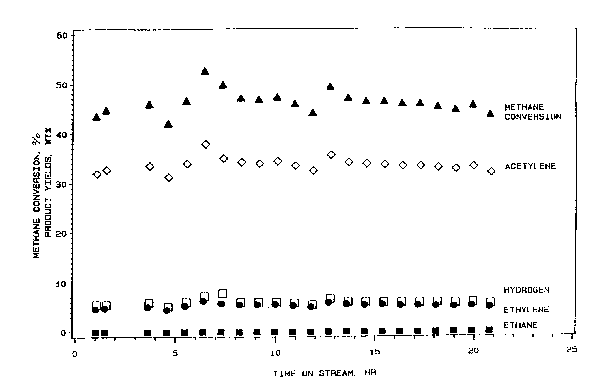
The methane conversion obtained is shown in Figure 1 as
a function of time. Figure 1 also shows that the
primary hydrocarbon products produced were acetylene
(an average of 33.6 wt.%) and ethylene (an average of
5.6 wt.~). Hydrogen ~an average of 6.1 wt.%) and small
amount of ethane (an average of 0.25 wt.%) were also
produced.
Example 2 - Effect of CH4/~2 Mole Ratio on Methane
Conversion ~sing Pulsed Microwave Radiation
Using the apparatus and procedure of Example
1 (except that 2.9 gm of iron wire was used and the
average power ranged from 7.5 to 10 watts), the meth-
ane/hydrogen mole ratio was decreased from 1:4 to 1:1.
The results of this test (as illustrated in Figure 2)
show that a reduction of the methane/hydrogen mole
ratio from 1:4 to 1:2 had little effect on methane
conversion. However, a further reduction to 1:1
resulted in a significant decrease in methane conver-
sion. This decrease proved to be irreversible as shown
~y the further contact with the methane/hydrogen
mixture (1:4 mole ratio) following 17 minutes of
regeneration in air.
~ o ~
8 --
Example 3 - Effect of Various Plasma Initiators on
Methane Conversion Using Pulsed Microwave
Radiation
Using the apparatus and procedure of Example
1, various plasma initiators were tested for their
effectiveness in converting methane. The results of
these tests (as illustrated in Figure 3) show that
tungsten, iron, or carbon (in the form of fibers) can
be used without any adverse effect on methane conver-
sion. However, in a companion experiment using silica
fibers as the plasma initiator, no methane conversion
was obtained.
Example 4 - Effect of Elevated Pressure on Product
Distribution Using Pulsed Microwave
Radiation
Using the apparatus and procedure of Example
1 (except that the methane/hydrogen mole ratio ranged
from 1:1 to 1:11 and methane flow rates ranged from 5
to 20 ml/min), tests were made to determine the effect
of pressure on product selectivity. The results of
these tests are shown in Figure 4 at various apparent
contact times, which is defined as follows:
Apparent Contact Time (sec) 5 1206 x T x CD x WHSV x (H2:Feed) + 1)
here FMW = Average molecular weight of the hydro-
carbon feed
P = Pressure, psia
T = Arbitrarily set at 373K
CD = lnitiator bulk den~ity, g/cc
H2:Feed = Hydrogen to methane mole ratio
WHSV = Weight hourly space velocity, w/w/h
3600 = Conversion from hours to seconds
1206 = Gas constant in tcm3)(psia)/(gm mole)(K).
~3~
The data in Figure 4 show that the product distribution
is relatively insensitive to apparent contact time, but
increasing pressure favors the formation of ethylene
rather than acetylene. Thus, this invention also con-
templates the products from methane conversion being
primarily ethylene and hydrogen at elevated pres~ures.
Example 5 - Bffect of Plasma Initiator Proximity on
Methane Conversion Using Pulsed Microwave
Radiation
The apparatus and procedure of Example 1 was
used to determine the effect of the proximity (or
interpoint distance) of plasma initiators on methane
conversion. In this example, the distance between
plasma initiators was varied from about 0.5 cm (close)
to about 1.0 cm (isolated). The results obtained (as
illustrated Figure 5) show that increased methane
conversion is obtained when the initiators are iso-
lated.
xam~le 6 - Metal Powders`and Filings are Ineffective
for Methane Conversion Using Pulsed
Microwave Radiation
A methane/hydrogen (1:4.2 mole ratio) mixture
flowing at 12.9 ml/min at about atmospheric pressure
was introduced into a quartz reactor and contacted with
0.2 g of nickel powder (from Alpha Products) having a
1.0 micron particle size. The reactor was irradiated
with microwave radiation centered at 2.45 GHz frequency
having 700 watts of power pulsed in an 50/50 on/off
cycle, the cycle length of which was about 22 sec. The
product stream was analyzed by gas chromatography and
showed essentially no conversion of methane to ace-
tylene and ethylene.
Following the same procedure, another experi-
ment using a methane/hydrogen (1:4.1 molQ ratio)
~ ~ 3 ~
-- 10 --
mixture flowing at 11.2 ml/min and iron powder (Fisher
I 60 grade) gave the same result.
Another experiment using o.4 g iron filings
(about 40 mesh) and the same conditions as the iron
powder again gave the same result.
The data in this example show that metal
powders and filings are ineffective initiators for
methane conversion.
Examp~e 7 - Effect of Number o~ Plasma Initiators on
Methane Conversion Using Pulsed Microwave
Radiation
The apparatus and procedure of Example 1 was
used to determine the effect of the number of plasma
initiators on methane conversion, except that 3.0 g of
tungsten wire was used. The results obtained (as
illustrated in Figure 6) show that higher methane
conversion is obtained with 6 rather than 4 plasma
initiators.
Example 8 - Conversion of Methane Using Continuous
Microwave Radiation
A methane/hydrogen mixture (1:4 mole ratio)
flowing at 25 ml/minute (milliliters/minute) at
atmospheric pressure was contacted with 0.37 g of a
straight tungsten wire ~approximately O.76 mm in
diameter and cut into about 47 mm lengths) in a reactor
of a straight piece of quartz tubing, 7 mm in internal
diameter. The part of the tube containing the wire was
inserted in a WR43~ microwave waveguide and positioned
approximately one-quarter waveguide wavelength from a
~-hort circuit plate. The reactor was then irradiated
with continuous microwave radiation centered at a 2.45
GHz frequency, with an average power of 16 watts. The
2~3~
11 --
methane conversion was calculated to be 65.2% using the
following equation:
% Methane Conversion = ~1 wt ~0%ethane ln the products~ 10~
After about 10 minutes, the primary hydrocarbon
products formed were acetylene (an average of 31.0
wt%), ethylene (an average of 5.6 wt%~. The product
stream also contained 42.3 wt% hydrogen (versus 33.3
wt% in the mixture fed to the reactor), small amounts
of ethane (0.25 wt%), and smaller amounts of higher
hydrocarbons. The product stream contained 20.05 wt%
methane (versus 66.6 wt% in the mixture fed to the
reactor).
xample 9 - Effect of Power and Flow Rate on Methane
Conversion Using Continuous Microwave
Radiation
Using the apparatus of Example 8, a meth-
ane/hydrogen mixture flowing at 250 ml/minute (50
ml/minute of methane and 200 ml/minute of hydrogen) was
introduced into the reactor. The average microwave
power was 19 watts. After about 40 minutes under these
conditions, the reaction products contained 35.7 wt%
hydrogen, 50.3 wt% methane, 1.6 wt% ethylene, 0.13 wt%
ethane, 12.1 wt% acetylene, and smaller amounts of
higher hydrocarbons. The methane conversion was
calculated to be 21.8%.
This experiment was repeated except that the
flow rate of the methane/hydrogen mixture into the
reactor was 638 ml/minute (128 ml/minute of methane and
510 ml/minute of hydrogen) and the average microwave
power was 52 watts. After about 5 minutes under these
conditions, the reaction products contained 34.5 wt%
hydrogen, 43.0 wt% methane, 1.9 wt% ethylene, 0.10 wt%
~ L~3~
- 12 -
ethane, 19.6 wt% acetylene, and smaller amounts of
higher hydrocarbons. The methane conversion was
calculated to be 34.4%.
A comparison of these data with the data in
Example 8 show that an increase in flow rate causes a
reduction in methane conversion, while an increase in
microwave power causes an increase in methane conver-
sion.
Example 10 - Effect of Elevated Pressure on Product
Distribution Using Continuous Microwave
Radiation
Using the apparatus of Example 8, a meth-
ane/hydrogen mixture flowing at 115 ml/minute (23
ml/minute of methane and 92 ml/minute of hydrogen) at
standard temperature and pressure was introduced into
the reactor operating at a total pressure of 355 kPa
absolute (or 36.5 psig). The average microwave power
was 16 watts. After about 5 minutes under these
conditions, the reaction products contained 55.8 wt%
hydrogen, 26.5 wt% methane, 6.1 wt% ethylene, 0.73 wt%
ethane, 10.9 wt% acetylene, and smaller amounts of
higher hydrocarbons. The methane conversion and the
weight ratio of acetylene to ethylene in the products
were calculated to be 40.0% and 1.79, respectively.
This experiment was repeated except that the
methane/hydrogen mixture, flowing at 270 ml/minute (54
ml/minute of methane and 216 ml/minute of hydrogen)
STP, was introduced into the reactor at a total pres-
sure of 694 kPa absolute (or 86 psig). The average
microwave power was 23 watts. After about 5 minutes
under these condition~, the reaction products contained
39.1 wt% hydrogen, 47.0 wt% methane, 7.9 wt% ethylene,
1.94 wt~ ethane, 4.2 wt~ acetylene, and smaller amounts
- 13 -
of higher hydrocarbons. The methane conversion and
weight ratio of acetylene to ethylene in the products
was calculated to be 22.8% and 0.53, respectively.
The data in this example show that an in-
crease in pressure affects the product distribution
and, in particular, increases the yield of ethylene at
the expense of the acetylene.