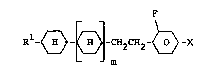Note: Descriptions are shown in the official language in which they were submitted.
-- 2 0 3 ~ O ~ ~
-- 1 --
Merck Patent Gesellschaft
mit beschrankter ~aftung
6100 D a r m s t a d t
Fluorinated Cyclohexyl Derivatives
The invention relates to fluorinated cyclohexyl deri-
vatives of formula I ~ -
F
{ m
wherein R1 denotes an alkyl residue with up to 12
carbon atoms wherein one or two non-
adjacent CH2-groups may also be replaced
by -O-, -O-CO-, -CO-O- and/or -CH=CH-,
m is 0 or 1, and
X is F, -CF3, -OCF3 or -OCHF2
and also to liquid crystalline media being a mixture of
at least 2 compounds, characterized in that at least one
compound is a fluorinated cyclohexyl derivative accord-
ing to formula I.
The invention was based on the object of discovering
new stable liquid crystal or mesogenic compounds which
are suitable as components of liquid crystalline media and,
in particular, have advantageous values for optical and
dielectric anisotropy combined with low viscosity and
high nematc~enity.
, ~ ~
:
.~
.,. ~ ,
.
, ~ , ,
20330~7
Similar bicyclohexyl derivatives are described in Euro~
pean Patent 0 125 563: ~ -
alkyl- ~ - ~ -CH2CH2- ~ -CN
alkY~ >~CH2CH2~~-F ;
C3H7- ~ ~ ~ -C~2CH2- ~ -CN
F F :;.
C3 ~ - ~ _ ~ -CH2CH2- ~ -CN ~ .
CH3 ~ ;
Similar cyclohexyl derivatives are described in German ~-
10 Patent 32 33 641:
alkyl ~ -CH2CH2- ~ - F
These compounds, however, still pose some problems in
the mixture development due to the occurrence of disturb-
ing smectic phases and to the fact that the strongly p~-
lar compounds exhibit high viscosities whereas the lowviscosity compounds have relatively low values for 1,
and/or 1. In-addition, the laterally substituted species
are not easily accessible.
It has now been found that fluorinated cyclohexyls of
formula I are highly suitable as components of liquid
crystalline media. In particular, they have especially
advantageous values of optical and dielectric anisotropy
~ and ~"). It is also possible to obtain stable
liquid crystal phases with a broad nematic mesophase
range including a good deep temperature behaviour and a
comparatively low viscosity with the aid of ~hese compounds.
~3~87~
Depending on the choice of the substituents, the compounds
of the formula I can be used as the base materials from
which liquid crystal media are predominantly composed;
however, it is also possible for compounds of the formula I
to be added to liquid crystal base materials of other
classes of compounds, for example in order to influence
the dielectric and/or optical anisotropy and/or the viscosity
and/or the nematic mesophase range of such a dielectric.
The compounds of the formula I are colourless in the pure
state and are liquid crystalline in a temperature range
which is favourably placed for electrooptical use.
They are very stable towards chemicals, heat and light.
The invention thus relates to the fluorinated cyclohexyl
derivatives of the formula I, to liquid crystalline media
with at least two liquid crystalline components, wherein
at least on component is a compound of the formula I and
to liquid crystal display devices containing such media.
Above and below, R1 and X have the meaning given unless
expressly indicated otherwise.
R1 i~ preferably alkyl, alkoxy, oxaalkyl, alkanoyloxy or
alkenyl and can exhibit a straight-chain or branched
structure.
Alkyl or alkoxy preferably are straight-chain and have
2, 3, 4, 5, 6 or 7 C atoms. Accordingly they are prefer-
ably ethyl, propyl, butyl, pentyl, hexyl, heptyl, ethoxy,propoxy, butoxy, pentoxy, hexoxy or heptoxy, also methyl,
octyl, nonyl, decyl, undecyl, dodecyl, tridecyl, tetra-
decyl, pentadecyl, methoxy, octoxy, nonoxy, decoxy, un-
decoxy, dodecoxy, tridecoxy or tetradecoxy.
., .: - -. . :.. . .-, . . . . . - .. ... ... , .......... .. . .- .. ~, .. :..... ..
.- ~. . - - . -, - .. , . - . . .. . ....
- 4 -
Oxaalkyl is preferably straight-chain 2-oxapropyl (= methoxy-
methyl), 2-(= ethoxymethyl) or 3-oxybutyl (= 2-methoxyethyl), -
2-, 3- or 4-oxapentyl, 2-, 3-, 4- or 5-oxahexyl, 2-, 3-, - --
4- 5- or 6-oxaheptyl, 2-, 3-, 4-, 5-, 6- or 7-oxaoctyl, ~ -
2-, 3-, 4-, 5-, 6-, 7- or 8-oxanonyl or 2-, 3-, 4-, 5-,
6-, 7-, 8-, or 9-oxadecyl.
Alkenyl is preferably straight-chain and has 2 or 10 C atoms.
It is accordingly, in particular, vinyl, prop-l- or prop-2- ; -
enyl, but-l-, -2- or -3-enyl, pent-1-, -2-, -3- or -4-enyl,
hex-l-, -2-, -3-, -4- or -5-enyl, hept-l-, -2-, -3-, -4-,
-5- or -6-enyl, oct-l-, -2-, -3-, -4-, -5- -6- or -7-enyl,
non-l-, -2-, -3-, -4-, -5-, -6-, -7- or -8-enyl or dec-1-,
-2-, -3-, -4-, -5-, -6-, -7-, -8- or -9-enyl.
Compounds of the formula I containing a branched
terminal group can occasionally be of importance because
of an improved solubility in the customary liquid crystal
base materials, but in particular as chiral doping substances ~-
if they are optically active.
Branched groups of this type as a rule contain not more
than one chain branching. Preferred branched radicals ~re
isopropyl, 2-butyl (= 1-methylpropyl), isobutyl (= 2-
methylpropyl, 2-methylbutyl, isopentyl, (= 3-methylbutyl),
- 2-methylpentyl, 2-ethylhexyl, 2-propylpentyl, 2-octyl,
isopropoxy, 2-methylpropoxy, 2-methylbutoxy, 3-methylbut-
oxy, 2-methylpentoxy, 3-methylpentoxy, 2-ethylhexoxy,
2-methylhexoxy, l-methylhexoxy, 1-methylheptoxy (= 2-octyl-
oxy), 2-oxa-3-methylbutyl, 3-oxa-4-methylpentyl, 4-methyl-
hexyl, 2-nonyl, 2-decyl, 2-dodecyl, 6-methyloctoxy, 6-
methyloctanoyloxy, 4-methylheptyloxycarbonyl, 2-methylbuty-
ryloxy, 3-methylvaleryloxy, 4-methylhexanoyloxy, 2-methyl-3-
oxapentyl and 2-methyl-3-oxahexyl.
.~.. -, -, ,; .-,.. -.- . :.- - . , - . , . .:
--~ 20330~7
In the case of compounds with a branched terminal
group R1, formula I includes both the optical antipodes
and racemates as well as mixtures thereof.
m is preferably l. X is preferably -OCF3 or -OCHF2 and
furthermore preferably -CF3.
Of the compounds of the formula I and subformulae thereof,
those in which at least one of the radicals contained
therein has one of the preferred meanings given are
preferred.
The compounds of the formula I are prepared by methods
which are known per se, such as are described in the
literature (for example in the standard works, such as
Houben-Weyl, Methoden der Organischen Chemie [Methods of
Organic Chemistryl, Georg Thieme Verlag, Stuttgart), and
15 in particular under reaction conditions which are known -
an~ suitable for the reactions mentioned. Variants which
are known per se and are not mentioned in more detail
here can also be used in this connection.
If desired, the starting materials can also be formed
in situ, ~uch that they are not isolated from the reac~
tion mixture but are immediately reacted further to give
the compounds of the formula I.
A preferred route for preparation starts from the easily
accessible Wittig reagent R1 ~ - ~ ¦-C~2P~Ph3Je, which
is reacted with 2-fluoro-4-X-benzaldehyde under the known
conditions for Wittig reaction. The cyclohexyl deriva-
tives of formula I are easily obtained by catalytic hydro-
genation (Pd-C) of the Wittig products.
, , ,
., - , .
: ~ , , ' ; : ' -
. ;. . . .
, ....... , . .
---` 20~3Q87 -:
An alternative route is shown in Scheme 1:
Scheme 1
R~ -C~=CH2 + Br- ~ -X :~
~ Heck reaction
R~ _CH=CH_ ~ -X
m
l~ ~2/Pd-C
Rl_~ ~ -C~12CH2 -~ -X
Other routes are apparent to the skilled worker. In
addition it is possible to follow the above shown routes
with a group X being different from the clesired one and
to introduce the desired group X in the f inal step, e.g.
conversion of -CN to -COO~ and finally to -CF3 ~ also being
possible to Use Br as a precurSor for CN3; or -OMe to -OH
and finally to -OCF3 or -OC~F2. All these steps and the
corresponding reaction conditions are known to the skilled
worker.
. " .. . .
,. : : : - ,
.. . . .
,. , ., , ,,~ . :
~.,~ :, : -- : , ,
2033087
In addition to one or more compounds for formula I the
liquid crystal media according to the invention prefer- -
ably contain 2-40 components and in particular 4-30 com-
ponents. Liquid crystal media being composed of one or
S more compounds of formula I and 7-25 other components
~are especially preferred.
These additional components are preferably chosen from
the nematic or nematogenic (monotropic or isotropic)
substances; in particular from the classes of azoxy-
benzenes, benzylideneanilines, biphenyls, terphenyls,phenyl or cyclohexyl benzoates, phenyl or cyclohexyl
cyclohexanecarboxylates, phenyl or cyclohexyl cyclohexyl-
benzoates, phenyl or cyclohexyl cyclohexylcyclohexane-
carboxylates, cyclohexylphenyIbenzoates, cyclohexyl-
phenyl cyclohexanecarboxylates, cyclohexylphenyl cyclo-
hexylcyclohexanecarboxylates, phenylcyclohexanes,
cyclohexylbiphenyls, phenylcyclohexylcyclohexanes,
cyclohexylcyclohexanes, cyclohexylcyclohexenes, cyclo-
hexylcyclohexylcyclohexene, 1,4-bis-cyclohexyIbenzenes,
4,4'-bis-cyclohexylbiphenyls, phenyl- or cyclohexyl-
pyrimidines, phenyl- or cyclohexylpyridines, phenyl- or
cyclohexyldioxanes, phenyl- or cyclohexyl-1,3-dithiane~,
1,2-diphenylethanes, 1,2-dicyclohexylethanes, l-phenyl-
2-cyclohexylethanes, 1-cyclohexyl-2-(4-phenyl-cyclohexyl)-
ethanes, 1-cyclohexyl-2-biphenylethanes, 1-phenyl-2-cyclo-
hexyl-phenylethanes, optionally halogenated stilbenes,
benzyl phenyl ethers, tolanes and substituted cinnamic
acids.
The 1,4-phenylene groups of these compounds may be
fluorinated.
,.,.. ,,: !. ~ ~. . . :
2~33Q87
- 8 -
The most important compounds which are possible consti~
tuents of liquid crystal media according to the invention
can be characterized by the formalae 1, 2, 3, 4 and 5:
R'-L-U-R" 1
S R'-L-COO-U-R" 2 ..
R'-L-OOC-U-R" 3
2 2
R'-L-C-C-U-R" S
In the formulae 1, 2, 3, 4 and 5 L and U may be equal or
different from each other. ~ and U independently from
each other denote a bivalent residue selected from the
group consisting of -Phe-, -Cyc-, -Phe-Phe-, -Phe-Cyc-,
-Cyc-Cyc-, -Pyr-, -Dio-, -G-Phe-, -G-Cyc- and their mirror
images; in this compilation of residues Phe denotes un-
15 substituted or fluorinated 1,4-phenylen, Cyc trans- 1,4- :
cyclohexylene or 1,4-cyclohexenylen, Pyr pyrLmidine-2,5-
diyl or pyridine-2,5-diyl, Dio 1,3-dioxane-2,5-diyl and
G 2-(trans-1,4-cyclohexyl)-ethyl, pyrimidine-2,5-diyl,
pyridine-2,5-diyl or 1,3-dioxane-2,5-diyl.
One of the residue L and U is preferably Cyc, Phe or ~yr.
U preferably denotes Cyc, Phe or Phe-Cyc. The liquid
crystal media according to the invention preferably con-
tain one or more components selected from the compounds of
formulae 1, 2, 3, 4 and 5 with L and U meaning Cyc, Phe
and Pyr, said liquid crystal media further containing
at the same time one ore more components selected from
the compounds of formulae 1, 2, 3, 4 and 5 with one of
the residues L and U denoting Cyc, Phe and Pyr and the
other residue being selected from the group consisting
of -Phe-Phe-, -Phe-Cyc-, -Cyc-Cyc-, -G-Cyc-, said liquid
'.~ :. , ; ,
;:;: . . . .
. .i, . . .
, . .... . . . . ~: .
,. - ~ . . . : ~
. ~ - . . . - . - .
~033087
crystal media containing in addition to this optionally
one or more components selected from the compounds of
formulae 1, 2, 3, 4 and 5 with L and U being selected
from the group consisting of -Phe-Cyc-, -Cyc-Cyc-,
-G-Phe- and -G-Cyc.
In a preferred subgroup of the compounds of formulae
1, 2, 3, 4 and 5 (subgroup 1~ R' and R" are independent-
ly from each other alkyl, alkenyl, alkoxy, alkenoxy with
up to 8 carbon atoms. R' and R" differ from one another
in most of these compounds, one of the residues usually
being alkyl or alkenyl. In another preferred subgroup of
the com~ounds of formulae 1, 2, 3, 4 and 5 (subgroup 2)
R" denotes -CN, -CF3, -F, -Cl or -NCS while R' has the
meaning indicated in subgroup 1 and is preferably alkyl
or alkenyl. Other variants of the envisaged substituents
in the compounds of formulae 1, 2, 3, ~ and 5 are also
customary. Many such substances are commercially available~
All these substances are obtainable by methods which are
known from the literature or by analogous methods.
The liquid crystal media according to the invention pre-
ferably contain in addition to components selected fro~
subgroup 1 also components of subgroup 2, the percentage
of these components being as follows:
subgroup 1: 20 to 90 %, in particular 30 to 90 %
25 subgroup 2: 10 to 50 %, in particular 10 to 50 %
In ~hese liquid crystal media the percentages of the com-
pounds according to the invention and the compounds of sub-
group 1 and 2 may add up to give 100 %.
;. , -: .
, .
., . - . :: .
2~33087
-- 10 --
The media according to the invention preferably contain -
1 to 40 %, in particular 5 to 30 % of the compounds
according to the invention. Media containing more than
40 %, in particular 45 to 90 % of the compounds accor-
ding to the invention are further preferred. The media
contain preferably 3, 4 or 5 compounds according to the
invention.
The media according to the invention are prepared in a
manner which is customary per se. As a rule, the com-
ponents are dissolved in one another, advantageouslyat elevated temperature. The liquid crystal media accor-
ding to the invention can be modified by suitable addi-
tives so that they can be used in all the types of liquid
crystal display devices. Such additives are known to the
expert and are described in detail in the liteL~ture
(H. Kelker/R. ~atz, ~andbook of Liquid Crystals, Verlag
Chemie, Weinheim, 1980). For example, it is possible to
add pleochroic dyestuffs to prepare colored guest-host
~y~tems or substances for modifying the dielectric aniso-
tropy, the viscosity and/or the orientation of the nematicphases.
The following examples are to be construed as merely
illustrative and not limitative. m.p. = melting point,
c.p. = clearing point. In the foregoing and in the fol-
lowing all parts and percentages are by weight and thetemperatures are set forth in degrees Celsius. "Custo-
mary work-up" means that water is added, the mixture
i~ extracted with methylene chloride, the organic phase
is seperated off, dried and evaporated, and the product
is purified by crystallization and/or chromatography.
~, .. , ... , . . : .
. ~ ..... , ~ ,., . . .. ' ' '- .: '
.,.. ,.............. , ~-
., ., , .. , :,: -- . -
. ~ ,. . . : - , . .
:; ~ . , .- .-- .,,: : . , .
203~087
Further are:
C: crystalline-solid state, S: smectic phase (the index
denoting the typ of smectic phase), N: nematic phase, ~ :
Ch: cholesteric phase, I: isotropic phase. The number :~
S being embraced by 2 of these symbols denotes the tem-
perature of phase change.
ExamPles for Production
Exam~le 1
Hydrogenation of 1-(4'-n-propylbicyclohexyl)-2-(2-
fluoro-4-trifluoromethylphenyl)-et~ene yields after
customary work up l-(4'-n-propylbicyclohexyl)-2-(2-
fluoro-4-trifluoromethylphenyl)-ethane, C 79.5 N 104 I.
Exa~mples 2 to 11
:: :
The following compounds are obtained analogously:
(2) 1-(4'-n-pentylbicyclohexyl)-2-(2-fluoro-4-trifluoro-
methylphenyl)-ethane
(3) 1-(4'-n-pentylbicyclohexyl)-2-(2-fluoro-4-trifluoro-
methoxyphenyl)-ethane
(4) 1-(4'-n-propylbicyclohexyl)-2-(2-fluoro-4-trifluoro-
methoxyphenyl)-ethane
(5) 1-(4'-n-butylbicyclohexyl)-2-(2-fluoro-4-trifluoro-
methoxyphenyl)-ethane
(6) 1-(4'-n-propylbicyclohexyl)-2-(2-fluoro-4-difluoro-
methoxyphenyl)-ethane
(7) 1-(4'-n-butylbicyclohexyl)-2-(2-fluoro-4-difluoro-
methoxyphenyl)-ethane
'7''~' 2033087
I
- 12
(8) 1-(4'-n-pentylbicyclohexyl)-2-(2-fluoro-4-difluoro-
methoxyphenyl)-ethane
(9) 1-(4'-n-pentylbicyclohexyl)-2-(2,4-difluorophenyl)-
ethane
(10) 1-(4'-n-butylbicyclohexyl)-2-(2,4-difluorophenyl)-
ethane
(11) 1-(4'-n-propylbicyclohexyl)-2-(2,4-difluorophenyl)-
ethane
Examples of compositions
ExamPle A
A liquid crystalline medium consisting of
15 % of 1-(4'-n-propylbicyclohexyl~-2-(2-fluoro-4-tri-
fluoromethylphenyl)-ethane and
85 % of a mixture containing
24 % p-(trans-4-propylcyclohexyl)-benzonitrile,
36 % p-(trans-4-pentylcyclohexyl)-benzonitrile,
25 % p-(trans-4-heptylcyclohexyl)-benzonitrile and
15 % 4-cyano-4'-(trans-4-pentylcyclohexy ~biphenyl
shows N 73.5 I.
.; : . - .
,,.. ,.~ ~, ,. ...... - . - .
,:,, . ., , . - .: : , -
,.,. ., : . . .
" . .. .. .
r; ~' . ~ . : '