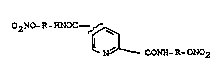Note: Descriptions are shown in the official language in which they were submitted.
HOECHST AXTIENGESEhLSCHAET HOE 90/F 010 Dr. SW/PP
De~cription 20~20B
Di(nitroxyalkyl)amides of pyridine-2,4- and ~2,5-
dicarboxyli~ acids, a process for ~he preparation there-
of, and the use thereof
Compounds which inhibit the enzymes pxol~ne hydroxyla~e
and lysine hydroxylase bring about ~ very ~elective
inhibition of collagen biosynthesi~ by influencing the
collagen-~pecific hydroxylation reaction~. In the cour~e
thereof, prote~n-bound proline or lysine i8 hydroxylated
by the enzymes proline hydroxylase or lysina hydroxyla5e.
If this reaction i~ suppressed by inhibitor6, the re~ult
is an insufficiently hydroxylated colla~en molecule which
is unable to function and can be released by the cells
into the extracellular ~pace only in a ~mall ~mount.
~oreover, the in~ufficiently hy~roxylated collaqen cannot
be incorporated in the ~ollagen matrix and very readily
undergoes proteolytic degradation. The consequence of
these effects i~ an overall reduction in the amount of
collagen deposited outside the cell~.
It is knvwn that the inhibition of proline hydroxylase by
known inhibitors ~uch as ~ dipyridyl results in
inhibition of Clq biosynthesi~ by macrophages (W. Muller
et al., FE3S Lett7 90 (1978), 218; Immunobiology 155
(1978), 47). Thi~ lead to the classical pathway of
complement activation becoming inoperative. ~hus, inhibi-
tors of proline h~droxylase also act a~ immunosuppre~-
~ants, for example in immune complex diEeases.
It is ~nown that the enzyme proline hydroxylase i8
e~ficien~}y inhibited by pyridine-2,4- and -2,5-dicarb-
oxylic acids (X. Ma~amaa et al., Eur. J. Biochem. 138
(1984) ~39-245). The~e compounds are, however, effective
inhibitors in cell culture only in very high concentra-
tions ~Tschank, G. et al., Biochem. J. 238 (1987~ 625-
633).
- 2 ~ 20~20~
DE-A 34 32 094 ~3i.ve~ a d~cription of die~tQrs of pyri-
dine-2, 4- and -2, 5-dicarbo~rlic aclds with 1-6 carbon
atom~ in the efiter alkyl moiety as pharma~ceuticals ~c~r
inhibiting proline hydro~ se ~n~ ly~ine hy~roxyla~e.
These lower nlkyl diesters ha re ~he diRadvantage, how-
ever~ th~t ill ~he body they are too rapidly clea~red to
the acids and do not reach ~heir ~te of ~ctioTl ~ n the
Cl311 in ~ufficiently high concentration and thu~ nre
poorly suited :Eor po6sible ~*ministration a8 phann~:eu~i-
cal~.
DE-A 37 03 959, D~:-A 37 03 962 and DE-A 37 03 963 de~-
criba in a ~eneral ~Eorm mixed ester/amide~, higher all~l
diesters and diamide~ of p~ridine-2, 4- ~nd -2, 5-dicar-
boa~lic acid~, which are effec~ive inhibitors of collagen
biosynthe~ ani~al model~.
~hus, DE-A 37 03 959 de~cribes, inker alia, the ~ynthe~i~
OI N,N'-bi8(2-methoxyethyl)pyridine-2,4-dic~rboxamideand
N,N'-bls(3-isopropoxypropyl)pyridine-2,4-dicarboxamide.
German Patent Applications P 38 26 4~1.4 and P 38 28
140.6 propose ~n improved proce~ for the preparation of
N,N'~bis(2-methoxyethyl)pyridine-2,4-di~arboxamide.
~erman Patent Application P 3924093.2 propo~e~ n~w N,~'-
bi~(alkoxyalkyl)pyridine-2,4-dicarboxamides.
Both pyridine-2,4- ~nd -2,5-dicarbox2mides (Hirakata et
al., J. pharm. So~. Japan 77 (19S7) 219 and H~rincJ et
elv. 37 ~1954) 147, 153) ~nd pyridinel~2,4- and
~2,5-dicarbohydrazides (Itai et al ., Bl . nation . hyg .
~abor. ~okyo, ?4 (1956) 115, 117 and Shinohara st ~1.,
Chem. High Polymer~ Japan, 15 (1958) 839) have already
been di6closed a8 agent~ for tuberc~losi~.
JP 53/28175 ~78/28175) describes N,~'-bis(2-nitroxy-
ethyl)pyridine-2,4- and -2,5-dicarboxamides as substancQs
with a vasodilator action.
~3~2~
_ 3 _
It has now ~een ~ound, ~nrpri~i~gly, th~t di~nitroxy-
~lkyl)amides ~f pyridine-2,4- and -2,5-dic~rboxylic
aci~s, of the fo~mula I
02NO~ NOC~,~
~ ~a~~ C:ONH-~.- ON02
in which
R is C1-Cj-alk~n~diyl,
and ~he phy~iol~gically tolera~ed ~allts, effactiv~ly
inhibit lys~na hydroxyl~e and proline hydroxylas~ in
animal ~odels.
Accordingly, the invention relate~ ~o a~ ~he u~e of
compounds of ~he for~ula I
02NO-R-HNoC_ ~ ~ (I)
N C4NH-R-~N02 ~-
.
~n whi~h
R is Cl C4-alkaned~yl,
and the physiologically tolerated 8alt~, for the prepar-
ation of a pharmaceutical which inhibit~ proline hy-
droxylase and ly~ine hydroxylase.
Tha inven~ion additionally rela~e~ to b) the co~pound3 of~he formula I
in w~ich
R is methylene, propylen~ or butylene,
and the phy~iologically tolerated ~alt~, for use a~
pharmaceuticals.
" 2~3420~
The i~ven~ion ~dditionally rel~tes ~o c) the compound~ of
the formul~ I
in which
R is methylene, propylene or butylene,
5 and the physiolog~cally tolerated salts th~reof.
The invention particularly r~late~ to ~he c~mpound~ o~
the formula I def~ned ~n a~, b) ~nd c) for u~e a~ fibro-
~uppres~ant~ and immuno~uppres~ants and for the ~nhi-
bi~ion of proline hydroxyla~e and ly~ine h~droxyla~e and
for influencing ~he metaboli~m of ~ollagen ~nd coll~gen~
like ~ubstances ~nd the biosynthesi~ of ~
All the ~aid alkyl radic~ls with more than 2 carbon atoms
can be both straight-chain and branched.
~he in~ention additionally relat28 to ~ proces~ for ghe
preparation of cGmpou~d~ of the formula I, which com- --
pri~es r~actin~ a csmpound of the formula I~
Y-C -r~
N ~Y
with a co~pound of the formula III
~2N-R-ONO~ (I$~)
where R has the ~eaning ~peci ied for for~ula I, ~nd ~ i8
halogen, hydroxyl or Cl--C~-alkoxy, or form~ toge~h2r with
the carbon~l group an active e3ter or a mixed anhydrida,
or compriEes nitrating ~ compound o~ tho formula IV
~3~2~6
H0- R- ~NOC f ~,
g IV)
~ N~~ t:O~ OH
in which
R ~ ~ as dafined above, ~d sub~equently con~ertirlg the
reaction product~ whare appropri~te .~ n~o their phy~io-
logi cally tolerated l~alt8 .
S The pr~par~tion of compounds of the formula I ~nd the
preparation of tho~e ~ar~ing sub~tances re~ir~d for
th~ which canno~ be bought i~ da~cribed in detai 1
hereinafter .
The compound~ according ~o the ~ nvention are prepzred
10 ~no~t ~traightforwardly by ~he two component~, ~he pyr~-
dine derivative of t~e formula ( II ) and the amine of 1the
formula (III), baing ~ixed in equimolar amoun~ or with
~n up to about 5-fold exces~ of III and reacted at
temperatures between -30 and 150C, prefer~bly at 20 to
15 100C, urltil the reaction i6 c:omple~e. ~he completion of
the reaction can be deter~ined, for esample, by thin-
layer ~hromatograp~y. One vari~nt of thi~ process ~o~-
pri~e~ u~ing ~ ~uitable 801vent ~uch as die~hyl ether or
dime~hoxyethane or ~etrahydrofuran, chlorinated hydro-
carbon~ such a~ methylene chloride, chloroform, tri- or
tetrachloroethyl~ne, benzene, toluene or else polar
solvent~ ~uch ~ dimethyl~or~amide, acetone, al~ohols
su~h a~ methanol or ethanol or dimethyl sulfo~ide. It ~a
al80 po~sible in this ca~e to u~e an exce88 of amine of
the formula (III), which c~n be up ~o about 5-fold
amount~. ~he t~mperatures for this reaction ar0 betwaen
room temperature ~nd the boilin~ point of the ~ol~ent,
with temperatures in the range ~rom room temperature to
130C being partlcularly preferred.
The reaction can likewise be carried out via a ~i~ed
- 6 - 2~2~
anhydride such a~ athyl ~hloroformate or ~ia an activated
e~ter ~uch as p~ranitrophenyl e~ter ~g= ClCH2-COO or
~O2-C~H~-O). Appropri~te methods ~re de~cxibed in the
literature.
It i~ al~o pos~ible, where appropriate, for the r~action
to be carxied out in the presence of b~e~. ~x~ple3 of
suitabl~ additional ba~es are carbonate~ or bicarbonat~
~uch a~ ~odium or potas~ium car~onate or ~odium or
potasfiium bicarbona~e, or ter~iary amines ~uch a~ tri-
e~hylamine, tributyl~ine, ethyldii~opropylamine or
hetQrocyclic amine~ ~uch a~ ~al~y~orpholin~, pyridlne,
quinoline or dialkylanilines.
Ome variant for the prQparation of the comp~unds o$ the
fonmula I comprises nitration of the correspondiny
hydroxyalkyldia~ides of pyridine-2,4- or -2,5 di-
carboxylic acid ~ IV) . Thi8 entail~ adding concentrat~d
nitric acid to the coxre~ponding hydroxyalkyldiamide~ at
reaction ~emperature~ ~rom -20C to +10C~ preferably at
-10C to 5C. The reaction t~me in thi~ ca~e i~
10-240 min, pr~ferably 20-90 min. The reaction product iB
sub~eguently neutralized where appropriate.
Where appropriate the produc~ can be worked up, for
example, by extraction or by chromatography, for example
on silica gel. The i~olated product can ba racry~tallized
and, where appropriat2, reacted with a auitsble acid ~o
gi~e a physLolo~ically toler2ted salt. ~ample~ of
~uitable acids are:
mineral acid~ such a~ hydrochloric and hydrobromic ~cid,
~nd ~ulfuric, phosphoric, nitric or perchloric acid or
organic acid~ such as formic, ~cetic, propionic, ~uc-
cLnic, glycolic, lactic, malic, ~artaric, citric, maleic,
fumaric, phenylacetic, benzoic, methanesulfonic, toluene-
~ulfonic, oxalic, 4-uminobenzoic, naphthalen3-lt5-disulf-
onic or a~corbic ~cid.
203420B
- 7 -
Those starting compounds of the formul~ (III) which
cannot ba bought can b~ ~ynthe~ized by proce~se~ known
from the liter~ture.
The 3tarting ~ompounds of ~he for~la (II) ~re obtained,
S for example, by conv~rt~ng pyridine-2,4- or -2,5-dicarb-
oxylic acid ~nto the corre~pondi~g p~ridine~2 t 4- or ~2,5-
dic~r~o~yl halide, praferably ~hloride (~y proce~e~
known fro~ the literature), preferably in tha pr~ence of
a catalyst such AS dimethylform~mide. Thi~ acid halide
10 can then be reacted, ~or ex~mplQ, either wLth ~ suitable
alcohol, for example par~nitrobanzyl alcohol, to giva ~he
co~re~ponding active ester, or else with lower alcohol~
~uch a~ ~ethanol or ethanol ~o give the corre~po~ding
esters. It i~ likewi~e a180 pos~ible for the pyridine-
2,4- or 2,5-dicarbo~ylic acid ini~ially ~o be conYerted
by addition of a 6uitab1e carboxylic acid vr of a carb-
oxylic es~er such as ethyl chlorovrmate into a ~ixed
anhydride which i~ then reacted with ~he amine~ ~III) to
give the products accord~ng to the invention. An approp-
riate method i~ likewi~e de~cribed in the literature.
~he starting compounds o~ the formula (IV) are obtained,for example, by reacting corre~ponding ~,N'-bis(alkoxy-
alkyl)pyridi~e-2~4- or -2,5-dicarboxamide~, preferably
the bis(methoxyalkyl)diamide by proce~se~ known fro~ the
literature, for ex2mple wi~h boron tribromide. The
preparation of the bi~(alkoxyalkyl)diamide~ i~ knowm and
de~cribed, or e~ample, in DE-A 3,703,~59. Thi~ entail~
reactin~ a reac~ive pyridinedicarboxylic acid derivativa,
for exsmple the pyIidinedicarbon~l ~hloride, with an
alkoxyalkylamine.
The compound~ o~ th2 formula I according to the in~ontion
have valuable pharmacological properties and di~pl~y, in
particular~ activity aE inhibitors of proline hydroxyla3e
and lysine hydro~ylase, as fibrosuppres~ant and immuno-
3s ~uppres~ant.
2~3~2~6
secause of the~ pharmacological propertie~ the co~-
pounds according to the invention are suitable for the
treatment of di~turbance~ of ~he metaboli~m of ~ollag~n
and collagen-like ~ub~tance~ an~ ~or the treatment of
S disturbances of the bio~ynthe~i3 of Clq.
Hence the i~ention furthermore rel~te~ to the u8e of the
~ompounds of the formula I ~ccording to ~he invention ~nd
of the phy3iologically tolerated ~altls thereof for the
tx~atment of the abovementioned metabo.lic dieorder~.
14 The compounds can be used as pharmaceuticals eithe~ ~lone
or mi~ed ~ith physlologically tslarated ~uxiliari~ or
excipient~. They can be ~dm$nistered or this purpo~e
orally in do~es of O.Ol - 25.Q mg/kg/day, pxefer~bly O.Ol
- 5.4 mg/kg~day or parenterally in dose~ of O.OOl - 5 ~/
kg/day, preferably 0~401 - 2.5 mg/kg~day, in particular
0.005 - 1.0 mg~kg/day. ~he do~e can al90 be increas~d in
aavere ca3es. How~vsr~ in many ca~es, low~r dose~ are
al~o sufficient. ~hese data rel~e to ~dul~s weighing
about 75 kg.
The invention ~dditionally e~braces the use of ~h~
compound~ according to the inv~ntion for the preparation
of pha~maceutical3 which are ~ployed ~or the ~reatment
and prophylaxi3 o~ the abovemen~ione~ me~abol~c dia-
order~.
The invent~on furthermore relates to pharmaceutical~
which contain ona or ~ore compound~ o~ the formllla.I
according to the inven~ion ~nd/or the phy~iologically
tolerated salts thereof.
The pharmaceuticals are prepared by proce~fies which are
known per se ~nd ~amiliar to tho~e skilled in the ~rt.
The pharmacologically active compounds (~ ~cti~e ~ub-
s~ance~ according to the invention are ffmployed a~
pharmaceutical~ either ax ~uch or, pre~erably, in com-
bination with ~uitable pharmaceutical ~uxiliaries or
- 9 - 2~206
excipients in the form of tablsts, coatsd tablet~,
cap~ules ~ ~UppoE~itorie~ ~ ~mulsions ~ su3pension~ or
solutions, in whi~h the content o~ ac~ive ~ aIIce~ i~ up
to abollt 9596, advantag20usly between 10 and 759~.
S ~:xample~ of suitablQ æuxill~Lrie~; or Q~ccipient~ for the
desired pharmaceutical formulation are, be~ides ~olvent~,
gel-formers, suppository ba~a~, t~blet auxiliarie~ ~nd
other act~ve E3ubstanae ~ehicle~, al~o ant~o~idan~,
dispersing agent~, 0mul~ifierE~, anti~oam z~5~ents" flavor
correctlves, pre~ervative~, ~olubilizers ~nd color~nt~.
The acti~e substance6 c:an be admini6tered sr~lly, paren-
ter~lly or rec~ally.
The a~tive compound3 are mixed with the additives ~uit-
able for this purpo~e, ~uch a8 excipients, Etabili~ers or
inert diluent~, ~nd conYerted by cu~tomary methods into
~uitab~e dosage forms ~uch a~ tablets, coated tablets,
hard gelatin cap~ules, aqueou~ alcoholic or oily suspen-
~ion~ or aqueou~ or oily ~olution~.
~ xamples of inert excipients which can be u~ed are gum
arabic, magnesia, ma~nesium ~arbona~e, potassium phos-
phate, lacto~e, gluco~e or starch, especially corn
starch. This preparation can be carried out both ~ dry
and as w~t ~ranules. Examples of ~uitable oily excipients
or ~ol~ent3 are qegetabla or animal 0~18 r BU~h a~ sun-
flower oil or fish liver oil.
For subcutaneous or intravenous administration, ~heactive compounds are converted in~o ~olution, su~pen~ion
or emulsion, if de~ired with the sub3tances suitable for
thi~ purpose, ~uch as ~olubilizers, emulsifier~ or other
auxiliaries. ~xamples of suitable ~olvents are phy~io-
logical saline or alcohols, for ex~mple ethanol, propan-
ol, glycerol~ a~ well as sugar solution~ ~uch as glucoQe
or mannitol solutions, or el~e a mi~ture of the various
solvent~ men~ioned.
lo- ~3~6
The invention 1~ explain2d in detail hereinaf~er by means
of axamples.
lr80r 1 S
bis-N,N~-(Metho~yathyl)amideofpyridine 2,4-dicarbosylic
aci~
~ON~ 2-C~2-~H3
N CffN~-CH2~C~2-OC~3
3 g of pyridine-2,4-dicarboxylic acid are introduced into
50 ~1 of toluene and 1 ml of D~F, ~nd 2.7 ~1 o ~hinnyl
chloride are added dropwi~e to the ~olut~o~. ~he ~ixt~re
i8 heated un~il no further evolutio~ of g~ i8 evid2nt
(about 2.5 h). The mixture i8 cooled, 5 ml of toluene are
di~illed out, and 4.6 ml of 2-methoxyethylamine and 5 ml
o~ trieth~lamine are added dropwi~e to the solution. ~he
~olution i~ ~t~rred ~t room temperature ~or 4 h and ~hQn
evaporated, the rQsidu2 i~ taken up in water and QX-
tracted 4 x with ~ethylene chlorlde. ~he ~o~bined orga~ic
pha~es are dried over m~gne3ium eulfate ~nd e~aporated.
The cruda produ~t i~ ~hromatographed on ~ilica gel
(solven~ ethyl acetate).
Nelting point: 42 44C
lH-~MR tCDCl3)s 6 = 1.2 (3H, tr); 3.3-3.8 (12H, gu. and
m); 7.9 (lH, m); 8.4-8.5 (1~, m3;
8.7-808 (1~, m);
U3~206
Precur30r 2 ~
bi~ -~, N ' ~ ydrc)xysthyl ) amld~e o f pyridi~e~2, 4 -
dicarboxylic acid
~:ONH- C~H2- ~2- ~
~ i
~:02~ 2- ~2- ~
0.5 g of bi~ ,N'-(2-methoxyethyl)am~de o~ pyridim3-2,4-
dicarboxylic ~cid (precur~or 1) i8 diB81:)1Ved ~n 10 ml of
dichlorome~hane and, at -78~C, boron tribromide ~11 ~1,
1 molar ~olution in dichlorometh~ne) ~3 added drop~ e.
After addition i~ co~plote, ~llow to reach room teDlper-
ature and then stir ~or 3 hour~. Pour in~o 100 ml of
10 ~atura~ed bicarbonate ~olution and ~actrwt 3 ae wi~h ~thyl
~cetate. The combined or~lanic 801~ent~ re dried with
magnesium sulfate ~nd e~aporated. The c:~ude produc~t i~
chromatographed on silica gel.
lY-~5R (CDCl3)s o ~ 1.5-2.2 (4LH, m); 3.4 (4H, m)7 3.6
(4H, m), 7.9-8.0 (lH, ~); 8.4-8.5
(lH, ~; 8.7-8.8 (lH, m)
~ca~pl~ 1
N,N'-Di(2-nitroxyethyl)amideofpyridine-2,4-dicarbo~ylic
ci~
.
CON~-CH2-CH2-`ON02
,. ~
N OONH-eX2-~H2~0NO~
1 g of di(2-hydroxyethyl)amide of pyridine-2,4-di-
carboxylic acid (precursor 2) i8 added at -10C to -5C
- 12 - 2~3~20~
to5ml of concentrated nitric acid. ~he mixture i8 al-
lowed to warm to 2C and then ~tirrad for 40 minutes. It
is pouxed into ice~w~ter and ~eutralized with sodium
carbonate. The ~olution $~ ex~racted three ~imes with
dichloromethane, and the organic pha~es are dried over
magnesium sulfata and evaporat~d. The rasidu~ cry~tal-
lizes from ether.
Yield: 750 mg
Melting points 85 - 88C