Note: Descriptions are shown in the official language in which they were submitted.
- .
2~3697~ -
., .
- 1
PS/5-17973/A
Microbicides
The present invention relates to novel N-(2~6-dinitro-3-chloro-4-trifluoromethylphenyl)-
4-amino-~fluoropyrimidine derivatives of formula I below. It relates also to thepreparation of those compounds and to agrochemical compositions that contain at least
one of those compounds as a tive ingredient. The invention relates also to the preparation
of the said compositions and to the use of the compounds or the compositions forcontrolling or preventing an attack on plants by phytopathogenic fungi.
' ,' . .-:
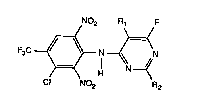
The compounds according to the invention have the general formula I :
N02 ~F
F3C ~ ,~ N N (I~
)=~ H
Cl No2 R2
wherem
R1 and R2 independently of each other are hydrogen, Cl-C4rL~c,vl or cyclopropyl; including
the acid addition salts of the compnunds of ~formula 1.
Dep*r~ing~on the number of carbon atoms indicated, alkyl shall be understood~as being,
for exampk,~the folbwing~straight-chain or branched groups: methyl, ethyl, propyl, butyl
and-also the isomers thereof,~for t,r~nple isoprnpyl,~isobutyl, sec-butyl or tert-butyl.
I}ie ca~ipnunds of formula I are solids at room t*~tnperatnre and are distinguished by very
valuable phytofungicidalproperties, They can therefore be used in the agricultural sector
or r*la~fields for cnntmlling phytopathogenic fnngi.
e inv*ntion relates~both to~the~free cortponnds of fo¢mula~I and to their addition salts
with inorganic and organic acids.;
. Y " . ; '
- . ,. , . : ., : .:; . : . . ., .
2036976
Salts according to the invention are especially addition salts witn, according ~o the
intended use, biocompatible inorganic or organic acids, for example hydrohalic acids, e.g.
hydrochloric, hydrobromic or hydriodic acid, and also sulfuric acid, phosphoric acid,
phosphorous acid, nitric acid, unsubstituted or halogenated fatty acids, such as acetic acid,
trichloroacetic acid and oxalic acid, or sulfonic acids, such as benzenesul~onic acid and
methanesulfonic acid, or alternatively addition salts with suitable salts, for example
magnesium chloride or calcium chloride.
The compounds of formula I are novel and as such represent a selection of products from
the general descriptions of European Patent Applications No. 139613, No. 248 348 and
No. 248 349. The compounds disclosed in the above-mentioned publications are described
as pesticides, in some cases also as fungicides, but those compounds do not always meet
the requirements madç of tnem in practice, especially when used against certain pests.
By contrast, the novel compounds of formula I prove to be effective especially against
specific genera of the following classes of fungus: Fungi imperfecti (e.g. Cercospora),
Basidiomycetes (e.g. Puccinia), Ascomycetes (e.g. Erysiphe and Venturia) and Oomycetes
(e.g. Plasmopara and Phytophthora). They therefore represent a valuable addition to the
compositions available in crop protection for controlling phytopathogenic fungi. For
practical field application purposes, they have, advantageously, curative, preventive and
also systemic properties and can be used for protecting numerous cultivated plants. With
the compounds of formula I it is possible to inhibit or des~oy the pests which occur on
plants or on parts of plants (fruit, blossoms, leaves, stems, tubers, roots) in different crops
of useful plants, while at the same time the parts of plants which grow later are also
protected from attack e.g. by phytopathogenic fungi. The compounds of formula I can also
be used as dressing agents for protecting seeds (fruits, tubers, grains) and plant cuttings
against fungus infections as well as against phytopathogenic fungi which occur in the soil.
The following compounds of formula I are preferred in respect of their mode of acdon:
N-(2',6'-dinitr~3'-chlor~4'-trifluoromethylphenyl)-4-arnino-(2,5-diethyl-6-fluoro)-
py~imidine;
N-(2',6'-dinitr~3'-chloro-4'-~ifluoromethylphenyl)-4-amin~(5-ethyl-6-fluoro)-
pyrimidine;
N-(2',6'-diniiro-3'-chloro-4'-trifluoromethylphenyl)-4-arnino-(2-ethyl-5-methyl-6-fluoro)-
pyrin~idine;
.. . ... .... .. . . . .. . . .
2~3697~
- 3 -
N-(2',6-dinitro-3'-chloro-4'-trifluoromethylphenyl)-4-amino-(2-cyclopropyl-6-fluoro)-
pyrimidine;
N-(2',6'-dinitro-3'-chloro-4'-trifluoromethylphenyl)-4-amino-(2-n-propyl-6-fluoro)-
pyrimidine;
N-(2',6'-dinitro-3'-chloro-4'-trifluoromethylphenyl)-4-amino-(2-isopropyl-6-fluoro)-
pyrimidine;
N-(2',6'-dinitro-3'-chloro-4'-~ifluoromethylphenyl)-4-amino-(5-ethyl-6-fluoro)-
pyrimidine;
N-(2',6'-dinitro-3'-chloro-4'-trifluoromethylphenyl)-4-amino-(2-cyclopropyl-6-fluoro)-
pyrimidine;
N-(2',6'-dinitro-3'-chloro-4'-trifluoromethylphenyl)-4-amino-(2,5-dimethyl-6-fluoro)- ~ .
pyrimidine;
N-(2',6'-dinitro-3'-chloro-4'-trifluoromethylphenyl)-4-amino-(2-methyl-5-n-propyl-6-
fluoro)-pyrimidine; .:
N-(2',6'-dinitro-3'-chloro-4-trifluoromethylphenyl)-4-amino-(2-methyl-5-isopropyl-6~ :
fluoro)-pyrimidine.
Of those compounds, the following compound is to be regarded as especially ::
advantageous: .~
N-(2',6'-dinitro-3'-chloro-4'-trifluoromethylphenyl)-4-amino-(2,5-diethyl-6-fluoro)-::
pyrimidine.
': .
The compounds of formllla I are prepared by reacting:
a compound of formula II
.
N2
3C~--Z (II)
Cl NO2
with a pyrimidine derivativc of fonnula III :
.
.. , -. .. .... : ,.; ~ . , . ;. . .. . . . . .. . ~ .. - ......... ..
.
203697~
R j=~F
Y ~ N ~III),
N~
F~;2 ,
wherein the substituents Rl and R2 are as defined under formula I and Z and Y are NH2,
halogen or SO2R in which R is Cl-C4aLIcyl or aryl, with the proviso that, when Z is
halogen or S02R, Y is NH2, and when Z is NH2, Y is halogen or S02R, if desired in
solvents that are inert towards the reactants, at temperatures of from -80C to +150C,
preferably at from -50C to ~30C, and, if desired, advantageously in the presence of
acid-binding agents.
Suitable acid-binding agents are organic and inorganic bases, for example tertiary amines, ~ -
such as trialkylamines (trimethylamine, ~iethylamine, tripropylamine, etc.), pyridine and
pyridine bases (4-dimethylaminopyridine, 4-pyrrolidylaminopylidine, etc.), oxides and
hydroxides, carbonates and hydrogen carbonates of aLlcali metals and alkaline earth metals,
and also alkali metal acetates.
Examples of suitable inert solvents or diluents are aliphatic and aromatic hydrocarbons,
such as benzene, toluene, xylenes, petroleum ether; halogenated hydrocarbons, such as
chlorobenæne, methylene chloride, ethylene chloride, chloroform, carbon tetrachloride, ~ -
tetrachloroethylene; ethers and ethereal compow~ds, such as diaLlcyl ethers (diethyl ether,
diisopropyl ether, tert-butyl methyl ether, etc.), anisole, dioxane, tetrahydrofuran; nitriles,
such as acetonitrile, propionitrile; N,N-diaL~ylated amides, such as dirnethylfolmamide;
dimedlyl sulfoxidç, ketones, such~as acetone, diethyl ketone, methyl ethyl ketone, and
mixtures of such solvents with one another.
The reaction of the compound of forrnula (Il) with the compound of formula (III) may also
be~camed out in an aqueous two-phase system in accordance with the generally known
principle of ~phase t~nsfer catalysis. ~ ~
The~following solvents,~for example, are suitable as the organic, water-immiscible phase:
aliphatic and aromatic hydrocarbons, such as pentane, hexane, cyclohexane, petroleum
ethe~r,~lign~in, benzene, toluene, xylenes, etc., halogenated hydrocarbons, such as dichloro-
methane, chloroform, carbon tetrachloride, ethylene dichloride, 1,2-dichloroethane,
~03697~
tetrachloroethylene, etc., or aliphatic ethers, such as diethyl ether, diisopropyl ether,
tert-butyl methyl ether, etc. Examples of suitable phase transfer catalysts are:tetraalkylammonium halide, tetraalkylammonium hydrogen sulfates or tetraalkylam-monium hydroxide, such as tetrabu~ylammonium chloride, brornide or iodide; ~riethyl-
benzylarnmonium chloride or bromide; cetyltrimethylarnmonium chloride, brornide or
iodide, etc. Phosphonium salts are also suitable phase transfer catalysts. The reaction tem-
peratures are generally from -30 to 130C, or the boiling point of the solvent or solvent
mixture.
Some of the compounds of formulae II and III are known, or they can be prepared in
accordance with known processes [Ser. Khim. Naulc. 1973 (6) 81-85 (CA: 80/~9913),
Yakugaku Zasshi 87 (11), 1315-1321 (1967) (CA: 68/114540) and D.J. Brown; The
Chemistry of Heterocyclic Compounds, The Pyrimidines Supplement II 1985,167].
The invention also relates to compositions that contain compounds of formula I as aetive
ingredient, especially crop protection compositions, and to their use in the agricultural
sector or related fields.
The present invention further embraces the preparation of those compositions, which
comprises homogeneously mixing the active ingredient with one or more compounds or
groups of compounds described herein. The invention furthermore relates to a method of
treating plants, which comprises applying thereto the novel compounds of forrnula I or the
novel compositions.
Target crops to be protected within the scope of the present invention comprise e.g. the
following species of plants:
cereals (wheat, barley, rye, oats, rice, maize, sorghum and related crops); beet (sugar beet
and fodder beet); pomes, drupes and soft frui~ (apples, pears, plums, peaches, almonds,
cherries, strawberries, raspberries and blackberries); leguminous plants (beans, lentils,
peas, soybeans), oil plants (rape, mustard, poppy, olives, sunflowers, coconut, castor oil
plants, cocoa beans, groundnuts); cucumber plants (cucumber, marrows, melons); fibre
plants (cotton, flax, hemp, jute); citrus fruit (oranges, lemons, grapefruit, mandarins);
vegetables (spinach, lettuce, asparagus, cabbages, carrots, onions, tomatoes, potatoes,
papr~ca); lauraceae (avocados, cinnamon, camphor), or plants such as tobacco, mlts,
coffee, sugar cane, tea, pepper, vines, hops, bananas and natural rubber plants, as well as
ornamentals.
~.
: ' ~ , ' ~ ' '`. .~' . . : ' .` ' . '
; .
.
203697~
The compounds of forrnula I are normally applied in the form of compositions and can be
applied to the crop area or plant to be treated, simultaneously or in succession, with further
compounds. These compounds can be fertilisers or micronutrient donors or other prepara-
tions that influence plant growth. They can also be selecdve herbicides, insecticides,
fungicides, bactericides, nematicides, molluscicides or rnixtures of several of these
preparations, if desired together with further carners, surfactants or other
application-promoting adjuvants customarily employed in formulation technology.
Suitable carriers and adjuvants can be solid or liquid and correspond to the substances
ordinarily employed in formulation technology, e.g. natural or regenerated mineral sub-
stances, solvents, dispersants, wetting agents, tackifiers, thickeners, binders or fertilisers.
A preferred method of applying a compound of formula I, or an agrochernical composition
which contains at least one of said compounds, is foliar application. The number of
applications and the rate of application depend on the risk of infestation by the
corresponding pathogen. However, the compounds of formula I can also penetrate the
plant through the roots via the soil (systemic action) if the locus of the plant is
impregnated with a liquid formulation, or if the compounds are applied in solid form to the
soil, e.g. in granular form (soil applicadon). In paddy rice crops, such granules may be
applied in metered amounts to the flooded rice ~leld. The compounds of fo~mula I may,
however, also be applied to seeds (coating) either by impregnating the seeds with a liquid
formulation containing a compound of formula I, or by coating them with a solid
formulation.
,
The compounds of fonnula I are used in unmodified form or, preferably, together with the
adjuvants conventionally employed in formulation technology, and are for this purpose
advantageously formulated in known manner e.g. into emulsifiable concentrates, coatable
pastes, directly sprayable or dilutable solutions, dilute emulsions, wettable powders,
soluble powders, dusts, granules, and also encapsulations in e.g. polymer substances. As
with the nature of the compositions, the methods of application, such as spraying,
atomising, dustdng, scattering, coadng or pouring, are chosen in accordance with the
intended objecdves and the prevailing circumstances. Advantageous rates of application
are normally from 10 g to 5 kg of active ingredient (a.i.) per hectare, preferably from 50 g
to I kg ai.~a, most preferably from 200 g to 600 g a.i./ha.
20~69~
The formulations, i.e. ~e compositions, preparations or mixtures containing.the compound
(active ingredient) of formula I and, where appropriate, a solid or liquid adjuvant, are
prepared in known manner, e.g. by homogeneously mixing and/or grinding the active
ingredients with extenders, e.g. solvents, solid camers and, where appropriate,
surface-active compounds (surfactants).
Suitable solvents are: aromadc hydrocarbons, preferably the fractions containing 8 to 12
carbon atoms, e.g. xylene rnixtures or substituted naphthalenes, phthalates such as dibutyl
phthalate or dioctyl phthalate, aliphatic hydrocarbons such as cyclohexane or paraffins,
alcohols and glycols and their ethers and esters, such as ethanol, ethylene glycol, ethylene
glycol monomethyl or monoeth yl ether, ketones such as cyclohexanone, strongly polar
solvents such as N-methyl-2-pyrrolidone, dimethyl sulfoxide or dimethylformamide, as
well as vegetable oils or epoxidised vegetable oils, such as epoxidised coconut oil or
soybean oil; or water.
The solid carriers used, e.g. for dusts and dispersible powders, are normally natural
minera1 fillers such as calcite, talcum, kaolin, montmorillonite or attapulgite. In order to
improve the physical properties it is also possible to add highly dispersed silicic acid or
highly dispersed absorbent polymers. Suitable granulated adsorptive carriers are porous
types, for example pumice, broken bdck, sepiolite or bentonite; and suitable nonsorbent
carriers are, for example, calcite or sand. In addition, a great number of pregranulated
materials of inorganic nature can be used, e.g. especially dolomite or pulverised plant
residues.
Depending on the nature of the compound of formula I to be formulated, suitable
surface-active compounds are non-ionic, cationic and/or~anionic surfactants having good
emulsifying, dispersing and wefflng p~dea The term "surfactants" will also be
understood as comprising mixtures of surfactants.
- ~
Bodl so called water-soluble soaps and also water-soluble synthedc surface-active
compounds are:suitable anionic:surfactants.
Suitable~ soaps are the alkali metal salts, aLlcaline earth metal salts or unsubstituted or
substituted ammonium salts of higher fatty acids (ClO-C22), e.g. the sodium orpotassium
salts~of oleic or steadc acid or of natural fatty acid mixtures which can be obtained e.g.
from coconut oil or tallow oil. Mention may a1so be made of fatty acid methyllaudn salts.
2036975
- 8 -
More f~equently, however, so-called synthetic surfactants are used, especially alkane-
sulfonates, fatty alcohol sulfates, sulfonated benzimidazole derivatives or aLIcylsulfonates.
The fatty alcohol sulfonates or sulfates are usually in the form of alkali metal salts,
aL~aline earth metal salts or unsubstituted or substituted ammonium salts and contain a
C8-C22aLlcyl radical, which also includes the aLlcyl moiety of acyl radicals, e.g. the sodium
or calcium salt of lignosulfonic acid, of dodecyl sulfate or of a mixture of fatty alcohol
sulfates obtained from natural fatty acids. These compounds also comprise the salts of
sul~ated and sulfonated fatty alcohoVethylene oxide adducts. The sulfonated benzimida-
zole derivatives preferably contain 2 sulfonic acid groups and one fatty acid radical
containing 8 to 22 carbon atoms. Examples of aLlcylarylsulfonates are the sodium, calcium
or triethanolamine salts of dodecylbenzenesulfonic acid, dibutylnaphthalenesulfonic acid,
or of a condensate of naphthalenesulfonic acid and formaldehyde.
Also suitable are colTesponding phosphates, e.g. salts of the phosphoric acid ester of an
adduct of p-nonylphenol with 4 to 14 mol of ethylene oxide. ~
. ,:
Non-ionic surfactants are preferably polyglycol ether derivatives of aliphatic or
cycloaliphatic alcohols, saturated or unsaturated fatty acids and aL1cylphenols, said
derivatives containing 3 to 30 glycol ether groups and 8 to 20 carbon atoms in the
(aliphatic) hydrocarbon moiety and 6 to 18 carbon atorns in the aL~cyl moiety of the
aLtcylphenols.
Further suitable non-ionic surfactants are the water-soluble adducts of polyethylene oxide
with polypropylene glycol, ethylenediaminopolypropylene glycol and allylpolypropylene
glycol containing 1 to 10 carbon atoms in the aLIcyl chain, which adducts contain 20 to 250
ethylene glycol ether groups and 10 to 100 propylene glycol ether groups. These com-
pounds usually contain 1 to 5 ethylene glycol units per propylene glycol unit. ' `~
Represen~ive examples of non-ionic surfactants are nonylphenolpolyethoxyethanols,
castor oil polyglycal ethers, polypropylene/polyethylene oxide adducts, tributylphenoxy-
polyethyleneethanol, polyethylene glycol and octylphenoxypolyethoxyethanol.
Fatty acid esters of polyoxyethylene sorbitan, e.g. polyoxyethylene sorbitan trioleate, are
also suitable non-ionic surfactants.
: ~ . . -
. _ .. , .. ... ,, ~ .. , . ~ , . . .. . . .. . . . . .
203697~
Cationic surfactants are preferably quaternary ammonium salts which contain, as
N-subsdtuent, at least one C8-C22aLltyl radical and, as further substituents, unsubsdtuted or
halogenated lower alkyl, benzyl or hydroxy-lower alkyl radicals. The salts are preferably
in the form of halides, methyl sulfates or ethyl sulfates, e.g. stearyltrimethylammonium
chloride or benzyldi(2-chloroethyl)ammonium bromide.
Further surfactants customarily employed in formuladon technology are known eo the
person skilled in the art or can be taken from the relevant specialist literature.
The agrochemical compositions usually contain 0.1 to 99 %, preferably 0.1 to 95 %, of a
compound of formula I, 99.9 to 1 %, preferably 99.9 to 5 %, of a solid or liquid adjuvant,
and 0 to 25 %, preferably 0.1 to 25 %, of a surfactant.
,: -
Whereas commercial products will preferably be formulated as concentrates, the end userwill normally employ dilute formulations.
The composidons may also contain further auxiliaries such as stabilisers, antifoams,
viscosity regulators, binders, tackifiers &S well as fertilisers or other acdve ingredients for
obtaining special effects.
,
The following Examples serve to illustrate the invention in greater detail, but do not
consdtute a limitation thereo
: (,
1. Prepara ion Examples:
l.l Preparadon of N-(2',6-dinitro-3'-chloro-4'-trifluoromethYlphenvl)-4-amin~
(2.5~di~divl-~fluoro)-pvrimidine (comp. nQ 3)
A solution of 7.2 g of potassium tert-butoxide in 50 ml of tetrahydrofuran is added
dropwise at ~5C, within a penod of half an hour, to a suspension of 5.1 g of 2,5-diethyl-
~amino-~fluoropyrimidine and 10.1 g of 1,3-dinitro-2,4-dichloro-5-trifluoromethyl-
benzene in 60 mI of ~tetrahydrofuran. When the addition is complete, the tempera ture of
the ~cQlouled reac~ion mixture i9 slowly allowu! to rise to room temperature. After
sti~ing for l2 hours at room temperature, the solvent is evaporated off under reduced
- pressure and the residue is taken up in 100 ml of ethyl acetate. The organic phase is
washed;with 2 x 150 ml of water, vith 3 x 150 ml of 10 % acetdc acid and with a further
.. , . . - ~:,.. .. , . . . , ,. . " , , .,,, :, .
203697~
- 10-
2 x 100 ml of water, dried over sodium sulfate and filteled, and the solvent is evaporated - .
off in vacuo. The residue is purified by chromatography over a silica gel column using
hexane/ethyl acetate (4:1), yielding the title compound in the form of white crystals; m.p.
111-112C.
The following compounds can be prepared analogously.
: . - .
~ ~ ';''''.
~ '
'
203697~
- 11
Table 1:
NO2 R, F
F3C ~ N ~(N
N ~
Cl N2 R2
_ _ .
No. Rl R2 physical data
1 H CH3 ~ -
2 CH3 H
3 CH2CH3 ~H2CH3 m.p. 111-112C
4 ~1 <I ` :
S C4~-n CtCH3)4 ~ .
6 CH(CH3)CH2cH3 C3H7-i m.p. 148-150C
7 CH3 CH3
8 H C4Hg-n
9 CH2CH3 H m.p. 79-80C
10 ~ CH2CH3 :: :
11 CH2CH2CH3 . ,; .
~12 C4~-t C4Hg-n
3 ~3H7-l ~ CH(CH3)C~I2CH
14; CH2CH3 C4H9-n
15~ H: H
16 CH3 ~ CH2CH3 : m.p. 98-99C
~17~ : ~ : H~
18 ~ c4H9-n m.p. 69-71C
~m.p. 13~138C
CH3 ~ C3~-n m.p. 82-86C
2036976
- 12-
Table 1: (continuation)
._
No. Rl R2 physical data
_
21 ~1 (~H3 :
22 CH2CH3 C3H7-i
23 C4Hg-t CH(CH3)CH2CH3
24 CH2CH2CH3 CH2CH3 m.p. 79-82C
25 C3H7-i
26 CH3 C3H7-i
27 H C4Hg-t
28 CH2CH3 C3H7~n m.p. 83-85C
29 <I C4Hg-t
30 CH(CH3)CH2CH3 C3H7-n
31 C3H7-i CH3 m.p. 98-100C ::
32 C4H9-n H - .
33 CH3 C4H9-n
34 H CH3
35 CH2CH3 ~<¦ m.p. 132-134C .s. . .
36 --~¦ C3H7~n
37 CII2CH2CH3 <
38 C4~-t C4Hg-t :
~39 CH2CH3 CH3 m.p. 118-121C :,:
40 CH3 l CH2CH(CH3)2
41 H C3H7~n m.p. 130-132C
42 ~ C3H7-i
43 CH(CH3)CH2CH3 ¦
44 C4H9~ CH(CH3)CH2CH3 __
~: , . .
: ~ :: .
2036976
Table 1: (continuation)
_
No. Rl R2 physical data
45 CH2CH3 CH2CH(CH3)2
46 H C3H7-i m.p. 128-129C
47 CH3 <I m.p. 111-113C
48 CH2CH2CH3 C3H7-n
49 <1 C4Hg-n
50 C4Hg-t CH2CH(CH3)2
51 C3H7-i C4Hg-t
52 C4Hg-n
53 CH3 CH(CH3)CH2cH3 .:
54 H CH2CH(CH3)2
55 CH2CH3 C(CH3)4 :
56 CH(CH3)C~I2CH3 CH3
57 <I CH2CH(CH3)2
58 CH2CH2CH3 CH(CH3)2
59 C4H9-t C3H7~n
60 C3H7-i CH2CH(CH3)2
61 CH2CH(CH3)2 H .
62 H CH(CH3)CH2CH3
63 CH3 C4Hg-n
64 C4H9-n CH2CH(CH3)2
65 CH2C~I(CH3)2 CH2C~I3 . .:
66 CH2CH3 CH~CH3)CH2CH3
67 C4~-t C3H7-i
68 CH2CH2CH3 C4Hg-n .
~ . .
: :
;: ' ' .
.. ., , ,,- ~ , ... ,, , ,, ., . , . :. .
2036976 -
- 14-
`
Table 1: (continuation)
No. physical data
69 C3H7-i C4Hg-n
70 ~1 CH(CH3)CH2CH3
71 CH(CH3)CH2CH3 <
72 C4Hg-t ~
73 CH2CH2CH3 CH3 m.p. 95-97C - . : .
74 CH(CH3)CH2C~3 C4Hg-n
75 C4H9-n C3H7-i : . . .
76 C3H7-i C3H7-i
77 C4Hg-t CH3
78 CH2CH(CH3~2 C3H7~n .::
79 CH(CH3)CH2CH3 CH(CH3)CH2CH3
80 C4Hg-n - C3Hrn
81 CH2CH(CH3)2 C3H7-i : .
82 CH2CH2CH3 CH2CH(CH3)2 .
83 C3H7-i C3H7-n
84 CH2CH(CH3)2 C4H~-n
85~ C4~-t CH2CH3
86 CH2CH(CH3)2 CH2CH(CH3)2 :
87 CH2CH(CH3)2 C4Hg-t
88 CH2CH2CH3 CH(C H3)CH2CH3
89 C4H9-n C~I3 m.p. 130-132C
90 CH(CH3)CH2CH3 C4Hg-t
91 CH2CH(CH3)2 --<¦
92 C3H7-i C~2CH3 . __ - ~`
"" ;-
, - . ; , , , , , . . , .~ .,. : .
2~36976
- 15-
Table 1: (continuation)
No. R2 physical data
93 CH2CH(CH3)2 CH(CH3)~2CH3
94 C4H9-t H
95 CH2CH2CH3 C4H9-t
96 CH(cH3)cH2cH3 CH2CH(CH3)2
97 C4H9-n CH2CH3 :
98 CH(CH3)CH2CH3 CH2CH3 . .
99 C3H7-i H
100 CH2CH(CH3)2 3
2. Formulation Examples for solid active ingredients of formula I (throughout, percenta~es
are bY wei~ht)
2.1. Wettable powders a) b) c)
a compound of Table 1 25 % 50 % 75 %
sodium lignosulfonate 5 % 5 %
sodiumlaurylsulfate 3 % - 5 %
sodium diisobutylnaphthalene-
sulfonate - 6 % 10 %
octylphenol polyethylene glycol
ether (7-8 mol of ethylene oxide) - 2 %
highly dispersed silicic acid 5 % 10 % 10 %
kaolin 62 % 27 % -
~: The active ingredient is thoroughly mixed with the adjuvants and the mixture is
thoroughly ground:in a suitable mill, affording wettable powders which can be diluted
with water to give suspensions of the desired concentration.
2.2. Emulsifiable concentrate
a compound of Table 1 10 %
2~3697~
- 16-
octylphenol polyethylene glycol
ether (4-5 mol of ethylene oxide) 3 %
calciumdodecylbenzenesulfonate 3 % :
castor oil polyglycol ether
(35 mol of ethylene oxide) 4 %
cyclohexanone 30 %
xylene mixture S0 % ~ '.
Emulsions of any required concentration can be obtained from this concentrate by dilution
with water.
2.3. Dusts a) b)
a compound of Table 1 5 % 8 %
talcum 9S %
kaolin - 92 %
Ready-for-use dusts are obtained by mixing the active ingredient with the callier and
grinding the mixture in a suitable mill.
2.4. Extruder granules
a compound of Table 1 10 %
sodium lignosulfonate 2 %
carboxymethylcellulose 1 %
kaolin 87 %
-
The active ingredient is mixed and ground with the adjuvants, and the n~ixture is subse-
quendy moistened with water. The mixture is extruded and then dried in a stream of air.
~, .
2.5. Coate granules
acom~ ndofTable l 3 %
polyethylene glycol (mol. wt. 200) 3 %
kaolin ~ 94 %
The finely ground active ingredient is uniformly applied, in a mixer, to the kaolin
moistened wlth polyethylene glycol. Non-dusty coated granules are obtained in this
manner.
~:~
:
'.,, ., -: . `.'' :~ ,` :: . ,'`, . :.' ' , , :. . , ' . : ' - ... , ' .
. : ~ '~ , ' ' . ' ' ,, . ~,' ' ' ' . ' ' :
203697~
2.6. Suspension concentrate
a compound of Table 1 40 %
ethylene glycol 10 %
nonylphenol polyethylene glycol
ether (15 mol of ethylene oxide) 6 %
sodium lignosulfonate 10 %
carboxymethylceUulose 1 %
37 % aqueous formaldehyde solution 0.2 %
silicone oil in the form of a 75 %
aqueous emulsion 0.8 %
water 32 %
The finely ground active ingredient is intimately mixed with the adjuvants, giving a
suspension concentrate from which suspensions of any desired concentration can be
obtained by dilution with water.
3. Biolo,eical Examples
Example 3.1: Action a~ainst Puccinia graminis on wheat
a) Residual-protecdve action
Wheat plants are sprayed 6 days after sowing with a spray mixture (0.02 % activeingredient) prepared from a wettable powder formulation of the test compound. After
24 hours the treated plants are infected with a uredospore suspension of the fungus. The
infected plants are incubated for 48 hours at 95-100 % relative humidity and about 20C
and then stood in a greenhouse at about 22C Evaluation of rust pustule development is
made 12 days after infection.
b) Svstemic acdon
Wheat~plants are watered 5 days after sowing with a spray mixture (0.006 % active
ingredient, based on the volume of the soil) prepared from a wettable powder formulation
of ~the~ test compound. After 48~ hours the treated plants are infected with a uredospore
suspension of the fungus. The infected plants are then incubated for 48 hours at 95-100 %
relative humidity and about 20C and then stood in a greenhouse at about 22C. Evalua-
tion of rusi~pustule development is made 12 days after infection.
,., ,. , . .. . ~ . . . , : . . . .... . . .. . .
203~7~
- 1 8 -
Compounds of Table 1 exhibit very good activity against Puccinia fungi. Compoundno. 46, inter alia, inhibits Puccinia infestation to 0 to 5 %. On the other hand, Puccinia
infestation is 100 % on untreated and infected control plants.
Example 3.2: Acdon a~ainst Cercospora arachidicola on ~roundnut plants
Residual-protective acdon
Groundnut plants 10-15 cm in height are sprayed with a spray mixture (û.006 % active
ingredient) prepared from a wettable powder formulation of the test compound, and
infected 48 hours later with a conidia suspension of the fungus. The infected plants are ~: -
incubated for 72 hours at about 21C and high humidity and then stood in a greenhouse
until the typical leaf specks occur. Evaluation of the fungicidal action is made 12 days
after infection and is based on the number and size of the specks.
Compared with untreated and infected control plants (number and size of the specks =
100 %), Cercospora infestation on groundnut plants treated with compounds of Table 1 is -
substantially reduced. Thus compounds nos. 3, 9, 16, 19 and 46 inhibit the occurrence of
specks almost completely (0 to 10 %) in the above test.
Example 3.3: Action a~ainst Ervsiphae ~aminis on barley
a) Residual-protective acdon
Barley plants about 8 cm in height are sprayed with a spray mixture (0.02 % active
ingredient) prepared from a wettable powder formulation of the test compound. The
treated plants are dusted with conidia of the fungus after 3 to 4 hours. The infected barley
plants are stood in a greenhouse at about 22C. The fungus infestation is evaluated after
10 days.
b) Svstemic action
A~spray mixture (0.006 % active ingredient, based on the volume of the soil) prepared
; from~a wettable powder formulation of the test compound is used to water barley plants
about 8 cm in height. Care is taken that the spray mixture does not come into contact with
the parts of the~plants above the soil. The treated plants are dusted 48 hours later with
conidia of the fungus. The infected barley plants are then stood in a greenhouse at about
22C and evaluadon of fungus infestation is made after 10 days.
Compounds of Table l exhibit good activity against Erysiphae fungi. Thus compounds
nos. 3, 16 and 46 inhibit fungus infestation on barley to less than 10 %. On the other hand,
:.. . .. ,, ,. . . . . .. ~ ,. :
203697~ :
- 19-
Erysiphae infestation is 100 % on untreated and infected control plants.
Example 3.4: Residual-protective action against Venturia inaequalis on apple shoots
Apple cuttings with 10-20 cm long fresh shoots are sprayed with a spray mixture (0.006 %
a.i.) prepared from a wettable powder formulation of the test compound. The treated plants
are infected 24 hours later with a conidia suspension of the fungus. The plants are then
incubated for 5 days at 90-100 % relative humidity and stood in a greenhouse for a further
10 days at 20-24C. Scab infestation is evaluated 15 days after infection. With compounds
of Table 1, infestation is substantially reduced. Thus compounds nos. 3, 9, 16, 19, 41 and
46 inhibit Venturia infestation to 5 %. On the other hand, infestation is 100 % on untreated
and infected control shoots.
,
Example 3.5: Action against Botrvtis cinerea on beans -
Residual-protective action
Bean plants about 10 cm in height are sprayed with a spray mixture (0.02 % active
ingredient) prepared from a wettable powder formulation of the test compound. After
48 hours the treated plants are infected with a conidia suspension of the fungus. The
infected plants are incubated for 3 days at 95-100 % relative humidity and 21C and then
evaluated for fungus infestation. Compounds of Table 1 inhibit fungus infestation very
strongly. On the other hand, Botrytis infestation is 100 % on untreated and infected bean
plants.
....
Example 3.6: kcdon against Phytophthora infestans on tomato plants
a) Residual~rotective action
After a cultivation period of 3 weeks, tomato plants are sprayed with a spray mixture
(0.02 % active ingredient) prepared from a wettable powder formulation of the test
compound. After 24 hours the treated plants are infected with a sporangia suspension of
the fungus. The infected plants are then incubated for S days at 90-100 % relative
humidity and 20C and then evaluated for fungus infestation.
:
b) Svstemic action
After a~cultivation period of 3 weeks, a spray mi?cture (0.006 % active ingredient, based on
the volume of the soil) prepared from a wettable powder formulation of the test compound
is used to water tomato plants. Care is taken that the spray mixture does not come into
contact with the parts of the plants above the soil. The treated plants are infected 48 hours
later with a sporangia suspension of the fungus. The infected plants are then incubated for
.. . . . . . . . . . .
203~976
- 20-
5 days at 90-100 % relative humidity and 20C and then evaluated for fungus infestation.
Compounds of Table 1 exhibit very good activity against Phytophthora in the above tests.
Compared with untreated and infected control plants in which infestation is 100 %,
compounds nos. 3, 9, 19, 41 and 49 inhibit fungus infestation almost completely (0 to
10%),
Example 3.7: Action a~ainst Plasmopara viticola on vines
a) Residual-protective action
Vine seedlings in the 4-5 leaf stage are sprayed with a spray mixture (0.02 % a.i.) prepared
from a wettable powder formulation of the test compound. After 24 hours the treated
plants are infected with a sporangia suspension of the fungus. Fungus infestation is
evaluated after incubation for 6 days at 95-100 % relative humidity and 20C.
b) Residual-curative action
Vine seedlings in the 4-5 leaf stage are infected with a sporangia suspension of the fungus.
After incubation for 24 hours in a humidity chamber at 95-100 % relative humidity and
20C, the infected plants are dried and sprayed with a spray rnixture (0.006 % active
ingredient) prepared from a wettable powder formulation of the test compound. After the
spray coadng has dried, the ~eated plants are again placed in the humidity chamber.
Evaluation oifungus infestation is made 6 days after infection. ~-
Compounds of Table 1 exhibit very good activi~ against Plasmopara viticola on vines; in
particular, compounds nos. 9, 19, 41 and 46 inhibit fungus infestation completely (0 to
5- ~ ;
. ...
., : .. ,, ~ . . ~ . , . , . . , , - . . . . . , - . ,, . . ... .,,, . - ,