Note: Descriptions are shown in the official language in which they were submitted.
Mo3548
LeA 27,610
A PROCESS FOR THE PRODUCTION OF
N~N'-BIS-~3-AMINOPHENYL~-UREAS
BACKGROUND OF THE INYEN~ION
Field of the Invention : This ~nvention relates to a
simple prooess for the product;on of
N,N'-bis-(3-aminophenyl)-ureas which is based on the reaction
of alkyl-substituted m-phenylenediamines with urea in water as
solvent. More specifically, the invention relates to the
process for the product~on of N,N'-~is-(3-aminophenyl)-ureas
corresponding to general formula (I3 which may optionally
contain small quantities of oligomeric ureas corresponding to
general for~ula (II~
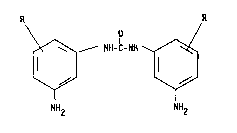
R R
\ O
~ NH-C-NH
NH2 NH2
R
/ NH-c-NH ~ [ NH-C-NH \ ~ ~NH2
NH2 R P~
_ _ n
(II)
wherein R is a linear or branched alkyl group containing 1 to 6
carbon atoms which may be ln the 2-, 4- and/or 6-positio...
Le A 27 610
,.,1... ~
~ 3
-2-
Brief Descr;ption of the Prior Art : It is known that
diaminodiphenyl ureas can be synthesked by phosgenation of
corresponding nitroanilines to form dinitrodiphenyl urea and
subsequent catalytic reduction to ~orm the diam;ne, cf. for
example the synthesis of III described by I.L. Khemel nitskaya
et al in ~h. Obsh. Khim 30 (2) (1960), page 6025 and the
synthesis of the N,N'-bis-~5-amino-2-methylphenyl)-urea IV
described by W.R. Turner and L.M. Werbel in J. Med. Chem. 28
(1985), page 1738.
p
~ NH-C-NH ~
H3C ~ I CH3 (III)
-,
NH2 NH2
CH3 CH3
NH C^NH ~
(IV)
~H2 N~2
Apart from the generally poor yields of the synthesis, the
main disadvantage thereof lies in the reduction step which is
~ost-intensive.
For the production of dia~inodiphenyl ureas such as
N,N'-bis-~4-amino-phenyl~-urea, another two-step synthesis is
the reaction of N-acetyl-p-phenylenediamine with urea, followed
by saponification of the protec$ive acetyl group, which is
described by H. Schiff and A. Ostrogovick in Liebigs Ann., 293,
Mo3548
r J; 1"' '~ 'J
-3-
18g6, pages 371 et seq. The disadvantage of this synthesis is
that monoacetylated diam;nes, which are generally not easy to
produce, have to be used as starting components.
Special p-phenylenediamines9 in which the reactivity of an
NH2 group is greatly reduced by suitable o-substituents, can be
directly reacted with phssgene to form the corresponding
aminocarbanilides. Suitable diamines are, for example,
~,S-diaminosulfonic acid (DRP 140613, Frl. 11, 1292) or
2,6-dichloro-p-phenylenediamine (~RP 268658, Frl. 11 164) Under
the conditions in question, other phenylenediamines almost
exclusively yield polyureas which are totally unsuitable as
reactive chain-extending agents.
US-PS 1,617,847 relates to the production of N,N'-bis-
(4-aminophenyl~-ureas by re~ction of p-phenylenediamine and
alkyl-substituted p phenylened;amines with urea in bulk or in
inert solvents, such as for example o-dichlorobenzene.
US-PS 2,503,797 des~ribes reaction of p-phenylenediamines
with urea in a~ueous solution However, it is specifically
pointed out and demonstrated by examples therein that high
yields of the corresponding low-oligomer m-phenylenediamines
are only obtained when 4 equivalents sulfuric acid are added.
For working up, the sulfuric acid salt of the urea which is
precipitated has to be converted with BaC12 into the
corresponding chlor~de from which the free base can then be
obtained.
To sum up, it may be said that all hitherto known
processes are either expensi~e or are only suitable for special
diamines, or only lead to the desired low molecular weight
ureas with p-substituted phenylenediamines as starting
compounds.
The problem addressed by the present invention was to
provide a simple synth~sis for ureas based on ortho-alkyl
substituted m-phenylenediamines.
Mo3548
SUMMARY OF_THE INVENTION
In accordance with the foregoing, the present
invention enco~passes a process for the production of an
N,N'-bis-(3-aminophenyl)-urea correspondir,g to yeneral formula
(I)
R NII-C-NH
NH2 NH2
wherein
R is a linear or branched alkyl group containing 1 to 6 carbon
atoms which is in the 2-, 4- and/or 6i-position,
comprising reacting a corresponding phenylenediamine with urea
in a molar ratio greater than 2:1, in water as a solvent.
The diaminodiphenyl ureas obtained by the process
according to the invention may be used in solid or dissolved
forms as chain-extending agents for the production of
polyurethane polyurea elastomers or pure polyurea elastomers.
The diaminodiphenyl ureas according to the inYention may also
be used, ~or example, as coupling components for diazo dyes, as
a catalyst ~or epo~y and phenolic resins and for other
reactions known per se involv;ng amines, such as amide or imide
formation and the like.
Two aspects of the invention are particularly ~ignifican~.
The process gives ureas of low molecular weight which are
suitable as chain-extending agents. Also, the percentage
content of monomeric starting amine in the ureas is reduced as
far as possible in order to eliminate any risk to health in the
handling of the product and the well-known adverse effect of
Mo3548
7 ~
free, aromatic low molecular weight-mines on the light
stability and color stability of the plastics produced
therewith.
~ETAILED DESCRIPTION OF THE INVENTION
It was surprising to find that, under the special reaction
conditions descr;bed in deta;l hereinafter, m-phenylenediamines
can be reacted with urea in aqueous solution to form good to
very good yields of low-oligomer diamincdiphenyl ureas. More
specifically, it was surprising to find that the reaction of
ortho-alkyl substituted m-phenylenedtamines with urea in water
as solvent, ln a molar ratio of phenylenediamine to urea
greater than 2:1 solves the problems set forth above.
Accordingly, the present ;nvention relates to a process
for the production of N,N'-bis-(3-aminophenyl)-ureas
corresponding to general formula tI)
R R
~ NH-C-NH
NH2 N~l2
in which
R is a linear or branched alkyl group containing 1 to 6 carbon
atoms which may be in the 2-, 4- and/or 6-positinn,
characterized 1n that the corresponding phenylenediamines are
reacted with urea in a molar ratio greater than ~:1 in water as
solvent.
The process of the invention is described in detail as
~ollows. Generally, the corresponding phenylenediamines such
as l-alkyl-2,4-diaminobenzenes, for example tolylene-2,4-
Mc3548
" ~, J 1:. ... ,!`
diamine, can be used in the process of the invention. Suitablecompounds may be used either individually or even in
combination with one another. Water is used as solvent.
The m-phenylenediamine and urea are used in a molar ratio
greater than 2:1, preferably in a molar ratio greater than 10:1
and more preferably in a molar ratio greater than 3:1 to 5:1.
If molar ratios of less than or equal to 2:1 are used~ products
having inadequate NH values, l.e. highly pre-extended ureas,
are obtained. In the particularly preferred range, products
having NH values approaching the theoretical are obtained.
Although an increase in ~he molar ratio is possible in
principle, this does not lead to any improvement in the product
and, in addition, does not make good economic sense.
The m-phenylenediamines are dissolved in the water in
quantities of 20 to 200 parts by weight, preferably in
quantities of 40 to 150 parts by weight and more preferably in
quantities of 80 to 120 parts by weight per 100 parts by weight
solvent. If the concentr~tion is excessively increased, the
reaction mixture becomes increasingly more difficult to stir on
account of the precipitating product. In addition, the product
contains considerable quantities of entra;ned starting amine
which can only be removed by expensive purifying steps. In the
preferred concentration range, NH values approaching the
theoretical are obtained. The urea is added to the solution of
the amine in water in the described molar ratio and the
reaction mixture is stirred at temperatures of 60 to 100C,
preferably under reflux.
The precipitated product is filtered off and washed with
water to remove residual quantities of starting amine. It is of
advantage in this regard to add water before filtration and to
carry out the filtration step in a steamheated nutsch filter to
prevent the residual am;ne from precipitating at low
tPmperatures. For the same reasons, it is also of advantage to
wash the product with hot water. The excess starting amine
Plo3548
~? ~ ~''~iig~ ',3 .';~,
-7-
recoverable from the mother liquor may be reused without
further purification.
After drying, the desired urea is obtained in an excellent
yield in the form of a finely crystalline solid which,
depending on the concentration ratios selected, has NH values
which are greater than 270 mg KOH/g ~theoretical 416 mg KOH/g~
and preferably greater than 360 mg KOH/g, the content of free
monomeric starting amine being less than 1% by weight and
preferably less 0.5% by weight.
The following Examples are intended to illustrate the
process according to the invention without limiting it in any
way (percentages are by we;ght, unless otherwise stated).
EXAMPLES
ExamDle 1
366 g (3 mol) 2,4-tolylened;amine (2,4-TDA) and 60 9
(1 mol) urea (molar ratio of 2,4-TDA to urea 3:1) were
dissolved in 400 ml water. The reaction solution was stirred
under reflux for 20 hours. 1,000 ml water were then added and
the reaction mixture was refluxed for another 30 minutes.
After filtration in a steam-heated nutsch filter, the residue
was washed twice with 300 ml hot (90C) water. The product was
then freed from adhering water in a vacuum drying cabinet.
Yield: 213 9 (79%, based on the urea used)
NH value (HClO4/glacial acetic acid) 369 mg KOH/g
Residual 2,4-TDA content (HPLC): 0.353~ by weight.
ComParison Example (from US-PS 2,503,797)
` 54 Parts by weight 1,3-diaminobenzene were dissolved
while stirring in 110 parts by volume 38.6% sulfuric acid. The
temperature of the solution rose to 65-70C. After the
add;tion of l9 parts by weight urea, the reaction mixture was
boiled under reflux for 32 hours. 120 Parts by weight water
were then added. The precipitated product was filtered off and
washed with 200 parts by weight cold water. The sulfuric acid
salt thus obtained may be converted into the free base by
standard methods. It is of advantage to convert the salt into
Mo3548
~' ' C`t ,~1 ~ ,. ; ;.
-~-
the corresponding ehloride with BaC12 before recovery of the
base.
No details of yield or analytical data are provided in the
Comparison Example. From the above examples, however, one
skilled in the art can appreciate the advantages of the claimed
process.
Although the invention hàs been described in detail
in the foregoing for the purpose of illustration, it is to be
understood that such detail is solely for that purpose and that
10 . variation can be made therein by those skilled in the art
without departing from the spirit and scope of the invention
except as it may be limited by the claims.
Mo3548