Note: Descriptions are shown in the official language in which they were submitted.
2~3~
-- 1 --
Novel pyrazole derivatives which are angiotensin II
receptor antagonists, their methods of ~reparation and
pharmaceutical compositions in which _ ey are pres~nt
05 The present invention relates, by way of novel
products, to the pyraæole derivatives of general for-
mula (I) below and to their salts.
The compounds in question have a very valuable
pharmacological profile insofar as they possess anta-
lo gonistic properties towards angiotensin II receptors.
They are therefore especially indicated for the treat-
ment of cardiovascular diseases and in particular for
the treatment of hypertension and the treatment of
cardiac insufficiency.
The present invention further relates to the
method o~ preparing said products and to kheir applica-
tions in therapeutics. It further relates to the novel
intermediates which enable said products to be synthe-
sized.
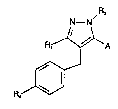
These pyrazole derivatives have general formula
(I)
R,
N-N
R, ~ A
f~
~.~
Formula (I)
in which:
R1 is a lower alkyl radical having 1 to 6
carbon atoms, a lower alkenyl radical having 2 to 6
2~3~
carbon atoms, a C~-c7 cycloalkyl radical or a C~-C7
cycloalkenyl radical;
R2 is the hydrogen atom, a lower alkyl radical
having 1 to 6 carbon atoms, a lower halogenoalkyl
05 radical having 1 to 6 carbon atoms, a C3-C~ cycloalkyl
radical, a group -(C~2)m-COOR5, a group -CH2-tCH2)m-OR5
or a group -CH2-(CH2)m-SR5, m being an integer Prom 0
to 5 and R5 being a hydrogen atom or a lower alkyl
radical having 1 to 6 carbon atoms;
A can be a group:
~(CH2)qOR~ R' being a hydrogen atom, a lower alkyl
radical having 1 to 6 carbon atoms or a C9-C7 cyclo-
alkyl radical and q being an integer from 1 to 5,
~tCH2)qL~ L being a halogen atom, preferably chlorine
or bromine, and q being as defined above,
-CHO, an acetal or a dioxolan,
-COOR', R' being as defined above,
-CONR''R''', R'' and R~i independently being a hydro-
gen atom, a lower alkyl radical having 1 to 6 carbon
atoms or a C3~C7 cycloalkyl radical, or being able to
form, with the nitrogen atom to which they are attach-
ed, a heterocycle such as pyrrolidine, piperidine,
morpholine, thiomorpholine or a piperazine,
-CN,
-tCHa)~CN, q being as defined above,
-(CH2)~COOR', R' and q being as defined above,
-(CH2)~CONR''R''', R'', R''' and q being as defined
abova,
-(CH~)~NR''R''', R~', R''' and q being as de~ined
above,
~(CH~)q~S~R~ R' and q being as defined above, or
-OR3, R3 being a hydrogen atom, a lower alkyl radical
having 1 to 6 carbon atom~, a C3-C~ cyc}oalkyl radical,
a group -(CH2)n-COOR~, a group -CH2-(CH2)~-CN, a group
-CH2-(CH2)~-O-R~, a group -CH2-(CH2)~-S-R~ or a group
2~3~2g
-- 3
-COR~, n being an integer from O to 5 and R~ beiny a
hydrogen atom or a lower alkyl radical having 1 to 6
carbon atoms, or R3 can be a group -(CH2)p-CO-NR7R8 or
(CH2)p-cH2-cH2NR7R8~ p being an integer from O to 5
05 and R7 and R~ independently being a hydrogen atom, a
lower alkyl radical having 1 to 6 carbon atoms or a
C3-C7 cycloalkyl radical, or bein~ able to form, with
the nitrogen atom to which they are attached, a hetero-
cycle such as pyrrolidine, piperidine, morpholine,
thiomorpholine or a piperazine; and
R4 can be a nitro or amino group or a group
-COORg, R~ being the hydrogen atom or a lower alkyl
radical having 1 to 6 carbon atoms, or R4 can be the
following radicals:
R900C~ NC~3 ~ N~3 ' o2N~3
N~ ~NH
N
CF,So,NH~3 , II,N~3 ' R,OOC/~
--NH--C~ --NH--C ~1 C NH
HO,S CF,SO,HN R900C
Y Y
2~3~
~,OOC ~ ' N
05 N~N,
in which Rg is as defined above, X and Y independently
being a hydrogen atom, a lower alkyl radical, a halogen
atomj an alkoxy radical or a trifluoromethyl radical.
In the description and the claims, lower alkyl
radical is understood as meaning a linear or branched
hydrocarbon chain havirlg from 1 to 6 carbon atoms. A
lower alkyl radical is, for example, a methyl, ethyl,
propyl, isopropyl, butyl, isobutyl, tert-butyl, pentyl,
isopentyl, hexyl or isohexyl radical.
C3-C7 cycloalkyl radical is understood as
meaning a saturated cyclic radical, preferably a cyclo-
propane, cyclobutane, cyclopentane, cyclohexane or
cycloheptane radical.
Lower alkenyl radical is understood as meaning
a linear or branched hydrocarbon chain having from 2 to
6 carbon atoms and possessing an unsaturation. A lower
alkenyl radical is, for example, an ethene, propene,
isopropene, butene, isobutene, pentene, isopentenle,
hexene or isoh~xene radical.
Ca-C7 cycloalkenyl radical is understood a~
meaning a cyclic radical possessing an unsaturation,
pre~erably a cyclobutene, cyclopentene, cyclohexene or
cycloheptene radical.
Lower halogenoalkyl radical having 1 to 6
carbon atoms is understood as meaning an alkyl radical
in which 1 to 6 hydrogen atoms have been substitut2d by
1 to 6 halogen atoms. A lower halogenoalkyl radical
is, ~or example, a trifluoromethyl radical or a 2,2,2-
~3~2~
trifluoroethyl radical.
Alkoxy radical is understood as meaning an 0-
lower alkyl group, lower alkyl being as defined above.
Heterocycle is understood as denoting a ring of
05 5 to 7 atoms containing from l to 3 heteroatoms selec-
ted from oxygen, sulfur or nitrogen, which is unsubsti-
tuted or substituted by a lower alkyl, lower halogeno
alkyl or lower alkoxy group or by a phenyl ring which
is unsubstituted or substituted by one of these groups.
In the description and the claims, halogen is
understood as meaning a chlorine, bromine, iodine or
fluorine atom.
Document EP-A-0323~41 to Du Pont de Nemours
describes pyrroles, pyrazoles and triazoles. These
compounds all possess a benzyl substituent on a nitro-
gen atom:
Z--Y
R,~N `k
~ A
Now, the Applicant has discovered, surprising-
ly, that in contrast to the information contained in
the Du Pont de Nemours document, it is not essential
for the benzyl substituent to be located on a nitrogen
atom, in particular in the l-position (or 2-position)
of the pyrazole ring, but that it is pvssible to obtain
very effective products when this benzyl substituent is
on a carbon atom in the 4-position o~ the pyrazole
ring. Furthermore, the Applicant has discovered t~at
the presence of a group A and especially of an oxygen
` 2~13~8
atom in the 3-position (or 5-position) of the pyrazole,
in conjunction with the benzyl substituent in the 4-
position, produces compounds which are particularly
active as angiotensin II receptor antagonists.
05 According to one embodiment, Rl is an n-propyl
group.
According to another embodiment, Rl is an n-
butyl group.
According to one embodiment, R2 is a methyl
group.
According to another embodiment~ R2 is a 2,2,2-
trifluoroethyl group.
According to one embodiment, A is an ethoxycar-
bonylmethyleneoxy group.
According to another embodiment, A is a di-
methylaminocarbonyloxy group.
According to another embodiment, A is a meth-
oxymethylene group.
According to ~nother embodiment, A is a 2-
hydroxyethoxy group.
According to one embodiment, R~ is a 2-carboxy-
3,6-dichlorobenzoylamino group.
According to another embodiment, R4 is a 2-
sulfobenzoylamino group.
According to another embodiment, R~ i~ a 2-
(tetrazol-5-yl)phenyl group.
The particularly pre~erred compounds oP the
invention are those selected from the products o~ the
~ormulae
2~3~2~
-- 7 --
CHJ
N--N~
~O~COOC2Hs
~NJ~J
l ll H
a COO=~OJ~N~
i S
SO~H
N N~CH'
~O~COOC~H,
~N~
3 0 1 ~N
`
2~3~8
,GH,
\~ ~CH
05 ,~,,1
.~
~N -
1 ,N
H N--N/~CF,
~--' ~CH,
.
~N~
N
N_N/~
N--~CF,
2 5 ~~o~f
~0~J
~N,
H--N
According to the invention, it will be possible
2 ~
- 9 -
to prepare the compounds of formula (I) according to
the ~ollowing scheme:
- the alkyl 3-oxoalkanoates of formula (II):
05 R,---C--CH~--C OOR~o
Formula (II)
in which Rl is as de~ined above and Rlo is a lower
alkyl radical, preferably methyl or ethyl, and
- th~ 1,3-diketones of formula (III):
R~ 8 - CH2~ CH~q- O-R~
15 O
Formula (III)
in which Rl, R' and q are as defined above,
will be prepared. These compounds of formulae (II) and
(III) can be prepared by classical methods such as the
Claisen reaction or the method using Meldrum's acid.
Methods of preparing this type of compound may be found
in the following references:
- OIKAWA Y.; SUGANO K.; YONEMITSU O.; J. Org. Chem.,
1978, ~3(10), 2087-~8,
- WIERENGA W.; SKULNICK H.I.; J. Org. Chem., 1979, 44,
310,
- HO~GHTON R.; LAPHAM D.; SYNI'HESIS, 1982, 6, 451-2,
- BRAM G.; VILKAS M.; Bull. Soc. Chim. France, 1964
(5), 945-51,
- BALYAKINA M.V.; ZHDANOVICH E.S.; PREOBRAZHENSKII
N.A.; Tr. Vses. Nauchn. Issled. Vitamin Inst., 1961,
7, 8-16,
- RENARD M.; MAQUINAY A.; Bull. Soc. Chim. Belg., 1946,
2~13~2~
_ , 98-105,
- BRUCE F.W.; COOVER H4W.; J. Am. Chem. Soc., 1944, 66,
2092-94, and
- EBY C.J.; HAUSER C.R.; J. Am. Chem. Soc., 1957, 79,
05 723-5.
Benzylation of the compounds of formula (II) or
(III) with compounds of formula (IV):
W
~
V ~
Formula (IV)
in the presence of a basa such as sodium or potassium
carbonate in acetone, a sodium or potassium alcoholate
in an alcohol, or sodium or lithium hydride in solvents
such as tetrahydrofuran, dioxane or dimethylformamide
for example, at a temperature of between 50 and 100 C,
or else in the presence of one equivalent of lithium
chloride or bromide and two equivalents of diisopropyl-
ethylamine under reflux in tetrahydrofuran, according
to the reference SUNG-EUN Y00; KYU YANG YI; Bull.
Korean Chem. Soc.~ 1989, 10(1), 112, will give the
compounds of formulae (V) and (VI):
R~COOR~o R~CH~q--OP~'
V~ V~
35 Formula (V) Formula (VI)
-
~3~
11 -
The compounds of formulae (V) and (VI) can al~o be
obtained by condensation of an aldehyde of ~ormula
(VII):
05 CHO
Formula (VII)
with a compound of formula (II) or formula (III),
followed by catalytic hydrogenation, for example in the
presence of Raney nickel, in a solvent such as an
alcohol, under pressure or at ordinary pressure if the
substituents pr~sent allow it.
In more general terms, methods of preparing the
compounds of formula (V) or formula (VI) will be found
in the following references:
- DURGESHWARI P.; CHAUDHURY N.D.; J. Ind. Chem. Soc.,
1962, 39, 735-6,
~ HEINZ P.; KREGLE~SKI A.; J. Prakt. Chem., 19G3,
21(3-4), 186-197,
- ZAUGG H.E.; DUNNIGAN D.A.; MICHAELS R.J.; SWETT L.R.;
J. org. Chem., 1961, 26, 644-51,
- KAGAN H.B.; HENG SUEN Y.; Bull. Soc. Chim. France,
1966 (6), 1~19-22,
- RATHKE M.W.; DEI~CH J.; Tetrahedron Lett., 1971 (31),
2953-6,
- BORRIES KUBEL; Liebigs Ann. Chem., 1980, 1392-1401,
- MARQUET J.; MORENO-MANAS M.; Chem. Lett., 1981, 2,
173-6,
- IOFFE T.; POPOV E.M.; VATSURO K.V.; TULIKOVA E.K.:
KABACHNIK M.I.; Tetrahedron, 1962, 18, 923-940, and
.
2 ~ 2 ~
- 12 -
- SHEPHERD T.M.; Chem. Ind. ~London), 1970, 17, 567.
In formula (IV), W is a halogen atom, prefer-
ably chlorine or bromine.
In the same ~ormula:
05 - V can be a nitro group, in which case the
derivative of formula (IV) is commercially available.
- V can be a group COORIl, R1l being a lower
alkyl or benzyl radical, in which case the derivative
of formula ~IV) will be prepared by chlorinating or
brominating a commercially available p-methylbenzoic
acid ester with N-chlorosuccinimide or N-bromosuccini-
mide, in a solvent such as carbon tetrachloride or
dibromoethane, according to the following reference:
- JULIA M.; CHASTRETTE F.; Bull. Soc. Chim. France,
1962 ~2), 2247.
- V can be a group
R~200C~
Rl2 being a lower alkyl or benzyl radical, in which
case the compounds of formula (IV) are prepared by
reacting a magnesium compound of p-bromotoluene with a
compound of the formula
CH,O
N
:30 ~ O
to give a compound of the formula
2~13~
13
CH, ~
1 11
05 ~
o
which is hydrolyzed to give the compound of the formula
CH~ ,~,
11
HOOC
Procedures for the three steps described above will be
found in the following referenca:
- MEYERS A.I.; MIHELICH E.D.; J~ Am. Chem. Soc., 1975,
97, 7383.
The acid is then esterified with an alcohol of
the formula Rl2OH, R~2 being as defined above.
These derivatives are then brominated or
chlorinated, for example with N-bromosuccinimide or N-
chlorosuccinimide, in a solvent such as carbon te~ra-
chloride or dibromoethane, to give the compounds of
formula (IV~ in which V is the group
~,
R,200C ~V
- V can be the group
2~3g~
- 14 -
NC
05
in which case the compound
CH~
l 11
HOOC ~
prepared above will be converted to the primary amide
by reacting the acid chloride (obtained with thionyl
chloride or phosphorus oxychloride) with ammonia, and
this amide will be converted to the nitrile by reaction
with phosphorus oxychloride in dimethylformamide or by
reaction with thionyl chloride. The nitrile obtained:
CH
l~ 11
~ ,~3
NC
will then be brominated or chlorinated under the same
conditions as the above ester to give the compounds of
formula ~IV) in which V is the group
NC
- V can be the group
-` 2~3~2~
- 15 -
o2N~3
05
in which case the compound
CICH~
~
l 11
02N/~
will be prepared by chloromethylating commercially
available 2-nitrobiphenyl according to the following
references:
- CA : 70 (25j : 114837d, and
- CA : 69 (2) : 3704t
to give the compounds of formula (IV) in which V is the
20 group
~3
02N
- V can be a group
,~3
Fl~2~ S
R12 being a lower alkyl or benzyl radical. The corres-
ponding compounds of formula ~IV) are obtained in the
~ollowing manner:
Starting ~rom the compound
`` 2 ~ 2 ~
-- 16 --
CH, ~\,
11
05 /~~S~
HO0C
whose preparation can be found in the ~ollowing refe-
rence:
- FISSELMANN H.; HABITCH H.; Ger. Offen. 1,092,929
~1960); CA : 57 : 5B94g,
the compounds of the formula
~H,
~ .
)~S>
OOC
will be obtained by esterification with an alcohol of
the formula R120~, R1~ being as defined above, by
classical methods Xnown to those skilled in the art.
These compounds are then treated with N-chloro-
succinimide or N-bromosuccinimide, in a solvent such as
carbon tetrachloride or dibromoethane for example, to
give the compounds of formula ~IV) in which V is the
group
R"00C S
R~2 being as defined above.
- V can be the group
2 ~
- 17 -
NC
05
in which case the corresponding compowlds of ~ormula
(IV) will be prepared in the following manner:
Starting ~rom the compound 3-(p methylph~nyl)-
thiophene-2 carboxylic acid, whose preparation is given
above, the amide compound is obtained by treatment with
thionyl chloride and then ammonia, said amide compound
then being dehydrated with thionyl chloride or phospho-
rus oxychloride, without a solvent or in dimethylforma-
mide, to give the nitrile compound
CH, ~
NC
This nitrile compound is then halogenated with
N-chlorosuccinimide or N-bromosuccinimide, in a solvent
such as carbon tetrachloride or dibromoethane, to give
the compounds of ~ormula (IV) in which V is the group
~3
NC S
- V can be the group
~S
NC~
in which case the corresponding compounds of formula
(IV) are synthesized in the following manner:
Starting from 4-chlorobutyrophenone of the
formula
05
CH, ~ CO-CH2- CH2~ CH2-CI
whose preparation can be fou~d in patent BE 577,977 of
lo15 May 1959, CA : 54, 4629c, the compound o~ the
formula
CH~ ~ C-C -CH2 CH,-CI
Cl CHO
will be obtained by treatment with phosphorus oxy-
chloride and dimethylformamide according to the con-
ditions described in the following re~erence:
- VOLODINA M.A.; TERENT'EV A.P.; KUDRYASHOVA V . A ~;
KABOSHINA L.N., Khim. Geterosikl. Soedim, 1967, 5-8.
This compound is then treated with sodium
sulfide, in a solvent ~uch aæ tetrahydrofuran under
reflux, to give the derivative
CH,
~S
I/ \
30 OHC ~
which is then converted in two ~teps to the nitrile
derivative by dehydration of the oxime formed from the
aldehyde and hydroxylamine. This dehydration may be
carried out for example with acetic anhydride to gi~e
2~3~28
the nitrile compound
CH, ~
~5 ~ S
~1 ~
NC ~
which may then be aromatized by treatment with bromine
in carbon tetrachloride and then with potassium tert-
butylate in tetrahydrofuran to give the compound
CH,
NC
This compound can then be chlorinated or bro-
minated with halogenating agents such as N-chloro-
succinimide or N-bromosuccinimide, in a solvent such as
carbon tetrachloride or dibromoethane, to give the
compounds of formula (IV) in which V is th~ group
~ S
NC
- V can ~e the group
R,IOOC~
Rl~ being as de~ined above. The corresponding com-
2~3~28
. .
- 20 -
pounds of formula (~V) may be prepared from the com-
pound of the formula
CH~
05
~S
NC~
by classical hydrolysis of the nitrile group and then
esterification of the acid obtained, or by direct con-
version of the nitrile group to the ester group by the
methods known to those skilled in the art, followed by
chlorination or bromination of the ester with N-chloro-
succinimide or N-bromosuccinimide, for example in
carbon tetrachloride or dibromoethane.
In formulae tv) and (VI~, Rl, R~o~ R' and q are
as defined above and V is as defined in formula (IV).
Some deriYatives of formula (V) and formula
(VI), when V is a 2-alkoxycarbonylphenyl, a 2-cyano-
phenyl, a 2-nitrophenyl, an alkoxycarbonylthiophene or
a cyanothiophene, are novel synthesis intermediates
which are claimed per se.
In formula (VII), V is as defined in formula
(IV), but this condensation method will only be used
when V possesses a functional group unaffected by
hydrogenation. In certain cases, these aldehydes may
be prepared ~rom the derivatives of ~ormula (IV)
according to reactions known to those skilled in the
art. The Sommelet reaction (Bull. Soc. Chim. France,
1918, ~4] 23, 95) or the nitropropane reaction (organic
Syntheses Collec., vol. IV, 932) may be mentioned.
Reaction of a hydrazine o~ formula (V~
, . . . .
gl 2 ~
- 21 -
H~N-NH-R2
Formula (VIII)
05 in which ~2 iS as defined above, with the compounds of
formula (V~, by simply refluxing in an alcohol for
example, will give the compounds of formula (IX):
/N-N
R, ~ OH
,~
V~
Formula (IX)
in which Rl, R2 and V are as defined above.
These compounds of formula (IX) are novel syn-
thesis intermediates which are claimed per se.
Alkylation of the compounds of formula (IX),
performed in the presence of a base such as potassium
or sodium carbonate in solvents such as acetone, butan--
2-one or dimethylformamide, or in the presence of a
sodium or potassium alcoholate in an alcohol, or in the
presence of sodium or lithium hydride in tetrahydro-
furan, with derivatives of formula (X):
~-R3
Formula ~X)
Z being a bromine, chlorine or iodine atom and R9 being
as de~ined above, gives the compounds of formula (XI):
2~3~
- 22 -
N-N
R~o~RJ
05
V~
For~ula (XI)
in which R~, R2, R3 and V are as def.ined above.
Likewise, reaction of a hydrazine of formula
tVIII)i as defined above, with a compound of formula
(VI), by simply refluxing in an alcohol for example,
will y7 ve a mixture of compounds of formula (XII~ and
formula tXII'):
R~(CHdq ,~\(Cll~q
25 VJ~ VJ~
Formula tXII) Formula (XII')
in which Rl, R2, R', q and V are as defined above.
The pure compounds of formula (XII) will be
obtained from this mixture by purification by chroma-
tography on silica, recryskallization or any other
method of puri~ication known to those skilled in the
art.
Another method of preparing the compounds of
2 ~ 2 ~
- 23 -
formula (XII) consists, in a first stage, in treating
the compounds of formula (VI) with hydrazine hydrate
under the same ~onditions to give the compounds of
formula (XIII):
05
N-N N-N
R, ~ OR' ~ (CH,)q OR
Formula (XIII)
in which R1, R', q and V are as defined above. These
compounds of formula (XIII) are then alkylated in the
presence of DBU (1,8-diazabicyclo[5.4.0]undec-7-ene),
in a solvent such as acetone or acetonitrile for
example, with halogenated derivatives of formula (XIV):
Z-R
Formula (XIV)
in which R2 is as defined above and Z is a bromine,
chlorine or iodine atom, to give a mixture of the com-
pounds of formula (XII) and formula (XII'), as defined
above, the compounds of formula (XII) then being ob-
~30 tained pure after purification as mentioned above.
This second method of preparation can be particularly
advantageous in the case where R2 is a lower alkyl
group, because it makes it possible to obtain a pre-
ponderant proportion of the compounds of formula (XII)
relative to the compounds of formula (XII').
-- . . .
2 ~
- 24 -
The compounds of formula (XII) in which R' is a
hydrogen will be prepared in two steps: Treatment of
the compounds of formula (XII) in which R' is a lower
alkyl group with boron tribromide will give the bromi-
05 nated derivatives of formula (XV):
N_N~RZ
R~/~\(CH~q Br
,D~
1 11
V~
Formula (XV)
in which Rl, R2, q and V are as defined above. Treat-
ment of these brominated derivatives of formula (XV~
with potassium or sodium carbonate under reflux in a
dioxane/water mixture makes it possible to obtain tha
compounds of formula (XII) in which R' is the hydrogen
atom.
The derivatives of formula (XI) or formula
(XII) in which V is a nitro group will be able to
undergo catalytic hydrogenation, for example in the
presence of Raney nickel, in an alcohol, at atmospheric
pressure or under pressure, to give the compounds of
formula (I) in which R4 is an amino group and A is a
group -(CH2)~-OR' or OR3, R', R3 and q being as defined
above.
Reaction of an appropriately substituted
phthalic anhydride with these derivatives will give the
compounds of general formula (I) in which R4 is the
group
2~3~
- 25 -
X
~ HN-C
05 HOOC ~
in which x and Y are as defined above and A i5 a group
~lCHa)q-OR~ or OR3, q, R~ and R3 being as defined
above, it then being possible for the acid obtained to
be esterified to give the group
O X
--HN- C~,~
R900G~
y
Likewise, reaction of the cyclic anhydride of
an appropriately substituted orthosulfobenzoic acid
with these amino compounds will give the compounds of
general formula (Il in which R~ is the group
z5 --HN--8~3
HO,S
y
and A is a group -(CH2)~-OR' or OR3, g, R', R3, X and Y
b~ing as defined above.
Likewise, reaction of N-(trifluoromethyl-
sulfonyl)anthranilic acid chloride, whose preparation
can be found in the following references:
CA 96 (13) : 103651z, and
2 ~ 2 ~
~ 26 -
CA 97 t7) : 55500w,
with these amino compounds will give the compounds of
general formula (I) in which Ra is the group
05 O
--HN--C~,
CF:~--S02--HNJ~
and A is a group ~(CH2)q~OR! or OR3, q, R' and R3 being
as defined above.
The compounds of formula (XI) or ~XII) in which
V is a group -COORl1 will be able to be hydrolyzed in
an acidic or basic medium, or hydrogenated in the case
where R~l is a benzyl so as not to affect the other
ester groups present, to give the compounds of formula
(I) in which R~ is a group -COOH and A is a group
-(CH2)~-OR' or OR3, q, R' and R3 being as defined
above.
After conversion to the acid chloride with
thionyl chloride or to a mixed anhydride with ethyl
chloroformate, these acid derivatives will be able to
give the compounds of general formula ~I) in which R~
is the group
o X
NH
R~O,C
Y
and A is a group -~CH2)~-OR' or OR~, q, R' and R~ being
as defined above, by reaction with anthranilic acid
derivatives of the formula
2 ~
- 27 -
H2
05 RDOOC~
in which X, Y and Rg are as defined above.
In the same way, the compounds of formula (XI)
or txII) in which V is a group
\f~
R,200C~
will be hydrolyzed, or hydrogenated in the presence of
a catalyst such as palladium-on-charcoal in the case
where R12 is a benzyl, to give the compounds of formula
(I) in which R~ is a group
HOOC ~
and A is a group ~(CH2)q-OR~ or OR3, q, R~ and R3 being
as defined above.
The compounds of formulae (XI) and (XII) in
which V is a group
NC~
will be able to react with one equivalent of sodium
nitride in a solvent such as dimethylformamide, in the
~33~
- 28 -
presence of an ammonium salt such as ammonium chloride,
or with a trialkyltin nitride under reflux in toluene
and then with gaseous hydrochloric acid in tetrahydro-
fuxan, to give the compounds of general formula (1) in
05 which R~ is a group
~,
11
N~/
N~ ,NH
N
and A is a group -(CH~)~-OR' or OR3, q, R' and R3 being
as defined above.
The compounds of formulae (XI) and (XII) in
which V is a group
~3
02N
will be able to undergo catalytic hydrogenation, for
example in the presence of Raney nickel, in an alcohol,
at atmospheric pressure or under pressure, to give com-
pounds of general formula (I) in which R~ is a group
HlN~J
and A is a group ~(CH2)q~0R~ or OR3, q, R' and R3 being
as defined above~
Reaction of trifluoromethanesulfonyl chloride
with the latter in a solvent such as chloroform or in
an aromatic solvent such as toluene, in the presence of
~3~
- 29 -
a base such as triethylamine or pyr.idine, or in pyri-
dine, will give the compounds of general formula (I) in
which R4 is a group
05 ~
CFJ--SO2--HN~J
and A is a group -(CH2)~-OR~ or OR3, q, ~' and R3 being
as defined above.
The compounds of formulae (XI) and (XII) in
which V is the group
NC ~ S
will be able to be treated with a trialkyltin nitride
under re~lux in toluene and then with gaseous hydro-
chloric acid in te~rahydrofuran to give the derivatives
of formula (I) in which R~ is the group
N~ ~S
N
and A is a group ~(CH2)q~OR~ or OR3, q, ~' and R3 being
as defined above.
The compounds of formulae (XI) and (XII) in
which V is the group
R"OOC ~ S
will be able to be hydrolyzed, or hydrogenated in the
~3~
~ 30 -
presence of a catalyst such as palladium-on-charcoal in
the case where Rl2 is a benzyl, to give the compounds
of formula (I) in which R~ is the group
05 ~
HOOC ~ S
and A is a group ~(CH2)q~0Rt or OR3, q, R' and R3 being
as defined above.
The compounds of formula (I) in which A is a
group CH20H will be able to be oxidized with a mild
oxidizing agent such as manganese dioxide, in a solvent
such as chloroform, to give the compounds of formula
(I) in which A is the group CH0, which will be able to
be converted, by the classical methods known to those
skilled in the ar~, to an acetal or a dioxolan by
heating with an alcohol or a diol, in the presence of
paratoluenesulfonic acid for example (Synthesis, 1981,
501).
More rigorous oxidation of these same aldehyde
compounds or of the alcohol compounds direct, for
example with oxidizing agents such as potassium per-
manganate, will give the compounds of formula (I) in
which A is the group C02H.
The compounds of formula (I) in which A is the
group CO~H will be able to be esterified by classical
methods of esterification to give the compounds of
formula (I) in which A is a group C02R', R' being a
lower alkyl radical.
The compounds of ~ormula (I) in which A is the
group C02H will also be able to be converted to the
amide in several steps, if necessary after steps for
protecting other functional groups, by conversion to
the acid chloride and then treatment with ammonia or
with an amine of the formula HNR''R''', R'' and R'''
2 ~
- 31 -
being as defined above, to give the compounds of
formula (I) in which A is a group CONR'tR'''.
The compounds of formula tI) in which A is a
group CONH2 will be able to be treated with an agent
05 such as thionyl chloride or phosphorus oxychloride to
give the compounds of formula (I) in which A is the
group CN.
The compounds of formula (I) in which A is a
group (CH2)~0R', R' being a lower alkyl radical and q
being an integer from 1 to 5, will be able to be
treated with boron tribromide in chloroform to give, if
necessary after steps for protecting and deprotecting
other functional groups, the compounds of formula (I)
in which A is the group (CH2)~Br.
The compounds of formula (I) in which A is the
group (CH2)~Br will be able to be treated with sodium
or potassium cyanide, in solvents such as alcohol, an
alcohol/water mixture, dimethyl sulfoxide or aceto-
nitrile, to give the compounds of formula (I) in which
A is the group tCH2)~CN, or with amines of the formula
HNR''R''', in which R'' and R''' are as defined above,
to give the compounds of formula (I) in which A is
(CH2)qNR~R~ or with thiols of the fo.rmula HS-R', R'
being as defined above, to give the derivatives of
formula (I) in which A is (CH2)~-S-R'.
The compounds of formula ~I) in which A is the
group ~CH2)~CN will be able to be hydrolyzed by the
classical methods of nitrile hydrolysis to give the
compounds of ~ormula (I) in which A is the group
(CH2)~CO2H.
These acid compounds will themselves be able to
be esterified by the classical methods of esterifica-
tion to give the compounds of ~ormula (I) in which A is
a group ~CH2)~CO2R', R' being a lower alkyl radical and
q being an integer from 1 to 5, or converted to an
2 ~ 3 ~f~
- 32 -
amide ~(C~2)q~CONR/~R~ as indicated above.
The compounds of formula (I) in which A is the
group ~CH2)qL~ L being a halogen and q being an integer
from 1 to 5, will be able to be synthesized by treating
05 the derivatives of formula (I) in which A is the group
(CH2)qOH with halogenating agents such as, for example,
thionyl chloride, phosphorus oxychloride or phosphorus
tribromide.
In the case where R4 possesses a functional
group which is not compatible with these reaction
sequences, the derivative of formula (XII) will be used
as the product for conversion, V subsequently being
converted to the group R~ in the manner mentioned
above.
It is possible to obtain addition salts of some
of the compounds of formula (I), especially pharmaceu-
tically acceptable addition salts. In particular, when
R2, R4 or A contains an acid group, there may be
mentioned the salts of sodium, potassium, calcium, an
amine such as dicyclohexylamine or an amino acid such
as lysine. When A or R4 contains an amine group, there
may be mentioned a salt of a mineral or organic acid,
such as, for example, the hydrochloride, methanesulfo-
nate, acetate, maleate, succinate, fumarate, sulfate,
lactate or citrate.
The novel compounds according to the invention
possess remarkable pharmacological properties as angio~
tensin II receptor antagonists and can be used in
therapeutics for the treatment of cardiovascular dis-
~
eases and in particular for the treatment of hyperten--
sion and cardiac insufficiency~
Thus the invention covers the pharm~ceutical
compositions which contain, as the active principle,
the drugs consistiny of a pharmaceutically effe.ctive
amount of at least one compound of formula (I) as
2 ~
- 33 -
defined above, as well as one of its pharmaceutically
acceptable addition salts if appropriate.
These compositions can be given by buccal,
rectal, parenteral, percutaneous or oculax administra~
05 tion.
These compositions can be solid or liquid and
presented in the pharmaceutical forms commonly used in
human medicine, such as, for example, simple or coated
tablets, gelatin capsules, granules, suppositories,
injectable preparations, percutaneous sys~ems and eye
lotions; they are prepared by the customary methods.
In said compositions, the active principle, consisting
of a pharmaceutically effective amount of at least one
compound of formula (I~ as defined above, or one of its
pharmaceutically acceptable addition salts, can be
incorporated in excipients normally employed in these
pharmaceutical compositions, such as talc, gum arabic,
lactose, starch, magnesium stearate, polyvidone,
cellulose derivatives, cocoa butter, semisynthetic
glycerides, aqueous or non-aqueous vehicles, ~ats of
animal or vegetable origin, glycols, various wetting
agents, dispersants or emulsifiers, silicone gels,
certain polymers or copolymers, preservatives, flavor-
ings and colors.
The invention also covers a pharmaceutical com-
position with antagonistic activity towards angiotensin
II receptors, which makes it possible especially to
favorably treat cardiovascular diseases, in particular
hypertension and cardiac insuf f iciency, said composi-
ti.on comprising a pharmaceutically effective amount o:f
at least one compound of formula (I) mentioned above,
or one of its pharmaceutically acceptable addition
salts, which may be incorporated in a pharmaceutically
acceptable excipient, vehicle or carrier.
The dosage varies especially accordinq to the
~3~
- 34 -
mode of administra~ion, the complaint treated and the
subject in question.
For example, for an adult with an average
weight of 60 to 70 kg, it can vary between 1 and 400 mg
05 of active principle administered orally in one or more
daily doses, or from 0.01 to 50 mg admi:nistered paren-
terally in one or more daily doses.
The invention also covers a method of preparing
a pharmaceutical composition, which comprises incor-
porating a pharmaceutically effective amount of atleast one compound of formula (I) as defined above, or
one of its pharmaceutically acceptable addition salts,
into a pharmaceutically acceptable excipient, vehicle
or carrier. According to a particular character.istic,
this pharmaceutical composition is formulated as
gelatin capsules or tablets containing from 1 to 400 mg
of active ingradient, or as injectable preparations
containing from 0.01 to 50 mg of active ingredient.
The invention also covers a method of thera-
peutic treatment for mammals, which comprises adminis-
tering to this mammal a therapeutically effective
a~ount of at least one compound of formula ~I) as
defined above, or one o~ its pharmaceutically accept-
able addition salts.
In animal therapeutics, the daily dose which
can be used should normally be between 1 and 100 mg per
kg.
Further characteristics and advantages of the
invention will be understood more clearly from the
following description of some Preparatory Examples,
which in no way imply a limitation but are given by way
of illustration.
2~3~3
- 35 -
Example_1: Ethyl 3-oxoheptanoate
Formula (II): Rl = n-butyl, Rlo = ethyl
05 70 g of Meldrum's acid are dissolved in 200 ml
of methylene chloride in the presence of 78.5 ml of
pyridine, the mixture is cooled to 0 C and 64.5 g of
valeroyl chloride are added dropwise at this tempera-
ture. When the addition is complete, the mixture is
left to stand at room temperature and stirred for two
hours. The solution is washed with a dilute solution
of hydrochloric a~id, dried over magnesium sulfate and
evaporated under vacuum to give 110 g of an oil, whi~h
is used as such for tAe next step. This oil is dis-
solved in 400 ml of absolute ethanol and the mixture is
refluxed for two hours and left to stand overnight at
room temperature. The ethanol is evaporated off under
vacuum and the oily residue is distilled under reduced
pressure to give 63.3 g of ethyl 3-oxoheptanoate in the
~orm of a liquid o~ b.p.20 = 115-120 C.
Example 2: Ethyl 3-oxohexanoate
Formula ~ Rl = n-propyl, Rlo = ethyl5
Prepared by the same procedure as Example l.
Liquid of b.p.20 - 95-100C.
~xample 3: Ethyl 2-(4-nitrobenzyl)-3-oxohepkanoa30
Formula (V): R~ = n-butyl, V = N02, Rlo =
ethyl
57.3 g of ethyl 3-oxoheptanoate are dissolved
in 300 ml of ethanol. A solution o~ sodium ethylate,
2 ~
- 36 -
prepared by adding 7.7 g of sodium to 50 ml of ethanol,
is added and the mixture is stirred for 20 minutes at
room temperature. 72 g of 4-nitrobenzyl bromide are
then added in portions and the mixture is subsequently
05 stirred for two hours at room temperature and then for
two hours under reflux. The ethanol is evaporated off
under vacuum and the residue obtained is taken up with
water and then extracted with chloroform. The organic
phase is dried over magnesium sulfate and concentrated
under vacuum. The oil obtained is taken up in a
mixture of ether and pentane and the crystals formed
are filtered off to remove the dibenzylated derivative
(m.p. = 135C); the mother liquors, ~oncentrated under
vacuum at 120C to remo~e the starting unsubstituted
keto-ester, give 69.2 g of ethyl 2-(4-nitrobenzyl)-3-
oxoheptanoate in the form of an oil, which is used as
such for the next step.
Example 4: Ethyl 2-(4-nitrobenzyl)-3-oxohexanoate
Formula (V): Rl = n-propyl, V - NO2, Rlo =
ethyl
Prepared by the procedure of Example 3, start-
ing from the ethyl 3-oxohexanoate prepared in Example
2.
Oil used as such for the next step.
Example 5: Ethyl 2-(2'-methoxycarbonylbiphenyl-4-yl)-
methyl-3-oxoheptanoate
Formula (V): R1 = n-butyl, V =
MeO2C/~
R1o = ethyl
2~3~28
- 37 -
Prepared by the procedure of Example 3, start-
ing from the ethyl 3-oxoheptanoate prepared in Example
1 and methyl (4~-bromomethylbiphenyl-2-yl)carboxylate.
Oil used as such for the n~xt step.
05
Preparation o~ methyl (~'-bromomethylbiphenyl-2-yl~-
carboxylate
A) Methyl (4'-methylbiphenyl-2-yl)carboxylate
1015 ml of acetyl chloride are added to 300 ml of
methanol cooled to 0 C. The mixture is stirred for 10
minutes at this temperature and 15 g of (4'-methylbi-
phenyl-2-yl)carboxylic acid (prepared according to
MEYERS A.I.; MIHELICH E.D., J. Am. Chem. Soc., 1975,
I5 97(25~, 7383, by reacting (4-methylphenyl)magnesium
bromide with 2-(2-methoxyphenyl)-4,4-dimethyl-1,3-
oxazolidine) are then added. The mixture is then
refluxed for 4 hours and the solvents are evaporated
off under vacuum to give 16 g of methyl (4'-methylbi-
phenyl-2-yl)carboxylate in the form of an oil, which is
used as such for the next step.
B) Methyl (4'-bromomethylbiphenyl-2-yl)carboxylate
16 g of methyl (4'-methylbiphenyl-2-yl)carhoxy
late, prepared in A), are dissolved in 120 ml of carbon
tetrachloride in the presence of 12.6 g of N-hromo-
succinimide and 0.5 g of benzoyl peroxide. The mixture
is refluxed for 6 hours, the crystals are filtered off
and the remaining solution is washed with a solution of
sodium bicarbonate and then evaporated under vacuum.
The residue is taken up with ether and the solution is
then filtered on charcoal and evaporated under vacuum
to give 14.5 g of methvl (4'-bromomethylbiphenyl-2-
yl)carboxylate in the form of an oil, which is used as
such ~or the next step.
- 38 -
Example 6: Ethyl ~-(2~-cyanobiphenyl-4-yl)methyl-3-
oxoheptanoate
05 Formula (V): Rl = n-butyl, v =
Rlo - ethyl
g of ethyl 3-oxoheptanoata, prepared in
Exa~ple 1, are dissolved in 300 ml of tetrahydrofuran.
31.6 g of 4'-bromomethyl-2-cyarlobiphenyl are added
together with 40 ml of N,N-diisopropylamine and 10 g of
lithium bromide. The mixture is refluxed or 15 hours
and then concentrated under vacuum, iced water and
dilute hydrochloric acid are added and the mixture is
then extracted with ethyl acetate. Tha organic phase
is washed with water and then dried and evaporated
under vacuum. The residue obtained is heated under
vacuum at 130 C in order to remove the residual ethyl
3-oxoheptanoate, giving 41 g of ethyl 2-(2t-cyanobi-
phenyl 4-yl)methyl-3-oxoheptanoate in the form of an
oil, which i8 used as such for the next step.
Preparation of 4'-bromomethyl-2-cyanobiphenyl
A) 4'-Methyl-2-cyanobiphenyl
18.5 g of (4'-methylbiphenyl-2-yl)carboxylic
acid, prepared as in Example 5 A), are refluxed in 60
ml of thionyl chloride for two hours. The thionyl
chloride is concentrated under vacuum, the residue is
poured into a 28~ solution of ammonium hydroxide, the
mixture is stirred for 30 minutes and the crystals
obtained are filtered off, washed with ether and then
dried to give 14.5 g of (4'-methylbiphenyl-2-yl)carbox-
amide in the form of crystals melting at 128C. These
~ 3~ _ .
?
cryst~ re ~akan u~ in 50 m~ o~ thionyl ~hlorl~a and
the mlxture 1~ re~lux~d ~or 3 hour~ and then ~naen~r~-
tad un~ar vaauum to ~lv~ 9 . ~ o~ 4'~ethyl-2=ay~nobi-
phe~yl in the ~orm o~ ory~a~ melti~ a~ 45-~6 C.
05
B) 4'-Bromo~t~yl-~-cyano~lph~nyl
7.~ 4'-m~thyl-?-~yanobiphenyl, prepa~d ln
A), ~re.~ o~ve~ in 1~0 m} o~ a~rbDn t~r~ch~o~ide in
t~ pr~38~nae o~ 7 . 3 g o~ N-br~mo~u~cinintidG ~nd O . 3 4~
o~ b~n20yl peroxid~. The mix~-ure i~ re:Elux~d ~or ~
hours, th~ cry~ are ~ D~ he ~emainin~
~olution 1~ conce~t~ted under ~auum and th~ r~ ue
i~ ~rystalli~d ~ro~ ~ther ~o glve 6.6 g of 4'-bromo-
me~hyl-2-~r~nobiphen~l ~n th~ ~orm of cry~al~ m~l~ing
15 ~ 115-118 C
~Xhm~ hyl~3~n-butyl-~ 14-~ltr~n~yl~
. hyd~oxypy~801
For~ula ~IX~: R~ ~ n-butyl, Ra ' CH~ ~ V
N0
~0 g oP ~hy~ 2-(4-nitrobenzyl)~3-oxoh~p~no~
at~, pr~p~rad in Ex~mpl~ 3, 4~ dlssolved in 150 ml o~
~t~nol, ~nd 4 ml o~ me~hylhyd~azina a~ ~dded. ~h~
mixture i~ r~luxa~ ~or ~ hou~. The. ~ hanol i&
ev~porAt~d o~ und~r va~uum, ~h~ r~ ue i~ tak~n Up
wi~h w~ter ~nd ~h~ ~x~raot~d wl~h ~hyl ~etate, ~h~
organiG pha~ hen wash~d ~ver~ tim~s w~th
dilute ~olu~ion o~ ~odlum hyA~oxi~ and the aom~lnad
a~ueou~ ~ractlon~ ar~ acid~ with eul~ur dioxide and
th~n extra~t~d wl~h chloro~orm. The ~hloro~orm pha~e
i~ ~risd o~ar m~ne6~um sul~t~ ~nd e~flporat~d under
vacuum ~o glve a re~i~Ue WhlCh cry~tAllize~ ~rom ~th~r.
~h~ ~ryR~ are ~iltered o~ and re~ry~t~ ed ~rom
.
203~2~
- 40 -
ethyl acetate to give 10~9 g of 1-methyl-3-n-butyl-4-
(4-nitrobenzyl)-5-hydroxypyrazole in the form of
crystals melting at 136C~
05 Example 8: 1-Methyl-3-n-propyl~4 (4-nitrobenzyl~-5~
hydroxypyrazole
Formula (IX): R~ = n-propyl, R2 = CH3, V =
NO2
Prepared by the procedure of Example 7, start-
ing from the ethyl 2-~4-nitrobenzyl)-3-oxohexanoate
prepared in Example 4.
Crystals melting at 174C.
Example 9: 3-n-Propyl-4-(4-nitrobenzyl~-5-hydroXy-
pyrazole
Formula (IX): R1 = n-propyl, R~ = H, V = N02
2~
Prepared by the procedure of Example 7, start-
ing from the ethyl 2-(4-nitrobenzyl)-3-oxohexanoate
prepared in Example 4 and hydrazine.
Crystals melting at 196C.
Example 10: 1-Ethoxycarbonylmethyl-3-n-propyl-4-(4-
nitrobenzyl)-5-hydroxypyrazole
Formula (IX): ~l = n-propyl, R2 = CH2C02Et,
V ~ N0z
Prepared by the procedure of Example 7, start
ing from ethyl hydrazinoacetate.
Crystals melting at 134C.
2~3~2~
- 4~ -
Example 11: 1-(2,2,2-Trî~luoroethyl)-3-n propyl-4-(4- -.nitrobenzyl)-5-hydroxypyrazole
Formula (IX): R1 = n-propyl, ~2 = CH2CF3,
05V = N02
Prepared by the procedure of Example 7, start-
ing from 2,2,2-tri~luoroethylhydrazine.
Crystals melting at 160C.
Example 12: 1-~ethyl-3-n~butyl-4-(2'-mathoxycarbonyl-
biphenyl-4-yl)methyl-5-hydroxypyrazole
Formula (IX~: Rl = n-butyl, R2 = CH3,
:15 ~
V= 1 11
CH,O,C ~
Prepared by the procedure of Example 7, start-
ing from the ethyl 2-(2' methoxycarbonylbiphenyl-4-
yl)methyl-3-oxoheptanoate prepared in Example 5.
Crystals melting at 108C.
Examplç_13: 1-~ethyl-3-n-butyl-4-(2'-cyanobiphenyl-4-
25yl)methyl-5-hydroxypyrazole
Formula (IX): R~ = n-butyl, R2 = CH3,
V= 1 11
30NC ~
Prepared by the procedure of Example 7, start-
ing from the ethyl 2-(2'-cyanobiphenyl-4-yl)methyl-3-
oxoheptanoate prepa~ed in Example 6.
35Crystals melting at 138~C.
~38~2~
- 42 -
Example 14: Ethyl [l-methyl-3 n-propyl-~-(4-nitro-
benzyl)pyrazol-~-yl]oxyacetate
Formula (Xl): R1 = n-propyl, R2 = methyl,
05 R3 = CH2C02Et, V = NO2
22.4 g of 1-methyl-3-n-propyl-4-~4-nitro-
benzyl)-5-hydroxypyrazole, prepared in Example 8, are
dissolved in 200 ml of acetone, and 8.7 g of sodium
~o carbonate and 9.2 ml of ethyl bromoacetate are added.
The mixture is refluxed for 5 hours and the solvents
are concentrated to dryness. The residue is taken up
with water and then extracted with ether. The organic
phase is dried over magnesium sulfate and evaporate~ to
dryness. The residue obtained is taken up with iso-
propyl ether and the resulting crystals are filtered
of f to give 9 g of ethyl [1-methyl-3-n-propyl-4-(4-
nitrobenzyl)-5-oxopyrazol-2-yl]acetate melting at 62C.
The mother liquors are concentrated and the oil
obtained is chromatographed on silica gel in a methy-
lene chloride/acetone eluent (90/10) to give lO g of
ethyl [l-methyl 3 n-propyl-4-(4-nitrobenzyl)pyrazol-5-
yl]oxyacetate in the form of crystals melting at
60-61 C.
Example 15: ~thyl [l-methyl 3-n-butyl-4-~4-nitro-
benzyl)pyrazol-5-yl~oxyacetate
Formula (XI): R1 = n-butyl, R2 = methyl,
~3 -- CH2C2Et l V = N02
Prepared by the procedure of Example 14.
Crystals melting at 68C.
2~3~28
- 43 -
Example 16: Ekhyl [3-n-propyl-4~ nitrobenzyl~pyrazol-
5-yl~oxyacetate
~ormula (XI): R1 = n-propyl, R2 = H, R3 =
05 CH2Co2Et~ V = N02
Prepare~ by the procedure o~ Example 14.
Crystals melting at 116C.
Example 17: ~ethyl ~1-methyl-3-n-p~opyl-4-(4-nitro-
benzyl)pyrazol-5-yl]oxyacetat~
Formula ~XI): R1 = n-propyl, R2 = methyl,
R3 = CH2C02Me, V = N02
Prepared by the procedure of Example 14.
Crystals melt.ing at 58C.
Example 18: Methyl Cl-l2,2,2-tri~luoroethyl)-3-n-
prupyl-4-(4-nitrobenzyl)pyrazol-5~yl]oxy-
acetate
~o~mula ~XI): R1 = n-propyl, R2 = CH2CF3,
R3 = CH2C02Me, V = N02
Prepared by the procedure of Example 14.
Crystals melting at 73C.
E~am~le 1~: Ethyl tl-methyl-3-n-butyl-4-(2'-methoxy-
carbonylbiphenyl-~-yl)methylpyrazol-5-yl3-
oxyacetate
For~ula (X~): Rl = n-~utyl, R2 = methyl,
-- CH2C02Et, V =
. ~.
2~3~8
- ~4 -
Prepared by the procedure of Example 14.
oi 1 used as such for the next step.
Example 20: Ethyl t1-methyl-3-n-butyl-4-(2~-cy~nobi-
05 phenyl-4-yl)~ethylpyrazol-s-yl~oxyacetate
Formula (XI3: R1 = n~butyl, R2 ~ methyl,
R3 = CH2CO2Et, V =
NC~
Prepared by the procedure of Example 14.
Oil used as such for the next step.
Example 21: Ethyl [l-methyl-3-n-butyl-4-(4-amino-
benzyl~pyrazol-5-yl]oxyacetate
Formula (I~ Rl = n-butyl, R2 = methyl, A =
OR3, R3 = cH2C02Et, Ra = NH2
3.4 g o~ ethyl [1-methyl-3 n butyl-4-~4-nitro-
benzyl)pyrazol-5-yl]oxyacetate, prepared in Example 15,
are dissolved in 50 ml of absolute ethanol in the
presence of 500 mg of Raney nickel. The mixture is
hydrogenated at atmospheric pressure and room tempera-
ture and, when the uptake of hydrogen has ceased, tha
catalyst is filtered off, the ethanol is evaporated off
under vacuum and the residue is taken up with pentane
to give 2.9 g of ethyl [1-methyl-3-n-butyl-4-(4-amino-
ben2yl)pyrazol-5-yl]oxyacetata in the form of crystals
melting at 65C.
.
~3~8
- 45 -
Example 22: ~khyl [1-methyl-3-n-propyl-~-(4-amino-
benzyl)pyrazol-5~yl]oxyacetate
Formula (I): Rl = n-propyl, R2 = methyl,
05 A = OR3, R3 = CH2CO2Et, R~ =
NH~
Prepared by the procedure of Example 21.
Crystals melting at 103C.
. "
Example_23: Methyl [1-methyl-3-n-propyl-4-(~-a~ino-
~enzyl~pyrazol-5-yl~oxyacetate
Formula (I): R1 = n-propyl, R2 = methyl,
A = OR3, R3 = CH2CO2Me, R~ =
NH2
Prepared by the procedure of Example 21.
Oil used as sUch for the next step.
Example 24: Methyl [1-(2,2,2-trifluoroethyl)-3-n-
propyl-4-(4-aminobenzyl)pyrazol-5-yl]oxy-
acekate
Formula (I): Rl = n-propyl, R2 = CH2CF3,
A = OR3, R3 = CH2CO2Me, R4 =
NHa
Prepared by the procedure o~ Example 21.
Crystals melting at 63~C.
3~42~
- 46 -
Examp~e 25~ thoxycarbonylmet~lyl-3-n-propyl 4-(4-
aminobenzyl)-5-hydroxyp~razole
Formula (I): Rl = n-propyl, Ra = CH2CO2Et,
05 A = OR3, R = H, R - NH
Prepared by the procedure of Example 21.
Oil used as such for the next step.
Example_26: Ethyl ~3-n-propyl-4-(~-aminobenzyl)pyrazol-
5-ylloxyacetate
F~rmula (I): Rl = n-propyl, R2 = ~, A =
OR3, R~ = CH2C02Et, R4 = NH2
Prepared by the procedure of Example 21.
oi l used as such for the next step.
Exampla ~7: E~hyl [l-methyl-3~n-butyl 4-(4-(2-carbo~y-
~enzoylamino)benzyl)pyrazol-5-yl]oxyacetate
Formula (I): Rl = n-butyl, R2 = methylj A =
OR3, R3 = CH~Co2Et,
CO2H
~H-C ~
3 g of ethyl [1-methyl-3-n-butyl~4-(4-amino-
benzyl)pyrazol-5-yl]oxyacetate, prepared in Example 21,
are dissolved in 50 ml of acetonitrile. 1.3 g o~
phthalic anhydride are added and the mixture is left to
stand at room temperature overnight. The crystals
obtained are filtered off, washed with isopropyl ether
`` 2~3~8
- 47
and dried to give 2.5 g of ethyl [1-methyl-3-n-butyl-4-
(4-(2-carboxybenzoylamino~benzyl)pyrazol-5-yl]oxy-
acetate in the form of crystals melting at 140-141 C.
05 The following Examples were prepared by the
same procedure:
Example 28: ~ethyl tl-methyl-3-n-propyl-4-(4-(2-car-
boxybenzoylamino)benzyl)pyrazol-5-yl]oxy-
ace$ate
For~ula (I) R1 = n-propyl, R2 = CH3, A =
OR3, R3 = CH2CO2Me,
o
NH--C~
HO2C
Crystals, in the form of the dicyclohexylamine
salt, melting at 173-174C.
Example 29: Ethyl tl-~ethyl-3-n-propyl-4-(4-(2-carboxy
b~nzoylamino)benæyl)pyrazol-5-yl]oxyacetate
Formula (I) Rl = n-propyl, R2 = CH9, A =
OR~, R3 = CH2CO2Et,
~4 =
HO,C
Crystals melting at 139-140C.
~-` 2~38428
-- 48 -
Example 30: ~thyl [1-methyl-3-n-propyl-4-(4-(2-carbOXy-
3,6-dichloro~enzoylamino)benzyl)pyrazol-5
yl]oxyacetate
05 Formula ( I ): Rl = n-propyl t ~a a CH3, A =
OR3, R3 = CHzCO2Et,
O Cl
NH--C~
R4 =
HO2C
Ct
From 3,6-dichlorophthalic anhydride.
Crystals, in the form of the dicyclohexylamina
salt, melting at 199-200C.
~amnl~ 31: ~e~hyl [1-~ethyl-3-n-propyl-4-(4-~2-car-
~oxy-3,6-dichloxobenzoyla~ino)benzyl)~
pyrazol-5-yl]oxyace~ate
For~ula lI): Rl = n-propyl, R2 = mathyl,
A = OR3, R3 = CH2CO2Me,
O Cl
Il I
NH - C~
HO2C
Cl
From 3,6-dichlorophthalic anhydride.
Crystals mel~ing at 150-151C.
:
.
-` 2038~28
~9
Example 32: ~ethyl Ll-(2,2,2-trifluoroethyl)~3-n-
propyl-4-(4-~2-carbo~y-3,6-dichlorobenzoyl-
amino)benzyl)pyrazol-5-yl]oxyacetate
05 Formula ~ Rl = n-propyl, R2 = CH2CF3,
A = OR3 ~ R3 = CH2COaMe,
O Cl
NH-C
~4= 1~
Ho2~ T
Cl
From 3,6-dichlorophthalic anhydride~
Crystals melting at 169-170C~
Example 33: ~ethyl [1-(2,2,2-trifluoroethyl)-3-n-
propyl-4-(4-(2-carboxybenzoyla~ino)benzyl)-
pyrazol-5-yl]oxyacetate
Formula ~ R~ = n-propyl, R2 = CH2CF3,
A = OR3, R3 = CH2CO2Me,
NH-C
HO2C
Crystals melting at 189-192C.
03~28
- 50 -
Example 34: [3-n-Propyl-4-(~ [2-carboxyben~oylamino)-
be~zyl)pyrazol-5-yl]oxyacetic acid
For~ula (I). Rl = n-propyl, Rz = H, A -
05 VR3, R3 = CH2C02H,
ll
NH--C~
1 0 HO2C
0.9 g of ethyl ~3-n-propyl-4-(4-aminobenzyl)-
pyrazol-5-yl~oxyacetate, prepared in Example 26, is
dissolved in 20 ml of acetonitrile in the presence of
0.45 g o~ phthalic anhydride. The mixture is left to
stand at room temperature overnight. The acetonitrile
is evaporated off under vacuum and the residue is taken
up in an ethyl acetate/ether mixture to give crystals,
which are filtered off. These crystals are dissolved
in methylene chloride and the solution is washed
several times with a 1 N solution of sodium hydroxide.
The combined aqueous phases are acidified by having
sulfur dioxide bubbled through them and the crystals
obtained are filtered off and dried to give 0.5 g of
[3-n-propyl-4-(4-t2-carboxybenzoylamino)benzyl)pyrazol-
5-yl]oxyacetic acid in the form of crystals melting at
170-171 C.
203~
- 51 -
Example 35~ Methyl-3-n-propyl-4~ (2-sulfobenzoyl-
a~ino)benzyl]pyrazol-5~yl]oxyacetic acid
Formula (I): R1 - n-propyl, Rz = CH3, A =
05 OR3, R3 -
NH--C
R~ = \~
1 O HOJS~
Prepared by the procedure of Example 34, start-
ing from the cyclic anhydride of orthosulfobenzoic
acid.
Crystals melting at 16~-170C.
Example 36: 4'-[1-~ethyl-3-n-butyl-5-hydroxypyrazol-4-
yl]methylbiphenyl-2-carboxylic acid
Formula (I): R1 = n-butyl, R2 = methyl, A =
OR3 ~ R~ = H,
\~ .
R" =
HO,C ~ ~
1.5 g of l~methyl-3-n-butyl-5-hydroxy-4-(2'-
methoxycarbonylbiphenyl-4-yl)methylpyrazole, prepared
in Example 12, are suspended in 15 ml of a 1 N solution
o~ sodium hydroxide and the suspension is stirred ~or
one hour at 40~C. The solution is washed with methy-
lene chloride and then acidified by having sulfur
dioxide bubbled through it and extracted with methylene
chloride. The organic phase is dried over magnesium
sul~ake and then evaporated under vacuum. The residue
38~
- 52 -
is diluted in ethyl acetate, extracted with a solution
of sodium bicarbonate and then acidified with sulfur
dioxide and the crystals obtained are filtared off and
then dried to give 1.1 g of 4'-[1-methyl-3-n-butyl-5-
05 hydroxypyrazol-4-yl]methylbiphenyl-2-carboxylic acid in
the form of crystals melting at 228-30 C.
Exam~le 37O tl-Methyl-3-n-butyl-4-(2'-carboxybiphenyl-
4-yl)methylpyrazol-5-yl]oxyacetic acid
Fo~mula (I): R = n-butyl, R2 = CH3, A =
OR3, R3 = CH2CO2H,
\~ :
R4 =
HO~C ~ ~
2.6 g of ethyl [1-methyl-3-n-butyl-4-(2'-
methoxycarbonylbiphenyl-4-yl)methylpyrazol-5-yl]oxy-
acetate, prepared in Example 19, are dissolved in 30 mlof ethanol. 1 g of sodium hydroxide pellets and 10 ml
of water are added and the mixture is stirred at room
temperature for two hours and then heated for 3 hours
at 50-55C. After cooling, the solution is diluted
with water and washed with ether and the aqueous phase
is acidified by having sulfu.r dioxide bubbled through
it and extracted with chloroformO The organic phase is
dried over magnesium sulfate and evaporated under
vacuum to give a residue which crystallizes from an
ethyl acetate/ether mixture to give 2 g of ~1-methyl-3-
n-butyl-4-(2'-carboxybiphenyl-4-yl)methylpyrazol-5-
yl]oxyacetic acid in the form of crystals melting at153-154 C.
2~38~28
- 53 -
Exampla 38: 1-Methyl-3-n-butyl-~-(4-nitrobenzyl)-5-
methoxycarbonyloxypyra201e
Formula (I): Rl = n-butyl, R2 = methyl, A =
05 OR3, R3 - Il-OMe, R~ = NO2
o
12 g of 1-methyl-3-n-butyl-4-(4-nitrobenzyl)-5-
hydroxypyrazole, prepared in Example 7, are dissolved
in 120 ml of 1,2-dichloroethane in ~he presence of 6 ml
of triethylamine. 3.3 g of methyl chloroformate are
added dropwise and the mixture is stirred for 2 hours
at room temperature and then for 4 hours under reflux.
After cooling, the solution is washed with water and
then dried over magnesium sulfate and concentrated
under vacuum. The oily residue is chromatographed on
silica gel in a methylene chloride/acetone eluent
t95/5) to give 6.6 g of 1-methyl-3-n-butyl-4~(4-
ni~robenzyl~-5-methoxycarbonyloxypyrazole in the form
of crystals melting at ~4-47C.
.
Example 39~ ethyl-3-n-butyl-4-~4-aminobenzyl)-5-
methoxycarbonyloxypyrazole
Formula (XI3: R~ = n-butyl, R2 = methyl,
R3 = I-OMe, V = NH2
Prepared by the procedure of Example 21.
oi l used as such for the next step.
~3~2~
- 54 -
Example ~0: 2-E[1-Methyl-3-n~butyl-5-hydroxypyrazol-4
yl]methylphenyl-4-yl]aminocar~onyl-3,6-
dichloroben~oic acid
05 Formula (I): Rl = n-hutyl, Ra = methyl, A =
OR3, R3 = H,
O Cl
NH--C~
R4 =
HOzC Cl
Prepared by the procedure of Example 27, start-
ing Prom 3,6-dichlorophthalic anhydride.
Crystals melting at 172-174C.
E~ample 41: 2-[~1-EthoxycarbDnyl~ethyl-3-n-propyl-5-
hydroxypyrazol-4-yl~methylphenyl-4-yl]-
a~inocar~onyl-3~6-dichlorobenzoic acid
Form~la (I): Rl - n-propyl, R2 = CH2CO2Et,
A Q OR3, R3 = H,
NH-C
R -
HOlC
Prepared by the procedure of Example 27, start~
ing Prom 3,6-dichlorophthalic anhydride.
Crystals melting at 150-153C.
2~38~L2~
55 -
Example 4?: Ethyl ~l-ethoxycarbonylmethyl-3-n-propyl-4-
(4-nitrobenzyl)pyx~zol-~5-yl]oxyacetate
For~ula (XI): Rl = n-propyl, R2 = CH2CO2Et,
05 R3 = CH2CO2Et, V = NO~
Prepared by the procedure of Example 14.
Oil used as such for the next step.
0 Example 43: Ethyl [l-ethoxycarbonylmethyl-3-n-propyl-4-
(4-aminobenzyl)pyrazol-5 yl]oxyacetate
Formula (I3: R1 = n-propyl, R2 = CH~CO2Et,
A = OR3, R3 = CH2CO2Et, R4 =
NH2
Prepared by the procedure of Example 21.
Oil used as such for the next step.
Example 44: 2-[(1-Ethoxycarbonylmethyl-3-n-propyl-5-
ethoxycarbonylmethox~pyra~ol-4-yl3methyl-
phenyl-4-yl~aminocarbonyl-3,6-dichloro-
be~zoic acid
Formula (I): R1 = n-propyl, R2 = CH2CO2Et,
A = OR3, R3 = CH2Co2Et,
O Ct
NH--C~
3 0 R" =
HO,C
Prepared by the procedure of Example 27. start-
ing from 3, 6-dichlorophthalic anhydride.
2~38~28
- 56 -
Crystals, in the form of the dicyclohexylamine
salt, melting at 189-l91~C.
~xample 45- 2-t(l-Methyl-3-n-propyl-~-ethoxycarbonyl-
05 methoxypyrazol-4-yl)methylphenyl~4-yl]-
aminocarbonylbenzenesulfonic a~id
Formula (I): Rl = n-propyl, R2 = methyl,
A = OR3, R3 =
11
NH--C~3
HO,S
Prepared by the procedure of Example 27, but
with the cyclic anhydride of orthosulfobenzoic acid.
Crystals meltinq at 203-20sC.
20 Example 46: N-[rl-~ethyl-3-n-butyl-4-(4-nitrobenzyl)-
pyrazol-5-yl~oxyacetyl]morpholine
Formula (XI): ~1 = n-butyl, R2 = methyl,
/ \ '
R~ = CHI- C-N O , V = NO2
Prepared by the procedure of Example 14, start-
ing from N-tchloroacetyl)morpholine.
Crystals melting at 98C.
Preparation o~ N-(chloroacetyl)morpholine
21.7 g of morpholine are dissolved in 250 ml of
methylene chloride and the solution obtained is cooled
2~3~428
- 57 -
with an ice/water mixture.
14.1 g of chloroacetyl chloride are added drop
wise with the temperature being kept at 0C, and the
mixture is then stirred for 3 hours at room temperature
05 and washed with a dilute solution of hydrochloric acid.
The organic phase is dried over magnesium sulfate and
evaporated under vacuum to yive 29.8 g of N-~chloro-
acetyl)morpholine in the form of an oil of sufficient
purity for the next step.
Example 47: N-[[l-Methyl-3-n-butyl-4-~4-aminobenzyl)-
pyrazol-5-yl]oxyacetyl]morpholine
Formula (I): Rl = n-butyl, R2 = methyl, A =
~
OR3, R3 = CH~- C-N O, R4 =
NH2
Prepared by the procedure of Example 21.
Crystals melting at 130C.
Example 48: N-[tl-Methyl-3-n-butyl-4-[4-(2-sulfo-
benzoylamino)benzyl]pyrazol-5-yl]oxy-
acetyl]morpholine
Formula (I): R1 = n-butyl, R2 = methyl~ A -
r~
OR3, R~ = CH,- C-N 0,
R4 = ~
~3
HQ~S
~038~2~
- 58 -
Prepared by the procedure of Example 27 using
the cyclic anhydride of orthosulfobenzoic acid.
Crystals melting at 236-237C.
05 Example 49: 2-[1-~ethyl-3-n-butyl-4-(4-nitrobenzyl)-
pyrazol-5-yl]oxyethanol
Formula (XI): R1 = n-butyl, R2 = methyl,
R3 = CH2CH20H, V = NO2
12 g of 1-methyl-3-n-butyl-4-(4-nitrobenzyl)-5-
hydroxypyrazole, prepared in ~xample 7, are dissolved
in 150 ml of butan~2-ons. 4.6 g of sodium carbonate
and 6 g of 2-bromoethanol are addad and the mixture is
refluxed for 12 hours. After cooling, the solution is
concentrated and then taken up with water and the pH is
rendered alkaline by the addition of dilute sodium
hydroxide solution. After extraction with methylene
chloride, the organic phase is dried over magnesium
sulfate and then evaporated to dryness. ~he residue
obtained is chromatographed on silica gel in an ethyl
acetate/acetone eluent (6/4) to give 5 g of 2
methyl-3-n-butyl-4-(4-nitrobenzyl)pyrazol-5-yl]oxy-
ethanol in the form of crystals melting at 81C.
Example 50: 2-[1-Methyl-3-n-butyl-4-(4-aminobenzyl)-
pyrazol-5-yl]oxy~thanol
Formula (I): Rl = n-butyl, R2 = methyl, A =
OR3, R3 = CH2CH20H, R4 = NH2
Prepared by the procedure o~ Example 21.
oi l used as such for the next step.
2~3~
- 59 -
Example 51; 2-~[1-~ethyl-3-n-butyl-5-(2-hydxoxyethoxy~-
pyrazol-4-yl~ethylphenyl 4-yl}aminocar-
bonylbenzenesulfonic acid
05 Form~la (I): R1 2 n-butyl, R2 - methyl r A =
OR3, R3 = CH2CH20H,
O
NH
1 0
HO,S
1'.
Prepared by the procedure of Example 27, but
using the cyclic anhydride o~ orthosulfobenzoic aci~.
~rystals melting at 170-171C.
Exam~le 52: 1-M~thyl-4-(4-nitrobenzyl)-3-n-propyl-5-
(N,N-dimethylcarbamoyl30xypyrazole
.
Formula (XI): Rl = n-propyl, R2 = methyl,
R = C - N~ , V = NO,
CH,
I
10 g o~ 1-methyl-~-(4-nitrobenzyl)-3-n-propyl-
5-hydroxypyrazole, prepared in Example 8, are dissolved
in 100 ml o~ methylene chloride and 5 ml of triethyl-
amine. 3.2 ml of N,N-dimethylcarbamoyl chloride are
addad dropwise and the mixture is then ref~uxed for 10
hours. A~ter cooling, the mixture is taken up with
water and washed with a solution o~ potassium bicarbo-
nate. The chloro~orm phase is dried over magnesium
sul~ate and then evaporated under vacuum to give 9.1 g
o.~ 1-methyl-4-(4-nitrobenzyl)-3-n-propyl-5-(N,N-di-
methylcarbamoyl)oxypyrazole in the form of crystals
2~3~2~
- 60 -
melting at 90C.
Example 53: 1-~ethyl-4-(4-a~inobenzyl)-3-n-propyl-5
(N,N-di~ethylcarbamoyl)oxypyrazole
05
~ormula (~): Rl = n-propyl, R2 = methyl,
A = OR3, R3 = C - N~
R4 = NH2
Prepared by the procedure of Example 21.
Crystals melting at 138C.
15 Example 54: 2-[[1-~ethyl-3-n-prop~1-5-(N,N-dimethylcar-
ba~oyl)oxypyr~zol-4-yl]methylpheny~-4-yl~-
aminocar~onylbenzenesulfonic acid
Formula (I): Rl = n-propyl, R2 = methyl,
CH,
A = OR~, R3 = C - N~
O CH,
R~ = NH
HO~S ~
Prepared by the procedure of Example 27 u~ing
the cyclic anhydride of orthosulfobenzoic acid.
Cry~tals melting at 210-2~C.
2~38428
~ample 55: 1-~ethyl~3-n-propyl-4-(4-nitrob~nzyl~-5-
~,N-diethylcarbamoyl)oxypyrazole
Formula (XI): R1 = n-propyl, R2 = methyl,
C,Hs
R = C--N~ , V = NO2
o C2Hs
Prepared by the procedure of ~xample 52.
lo Oil used as such for the next step.
Example 56: 1-Methyl-3-n-propyl-4-[4-amino~enzyl)~5-
(N,N-diethylcarbamoyl~oxypyrazole
Formula (I): Rl = n-propyl, R2 = methyl,
,c2Hs
A = OR3, R3 = C - N~
O C~,
R4 = N~2
Prepared by the procedure of Exampl~ 21.
Crystals melting at lOO~C.
~xample 57: 2-[[1-Methyl-3-n-propyl-~-(N,N-diethylcar-
bamoyl)oxypyrazol-~-yl]methylphenyl-4-yl]-
aminocarbonylbenzenesul~onic acid
Formula (I): R1 = n-propyl, R2 = methyl,
A = OR3, R~ N~
C2H,
o
NH--C~
HO~S~
2~3~
- 62 -
Prepared by the procedure o~ Example 27 using
the cyclic anhydride of orthosulfobenzoic acid.
Crystals melting at 216-217C.
05 Example 58: 1-(2,2,2-Trifluoroethyl)-3-n~-propyl-5-(N,N-
dimethylcarba~oyl)oxy-4-~4-ni.trobenzyl)-
pyrazole
Formula (XI): R1 = n-propyl, R2 = CH2CF3,
CH,
R9 = 11--N~ , V = NO2
o CH,
Prepared by the procedure of Example 52.
Crystals melting at 70C.
~x~ple ~ 2~2,2-Trifluoroethyl)-3-n-propyl-5-(N,~-
di~ethylcarbamoyl)oxy-4-(4 aminobenzyl)-
pyrazole
Formula (I): R1 = n-propyl, R2 = CH2CF3,
,CHJ
A = OR3, R3 = Il-N~
O CH,
R4 = NH2
Prepared by the procedure of Example 21.
Crystals melting at 82~C.
`` 2~3~2~
- 63
Example 60: 2~ (2,2,2-Trifluoroethyl)-3-n-propyl-5-
(N,N-dimethylcarbamoyl)oxypyrazol-4-yl]-
methylphenyl-4-yl]aminocarbonylbenzene-
sul~onic acid
05
~ormula (I~: Rl = n propyl, ~2 = CH2CF3,
A = OR3, R3 = C - N~ ,
R a NH
HO~S ~
Prepared by the procedure of Example 27 using
the cyclic anhydride of orthosulfobenzoic acid.
Crystals melting at 159-161~C.
Ex~mp~e 61~ 1-(2,2,2-Tri~luoroethyl~-3-n-propyl-4-~4-
nitrobenzyl)-5-~2-hydroxyethoxy)pyrazole
Formula (XI): Rl = n-propyl, R2 = CH2CF3,
R3 = CH2CH~o~, V = NO2
. ~
Prepared by the procedure of Example 49.
Crystals melting at 71C.
xa~le 6?: 1-(2,2,2-Trifluoroe~hyl)-3-n-propy~-4-(4-
aminobenzyl)-5-(2-hydroxyethoxy)pyra201e
For~ula (I): ~1 = n-propyl, R2 = CH2CF3,
A - OR3, R3 = CH2CH20H, R4 =
NH2
Prepared by the procedure of Example 21.
2~3~2~
- 6~ -
Oil used as such for the next step.
Exam~le 63: 2-[[1-(2,2,2-Trifluoroethyl)-3-n-propyl-5-
(2 hydroxyethoxy)pyrazol-4-yl]me~hylphenyl-
05 4-yl]aminocarbonylbenzenesul~onic acid
For~ula (I): Rl = n-propyl, R2 = CH2CF3,
A = OR3, R3 = C~2~Hz~H,
R~ = NH
HO~S ~
Prepared by the procedure of Example 27 using
the cyclic anhydride of orthosulfobenzoic acid.
Crystals melting at 211 3C.
Example 64: 1-(2-~sthoxyphenyl)-4-tl-~e~hyl-3-n-propyl
4-(4-nitrobenzyl)pyrazol-5-yl30xyacetyl-
piperazine
F~rmula ~XI~: Rl = n-propyl, R2 = methyl,
CH,O
R9 = C~ C-N N ~ ,
V = NO~
Prepared by the procedure o~ Example 14, start-
ing from l-chloroacetyl-4-(2-methoxyphenyl)piperazine.
Oil used as such ~or the next step.
1-Chloroacetyl-4-(2~methoxyphenyl)piperazine is
prepared by the procedure used to synthesize N-chloro-
acetylmorpholine, described in Example 460
~3~
- 65 -
Example 65~ 2-~ethvxyphenyl)-4-[1-methyl-3-n-propy~-
4-(4-aminobenzyl)pyrazol-5-yl]oxyacetyl~
piperazine
05 Formula (I): Rl - n-propyl, R2 ~ methyl,
A = OR3,
CH,O
R3 = CH2- C~N N
R~ = NH2
Prepared by the procedure of Example 21.
Crystals melting at 145C.
Example 66: 1~(2-Metho~yphenyl~-~-E[1-~ethyl-3-n-
propyl-4-[4-(2-sulfobenzoylamino)benzyl]-
pyraæol-5-yl~oxyacetyl]piperazine
Formula (I): Rl = n-propyl, R2 = methyl,
A = OR~,
CHO
R3 = CH2-C N N~,
R~ = NH~3
Prepared by ~he procedure of Example 27 using
~he cyclic anhydride of orthosulfobenzoic acid.
Crystals melting at 226-227C.
203~2~
- 66 -
Z: Ethyl 2-(2~-cyanobiphenyl-4-yl)methyl-3-
oxohexanoate
~,
05 ~ormula ~V): Rl ~ n-propyl, V =
NC
Rlo = ethyl
Prepared by the procedure of Example 6.
Oil used as such for the next step.
Examp~e 68: 1-Me~hyl-3-n-propyl-4-(2'-cyanobiphenyl-4-
yl)methyl-5-hydroxypyrazole
For~ula (IX): Rl = n-propyl, V =
NC
R2 = met~yl
Prepared by the procPdure of Example 7.
Crystals melting a~ 1~4C.
Example 69: Ethyl rl-methyl-~-n-propyl-4-(2'-cyano~i-
phenyl-4-yl)methylpyrazol-5-yl]oxyacetate
~or~ula (~ Rl = n-propyl, R2 = methyl,
R3 = CH2CO2Et, R4 =
NC
Prepared by the procedure of Example 14.
Oil used as such for the next step.
" ~03~2g
- 67 -
Example 70: Ethyl [l-methyl-3-n-propyl-4-[2'-(tetrazol-
5-yl)biphenyl 4-yl]methylpyrazol-5-yl~o~y-
acetate
05 Formula (I): Rl = n propyl~ R2 = methyl,
A = OR3, R3 = CH2CO2Et,
R4 =
N/ =~
N H
11 g of ethyl [1-methyl-3-n-propyl-4-(2'-cyano-
biphenyl-4-yl)methylpyrazol-5-yl]oxyacetate, prepared
in Example 69, are dissolved in 75 ml of toluene.
6.5 g of trimethyltin nitride are added and the mixture
is refluxed for 24 hours. The solvent is evaporated
off under vacuum and the residue is chromatographed on
silica gel in a chloroform/methanol eluent (9/1) to
give 6~7 g of ethyl ~1-methyl-3-n-propyl-4-[2i-(tetra-
zol 5-yl)biphenyl-4-yl]methylpyrazol-5-yl]oxyacetate in
the form of crystals melting at 187-189C.
Exa~ple 71: 1-Methyl-3-n-propyl-5-hydroxy-4-t2'-(tet-
razol-5-yl)~iphenyl-4-yl]methylpyrazole
Formula (I): Rl - n-propyl, R2 = methyl,
A = OH, R4 =
N
N\~N'HN
5 g of 1-methyl-3-n-propyl-4-(2'-cyanobiphenyl-
4-yl)methyl-5-hydroxypyrazole, prepared in Example 68,
~Q3~4~8
- 68 -
are dissolved in 50 ml of toluene. 4 g of trimethyltin
nitride are added and the mixture is refluxed for 14
hours. After cooli~g, ~0 ml of methanol and 10 ml of
chloroform are added and the crystals formed are fil-
05 tered off and then taken up in 40 ml of ~oluene and 10ml of tetrahydrofuran. Gaseous hydrochloric acid is
bubbled through the reaction mixture for 10 minutes and
the mixture is then stirred for 1 hour at room tempera-
ture. The solvents are ~vaporated off under vacuum and
the residue is taken up with a dilute sol~tion of
sodium hydroxide and then washed with ethyl acetate.
The aqueous phase is neutralized by having sulfur di-
oxide bubbled through it and the residue crystallizes
slowly from water ~o give, after washing with acetone,
3.1 g of 1-methyl-3-n-propyl-5-hydroxy-4-[2'-(tetrazol-
5-yl)biphenyl-4-yl]methylpyrazole in the form of crys-
tals melting at 150-153C.
Example 72: 1-Methyl-3-n~butyl-5-hydroxy-4-[2'-(tet-
razol-5-yl)biphenyl-4-yl~methylpyrazole
Formula (I): R1 = n-butyl, R~ = methyl,
A = OH, R~
N
N~N~H
Prepared by the procedure of Example 71.
Crystals melting at 176-7C.
2~3~28
-- 6g
Exa~ple 73: 1-(2,2,2-Trifluoroethyl)-3-n-propyl-5-
hydroxy-4-(2'-cyanobiphenyl-4-yl)methyl-
pyrazole
05 For~ula (IX)~ Rl = n-propyl, R2 = CH2CF3,
\~ .
NC ~
Prepared by the procedure of Example 7, start-
ing from 2,2,2-trifluoroethylhydrazine.
Crystals melting at 154C.
Bxample 74: 1-(2,2,2-Trifluoroethyl)-3-n-propyl-5-
hydroxy-4-[2'-ttetrazol-5-yl)biphenyl-4-
yl]methylpyrazole
Formula (I): Rl = n-propyl, R2 = CH2CF3,
A = OH, R4 =
N
N\~N'N
Prepared by the procedure of Example 71.
Crystals melting at 218-220C.
Example 75: 2-tl Methyl-3-n-butyl-4-~2'-cyanobiphenyl-
4-yl)~ethylpyra~ol-5-yl]oxyethanol
Formula (XI): Rl = n-butyl, R2 = methyl,
R3 = CH2CH20H~ V ~
NC
.
.~
2~3~2~
- 70 -
Prepared by the procedure of Example 49.
Oil used as such for the next stap.
Example 76~ ~ethyl-3-n-butyl-4-[2~tetrazol-5-
05 yl)biphenyl-4-yl]methyl~yrazol-5-yl]oxy-
etha~ol
Yormula (~): R1 = n butyl, R~ = methyl,
A = OR3, R3 = CH2CH20H,
R~ =
N
N~H
Prepared by the procedure of Example 70.
Crystals melting at 105-8~C.
Example 77: Ethyl [1-(2,2,2-triflu~roethyl)-3-n butyl-
4-(2'-cyanobiphenyl-4-yl)methylpyrazol-5-
yl lo~yac~tate
Formula (XI): R~ = n-butyl, R2 = CH2CF3,
R3 = CH2CO2Et, V =
NC
Prepared by procedure 14.
Oil used as such ~or the next step.
03~2~
Example 78: Ethyl [1-(2,2~ trifluoroethyl)-3 n-~utyl-
4-~2'-(tetrazol-5-yl)biphenyl-~-yl]methyl-
pyrazol-5~yl]oxyacetate
For~ula (I): Rl = n-butyl, R2 = CH2CF3, A =
OR3, R3 = CH~CO2Et,
N~
~ _
N''H
Prepared by the procedure of Example 70.
Crystal~ melting at 140 1C.
Example 79: 1-(2,2,2 Trifluoroethyl)-3-n-butyl-4-~2'-
cyanobiphenyl-4-yl)meth~1-5-(N,N-di~ethyl-
aarb~moyl)oxypyrazole
Pormula (XI): R1 = n butyl, R2 = CH2CF3,
CH
ll CHJ ~CJ~
Prepared by the procedure of Example 52.
oi 1 used as such for the next step.
203~2~
- 72 -
Example 800 1-(2,2,2-Trifluoroethyl)-3-n-butyl-4-[2'~
(tetrazol-5-yl)biphenyl-4-yl]methyl-5-
(~,N-di~ethylcar~a~oyl)oxypyrazole
05 Form~la (I): Rl = n-butyl, R2 = CHzCF3~ A =
CH,
OR3, R9 = C - N~ ,
Il CH,
R"
N~ ~/
N~ N
N~ H
Prepared by the procedure of Example 70.
Crystals melting at 151-2C.
Examp~e 81: 1-Met~yl-3-n-butyl-4-(2'-cyanobiphenyl~4-
yl~methyl-5-(N,~-di~ethylcarbamoyl)oxy-
pyrazole
For~ula (XI): Rl = n-butyl, R2 ~ methyl,
CH
Prepared by the procedure of Example 52.
Oil used as such for the next step.
,
" ' ~ ' ~ .
---` 20~42~
- 73 -
Example 8?: 1-Methyl-3-n-butyl-4-[2'-~tetrazol-5-yl)bi-
phenyl-4-yl]~ethyl-5~(N,N-di~ethylcarba-
moyl)oxypyra201e
05 Formula (I): R1 = n butyl, R2 = methyl,
~CH~
A = OR3, R3 = C - N~
R.s = ¦ ¦¦
I
N~ ~N
N H
Prepared by the procedure of Example 70.
Crystals melting at 160C.
Examp~e 83: ~thyl [1-(2,2,2-tri~luoroethyl~-3-n~propyl-
4-(2'-cyanobiphenyl-4-yl)methylpyrazol 5-
yl~oxyacetate
Formula (XI): Rl = n-propyl, R2 = CH2CF3,
R3 = CH2C02Et, V =
NC
Prepared by the procedure of Example 14.
Oil used as such ~or the next step.
38~2~
- 74 -
Example 84: ~thyl ~1-(2~2,2-trifluoroethyl~-3-n-propyl-
4-[2'-~tetrazol-5-yl)~iphenyl-4-yl]methyl-
pyrazol-5-yl]oxyaaetate
05 Formula ~ Rl = n-propyl, R2 = CH2CF3,
A = OR3, R3 = C~2CO2Et~
\~
R~ - l IJ
N
N'H
Prepared by the procedure of Example 70.
Crystals melting at 146C.
Example 85~ ethyl-3-n-propyl-4-(2'-cyanobiphenyl-4-
yl)methyl-5-(N,N-dimethylcarbamoyl)oxy-
pyrazole
Formula (XI~ Rl = n-propyl, R2 = methyl,
~CH,
Prepared by the procedure of Example 52.
Oil used as such ~or the next step.
---` 2~13~28
- 75 -
Examp1e 86: 1-Methyl-3~n-propyl-4-~2'-ttetrazol-5-
yl)biphenyl-4-yl]methyl-5 (N,N-di~ethyl-
carba~oyl)oxypyrazole
05 Formula (I): R = n-propyl, R2 = methyl,
CH,
A = OR3 ~ R3 = C - N
O CH,
p~4= l ll
N
N~N~H
Prepared by the procedure of Example 70.
Crystals melting at 103-5C.
Example 87: 2-jl-Methyl-3-n~propyl-4-(2'-cyanobiphenyl-
4-yl)~ethylpyrazol-5~yl]oxyethanol
~0
Formula (XI3: Rl = n-propyl, R2 = methyl,
R3 = CH2CH2OH, V =
NC
Prepared by the procedure of Example 4~.
Oil used as such for the next step.
, :
`
-`` 2~3~28
- 76 -
Example 88: 2-~l~Nethyl-3-n-propyl~ 2'-~tetra201-5-
yl)~iphenyl-~-yl~methylpyrazol-5-yl]oxy-
ethanol
05 Formula (I~: Rl = n-propyl, ~2 = methyl,
A = OR3, R3 = CH2CH20H, `
~`~
R". =
N~ N
N H
Prepar~d by the procedure of Example 70.
Crystals melting at 120-122C.
Example 89: Ethyl [l-methyl~3-n-butyl-4-(2~-cyanobi-
phenyl-4-yl)methylpyra~ol-5-yl]oxyacetate
~ormula ~XI): Rl = n-butyl, R2 = methyl,
R3 = CH2~02Et, V =
NC
Prepared by the procedure of Example 14.
Oil used as such for the next step.
-" 2~3~28
Example 90: Ethyl ~l~methyl-3-n-butyl-4-~2'-(tetra~ol-
5-yl~biphenyl-4-yl}methylpyrazol-5-yl~oxy-
acetate
05 Formula (I): Rl = n-butyl, R2 = methyl, A =
OR3, R3 = CH2CO2Et,
R" =
N~
N H
Prepared by the procedure of Example 70.
Crystals melting at 142-3C.
Example 91: 2-[1-(2,2,2-Trifluoroethyl)-3-n-propyl-4-
~2'-cyanubiphenyl-4-yl)methylpyrazol-5-yl]-
oxyethanol
Formula ~XI~: R - n-propyl, R2 = CH2CF3,
R3 = CH2C~2OH, V = ¦ ¦¦
NC
Prepared by the procedure of Example 49.
Oil used as such for the next step.
~3$~2~
- 78 -
Example g2: 2~ (2,2,2-Trifluorethyl)-3 n-propyl 4-
[2'- r tekrazol-5~yl)biphenyl 4-yl~ethyl-
pyrazol-5-yl]oxyethanol
05 Formula ~ Rl a n-propyl, R~ = CH2CF3,
A = OR3, R3 = CH~CH20H,
R =
N
1 0 N~H
Prepared by the procedure of Example 70.
Crystals melting at 167C.
Example 93~ ethoxyoctane-2,4-dione
Formula (III): Rl = n-butyl, R' = CH3 t
q = 1
200 ml o~ hexan-2-one and 102 ml of methyl
methoxyacetate are added dropwise to ~00 ml of tolu~ne
containing 26.9 g of divided sodium, with heating. The
temperature during the addition is kept between 60 C
and 70 C. When the addition is complete, the mixture
is stirred for 2 hours at 55C. After cooling, 20 ml
of methanol are a~ded. The mixture is stirred and then
taken up with a dilute solution of sodium hydroxide and
the aqueous phase is washed with ether and then acidi-
fied with dilute hydrochloric acid and extracted withether. The organic phase is evaporated under vacuum at
30 C after drying over magnesium sulfate and the resi-
due is distilled under reduced pressure to give 98.6 g
of l-methoxyoctane-2,4-dione in the form of a liquid of
3s b.p.l5 = 115-120C.
-`` 2~3~28
- 79 -
Example 94~ ethoxyhep~ane 2,4-dione
Formula (III~: Rl = n-propyl, R' = CH3,
q = 1
05
Prepared by the procedure of Example 93, start-
ing from pentan-2-one.
Liquid of b.p. 20 = 100-110 C .
10xample 95: 1-~ethoxy-3-(~-nitroben~yl]heptane-2,4-
dione
Formula (VI): Rl = n-propyl, R' = CH3, V =
NO~, q = 1
135 g of 1-methoxyheptane-2,4-dione, prepared
in Example 94, are dissolved in 1.5 liters of tetra-
hydrofuran. 123.3 g of 4-nikrobenzyl bromide, 197 ml
of N,N-diisopropylethylamine and 49.5 g of lithium
bromide are added and the mixture is refluxed for 15
hours. The solvent is evaporated off under vacuum and
the residue is taken up with dilute hydrochloric acid
and extracted with ethyl acetate. The organic phase is
evaporated to dryness and the crystals obtained are
washed with ether several times and then methanol, with
heating. The filtrates are combined and evaporated to
dryness and the residue is then taken up with isopropyl
ether. Tha crystals obtained are washed with isopropyl
ether and dried at 30 C under vacuum to give 85.7 g of
3-(4-nitrobenzyl)-1-methoxyheptane-2,~-dione in thle
form of crystals melting at 55C.
~ he following two Examples were prepared by the
same procedure:
`` 2V38~28
- 80 -
~xample 96~ ethoxy-3~ cyanobiphenyl-4-yl)~ethyl-
heptane-2,4-dione
Formula ~VI): Rl = n-propyl, ~ - CH3,
05
V = ~ 3 , q = l
NG
Oil used as such for the next step.
Example 97: 1-Methoxy-3-(2'-cyanobiphenyl 4-yl~methyl-
octane-2,4-dione
Formula ~VI): R1 = n-butyl, R' = CH3,
V = ~ , q = 1
NC
Oil used as such for the next step.
Example 98: 3-n-Propyl-4-(4-nitrobenzyl)-5-~ethoxy-
methylpyrazole
For~ula (XIII): R1 = n-propyl, R' = CH3,
q = 1, V = NO2
20 g of 3 (4-nitrobenzyl)-l-methoxyhsptane-2,4-
dione, prepared in Example 95, are dissolved in 400 ml
of ethanol, 4 ml of hydrazine hydrate are added and the
mixture is refluxed for 2 hours. The solvent is evapo-
rated off to dryness under vacuum, the residue is taken
up with isopropyl ether and the crystals obtained are
filtered off to give 19.5 g of 4-~4-nitrobenzyl)-3-n-
propyl-5-methoxymethylpyrazole in the form of crystals
melting at 124~C.
` 2~38~28
- 81 -
The following two Examples were prepared by the
same procedure:
Ex~mple 99: 3-n-Propyl-4-~2'-cyano~iphenyl-4-yl)methyl-
05 5-methoxy~ethylpyrazole
For~ula (XIII): R1 = n-propyl, R' = CH3,
V=~, q=l
Oil used as such for the next step.
Example 100: 3-n-Butyl-4-(2'-cyanobiphenyl-4-yl)methyl-
5-methoxy~ethylpyrazole
Formula (XIII): R~ = n-butyl, R' = CH3,
v= ~ ,q=l
NC ~
Oil used as such for the next step.
Example 10.~ (2,2,~-Trifluoroethyl)-3-n-butyl-4-(2'--
cyanobiphenyl-4-yl)methyl-5-methoxy3ethyl-
pyrazole
Formula (XII3: R1 = n-butyl, R2 = CH2CF~,
R3 = CH3, q = 1,
~
V= I I
NC ~
Prepared by the procedure of Example 98, start-
ing from 2,2,2-trifluoroethylhydrazine.
.
2~3~42~ `
- 82 -
After chromatography on silica gel in an ethyl
acetate/cyclohexane eluent ~2/8), the desired product
is obtained in the form of an oil, which is used as
such for the next step.
05
Example 102~ 1-Methyl-3-n-propyl-4-(~-nitrobenzyl)-5-
methoxymethylpyrazole
Formula (XII): R1 = n-propyl, R2 = methyl,
10R' = methyl, v = N02, q = 1
5 g of 3-n-propyl-4-(4-nitrobenzyl)-5-methoxy-
methylpyrazole, prepared in Example 98, are dissolved
.in 50 ml of acetone in the presence of 3 ml of methyl
15iodide and 3 ml of DBU ~1,8-diazabicyclo[5.4.0~undec-7-
ene). The mixture is heated for 8 hours at 45 C and
the solvent is then evaporated off. The residue is
ta~en up with dilute hydrochloric acid and then extrac~
ted with ether. The organic phase is dried over mag-
nesium sulfate and then evaporated under vacuum to give
an oily residue, which is chromatographed on silica gel
in an ether/cyclopentane eluent (7/3) to give 2.5 g of
l-methyl-3-n-propyl-4-(4-nitrobenzyl)-5-methoxym0thyl
pyrazole in the form of crystals melting at 73C.
The following two Examples were prepared by the
procedure of Example 102:
2~38~28
- 83 -
xample 103: 1-Methyl-3-n-propyl-4-(2'-cyanobiphenyl-
4-yl~methyl-s-methoxymethylpyrazole
Formula ~XII)~ Rl = n-propyl, R2 = methyl,
05
R' = methyl, V =
NC
q = 1
Oil used as such for the next step.
Example 104: 1-Methyl-3-n-~utyl-4-(2'-cyanobiphenyl-
4-yl)methyl-5-methoxymethylp~razole
FQr~U1a ~X~ Rl - n-~utyl, R2 = methyl,
R~ = methyl, V =
NC~ ~
q = 1
Oil used as such for the next step.
Example 105: 1-Methyl-3-n-propyl-4-(4-aminohenzyl)-5-
methoxymethylpyrazole
Formula (I). Rl = n-propyl, Ra = methyl,
A = CH20CH~, R4 = ~H2
Prepared by the procedure of Example 21.
Oil used as such for the next step.
`` 2 ~
- 84 -
Example 106: 3-n-Propyl-4~ aminobenzyl) 5-methoxy-
methylpy~azole
Formula (~ = n-propyl, R2 = H, A =
05 CH2OCH3, R~ = NH2
Prepared by the procedure of Example 21.
Crystals melting at 126C.
Example 107: 2-[(1-~ethyl-3-n-propyl-5-~ethoxymethyl-
pyrazol-4-yl~methylphenyl-~-yl]aminocar-
bonylbenzenesulfonic acid
Formula (I): Rl = n-propyl, R2 - methyl,
O
A = cH2OCH3, R4 =
HO,S
Preparad by the procedure of Example 27 using
the cyclic anhydride of orthosulfobenzoic acid.
Crystals melting at 126C.
25 ~ sLl~: 2-[(3-n-Propyl-5-methoxymethylpyrazol-4-
yl)m~thylphenyl-4-yl]a~inocarbonylbenzene-
sulfonic acid
Formula ~ Rl = n-propyl, R2 = H, A =
O
CH2OCH3, R~ =
HOJS
`` 2~3~2~
- 85 -
Prepared by ths procedure of Example 27 using
the cyclic anhydride of orthosulfobenzoic acid.
Crystals melting at 253-256C.
05 Example 109: 1-Methyl-3-n~prop~1-4-(4-nitr~benzyl~-5-
br~momethylpyrazole
Yormula (I): Rl = n-propyl, R2 = methyl,
A = C~I2Br, R4 = N02
4.5 g of 1-methyl-3~n-propyl-4-(4-nitrobenzyl)-
5-methoxymethylpyrazole, prepared in Example 102, are
dissolved in 140 ml o chloroform. 2.8 ml of boron
tribromide are added dropwise at a temperature of
between 0 C and 5 C and the mixture is subsequently
stirred for one hour at this temperature and then for 2
hours at room temperatura. A dilute solution o~ sodium
hydroxide is added to the mixture and t~e organic phase
is decanted, dried and evaporated under vacuum to give
5 g of 1-methyl-3-n-propyl-4-(~-nitrobenzyl)-5 bromo-
methylpyra201e in the form of an oil, which is used as
such for the next step.
Example 110: 3-n-Propyl-4-(4-nitrobenzyl)-5-bro~o
methylpyrazole
Formula ~ R - n-propyl, R2 a ~ t A =
CH2Br, R~ = N0z
Prepared by the procedure of Example 109.
Crystals melting at 120C.
-`- 2~3842~
- 86 -
Example 111: 1-Methyl-3-n-propyl-4-~4-nitrobenzyl~-5-
hydroxymethylpyrazole
Formula ~XI~): R1 = n-propyl, R2 = methyl,
05 R' = H, V = N02, q = 1
6.5 g of 1-methyl-3-n-propyl-4-(4-nitrobenzyl)-
5-bromomethylpyrazole, prepared in Example 109, are
dissolved in a mixture composed of 50 ml of dioxane and
1~ 50 ml of water. 5 g of sodium carbonate are added and
the -mixture is refluxed for 3 hours. The solvents are
evaporated off under vacuum and the resldue is taken up
with water and extracted with ether~ The organic phase
is dried over magnesium sulfate and ~hen evaporated
under vacuum to give a residue, which crystallizes from
isopropyl ether t.o give 3.8 g of 1-methyl-3-n-propyl-4-
(4-nitrobenzyl)-5-hydroxymethylpyrazole in the form of
crystals melting at 134C~
Exam~le 112: 3-n-Propyl 4-(4-nitrobenzyl~-5-hydroxy-
methylpyrazole
Formula ~XIII): Rl = n-prcpyl, R' = H,
V = N2~ q = 1
Prepared by the procedure of Example 111.
Crystals melting at 125C.
Exam~le 113~ ethyl-3-n-propyl-4-(4-aminobenzyl)-5-
hydroxymethylpyrazole
Formula (I): R1 = n-propyl, R2 = methyl,
A = CH20H, R~ = NH2
Prepared by the procedure of Example 21.
2~3$~28
- ~7 -
Crystals melting at 133C.
Example 114: 3-n-Propyl-4-(4-a~ino~enzyl~-5-hydroxy-
methylpyrazole
05
Formula ~ R1 = n-propyl, R2 = H, A =
CH20H, R4 = NH2
Prepared by the procedure of Example 21.
Oil used as such for the next step.
Example 115: 2-[~1-Nethyl-3-n-propyl-5-hydroxymethyl-
pyrazol-4-yl)~ethylphenyl-4-yl]aminocar-
bonylbenzenesulfonic acid
For~ula (I): R1 = n propyl, R2 = methyl,
o
A = CH20H, R4 =
HO,S
Prepared by t~e procedure of Example 27 using
the cyclic anhydride of orthosulfobenzoic acid.
Crystals melting at 255-257C.
Example 116: 2-[(3-n-Propyl-5-hydroxymethylpyraz~1-4-
yl)methylphenyl-4-yl]aminocarbonylbenzene-
sulfonic acid
For~ula (I): R1 = n-propyl, R2 = H, A =
o
CH20H, R~ =
HO,S
2~38~2~
88 -
Prepared by the procedure of Example 27 using
the cyclic anhydride of orthosulfobenzoic acid~
Crystals melting at 168-170C.
05 Example 117: 1-~ethyl-3-n-propyl-~-~2'-~tetrazol-5-
yl)biphenyl-4-yl]methyl-5-methoxymethyl-
pyrazole
Formula ~ Rl = n-propyl, R2 = methyl,
A = CH20CH3, R~
r~
N~`N''N
5
Prepared by the procedure of Example 710
Crystals melting at 128-130C.
~rnlQ~ 1 Methyl-3-n-butyl-4-[2'-(tetra201-5-yl)-
~iphenyl-4-yl]methyl-5-me~hoxymeth
pyrazole
Formula (X): Rl = n-butyl, Rz = methyl,
A = CH20CH " R~ =
N
N~ N
Prepared by the procedure of Example 71.
Crystals melting at 134-135C.
` 2~3~28
~9
le 119~ 2,2,2-Trifluoroethyl~-3-n-butyl~4-~2'-
(tetrazol-5-yl)biphenyl~4-yl]methyl-5-
~etho~ymethylpyrazole
05 For~la (I): Rl = n-butyl~ R2 = CH2CF~,
A = CH2OCH3 ~
r~
N~N,N
Prepared by the procedure of Example 71.
Crystals melting at 172-173C.
15 Example 120: Ethyl 3-oxo-5-~ethylhexanoate
~ormula (II~: R~ = Z-methylpropyl, Rlo =
ethyl
Prepared by the procedure of Example 1.
Liquid of b.p.1o = 100-110 C.
Example 121: Ethyl 2-(2'-cyanobiphenyl 4-yl)methyl-3-
oxo-5-~ethylhexanoate
For~ula ~V): R1 = 2-methylpropyl, Rlo =
ethyl, V =
NC
Prepared by the procedure of Example 6.
Oil used as such for the next step.
`"` ~03~28
- 90 -
Examp~e 122: l~Methyl~3-(2-methylpropyl)-4 (2'-cyano-
biph~nyl-4-yl)methyl-5-hydroxypyrazole
Form~la (IX): Rl = 2-methylpropyl, R2 ~
05
methyl, V =
NC~
Prepared by the procedure of Example 7.
Cry~tals melting at 163C.
Example 1~3: 1-(2~2,2-Trifluoroethyl)-3-(2-methyl-
pro W 1~-4-(2'-cyanobiphenyl-4-yl)methyl~
5-h~droxypyrazole
Formula (IX): Rl = 2-methylpropyl J R2 =
CH2CF3, V = ~3
NC
Prepared by the procedure of Example 7.
Oil used as such for the next step.
~ Example 124: E~hyl ~l~m~thyl-3-(2-methylpropyl)-4-~2~-
: 25 cyanobiphenyl~4-yl)methylpyrazol-5-yl]-
oxyacetate
~or~ula (XI): Rl = 2-methylpropyl, Rz =
methyl, R~ = CH2CO2Et,
V= L 11
NC ~;~"
Prepared by the procedure of Example 14.
Oil used as such for the next step.
- 203~42~
-- 91 --
E~ample 125: E~hyl [l-methyl-3-(2-methylpropyl)~4-[2'-
(tetrazol-5-yl)biphenyl~4~yl]methylpyra-
zol-5-yl~o~yacetate
05 E~or~ula (T): Rl = 2-methylpropyl, R~ =
methyl, A = OR9, R3 =
CH2co2E
Ra~
}O N~
N\~N~H
Preparsd by the procedure of Example 70.
Crystals melting at 172 173C.
~xample 126: Ethyl ~1-(2,2,2-trifluoroethyl)-3-(2-
mekhylpropyl)-4-~2'-cyanobiphenyl-4-yl~-
methylpyrazol-5-yl]oxyacetate
2~
For~ula (XI): Rl = 2-methylpropyl, R2 ~
CH2CF3, R3 = CH2C02Et,
V= ~
NC `~"
Prepared by the procedure of Example 14.
Oil used as such for the next step.
293~2~
g ;~ -- '
Exampla 127: Ethyl L 1- ( 2,2,2-trifluoroethyl)-3-(2-
~ethylpropyl)-4-[2'-(tetrazol-5 yl~bi-
phenyl-4-yl]methylpyrazol-5-yl]oxyacetate
05 Formula (I): Rl = 2-methylpropyl, R2 =
CH2CF3, A = OR3, R3 =
CH2CO2E~ R4 =
I
N~H
Prepared by the procedure of Example 70.
Crystals melting at 114-115C.
Example 1~: 2-[1-(2,2,2-Tri~luoroethyl)-3-(Z-methyl-
propyl)-4-(2'-cyanobiphenyl-4~yl)~ethyl-
pyra201-5-yl ]oxyethanol
~ormula ~XI): Rl = 2-methylpropyl, R2 =
CH2CF3, R3 = CH2CH2OH,
NC ~
Prepared by the procedure of Example 49.
Crystals melting at 116C.
2~3~
- 93 -
xample 129: 2~ (2,~,2-Trifluoroethyl)-3-(2-~ethyl-
propyl~-4-[2'-(tetra201-5-yl)biphenyl-4-
yl]methylpyrazol-5-yl~oxyethanol
05 Formula ~ R1 = 2-methylpropyl, R2 =
CH2CF3, A = OR3, ~3 --
CH2CH ~OH,
R4 =
N~/
N~H
Prepared by the procedure of Example 70.
Crystals melting at 145-146~C.
Example 130: N-~ Methyl~3-n-butyl-4-[4-~2-trifluoro-
~methylsulfonylamino~nzoyl~aminobenzyl]-
pyra201-5-yl]oxyacetyl]morpholinQ0
Formula ~ Rl = n-butyl, R2 = methyl,
A = OR3, R3 = CH2- C-N 0,
0
R NH ~3
CF,SO2N
4.3 g oP N-[[1-methyl-3-n-butyl-4-(4-amino-
benzyl)pyrazol-5-yl]oxyacetyl]morpholine, prapared in
Example 47, are dissolved in 20 ml of chloro~orm in the
presence of 1.54 ml of triethylamine. A solution of
3.52 g of N-trifluoromethylsulfonylanthranilic acid
2038~28
- 94 -
chloride (prepared according to the references CA :
96(13) : 103651z and CA : 9~(7) : 55500w) in 10 ml of
chloroform is added dropwise at room temperature and
the mixture is then heated for 2 hours at 50C and left
05 to stand overnight at room temperature. The solution
is washed with dilute hydrochloric acid, dried and
evaporated under vacuum. The residue is taken up with
methylene chloride and extracted with a dilute solution
of sodium hydroxide. The aquevus phase is acidified to
pH 2 and extracted with ethyl acetate, the organic
phase is dried over magnesium sulfate and then evapora-
ted to dryness and ths oil obtained crystallizes from
ether to give 4.6 g of N-[[1-methyl-3-n-butyl-4-[4-(2-
trifluoromethylsulfonylaminobenzoyl)aminobenzyl]pyra-
zol-5-yl]oxyacetyl]murpholine in the form of crystals
melting at 190C.
Example 131- Ethyl 2- f 2'-nitrobiphenyl-4-yl)methyl-3-
oxohexanoate0
~ormula (V): Rl = n-propyl, R1o = ethyl,
V= 1 11
O,N
Prepared by the procedure of Example 6, start-
ing from 2'-nitro-4-chloromethylbiphenyl.
oi l used as such for the next step.
2'-Nitro 4-chloromethylbiphenyl was prepared by
chloromethylating 2-nitrobiphenyl according to the
following references:
- CA 7Q(25) : 114837d, and
- CA 69(2) 3704t.
2~3~
- 95 -
Exam~le 132: 1-Methyl-3-n-propyl-4-(~'-nitrv~iphenyl~
4-yl3~ethyl-5-hydroxypyrazole
Formula (IX): Rl = n-propyl, R2 = methyl,
05 ~
V= 1 11
02N~
Prepared by the procedure of Example 7.
Crystals melting at 179-182C.
Example 133: ~e~hyl [1-methyl-3-n-propyl-4-(2'-nitrobi-
phenyl-~-yl)methylpyrazol-5-ylJoxyacetat~
Formula ~XI): Rl = n-propyl, R2 = methyl.,
R3 = CH2CO2Me,
R4 =
N~
N/,h
N ~
Prepared by the procedure of Example 14.
Oil used as such for the next step~
Example 134: ~ethyl [1-~ethyl-3-n-propyl~~-(2'-aminobi-
phenyl-4-yl)m~thylpyrazol-5-yl]oxyacetate
Formula (I): Rl = n-propyl, R2 = methyl,
A = OR3, R3 = CH2CO2Me,
~,
R" =
H2N~/
Prepared by the procedure of Example 21.
2~842~
- 96 -
Oil used as such for the next step.
Example 135: Methyl [l-methyl-3-n-propyl-4-l2~-tri-
fluoro~ethylsulfonylaminobiphenyl-4-yl)-
05 methylp~r~zol-5-yl]oxyacetate
Formula ~ Rl = n-propyl, Ra = methyl,
A = OR3, R3 = cH2~O2Me~
\r~ .
P~
CF,SO2NH~/
1 g of methyl [1-methyl-3-n-propyl-4-(2'-aminO-
biphenyl-4-yl)methylpyrazol-5-yl]oxyacetate, prepared
in Example 134, is dissolved in 30 ml of chloroform in
the presence of 0.4 ml of triethylamine; the mixture is
cooled to 0C and 0.43 ml of trifluoromethanesulfonic
anhydride is added dropwise. The mixture is then stir-
red for one hour at room temperature and the organic
phase is washed with water, dried over magnesium sul-
fate and evaporated under vacuum. The residue o~tained
crystallizes from isopropyl ether to give 0.7 g of
methyl E1-methyl-3-n-propyl-4-(2'-trifluoromethylsul-
fonylaminobiphenyl-4-yl)methylpyrazol-5 yl]oxyacetate
in the form of crystals melting at 114-116C.
Example 136: 2-tl-Methyl-3-n-propyl-4-(2'-carkethoxy
biphenyl-4~yl3methylpyrazol-5-yl]oxy-
ethanol
Formula (XI): Rl = n-propyl, R2 = methyl,
R9 = CH2CH2OH,
V = 11
EtO2C ~ ~
`` 2~3~8
- 97 -
Prepared by the procedure of Example 49.
Oil used as such for the next step.
Example 137: 2-[1-Methyl-3-n-propyl-4-(2'-carboxy-
05 biphenyl-4-yl)methylpyrazol-5-yl~oxy-
ethanol
Formula (I): Rl = n-propyl, R2 = methyl,
A = OR3, R3 = CH2CH2OH,
Ra =
)~O,C~
Prepared by the procedure of Example 37.
Crystals melting at 168-170C.
Example 138: ~thyl 2-~-(2-cyanothien-3-yl)benzyl]-3-
oxohe~anoate
Forcula tv): R1 = n-propyl, Rlo = ethyl,
V = ~
NC S
Prepared by the procedure of Example 6, start-
ing from 4-(2-cyanothien-3-yl)benzyl bromide.
Preparation of 4-t2 cyanothien-3-yl)benzyl bromide
A) 3-(4-Methylphenyl)thiophene-2-carboxylic acid
g of ethyl 3-~4-methylphenyl)thiophene-2-
carboxylate, prepared according to the reference
FISSELMANN H.; HABITCH H.; Ger. Offen. 1,092,929
(1960), CA : 57, 5894g, are dissolved in 580 ml of
ethanol and 50 ml of water. 6.2 g of sodium hydroxide
- 98 -
pellets are added and the mixture is refluxed for 2
hours, poured into water and acidified with concentra-
ted hydrochloric acid. The crystals obtained are fil-
tered off and dried to give 20.7 g of 3-(4-methyl-
05 phenyl)thiophene 2-carboxylic acid in the form of
crystals melting at 172C.
B) 3-(4-Methylphenyl~thiophene-2 carboxamide
20.7 g of 3-(4-methylphenyl)thiophene-2-car-
boxylic acid, prepared in A), are dissolved in 200 mlof anhydrous toluene with 10.4 ml of thionyl chloride.
The mixture is refluxed for three hours and then eva-
porated to dryness. The oily residue is added dropwise
to a 28% solution of ammonia at 25C. After stirring
for one hour, the mixture is extracted with chloroform
and the organic phase is washed with water, dried over
magnesium sulfate and evaporated under vacuum to give
18 g of 3-(4-methylphenyl)thiophene-2-carboxamide in
the form of crystals melting at 128-130 C.
C) 3-(4-Methylphenyl)thiophene-2-carbonitrile
18 g of 3-(4-methylphenyl)thiophene-2-car-
boxamide, prepared in B), are dissolved in 36 ml of
phosphorus oxychloride and the mixture is refluxed for
one hour. The phosphorus oxychloride is concentrated
under vacuum and the residue is taken up in water and
ethyl acetate. The oryanic phase is extracted, dried,
filtered over animal charcoal and then evaporated under
vacuum to give 12 g of 3-(4-methylphenyl)thiophene-2-
carbonitrile in the form of an oil, which is used assuch for the next step.
D) 4-(2-Cyanothien-3-yl)benzyl bromide
12 g of 3-(4-methylphenyl)thiophene-2-carbo-
nitrile, prepared in C), are dissolved in 100 ml of
`` 2~3~2~
99
carbon tetrachloride. 11.3 g of N-bromosuccinimide and
10 mg of benzoyl peroxide are added. The mixture is
refluxed until all the succinimide has come out of
solution. ~he crystals are filtered off and the sol-
05 vent is concentrated under vacuum to give 16 g o~ 4-(2-
cyanothien-3-yl)benzyl bromide in the form of an oil,
which is used as such for the next step.
Example 139: 1-~ethyl-3-n-propyl-4-~4-(2-cyanothian-
3-yl)benzyl3-5-hydroxypyrazole
~ormula (IX): R~ = n-propyl, R2 = methyl,
V = ~3
NC S
Prepared by the procedure of Example 7.
Crystals melting at 110-115C.
Example 140: Ethyl ~ thyl-3-n-propyl-4-~4-(2-cyano-
thien-3-yl)benzyl~pyrazoI-5-yl]oxyacetate
Form~la (XI): Rl = n-propyl, R2 = methyl,
R3 = CH2C02Et,
V = ~
NC S/
Prepared by the procedure of Example 14.
Oil used as such for the next step.
~3~
- 1~0 -
Example 141: ~thyl ~1-methyl-3-n-propyl-4-[4-[~-(tet-
razol-5-yl~thien-3-yl]benzyl~pyrazol-5
yl]oxyacetate
05 For~ula (I): ~1 = n-propyl, R2 - methyl,
A = OR3, R3 - CH~CO~Et,
R.
N~
N~ ,N
N t~
Prepared by the procedure of Example 71.
Crystals melting a~ 201C.
~xam~le l~: Eth~l 2-t4-(~-carbethoxythien-3-yl)-
benzyl]-3-oxohexanoate
For~ula (~: Rl - n-propyl, R1o - ethyl,
2~
V= ~3
~tO~C S
Prepared by the procedure of Example 6, start-5 i~g from 4-(2-carbethoxythien-3-yl)benzyl bromide.
Oil used as such for the next step.
Preparation of 4-~2-carbethoxythien-3-yl)benzyl bromide
43.4 g of ethyl 3-(4-methylphenyl)thiophene-2-
carboxylate, prepared according to the reference
FISSELMANN H.; HABITCH; Ger. Offen. 1,092,929 (~960);
CA : 57, 5894g, are dissolved in 250 ml o~ carbon
tetrachloride. 33 g of N-bromosuccinimide and 10 mg of
benzoyl peroxide are added, the mixture is refluxed for
~03~2~
-- lo~ --
one hour, the crystals of succinimide are filtered off
and the solvent is evaporated of~ under vacuum to give
56.6 g of 4-(2-carbethoxythien-3-yl)benzyl bromide in
the form of crystals melting at 55C.
05
Example 143: 1-~ethyl-3-n-propyl-~-[4-~2-carbethoxy-
thien-3~-yl)benzyl]-5-hydroxypyrazole
For~ula (IX): Rl - n-propyl, R2 = methyl,
V= ~3
EIO,C S
Prepared by the procedure of Example 7.
Crystals melting at 130-132C.
~BLQ~ 2-tl-~ethyl-3-n-propyl-4-[4-(2-carbeth
thien-3-yl)benzyl~pyrazol-5-yl]oxyethanol
Fo~m~la (XI): Rl = n-propyl, R~ = methyl,
R3 = CH2CH20~,
V= ~3
E~02C S
Prepared by the procedure of Example 49.
Oil used as such for the next step.
Example 145: 2-[1-Methyl-3-n-propyl-4-~4--(2-carboxy-
thien-3-yl)benzyl]pyrazol-5-yl~oxyethanol
Formula (I): R1 = n-propyl, R2 = methyl,
A = OR3, R~ = CH2CH20H,
3S V =
HO,C S
-`` 2~3~28
~ 102 -
Prepared by the procedure of Example 37.
Crystals melting at 118-120C.
Example 146: 1-(2-Methoxyphenyl)-4-~1-(2,~,2-triflu~ro-
05 ethyl)-3-n-propyl-4-~2'-cyanobiphenyl-4-
yl)methylpyrazol-5-yl3oxyacetylpiperazine
For~ula (XI~: Rl = n-propyl, R2 = CH2CF3,
CH,O
R3 = CH,CO N N
V= ~
Prepared by the procedure of Example 64.
Oil used as such for the next step.
Example I47~ 2-Methoxyphenyl)-4-[1-(~,2,2~trifluoro-
ethyl)-3-n-propyl-4-[2'-(tetrazol-5-yl)-
biphenyl 4-yl]me~hylpyrazol-5~yl]0xy-
acetylpiperazine
: 25 Formula (~): Rl = n-propyl, R2 = CH2CF3,
A = OR3,
R3 = CH2CO N N
CH,O
R~ =
~N_N
::
~138~8
- 1~3 -
Prepared by the procedure o~ Example 70.
Crystals melting at 158-160C.
Example 148: N-[~1-(2,2,2-Trifluoroethyl)-3-n-propyl-
oS ~-~2'-cyanobiphanyl-4-yl~ethylpyrazol-5-
yl]o~yacetyl]~orpholine
Formula (XI): Rl = n-propyl, R2 = CH2CF3,
R3 = C~O N 3
V= 1 11
NC
Prepared by the procedure of Example 46.
Oil used as such for the next step.
Example 149: N-[[1-(2,2,2-Trifluoroethyl)-3-n-propyl-
4-[2'-(tetrazol-5-yl)biphenyl-4-yl]-
m~thylpyrazol 5-yl~oxy~cetyl]morpholine
Formula (I): Rl = n-propyl, R2 = CH2CF3,
A = OR3,
R3 = C~CO N o ,
R4 =
~N
N N
Prepared by the procedure of ~xample 70
Crystals melting at 129-31C.
- 2~3~28
- 104 -
Example 150: 1-(2,2,2-Trifluoroethyl~-3-n-bU~yl-5-
hydroxy-~-~2'-(tetrazol-5-yl)biphenyl-4-
yl]methylpyrazole
05 Formula (I): Rl = n-butyl, R2 = CH2CF3,
A = OH, ~4 = l ll
r~
1 0 N~N~H
Prepared by the procedure of Example 71.
Crystal~ melting at 210-11C.
Example 151: 1-~ethyl-3-n-butyl-~-(2~-cyanobiphenyl-4-
yl)~ethyl-5-bromomethylpyrazole
Form~la (I): R~ = n-butyl, R2 = methyl,
A = CH2Br, Ra =
NC ~
Prepared by the procedure of Example los,
starting from the l-methyl-3-n-bu~yl~4-~2'-cyanobi-
phenyl~4-yl)methyl-5-methoxymethylpyrazole prepared in
Example 104.
Oil used as such for the next step.
" ~3~2~
- 105 -
Example 152: 1-~ethyl-3-n-butyl-~-(2'-cyanobiphenyl-4-
yl)~ethyl-5-hydroxymethylpyrazole
Formula ~XII): Rl = n-butyl, R2 = methyl,
05
R ' = H, V =
NC~:/
q = 1
~0 Prepared by the procedure of Example 111,
Oil used as such for the next step.
Example 153: 1-Me~hyl-3-n-~utyl-4-[2'-(tetrazol-5-
yl~biphenyl-4-yl~ethyl-5-hydroxymethyl
pyra201e
For~ula (I): Rl = n-butyl, Rz = methyl,
A = CH20H, Ra =
N
N~H
Prepared by the procedure o~ Example 71.
Crystals melting at 110-12C.
Example ~54: l-~ethyl-3-n-~utyl-4-(2'-cyanobiphenyl-4-
yl)methyl-5-formylpyraxole
Formula (I): Rl = n-butyl, R2 = methyl,
A = CH0, R4 =
~K:
2.3 g vf 2-nitropropane are dissolved in a
``` 2~3~28
- 106 -
solution of sodium methylate prepared from 0.7 g of
sodium and 40 ml of me~hanol. 10 of 1-methyl-3-n-
butyl-4-(2'-cyanobiphenyl-4-yl)methyl-s-bromomethyl-
pyrazole, prepared in ~xample 151, are dissolved in 3005 ml of methanol and this solution is ad~ed dropwise to
the reaction mixture. The mixture is stirred for two
hours at 50 C, water is then added and the resulting
mixture is extracted with ethyl acetate. The organic
phase is evaporated to dryness under vacuum and the
residue obtained is chromatographed on silica gel to
give 4.3 g of 1-methyl-3-n-butyl-4-(2'-cyanobiphenyl-4-
yl)methyl-5-formylpyrazole in the form of an oil, which
is used as such for the next step.
Example 155: 1-Methyl-3-n-butyl-4-(2'-cyanobiphenyl-
4-yl)methyl-5-(dioxolan~2~yl)pyrazole
Formula (I): Rl = n-butyl, R2 - methyl,
A = C~
R = ~
4.3 g of 1-methyl-3-n-butyl-4-(2'-cyanobi-
phenyl-4-yl)methyl-5-formylpyrazole, prepared in
Example 154, are dissolved in 50 ml of toluene. 1 g of
ethylene glycol and 10 mg of paratoluenesulfonic acid
are added. The mixture is brought to the reflux tem-
perature and the water is removed for 3 hours by means
of a Dean-Stark apparatus. The mixture is subsequently
taken up with water and extracted with ether and the
extract is then dried over magnesium sulfate and eva-
porated under vacuum to give 4 g o~ 1-methyl-3-n-butyl-
-`` 2~3~
- 107 -
4-(2'-cyanobiphenyl-~-yl)methyl-5-(dioxolan-2-yl)-
pyrazole in ths form of an oil, which is used as such
for khe next step.
05 Example 15 6: 1-~ethyl-3-n-butyl-4- L 2 ~ - ( tetrazol -5-yl ) -
biphenyl-4-yl]methyl-5-(dioxolan-~-yl)-
pyrazol~
Formula (I): Rl = n-butyl, R2 = methyl,
o
A = CH
0
R4
N~ N
N''H
Prepared by the procedure of Example 70.
Crystals melting at 144 6C.
Example 157: 2-~1-(2,2,2-Trifluoroethyl)-3-n-butyl-4-
(2'-cyanobiphenyl-4~yl)methylpyrazol-5-
ylloxyethanol
~ormula (XI): Rl = n-butyl, ~z = CH7CF3,
R3 = CH2CH20H,
V= 1 11
NC ~
Prepared by the procedure of Example 49.
oi l used as such for the next step.
2~3~ 8
- 108 -
Example 158: 2~ (2,2,2-Trifluoroethyl)-3-n-butyl-4-
~2'-(tetrazol-s-yl)biphenyl-4-yll~ethyl-
pyrazol-5-yl]oxyeth~nQl
05 Formula (I): Rl = n-butyl, R2 = CH~CF~,
A = OR3, R3 - CH2CHzOH,
R4 =
N~N~N
Prepared by the procedure of Example 71.
Crystals melting at 107-10C.
e_L~: Ethyl 3-oxo-2-[4-(3-cyanothien-2-yl]-
banzyl]heptanoate
For~ul~ (V): Rl = n-butyl, Rlo = ethyl,
` ~ S
NC ~
1.1 g of ethyl 3-oxoheptanoate, prepared in
Example 1, 1.2 g of 4-(3-cyanothien-2-yl)benzyl bro-
mide, 0.6 g of lithium bromide and 2.2 ml of diiso-
propylethylamine are added to 15 ml of tetrahydrofuran.
The mixture is stirred under reflux for 20 hoursj taken
up with water and extracted with ether. The organic
phase is dried over magnesium sulfate and then evapora-
ted. The excess ethyl 3-oxoheptanoate is evaporated
off using a vane pump and 1.5 g of ethyl 3-oxo-2-[4-(3-
cyanothien-2-yl)~enzyl]heptanoate are obtained in the
form of an oilj which is used as such for the next
step.
2 ~ 2 8
- 109
Preparation of 4-(3-cyanothien-2-yl)benzyl bromide
A) 4'-Methyl-4-chlorobutyrophenone
53 ml of toluene and 70O5 g of 4-chlorobutyroyl
05 chloride are dissolved in 100 ml of methylene chloride
and the solution is added at 10C to a suspension of
74 g of aluminum chloride in 200 ml of methylene chlo-
rideO The temperature is then allowed to rise for a
quarter of an hour and the mixture is treated with iced
water. The organic phase is dried over magnesium sul-
fate and evaporated under vacuum to give 96.9 g of 4'-
methyl-4-chlorobutyrophenone in the form of an oil,
which is used as such for the next step.
B) ~-Chloro-B-(2-chloroethyl)cinnamaldehyde
130 ml of phosphorus oxychloride are added
slowly, at 0 C, to 130 ml o~ dimethylformamide, and a
solution of 117.5 g of 4' me~hyl-4-chlorobutyrophenone,
prepared in A), in 50 ml of dimethylformamide is then
added dropwise. The mixture is subsequently stirred at
room temperature for one hour and then at 50 C for 2
hours and at 70~C for 1 hour. The mixture is then
poured on to ice and taken up with ether and the ether
phase is washed with a saturated solution of sodium bi-
carbonate, dried over sodium sulfate and evaporatedunder vacuum to give 133.8 g of ~-chloro-B-~2-chloro-
ethyl)cinnamaldehyde in the form of an oil, which is
used as such for the next step.
30C) 2-(4-Methylphenyl)-4 r 5-dihydrothiophene-3-carbox-
aldehyde
15.9 g of Q-chloro-B-(2-chloroethyl)cinnam-
aldehyde, prepared in B), and 22 g of sodium sulfide
(9H2O) are added to 200 ml of THF. A sufficient amount
of water is added for all the sodium sulfide to pass
2Q3~28
- 110 -
into solution, and the mixture is subsequently refluxed
for 3 hours, cooled and then takan up with ether. The
organic phase is decanted, wa~hed with water and then
dried over magnesium sulfate and evaporated under
05 vacuum to give 13.5 g of 2-(4-methylphsnyl)-~,s-di-
hydrothiophene-3-carboxaldehyde in the form of an oil,
which is used as such for the next step.
D) 2-(4 Methylphenyl)-3-cyano-4,5-dihydrothiophene
~0 15 g of 2-(4-methylphenyl)-4,5-dihydrothio-
phene-3-carboxaldehyde, prepared in C), and 6.5 g of
hydroxylamine hydrochloride are mixed with 40 ml of
ethanol and 10 ml of water. A solution of 4.7 g of
sodium carbonate in 10 ml of water is added. The mix-
ture is stirred at room temperature for half an hour
and then ~xtracted with ether. The ether phase is
washed with water and ~hen dried over sodium sulfate
and evaporated under vacuum to give 15~2 g of a gummy
yellow residue. This re~idue is added to 13 ml of
acetic anhydride and the mixture warms up slightly,
turns brown and becomes liquid. The mixture is sub-
sequently refluxed for 1 hour and then poured on to ice
and extracted with methylene chloride, the extract is
washed with a saturated solution of sodium bicarbonate,
the organic phase is then dried over magnesium sulfate
and evaporated under vacuum and ~he residue obtained is
chromatographed on æilica gel in methylene chloride to
give 10 g of 2-(4-methylphenyl~-3-cyano-4,5-dihydro-
thiophene in the form of an oil, which is used as such
for the next step.
E) 2-(4-Methylphenyl)-3-cyanothiophene
49.9 g of 2-(4-methylphenyl)-3-cyano 4,5-di-
hydrothiophene, prepared in D), are dissolved in 200 ml
of car~on tetrachloride, the mixture is heated to the
2038~28
-- 111 --
reflux temperature and, after two hours, a solution of
11 g of bromine in 200 ml of carbon tetrachloride is
added dropwise. Reflux is continued until the evolu-
tion of hydrobromic acid has ceas~d, and the solvent is
05 then evaporated off under vacuum. The residue is taXen
up in 200 ml of anhydrous tetrahydrofuran, and 28 g of
potassium tert-butylate are added. The mixture is re-
fluxed for one hour and then cooled, water and sodium
chloride are added and the mixture is extr~cted with
ether. The organic phase is evaporated under vacuum to
give 31.8 g of 2-(4-methylphenyl~-3-cyanothiophene in
the form of an oil, which is used as such for the next
step.
F) 4-(3-Cyanothien-2-yl)benzyl bromide
24.5 g of 2~(4-methylphenyl)-3-cyanothiophene,
prepared in E), are dissolved in 200 ml of carbon
tetrachloride. 21.9 g of N-bromosuccinimide and 0.1 g
of benzoyl peroxide are added. The mixture is refluxed
for 24 hours. The crystals of succinimide are filtered
off and the solvent is evaporated off under vacuum.
The residue is taken up in a mixture of hexane an~
ethyl acetate and the solution is kept in a refriqera-
tor for 24 hours. The crystals formed are filtered off
to give 14 g of 4-(3-cyanothian-2-yl)benzyl bromide in
the form of crystals melting at ~0C.
Example 160: 1-Methyl-3-n-butyl-4-[4-(3-cyanothien-2-
yl)benzyl]-5-hydroxypyrazole
Formula (IX): Rl = n-butyl, R2 = methyl,
V= ~
-- 2~38~28
- 112
1.4 g of ethyl 2-[4-(3 cyanothien-2-yl)benzyl]-
3-oxoheptanoate, prepared in Example 159, are dissolved
in 10 ~1 o~ ethanol. 2.2 ml of methylhydrazine are
added, the mixture is re1uxed for 13 hours, water is
05 added and the resu~ting mixture is extracted with ether
and then with ethyl acetate. The organic phases are
combined and evaporated under vacuum to give an oily
residue, whicht after chromatography on silica gel in a
methylene chloride/methanol eluent (95/5), gives 0.8 g
1~ of 1-methyl~3-n-butyl-4-~4-(3-cyanothien-2-yl)benzyl]-
5-hydroxypyrazole in the form of crystals melting at
120 C.
Example 161: ~-Methyl-3-n-propyl-4-(2'-cyanobiphenyl-
4-yl)methyl-5-~,N-diethylcar~amoyl~oxy-
pyrazole
Formula (IX): R = n-propyl, R2 = methyl,
C,H,
R3 = ll N~
o C2
~.
V= 1 1~
NC
Prepared by the procedure of Example 52.
Oil used as such for the next step.
2~38~28
- 113 -
xampl~_162: 1-Methyl-3-n-propyl-~ [2'-(tetrazol-5-yl)-
biphenyl-4 yl]methyl-5-(N/N diethylcar-
bamoyl)oxypyrazole
05 Formula (I): Rl = n-propyl, R2 = methyl,
A = OR3,
,~,H5
R3 = 1I N~
o C2H,
R~ =
N
/ \
N~ ~N
N H
Prepared by the procedure of Example 70.
Crystals melting at 134-135~C.
Exam~le 163: N-~[l ~2,~,2-Tri~luoroethyl)-3-n-propyl-
4-(2'-cyanobiphenyl-4-yl)methylpyrazol-5-
yl30xyacetyl]thiomorpholine
Formula (XI): Rl = n-propyl, R2 = CH~CF3,
R3 = C~CO N S ~
V= 1 11 .
NC
Prepared by the procedure of Example 46, start-
ing from N~(chloroacetyl)thiomorpholina.
Oil used as such for the next step.
~`` 2~3~28
- 114 -
Example 164- N-ttl-(2,2,2-Trifluoroethyl)-3-n-propyl-
4-[2'-[tetrazol-5-yl)biphenyl-4-yl]methyl-
pyrazol-5-yl~oxyacetyl]thio~orpholine
05 Formula ~ R1 = n-propyl, R2 = CH2CF
A = OR3,
r~
R~ = CHICO N ~ S,
\~ `
R =
N~H
Prepared by the procedure of Example 70.
Crystals melting at 127-128C.
Example 165: 1-(2-Methoxyphenyl)-4-[1-methyl-3-n-
propyl-4~(2~-cyanobiphenyl-4-yl)methyl-
pyrazol-5-yl]oxyacetylpiperazine
ForMula (XI): Rl = n-propyl, R2 = methyl,
~ R3 = CH2CO N ~ N
CHJO
V= ~3
NC
Prepared by the procedure of Example 64.
Oil used as such for the next step.
- 2~3~28
Example 166: 1 ~2-~ethvxyphenyl)-4-[l-methyl-3-n~
propyl-4-~2~-(tetrazol~5-yl~biphenyl-4-
yl]m~thylpyrazol-5-yl~oxyacetylpip~razine
05 Formula (I): R1 = n-propyl, R2 = methyl,
A = ~3
R3 = CH,C0 N N
lo CH,0
V= l 11
N~/
N~, N
1 5 N~H
Prepared by the procedure of Example 70.
Crystals melting at 139-141C.
Example 167: 3-n-Propyl-l-methyl-4-~2'-cyanobiphenyl~
4-yl)nethyl-5-(2-dimethylaminoethoxy)-
pyrazGle
Formula ~XI): R1 = n-propyl, R2 = methyl,
C~13
R3 = CH2CH2N~
CH,
V ~
g of 3-n-propyl-1-methyl-4-(2'-cyano~i-
phenyl-4-yl)methyl-5-hydroxypyrazole, prepared in
Example 68, are dissolved in 100 ml of butan-2-one in
the presence of lO g of sodium carbonate and 8.6 g of
2l338~8
- 116 -
N,N-dimethyl-2-chloroethylamine hydrochloride. The
mixture is refluxed ~or 20 hours and then concentrated
under vacuum, taken up with water and extracted with
ether. The ether phase is washed with water, dried and
05 then evaporated under vacuum. The residue is chromato-
graphed on silica gel in a chloroform/methanol eluent
~95/5) to give ~.6 g of 3-n-propyl-1-methyl-4-~2'-
cyanobiphenyl-4-yl)methyl-5-(2-dimethylaminoethoxy~-
pyrazole in the form of an oil, which is used as such
for the next step.
Example 168: 3-n-Propyl-1-methyl-4-[2'-(tetrazol-5-
yl)biphenyl-4-yl~me~hyl-5-(2-dimethyl-
a~inoethoxy)pyra~ole
Formula (I): Rl = n-propyl, R2 = methyl,
A = OR3,
CH,
R3 = C~C~N~
CH,
~,
Ra = 1 IJ
N
N~ ~N
N H
Prepared by the procedure of Example 70.
Crystals melting at 88-90C.
' "
2~3~28
- 117 -
Example 169: N-[2-[1-Methyl-3-n propyl-4-~2'-cyanobi-
phenyl-4-yl)~ethylpyraxol-5-yl~oxyethyl~-
~orpholine
05 Formula (XI): R~ = n-propyl, Ra ~ methyl,
r~ .
R~ = C~ CH2N ~ O ,
V= 1 1
NC~
Prepared by the procedure of Example 167,
starting from N-(2-chloroethyl)morpholine.
Oil used as such for the next step.
E~ample 170: N- L 2~ ethyl-3~n-propyl-4-C2'-(tetrazol-
5-yl)biphenyl-4-yl]m~thylpyrazol-5-yl~-
oxyethyl~morpholine
Formula (I): Rl = n-propyl, R2 = methyl,
A = OR3,
r~
R3 = C~CH2N ~ O ,
R4 =
/
N~ N
N~H
Prepared by the procedure of Example 70.
Crystals melting at 132-134C.
21~3~8
- 118 -
~xample 171: 1-(2,2,2-Trifluoroe~hyl) 3-n-~utyl-4-[2'-
cyanobiphenyl-4-yl)methyl-5-hydroxymethyl-
pyrazola
05 Formula (XII): Rl = n-butyl, Rz = CH2CF3 r
R' = H,
V = ~ 3 , q = l
NC
Prepared by the procedure of Example 111,
starting from the 1-(2,2,2-tri~luoroethyl)-3-n~bu~yl-4-
(2'-cyanobiphenyl-4-yl)methyl-5-bromomethylpyrazole
prepared by the procedure of Example lo9.
Oil uæed as such for the next step.
Exa~ LjL7~: 1-(2~2~2-Trifluoroethyl) 3-n-butyl-4-[2'--
(tetrazol-5-yl)~iphenyl-4-yl]methyl-5-
hydroxymethylpyrazole
F~rmula ~ R~ = n-butyl, R2 = CH2CF3,
A = CH20H,
R.,~
N~
N\~N~N
Prepared by the procedure of Example 71.
Crystals melting at 114-117C.
-
2~8~28
-- 119 --
Example 173: 1-(2,2,2-Trifluoroe~hyl~-3-n propyl-4-(2 7 -
cyanobiphenyl-4-yl)methyl-5~ethoxym~thyl-
pyrazole
05 ~ormula (XII): Rl = n-propyl, R2 = CH2CF3,
R' = CH3, ~ = 1,
~ .
V= 1~ IJ
Nc~V
Prepared by the procedure of Example 101.
oi 1 used as such for the next step.
Example 174: 1-(2,2,2-Trifluoroethyl)-3-n-propyl-4-[2'-
(tetrazol-5-yl~biph~nyl-4-yl]~e~hyl-5-
methoxyme~hylpyrazole
Formula (I): R~ z n-propyl, R2 - CH2CF3,
A = CH20CH~, ~4 =
N
N\~ ,N
Prepared by the proeedure of Example 71.
Crystals melting at ~77-178C.
2 ~
- 120 -
Example 175: N-[t1-Methyl-3-n-butyl-4-(2'-cyanobi-
phenyl-4-yl)methylpyrazol-5-yl]oxyac~tyl]-
m~rpholine
05For~ula (XI): R1 = n-butyl, R2 = methyl,
r~
R9 = C~CO N ~ O I
V= ~
NC
Prepared by the procedure of Example 46.
Oil used as such for the next step.
Example 176- ~r [1-Methyl-3-n-propyl-4 (2'-cyanobi-
phenyl-4-yl~me~hylpyrazol-~-yl~o~yac~t~
morpholine
20Pormula ~XI~- Rl = n-propyl, R2 = methyl,
R3 = C~cO N ~ O ,
V = l 11
NC ~
Prepared by the procadure of Example 46.
Oil us~d as such for the next step.
2~3~'~28
- 121 -
Example 177: N-[[l-~ethyl-3-n-butyl-4-[2'-~tetrazol-5-
yl)biphenyl-4-yl~methylpyrazol-5-yl~oxy-
acetyl~orpholine
05 Formula (I): Rl = n-butyl, R2 = methyl,
A = OR3,
~3 = CH2CO N 3 ,
R" =
I
N~ ~N
N H
Prepared by the procedure of Example 70.
Crystals meIting at 140-141C.
Exa~ple 178: N-[[l-~ethyl-3-n-propyl-4-C2'-(tetrazol 5-
yl)biphenyl-4-yl]methylpyrazol-5-yl]oxy-
acetyl]norpholine
Formula ~ R~ propyl, ~2 = methyl,
A = OR3,
A
R~ = C~ ~0 N O ,
R~ =
N~N~N
Prepared by the procedure of Example 70.
Crystals melting at 106-110~C.
-
34~
- 122 -
Example 179: 1-(2,2,2-Trifluoroethyl~-3-n-propyl~4-~2t-
cyanobiphenyl-4-yl)~ethyl-5-h~drox~methyl-
pyrazole
05 Form~la (XII~: Rl = n-propyl, R2 = CHaCF3
Rt = H,
V = X 3 ' q = l
NC
Prepared by the procedure of Exa~ple 111,
starting from the 1-(2,2,2-trifluoroethyl)-3-n-propyl-
4-(2'-cyanobiphenyl-4-yl~methyl-5-bromomethylpyrazole
prepared by the procedure of Example 109.
Oil used as such for the next step.
Exam~le 180: 1-(2,2,2-Trifluoroethyl)-3-n-propyl-4-[2'-
~tetrazol-5--yl~bipheny:L-4~yl~ethyl-5-
hydroxymethylpyrazole
~0
Formula (I3: Rl = n-propyl, R2 = ~H2CF
A = CH20H,
~,
R4 = ¦ ~¦
/ ~
N~N'~
Prepared by the procedure of Example 71.
Crystals melting at 110-2C.
2~38~8
- 123 -
xample 1~ Methyl-3-n-propyl-4-(2'-cyanobiphenyl-4-
yl)methyl-5-hydroxymethylpyra201e
For~ula (XII): R1 = n-propyl, R2 = methyl,
05
R' = H, V =
q = 1
Prepared ~y the procedure of Example lll.
Oil used as such for the next step.
Example 182. l-~ethyl-3-nrpropyl-4-[2'-(~etrazol-5-yl~-
biphenyl-4-yl]methyl-5~hydroxy~ethyl-
pyrazole
Formula (I): Rl = n-propyl, R2 = methyl
A - CH2oH~ R4 =
N
N\~N~N
Prepared by the procedure of Example 71.
Crystals melting at 136-9~C.
2~3~2~
- 124 -
Example 183: 1-(2-~e~hoxyphenyl)-4-~1-methyl-3-n-butyl-
4-(2'-cyanobiphenyl-~-yl)~ethylpyra201-5-
yl]oxyacetylpiperazine
05Formula (XI): Rl = n-butyl, R2 = methyl,
R9 - CH2CO N N
CH~O
~
v= 1 11
NC "'~
Prapared by the procedure of Example 64.
15Oil used as such for the next step.
Example 184~ 2-~ethoxyphenyl)-4 [1-methyl-3-n-butyl-
4-r2~-(tetrazol-5-yl)biphenyl-4-yl~eth
pyrazol-5-yl]oxyacetylp.ip~razine
Formula (I): R1 = n-butyl, R2 = methyl,
A = OR3,
R~3 = CH,CO N ~ N
CH30
N--
N~ ~N
Prepared by the procedure of Example 70.
Crystals melting at 153-5C.
- 2~38~28
- 125 -
Example 185: N-[[l-Me~hyl-3-n-propyl-4-~2'-cyanobi~
phenyl-4-yl)methylpyrazol-5-yl]oxyacetyl]-
thiomorpholin~ -
05 For~ula (XI)- R = n-propyl, R2 = methyl,
R, = t:H2G N 5,
V= ~
~C
Prepared by the procedure of Example 46, s~art-
ing from N-(chloroacetyl)thiomorpholine.
Oil used as such for the next s~ep.
Example 186: ~-[[1-~e~hyl-3~n-propyl-4-[2'-~t~tra~ol-5-
yl)biphenyl-4-yl]methylpyrazol-5-yl]ox~r-
acetyl]thiomorpholine
For~ula (I): Rl = n-propyl, R2 - mathyl,
A = OR3,
r~
R3 = C~C N ~ 5 ,
R =
N~ N
N~H
Prepared by the procedure o~ Example 70.
Crystals melting at 140-1C.
-" 2038~8
- 126
TAE~LE
CH,
N--N
~ O CH2 C02 Et
05 Example 27 `~ UP ~21-1
[~H N--N'CH'
COOH~~O CH2 CO2 Me P 2~1-6
Example 28 T u
~H CH~
COOH N--N'
~O CH2 CO2 Et
Example 29 O ,~3J UP 221-11
~N
~COOH N N,CH,
2 0 ~O CH2 C 2 Et
Exanple 30 ~ ~ UP 221-13
COOH
Cl N N,CH~
~kOCH2CO,Me
Cl O ~J
Example 31 ~ J~ UP 221-12
~COOH
Cl
2~3~2~
- 127 -
rCF,
~ OcH2co2M~
05 Example 32 ¦ UP 221-7
~N~
H CFJ
~COOH N--N
Cl /~0 CH, CO2 h~e
Example 33 O ~ UP 221-4
COOH N-NH
~ ~ OGH2CO2H
Example 34 o ~ UP 221-16
~ N
~ COOH N-N
~OCH,CO2H
Example 35 ~ ~ UP 221-1
~ so,HH ,CH~
N--N
~OH
Example 36 ~ UP 221-15
~ COOH
---" 2~3~2~
128 --
,CH,
~` ,~0 CH2 CO, H
05 Example 37 ~ ~J UP 221-17
b~COOH N--N'CH'
,l~OH
Exnmple 40 ~ J UP 2~1-18
COOH N_N CO2Et
~5 ~O~l
Example 41 ~ ~0J UP 221-19
2U COOH CO2Et
~~0 CH2 CO2 Et
Example 44 ~N~J UP 221-20
l 1I H
~COOH ,CHJ
~ ~, ,4~0 CH2 C02 Et
Example 45 O ~ UP 221-21
3 [~N~
SO,H
" 2~38~28
-- 129 --
,CH,
~0 Cl~ C N~O
05 Example 48 ~ UP 221-22
~N~J
SO,H N--N~CH~
~ ~0CH2CH~OH
Example 51 ,~J UP 221-23
f~N
~SO,H ,CH,
~~0 CO N'
Example 54 0 ~ UP ~21-27
~N
~60,H OCO~
Example 57 0 ,~3J UP 221-28
~N
1 ll~ H
~V SO,H rCFJ
N--N ,~"
~0 CO N"
Example 60 ~ UP 221-30
3
SO,H
.
.~ ' ,.
,
-` 2~3~28
-- 130 --
rCFJ
N--N
~O CH2 CH2 OH
O ~
05 Example 63 ~ ,,l~J UP 221-32
o CH2 C N~
Example 66 ~N~J UP 221-3 3
W `SO,H N--N
~0 CH2 CO2 Et
~
Exampla 70 ~ UP 221-34
~N~
N
H N -N'
~OH
Example 71 ~J UP 221-43
~ ll
~N
N~N' CH,
H N - N
Exa~nple 72 ~0~ ~ UP 221-59
~N~
N
N~
` ~038~28
- 131 -
rCF,
~ OH
05Example 74 ~ UP 221-56
~N CH
~ OCH,CH20H
Example 76 ~ UP 221 57
~ N~
H~N N-N r CF~
~ OCH2COjEt
Example 78 ~ UP 221-54
N
N_~' r CF,
~ N-N CH,
~_~0 CO ~C
Example 80 ~ UP 221-55
~ ;
,, ~ ~ - ;
-`` 203~2g
- 132 -
~0 CO N~H
05 Example 82 (~/ 13P 221-51
N
H--N'N CF,
N~N
~0 CH2 1::0, E~t
Example 84 ~J UP 221-44
,~W
~N
N "N
Bxampl~ 86 ~~OC0 N~ U}? 221-45
@$~
N
H--N' CH,
N--N
Example 88 ~OCH2CH~OH UP 221-46
~,~J
~N
HN--N
- 2~38~2g
- 133
N N,CH3
~0 CH, l ;2 Et
05 Example 90 ~J UP 221-47
,~
~N
H N--N
/~0 CH2 CH, OH
Example 92 ~ UP 221-50
~
N~ "N ,CH,
H N N--N
Example 107 ~OCH~ UP 221-38
~S0 H ,H
2 5 ~OCH,
Example 10~ o ~ UP 221-36
~NJ~
~SO,H N_N,CH,
3 0 ~C~H
l~xample 115 ~ ~ UP 221-~2
3 5 SO,H
2~3~28
- 134 -
N-N'
~ OH
Example 116 0 ~ UP 221-40
05 ~ N ~
~ OCH,
Example 117 ~ UP 221-48
~
N
N-N' N N,CH,
~ OCH,
Example 118 ~ UP 221-52
~j
` ~ N
H-N N-N ~ CF,
~ OCH,
Example 119 ~ UP 221-53
~ N~
N
Example 125 N_N~ UP 221-58
~ OCH,CO2Et
~
,~
~N~ .
N
N_N'
2~3~8
-- 135 -~
rCF~
N--N
~ ~--O CH2 CO2 Et
Example 127 T UP 221-61
05
~N
N_N~
N--N
~O CH2 CH2 OH
Example 129 ~ UP 221-60
~N~
H N
C~,
N--N 11 A
~OCH2C N 0
Example 130 ~l UP 221-~5
G~H
NH S02CF~
CH~
N--N
~OCH2COzM~
Example 135 ~l UP 2 21-26
~
NH SO, CF,
2~38~
-- 136 --
CH,
N--N
Example 137 ~OCH2CH,OH UP 221-29
05 f~J
CO2H
CH,
N--N
~~ ~0 CH2 CO, Et
Example 141 `~ UP 221-35
~
N~N~r CH,
N--N
E~ample 14~ /~0 CH~ CH, ~H UP ~21-39
~
: ~ , ,
CO,H
~~ C~C0 N N~
Example 147 ~ UP 221-62
~N
N~N~N
.
2~38~2~
-- 137 --
--CF,
~OCH~CO N~ ~O
Example 149 ¦ UP 221-63
05
~N
N "N rCF
n J~OH
Example 150 ,~,J UP 221-64
f~
~N
H~N"N ,CH,
Example 153 ~OH UP 221-65
~J
~N
N "N
H~N ,GHs
N--N
O~
Exampl~ 156 ~J UP 221-66
~N
H~N"N
203~2~
138 --
CF,
N--N
~---0 CH2 ~H2 OH
Exampla 158 ~3J UP 221-67
05 ~
N , N C2H,
/^ ~0CON~
EYaDP1e 162 ~ UP 22L--68
H N N--N ~
Example 164 /~OCH2CO N~S UP 221-69
~
~r~ h ,CH, C~,O
Example 166 ~/~-OCH2CON N~3 UP 221-70
1~ .
G~
N~,N~,N
38~2~
- 139 -
CH,
~ OCH,CH,N~
Example 168 ~ CH, UP 221-71
0~
~N
N , N CH
~OCHzCH2 N O
Example 170 ~ UP 221-72
~J
~N
~`N' N--N--CF~
Example 172 ~ Otl UP 221-73
~
,: HN~N'~N rCF,
~ OCH,
Example 174 ¦ UP 221-74
f~
~N
H`N"N
2~33 ~28
- 140
,CH~
N - N ,_~
~O CH2CO N O
~ . ' .
05 Example 177 l ll UP 221-76
~N
N`N~N CH,
N - N / - - ~
~O CH2CO N O
Example 178 J UP 221-77
~ ',.
W~N
H``N"N
rCF~
N - N
Example 180 ~OH UP 221-78
~ .'
~ CH,
N -N
Example 182 ~OH UP 221-79
~N
N~N
203~28
-- 141 --
~H,
~ ~ O CH2CO N\lN $~
05 Example 1~4 ~ OCH UP 221-~0
,~
~N~
N~ "N
H N
,CHJ
N~N ~
Example 186 ~--OCH,CON S UP 221-81
~N
N`N~N
2~3~28
142 -
PHARMACOLOGY
I. Principle
05 The affinity o the products of the Examples
for angiotensin II receptors is evaluated ~y the
techni~ue of displacing a radioligand specifically
~ound to rat adrenal angiotensin II receptors.
II. Procedure
An aliquot of a rat adrenal gland homogenate
incubates in the presence of a single concentration of
~`25I]-SIAII (Sarl,Tyr~,Ile~-angiotensin II), which is
an angiotensin II receptor antagonist, and two concen
trations of competing agents (10-5 M, 10-7 M) for 60
min at 25C.
The reaction is completed by the addition of a
buffer, followed by rapid filtration through glasspaper
filters. The non-specific binding is determined in the
presence of angiotensin II.
III. Expression of the results
The results are expressed, for the concentra-
tions tested, as the percentage displacement of the
radioligand specifically bound to the adrenal angio-
tensin II receptors.
20~28
- 143 -
IV. Results
_
Product of ~ displacemant of the labeled ligand
Example .
05 lE-5 M lE-7 M
_ _ _ _,
27 92 39
28 82 34
29 80 ~0
96 63
31 93 60
32 98 52
33 94 26
48
36 62 10
37 58 9
83 0
41 85 2
44 96 9
48 92 58
51 83 60
54 77 62
57 80 60
84 49
63 91 ~4
66 92 63
67 49
71 58 24
74 66 58
78 73 58
69 48
82 71 47
84 65 51
86 60 38
64 40
92 75 70
107 83 62
108 86 19
115 80 57
116 82 12
117 61 35
118 67 35
119 70 54
130 74 33
135 65 34
137 63 5
141 64 9
145 53 0
"` 2~3~28
- 144 -
TOXICOLOGY
The products o~ the Examples described have an
excellent ~olerance after oral administration~
05Their 50% lethal dose in rats was found to be
greater than 300 mg/kg.
CONCLUS ION
~ .
10The products of the Examples described have a
good affinity for angiotensin II receptors. In this
respect, they will be able to be used bene~icially for
the various pathological conditions in which angio-
tensin II is involved, in particular in the treatment
of arterial hypertension and cardiac insuf~ciency, in
dosages of 1 to 400 mg by oral administration and 0.01
to 50 mg by intravenous administration, in one or more
dosage units per day.