Note: Descriptions are shown in the official language in which they were submitted.
~ 3 ~
ANTIBODY COATED CRYSTAL CHEMICAL SENSOR
BACKGROUND
The present invention relates generally to chernical sensors, and more particu-
larly, to a chemical sensor that employs antibody coated oscillators.
There exists a need for chemical sniffers that have high selec~vity, low false
signal rate, and high sensitivity, on the order of one part in 108 - 109. Sensitive chemi-
5 cal detectors are desired for drug and contraband detection, and explosives detection,including plastic explosives and dynamite. Additionally, chemical sensors may be used
in the environmental cleanup and hazardous material field. Chemical detectors exist
that are highly specific, including devices such as spectrum analyzers, for example, but
such devices have relatively low sensitivity levels. Chemical detectors also exist that
10 are very sensitive, but these are not specific enough to one chemical (low selectivity),
thus increasing false signal rates.
Dr. George Guilbault, at the University of New Orleans, has experimented with
coating quartz crystal oscillators with antibodies. His research is discussed in a paper
entitled "Immobilization Methods For Piezoelectric Biosensors," published in
15 BiolTechnology, ~ol. 7, April 1989, pages 349-351. However, these oscillators must
be corrected for temperature, pressure, and humidity. The experiments must be done
in a laboratory because of sensitive corrections that are required. These corrections re-
sult in a decreased sensitivity in such devices.
Accordingly, it would be an advantage in the chemical detection art to have a
20 detector that is both selective and highly sensitive and that may be employed outside
controlle,d laboratory conditions.
Sl~MMARY OF THE IN~ENTION
The present invention employs specific antibodies deposited on a high Q crystal
oscillator to detect the change in frequency when particulates that the antibodies are spe-
ci~lc to become trapped by the antibodies. The trapped particulates change the effective
5 mass of the crystal and hence change the oscillating frequency thereof. In particular,
two crystals are used, one that has been coated with the antibodies, and one that is un-
coated.l This permits detection of the absolute frequency difference between the oscil-
lating frequencies of the coated oscillator, before and after exposure to the chemical.
Specific antibodies deposited on the crystal oscillator detect the change in fre-
10 quency as chemical particulates become trapped by the antibodies and change the effec-
tive mass of the crystal. Two oscillating crystals are used, one that has been coated
with the antibodies, and one that is uncoated. This permits detection of frequency dif-
ferences between the oscillating frequencies of the two crystals, thus eliminating pres-
sure~ temperature, and humidity corrections that conventionally must be made.
An alternative embodiment of the present invention employs a mass loading
technique that only allows the chemical particulates to adsorb onto the crystal in specific
nodal locations of a harmonic of the primary frequency. The ratio of the amplitudes of
the harmonics are then measured. This technique may be used with the two crystal ap-
proach, or because of the stability of this ratio under changing temperature, pressure,
and humidity conditions, may be used without the uncoated reference crystal. How-
ever, more complex scanning electronic circuitry is required to locate the peaks (shift-
ing in frequency).
The chemical sensor of the present invention maintains a high specificity by
using antibodies which are specifically related to a particular chemical, such as TNT,
DNT, cocaine or marijuana, for example. The sensor achieves relatively good sensi-
tivity by using two high Q oscillators and also eliminates drift problems due to tempera-
ture, pressure and hurnidity that typically occur with conventional sensors. Additional-
ly, the present chemical sensor is rugged and is able to fit in a small package, whereas
many conventional sensors will not.
A variety of crystal oscillators, including quartz and sapphire may be used.
Furthermore, the crystals may be cooled~to liquid helium temperature, which substan-
tially increases the Q of quartz by a factor of over ten. Also, the use of an out-of-phase
scheme for each crystal oscillator and integrating from minimum to maximum permits
the possibility of reaching the photon noise limit.
2~3~
BRIEF DESCRIPTION OF THE DRAWINGS
The various features and advantages of the present invention may be more read-
ily understood with reference to the following detailed description taken in conjunction
with the accompanying drawings, wherein like reference numerals designate like struc-
5 tural elements, and in which:
FIGS. la and lb illustrate an antibody coated dual crystal chemical sensor in
accord~nce with the principles of the present invendon;
F~G 2 shows curves illustrating an out-of-phase detection scheme that may be
employed with the sensor of FIG 1;
FIG 3 shows a second embodimens of a sensor in accordance with the princi-
ples of the present invention; and
FIGS 4a and 4b show a chemical detection scheme using the sensor of FIG 3
DETAILED DESCRIPTION
Biologically produced molecules that bind with specific molecules, including
DNT, TNT, marijuana and coeaine, for example, are called antibodies. There are two
types of antibodies: monoclonal and polyclonal, Monoclonal andbodies only interact
with speci~lc molecules and may be used to obtain high specificity. Polyclonal antibod-
ies bind to several related molecules, including DNT and TNT, for example, Antibod-
20 ies currently exist for DNT, TNT, marijuana, cocaine, and other ehemieals of interest.
A surfaee eovered with antibodies may typically bind with up to 1013 molecules/cm2,
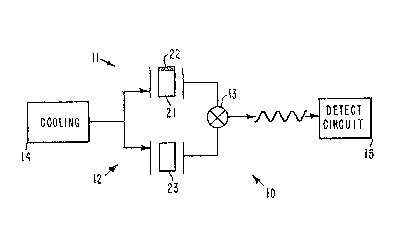
With reference to FIGS. la and Ib, an antibody eoated dual crystal ehemical
sensor 10 in accordance with the principles of the present invention is shown. The
sensor 10 comprises a coated piezoelectric crystal oscillator circuit 11, one example of
25 which is the circuit shown in FIG. lb. The coated piezoelectric erystal oscillator circuit
11 may be comprised of a coated piezoelectIic crystal 21 made of a material such as
quartz, or sapphire, for example, having its outer surface coated with either polyclonal
or monoclonal antibodies 22. Typical polyclonal or monoclonal andbodies 22 may be
employed to bind molecules of chemicals comprising DNT and TNT, for example.
30 Antibodies for TNT, DNT, morphine and other chemicals are available from IRT
Corporation in San Diego
A second substantially identical crystal oscillator circuit 12 comprising an un-coated crystal 23 is disposed adjacent to the first oscillator circuit 11. Outputs of the
two circuits 11, 12 are coupled to a frequency mixer 13, for example, that is adapted to
35 mix the output signals from the two oscillator circuits 11, 12 to produce a detectable
beat frequency signal. Cooling of the crystals 21, 23 may be provided by cooling ap-
paratus 14, such as a cryogenic refrigerator, for example The beat frequency signal
output from the mixer 13 is coupled to a detection circuit I S that is adapted to process
the beat frequency signals to produce a signal indicative of the detection of a chemical
of interest.
With reference to FIG. 1, the two crystal oscillators 21, 22 are part of the re-
S spective crystal controlled oscillator circuits 11, 12 that are adapted to control the oscil-
lating frequencies thereof such that they oscillate at substantially the same frequency.
The electronics associated with the crystal controlled oscillator circuits 11, 12 are gen-
erally well known, and each may comprise the oscillator circuit 11 shown in FIG. 1 b,
for example. The oscillator circuit 11 shown in FIG. lb includes the coated crystal 21,
10 a transistor 24 having its drain and base coupled across the crystal 21 and wherein the
base is coupled by way of a resistor 25 and capacitor 26 in seAes to the positive termi-
nal of a voltage source 27. The source of the transistor 24 is coupled by way of a re-
sistor 28 and capacitor 29 in parallel to the positive terminal of the power source 27.
Finally, the negative terminal of the power source 27 is coupled through a capacitor 30
15 and inductor 31 in parallel to the drain of the transistor 24.
With reference to FIG. 2, the second uncoated crystal oscillator 23 acts as a
control for the coated crystal oscillator 21, in that it provides reference data for frequen-
cy computations made on the coated crystal oscillator 21. l'he two crystal oscillator
circuits 11, 12 are also coupled to the detection circuit 15 that is adapted to detect the
20 oscillating frequencies of each of the crystal oscillators 21, 23 and produce a signal
indicative of the frequency difference between the frequency of oscillation of the first
crystal oscillator 21 before and after exposure to a chemical of interest that the antibod-
ies 22 are adapted to adsorb.
In operation, the crystal oscillator 21 is exposed to air or water contarninated25 with a chemical that is to be detected. The mass increase of chemical particulates bound
by the antibodies 22 on the coated crystal oscillator 21 is detected through a frequency
shift of the output signals from the coated oscillator 21. The frequency shift is given
by the equation ~f/f = -1/2 ~m/m, where f is the frequency of oscillation of the coated
oscillator 21, ~f is the change in frequency after exposure to the chemical of interest, m
30 is the mass of the coated oscillator 21 and ~m is the change in mass of the coated oscil-
Iator 21 result~ng from adsorption of the chemical by the antibodies 22 on the surface
thereof.
Quartz crystal oscillator normal mode frequencies, for example, range from 104
toaboutSx 107Hz. A0.1 mmthickdiscofquartzhasaf~=2x 107Hz,andhas
35 higher order harmonics of up to 2 x 108 Hz. The Q of a quartz cr)~stal is quite high and
may be greater than or equal to 106.
$ ~
The mass of the maximum amount of the chemical adsorbed by the first crystal
oscillator 21 is given by the equation am = n x M x mH, where n is the added molecule
density (1013 molecules/cm2), M is the molecular weight of the absorbed molecule, and
mH is the mass of a hydrogen atom. For a material such as 'l'NT, for example, where
M - 220; am _ (1013 molecules/cm2) x (220 molecule~l) x (1.7 x 10-24 grams) = 3.7 x
10-9 gm/cm2. A quartz crystal disc haYing a 0.1 mm thickness has mass m_ 0.01 cmx 2.2 gtams/cm3 = 0.022 gmlcm2. Therefore, the frequency shift for the coated crystal
oscillator 21 made of quartz and having an operating frequency of f = 20 MHz is ~f =
(-1/2 ~m/m)f - (-1/2)(3.7 x 10-9 gmlcm2 / 2.2 x 10-2 gm/cm2) / (2 x 107 Hz = 1.7 Hz.
This magnitude of sensitivity is somewhat difficult to measure due to tempera-
ture, pressure and humidity changes lhat affect the absolute vibrational frequency of the
crystal oscillators 21, 23. In accordance with the principles of the present invention,
by employing two crystal oscillators 21, 23, and having the first crystal oscillator 21
coated with antibodies 22 and the second crystal oscillator 23 as a reference, ~f may be
measured by detecting the beat frequency between the two crystal oscillators 21, 23.
The sensor rnay be electronically nulled to ignore the mass of the antibody alone, with-
out any adsorbed chemical particulates. There is an uncertainty (~) in the beat frequen-
cy, and for f = 2 x 107 and Q 3 1.3 x 107, is given by~ f) 3 f/Q = 1.5 Hz.
However, this uncertainty may be substantially reduced by increasing the Q of
the coated crystal oscillator 21, thus making the measurement easier. The Q of the
coated crystal oscillator 21 may be increased by the following techniques. The coated
crystal oscillator 21 may be cooled to liquid helium temperature, as illustrated in FIG.
la by the cooling apparatus 14, such as a cryogenic refrigerator, for example. Cooling
of the crystal oscillators 21, 23 increases which substantially increases the Q of quartz
by a factor of over ten. A crystal oscillator that has a substantially higher Q, such as
sapphire, may be employed. Also, an out-of-phase detection scheme may be employed
for each of the crystal oscillators 21, 23 which comprises integrating the output fre-
quency signals from each of the crystal oscillators 21, 22 during the measurement pe-
riod from minimum to maximum, which increases the probability of reaching the pho-
ton noise limit.
The details of the out-of-phase scheme are as follows, and shall be described
with reference to FIG. 2. It is possible to increase the detection capability of ~f = (fl-
f2) of the detection circuit 15 beyond its Q determined bandwidth. The two crystal
controlled oscillators 21, 23 have similar (almost identical) Q are operated in opposite
phase as illustrated in FIG. 2. The Fourier components in the first half of the reso-
nance curve will produce a net charge (N) in the detection circuit 15. The charge (N) is
proportional to the recording time. There is, however, a statistical noise equal to Nl/2
'
~3~
associated with the random distribution of the number of electrons, cornmonly referred
to as shot noise. Therefore, the accuracy of determining the best frequency is propor-
tional to the square root of time.
Specifically the number of charges Nl and N2 produced by the oscillators 21,
5 23 in a frequency interval df is given by:
Nl(f)df = Btdf ~YN(f)dt
(f_fl)2+(~f)2
N2(f)df = Btdf +YN2(f)dt
(f-f2)2+(~f)2
where ~f is the line width of the oscillation, t is the measurement time, ~ is Poisson
noise on the signal N(f)df, and B is a constant. The difference is given by D(f)df =
(N1-N2)df. If df < ~f ~f, where df = f2-fl, to get D(f)df > 2YN1(f)df, and
~> I "
~ ~f 3
It is therefore possible to detect partial covering of the antibody surface with the chemi-
cal of interest.
The number of molecules n collected per unit area by the antibody covered sur-
face is n = ng v~t where ng is the number density of the molecules in air, v is the ther-
mal velocity, ~ is the sticking coefficient and t is the time. Numerically, for TNT and
DNT, their molecular weights are about 200 and v = 2 x 104 cm/sec at room tempera-
ture. Therefore, to collect 10l3 molecules per square centimeter, the time required is t _
50/~ seconds, and where ~ is one of the quantities to be determined.
The dual crystal chemical sensor 10 employing an antibody-coated crystal oscil-
- lator 21 in accordance with the present invention is a very sensitive device. It is sensi-
tive enough to detect the presence of the cited chemicals when the surface of the coated
crystal oscillator 21 is only partial covered with the respective chemical. Furthermore,
for some antibodies the reaction is reversible, thus allowing repeated use of the same
coated crystal oscillator 21 for sensing different chemicals.
A second embodiment of the. sensor 10' of the present invention is shown in
FIG. 3 and employs the use of a mass loading of the chemical to be detected at specific
locations 40, 41 on the crystal oscillator 21'. The crystal oscillator 21' is shown con-
figured in the shape of a tuning fork. There is, however, no restriction to the use of
tuning-fork shaped oscillator crystal shown in FIG. 3, for clearly, other shaped crystals
may be used. These locations 40, 41 are chosen to correspond to nodal locations for a
particular higher frequency but a non-nodal location for the fundamental. By restricting
the chemical under detection to only these locations 40, 41 by: (1) only coating the an-
tibodies 22 at these locations 40, 41; (2) placing a baffle 43 above the crystal oscillator
~ 35 21 (shown in phantom to the right of the oscillator 21' in FIG. 3 with windows 44, 45
.;
~.~ ., - .
~IrJ3~
located above the locations 40, 41) which only allows exposure in the desired locations
40, 41 or (3) both (1) and (2).
The advantages gained by the sensor 10' of FIG. 3 is that the ratio of the N(h
harmonic to the fundamental (or a lower halmonic than the Nth) is detected. This ratio
S is independent of temperature and pressure. Therefore it is only necessary to measure
the change in arnplitude ratio of the harmonics of the sensor 10' of FIG. 3.
lFIGS. 4a and 4b show detection spectra generated using the sensor 10' shown
in FIG. 3. FIG. 4a shows the detected spectrum without the presence of any chemical
to be detected. FIG. 4b shows the same spectrum with the detectable chemical ad-
10 sorbed on the surface of the coated crystal oscillator 21 of the sensor 10'. The massloading reduces the lower harmonic frequency oscillating amplitude. The resolution
may be read out by detecting the ratio of the harmonics chosen, illustrated in FIG. 4b as
the first and third harmonic.
Because a ratio of the harmonic arnplitudes are measured instead of absolute fre-
15 quencies, a single coated crystal oscillator 21 may be employed without the referencecrystal oscillator and still achieves the temperature, pressure, and humidity insensitivity.
However using a reference crystal oscillator with the coated crystal oscillator 21' allows
for simpler detection electronics to be used.
Thus there has been described new and improved chemical sensors that employ
20 antibody coated oscillators. It is to be understood that the above-descdbed embodi-
ments are merely illustrative of some of the many specific embodiments which represent
applications of the principles of the present invention. Clearly, rlumerous and other ar-
rangements can be readily devised by those skilled in the art without departing from the
scope of the invention.
, ~