Note: Descriptions are shown in the official language in which they were submitted.
2 0 4 0 9 2 0 PATENT
- 102-24
CAPILLARY INOCULATOR AND ASSEMBLY
FOR INOCUL~TING MULTIPLE TEST SITES
AND METHOD OF INOCULATING TEST SITES THER~wl~ln
P-1459 BACRGROUND OF '1'~ INVENTTON
1. Field of the Invention.
The present invention relates generally to an
1 inoculator for inoculating test sites with small volumes
of a liquid or suspension. The inoculator may be used to
divide a liquid sample into small, discrete volumes and
dispense these volumes.
2. Brief description of the prior art.
Many laboratory procedures in the fields of
microbiology and chemistry require the division of a
liquid sample into small portions of known, and usually
equal volumes. The most commonly used method for dividing
and depositing such samples is referred to as single-
channel pipetting. In this method, the liquid sample isdrawn into a disposable tube or pipette and dispensed as a
single volume or in smaller, usually equal increments.
The technician must position the tip of the pipette over
each test site and dispense an appropriate amount of the
sample into or upon it. Care must be taken to insure the
tip does not contact the test site materials as it may
become contaminated. In addition, the volume of liquid
in the pipette may be lost if this occurs.
3 Inoculation of multiple test sites can be a tedious
operation when using a single channel pipette. A
multiple-channel pipette assembly including multiple tips
can be employed to reduce the ~number of procedures
necessary to inoculate all of the sites, but tip
contamination would still be a concern and the positioning
of the tips is more difficult. Single and multiple-
channel pipettes are generally not used for depositing
- 2 ~ 20~0920
1 very small liquid samples in the 3-5 microliter range due
to poor repeatability.
A second group of devices used to deposit small and
usually equal volumes of a liquid sample are devices
generally known as Steers Replicators. Such replicators
include a hand-held body including an array of pins
protruding downwardly from the body and arrayed as
required for the intended inoculation procedure. Each pin
has a blunt, concave or slotted tip. When the tips of the
pins contact the liquid sample, the bottom surfaces
thereof each retain a specifi~ volume of liquid due to
surface tension. These tips are then moved into contact
with the test sites which are inoculated as the liquid is
transferred thereto. The actual amounts of liquid
transferred to the test sites are dependent upon the
materials from which the pins are made, the liquid surface
tension, the materials present at the test sites, and
evaporation to room air. Replicators of this type may
also tend to entrap air as the geometries of the tips
often do not allow the air to escape as the liquid adheres
thereto. In addition to being rather cumbersome and
providing only fair accuracy, the liquid sample to be
transferred by the replicator must first be poured into a
flat tray so that the pins can be dipped therein. The
tray must then be carefully disposed of without spilling.
The replicators are most commonly made from stainless
steel and are therefore not disposable. They accordingly
must be sterilized once the test sites have been
inoculated.
Various disposable inoculation devices have been
proposed for inoculating test sites which contain various
substrates used for identifying microorganisms. One such
device is disclosed in U.S. Patent No. 4,808,316. This
device includes a substantially rigid, planar frame, a
plurality of test wells, a filling manifold in fluid
communication with the test wells, and a venting manifold
in fluid communication with the test wells. The test
wells each contain an appropriate reagent which is
~ 3 ~ 2040920
1 reactive with a fluid sample deposited therein. The fluid
sample is supplied to the test wells by a gravity feed
through the filling manifold.
Vacuum filling techniques for filling test wells
within cards are disclosed in U.S. Patent Nos. 3,957,583,
4,018,652, 4,116,775, 4,207,394, and 4,318,994. Each of
the test wells contains some combination of culture
medium, antibiotic, and indicator reagents. Microorganism
suspensions are drawn into the test wells by evacuating
most of the air from the cavities. Then the liquid is
drawn in when ambien~ pressure is restored. Light
transmission readings of the test wells are made through
the thickness of the panel.
One of the problems encountered in running medical
tests is that the quantity of specimen is frequently
small. There is accordingly a need for miniaturized
systems for delivering small volumes of specimen to a test
panel.
One such system has recently been developed which
involves the use of a plurality of disk-shaped or other
shaped supports having a dried reagent deposited thereon.
The supports may be made from alpha cellulose, pH
neutralized glass fiber, or other such absorbent
materials. The supports may be secured to a carrier such
as a card or other surface support, or positioned within
test wells formed within a tray. In either instance, it
is important to inoculate each support site with a precise
volume of analyte so that the test is accurate and
repeatable site-to-site and test-to-test.
3o
SUMMARY OF THE INVENTION
An inoculator in accordance with the present
invention comprises a carrier including a base and side
walls which define a reservoir, and a plurality of
capillaries extending through the base of said carrier and
in fluid communication with the reservoir. Each of the
capillaries is preferably formed as a bore within and
extending through the base.
~ 4 ~ 204092~
1 The base of the carrier preferably includes a
plurality of projections through which the capillaries
respectively extend. The projections are preferably
formed with tapered ends such as the conical ends normally
used for the delivery end of a pipette to provide superior
liquid retention within the capillaries while facilitating
liquid transfer to the test sites. The use of projections
allows the capillaries to be filled by immersing them in a
reservoir. If filling is to be accomplished in such a
manner, the carrier need not include a reservoir formed as
an integral part thereof. An absorbent trap is preferably
provided adjacent to the reservoir, if incorporated with
the carrier, for absorbing excess liquid.
Finally, an inoculating assembly is provided by the
present invention which comprises a carrier including a
plurality of capillaries extending therethrough and a test
device positioned adjacent to the carrier, the device
including test sites in registry with the respective
capillaries. Each of the test sites preferably includes
an absorbent support which is capable of drawing liquid
from the respective capillaries by wicking action. A
compressible member is preferably mounted between the
carrier and device for separating the ends of the
capillaries from the test sites prior to initiating the
inoculation procedure. Pressure exerted upon the carrier
urges the ends of the capillaries into contact with the
test sites, thereby causing the transfer of liquid
thereto. The carrier preferably includes a plurality of
projections through which each capillary extends. Each
projection is preferably formed with a tapered end which
is positioned in opposing relation to one of the test
sites.
A method in accordance with the present invention
includes the steps of providing a carrier including a
plurality of capillaries extending therethrough, providing
a liquid within each of the capillaries, aligning the
capillaries with a plurality of test sites, and causing
the liquid within each of the capillaries to be
- 5 - 20~0920
1 transferred to the respective test sites. Such liquid
transferral is preferably accomplished by moving the
carrier towards the test device until the capillaries
contact the respective test sites. Each test site is
preferably provided with an absorbent substrate which
draws the liquid from each capillary via wicking action.
A method is also provided for filling an inoculator
of the type including a carrier and a plurality of
capillaries extending through the carrier, thereby
providing a plurality of discrete liquid samples. The
method includes the steps of contacting the capillaries
with a liquid and substantially completely filling each of
the capillaries. The capillaires are partially or
entirely filled via capillary action. By completely
filling the capillaries in such a manner, whether by
capillary action or a combination of gravity feed and
capillary action, the volume of liquid entering each
capillary is precisely controlled.
BRIEF DESCRIPTION OF '1'~: DRAWINGS
Fig. 1 is an exploded, isometric view of an
inoculator and test panel assembly according to the
invention;
Fig. 2 is a top plan view of the inoculator shown
in Fig. l;
Fig. 3 is a side elevation view of the inoculator
shown in Figs. 1 and 2;
Fig. 4 is a sectional view of the inoculator test
panel assembly in a first position with respect to each
other;
Fig. 5 is a sectional view of the inoculator/test
panel assembly in a second position with respect to each
other;
Fig. 6 is a sectional view of an alternative
embodiment of an inoculator according to the invention
positioned above a test panel; and
Fig. 7 is a sectional view thereof showing the
inoculator engaging the test panel.
- 6 - 204092~
DETAILED DESCRIPTION OF 1~; INVENTION
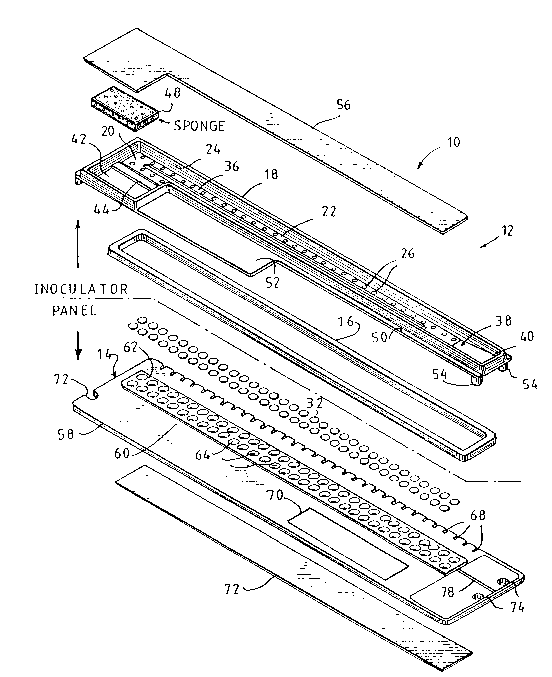
An assembly 10 for inoculating test sites with
accurately reproducible, and optionally equal volumes of a
suspension or other liquid sample is provided by the
invention. The assembly includes an inoculator 12 and a
test device which may be in the form of a panel 14 which
is matched to the inoculator. A compressible member such
as a foam gasket 16 is positioned between the inoculator
12 and test panel 14, and separates them by an appropriate
distance when the gasket is in the "relaxed" position.
The inoculator includes a generally trough-shaped
carrier 18 which is preferably of integral construction
and molded from a polymeric material such as transparent
polystyrene resin. It is important that the resin be of
high purity and that no silicone release agents are used
in the molding process. This is necessary to provide an
inoculator having clean surfaces of controlled
hydrophobicity, the importance of which shall be explained
below.
The carrier 18 has a base 20 including a
substantially flat upper surface 22, peripheral side walls
24 projecting from the upper surface 22, and two rows of
capillaries 26. The base and side walls define a
reservoir or trough. Each of the capillaries extends
through the base and includes an upper opening adjoining
the upper surface 22 of the carrier base and in fluid
communication with the reservoir. Filling of the
capillaries is facilitated if they are substantially
tangent to the side walls 24.
Two rows of projections 28 extend from the lower
surface of the carrier base 20. Each projection includes
a tapered end 30 adjoining the bottom opening of each
capillary. These tapered ends of the projections, which
with the carrier base 20 and projections define the walls
of the respective capillaries 26, are useful in
maintaining liquid within the capillaries. While flat-
bottomed projections or even no projections could be
employed, liquid would be encouraged to run through the
2040920
1 capillaries and form hanging drops on the respective flat,
bottom surfaces. The preferred tapered ends require the
liquid to run back up each bevelled edge, something which
is not likely to occur. A second advantage of the
tapered projection ends is that they increase the
likelihood of the capillaries properly contacting test
sites with which they may be brought into engagement, a
process described in greater detail below. It is
important that the bottom surfaces of the tips of the
projections not be significantly more wettable than the
i~ner walls of the capillaries as the inoculum may run
through the capillaries, thereby potentially contaminating
the work area or the test sites.
The capillaries in a single inoculator may have the
same or different volumes ranging between about one to
twenty-five microliters each. The inoculator 12 shown in
Figs. 1-5 is designed to inoculate sixty-four disk-shaped
supports 32 with equal volumes of a liquid or suspension.
(The terms liquid and suspension are used interchangeably
herein). The supports may be in the form of absorbent
disks, hydrophilic membranes, or other substrates
depending upon the tests which are to be conducted.
Microwells may also be inoculated with liquid from the
respective capillaries. Each capillary includes tapered
interior walls 34 tapering from about a 0.055" (0.140 cm)
diameter at the upper surface 22 of the carrier base to
about a 0.040" (0.102 cm) diameter at the open end of each
projection 28. The length of each capillary is about
0.12" (0.30 cm), and the volume thereof is accordingly
about 3.5 microliters.
The capillaries of the inoculator 12 used in
accordance with the preferred embodiment of the invention
are provided with such tapered, preferably conical
interior walls 34 to facilitate the process of molding the
inoculator. A long, thin core pin on the scale of the
capillary cannot be pulled out of the molded part without
damage thereto and erosion of the pin unless it has a
draft angle. Although it is not required in the
=~- 204092~
1 inoculator 12 shown and described herein, a capillary
which is larger at the top than at the bottom will
reliably hold a greater volume of liquid than one which is
of uniform diameter. The range of acceptable capillary
diameters depends on the material properties of the
capillaries and the liquid held therein, the acceleration
range over which the capillaries are expected to retain
the liquid by surface forces, the method by which the
inoculator is filled with liquid, and the method used for
removing liquid from the inoculator. In the preferred
embodiment of the invention, the liquid is removed from
each capillary 26 by wicking into a small absorbent
support containing the reagents with which the liquid is
to react. Using this removal process, the minimum
diameter of the bottom of the capillary must be a few
times larger than the effective pore size of the
absorbent support. For a particular support as described
below, this would permit a minimum diameter of 0.001 inch
(0.00254 cm). Such a small diameter is, however, very
difficult to mold and would unduly slow the desired
removal of the liquid. The liquid might, in other
applications, be removed by applying pressurized air or
other pressurized gas to the top of the capillaries, or by
deliberate acceleration of the liquid-filled device.
With these removal methods there are no definite lower
limits to the capillary diameter except the limits imposed
by the method of manufacture of the device and the
required speed of liquid removal.
The maximum diameter of the bottom of each
capillary 26 is determined by the need to retain the
liquid in the capillary during the accelerations
associated with any desired or likely motions of the
inoculator 12 between the time the capillaries 26 are
filled and the time the removal of the liquid is desired.
The resulting inertial forces must be overcome by the
surface tension forces at the surface of the liquid. In
the preferred embodiment of the invention, an aqueous
liquid containing a low concentration of wetting agent is
9 2040920
l held in polystyrene capillaries, each of which has a
length a few times (i.e. 2-4) its average diameter.
Manual -manipulation of the filled device is required to
expel the liquid. The practical upper limit of the
diameter of each capillary is about 0.1 inch (0.254 cm).
With more gentle motion of the inoculator, a more wettable
capillary material, and a liquid of higher surface
tension, a larger diameter could be used. These maximum
diameters depend also on the length of the capillary and
the direction and magnitude of the acceleration. The
force of gravity is generally the minimum force which must
be resisted.
The maximum diameter of the top, or liquid entrance
end, of the capillaries is limited by the volumetric
accuracy desired when the capillaries are filled by
flowing a liquid across them, which is the preferred
filling method employed for the inoculator disclosed
herein. If the diameter becomes too large, volumetric
accuracy suffers due to the variation in the liquid
surface shapes left as the flowing liquid breaks away from
that liquid which is retained in the particular capillary.
The capillaries may, if desired, be filled from the
bottom by capillary rise. The projections are immersed in
a liquid for a sufficient time to allow the capillaries to
completely fill. The capillaries are preferably
simultaneously filled. In this case, the maximum diameter
of the top (and all other portions of each capillary) is
imposed by the requirement that the surface tension of the
liquid, its contact angle with the material of the
capillary, and the diameter ensure that each capillary is
filled to the top. Volumetric accuracy is thereby
provided.
A transparent polystyrene carrier as employed
herein, used in conjunction with microbiological
suspensions for inoculating absorbent supports, functions
successfully where each capillary has a volume between
three and ten microliters. Using different materials
and/or design parameters, capillary volumes ranging from
- lo 2040920
1 1-25 microliters could be employed. Generally speaking,
the capillaries are designed so that the volume of liquid
retained by each is the same upon filling and remains the
same for up to ten minutes before transfer without
accidental release or significant evaporation. Using the
parameters described above, these objects can be
successfully attained.
Referring to Figs. 1, 2 and 4, the inoculator 12
includes a carrier designed as a long, narrow trough
having an elongate center ridge 36 which separates the two
rows of capillaries 26. (The ridge 36 should be omitted
if it causes difficulty in filling the capillaries). A
fill area 38 which is devoid of capillaries is provided at
one end of the carrier. The fill area 38 includes an
inclined upper surface 40 which adjoins the upper surface
22 of the carrier base 20 at one end and adjoins the
peripheral side walls 24 at three surfaces thereof.
Liquid deposited on the upper surface 40 of the fill area
accordingly tends to move toward the upper surface 22 of
the carrier base 20 which includes the upper openings of
the capillaries 26.
An excess inoculum trap 42 is integrally formed
with the carrier base 20 in offset relation to the
remainder of the reservoir. A second ridge 44 including
an inclined surface 46 adjoining the main reservoir
portion separates this reservoir portion from the trap 42.
A sponge 48 or other absorbent material is positioned
within the trap. Any absorbent material may be employed
in the trap as long as it does not release any dust or
fiber debris into the capillary area and it is not
affected by the inoculum.
A pair of laterally extending flanges 50 project
from each side of the carrier base 20. One of the flanges
includes a relatively large area 52 which may be used for
gripping the inoculator and/or applying an identification
label thereto.
A plurality of feet 54 project downwardly from the
bottom surface of the carrier 18. The feet serve to raise
20~0920
-- 11 --
l the capillary tips out of contact with the work surface
and to act as locators to align the inoculator with the
test panel if it is attached to a panel as described
later.
A transpar;ent cover tape 56 made from adhesive-
backed clear MYLAR or any other liquid-impervious material
is secured to the upper edges of the peripheral wall 24 of
the carrier 18. The tape covers all portions of the
carrier with the exception of the fill area 38.
The structure and configuration of the device to be
- inoculated by the i~oculator 12 according to the invention
depends upon the particular application of the device.
Several such devices are disclosed in commonly assigned
U.S. Application Serial Number 209,677 filed June 20, 1988
and entitled "Device For Enhancing Fluorescence And
Kinetics And Methods of Using~The Device",
(Canadian Serial Number 597,484, filed April 21, 1989).
The test panel 14 as shown in the figures is
molded from polypropylene or other suitable polymeric
material. As shown in Figs. 1 and 4, it includes a
substantially flat, rectangular body 58 including an
elongate ridge 60 which has a flat upper surface 62.
Sixty-four cylindrical test wells 64, each having an upper
opening adjoining the upper surface 62 of the elongate
ridge 60 and a lower opening adjoining the bottom surface
66 of the test panel, are provided. Each well has a
larger diameter than the diameter of the projections 28
extending from the inoculator. The depth of the wells 64
is less than the length of these projections. optional
position reference notches 68 are provided in the
longitudinal edge of the test panel nearest the wells,
each notch being aligned with one of the respective
wells. A panel data label 70 may be applied to the upper
surface of the panel.
A transparent, liquid-impermeable adhesive tape 72
is secured to the bottom surface 66 of the test panel 14.
The tape may be an acrylic, adhesive-backed strip of clear
MYLAR or other suitable polymeric material. As shown in
Trademark
20~0920
- 12 -
1 Fig. 4, the tape 72 is secured to the bottom surface 66 of
the test panel 14. The tape 72 provides an adhesive
bottom for each of the cylindrical wells 64. The disks or
membranes 32 are secured, respectively, to the adhesive
bottoms of the cylindrical wells 64, and comprise the
test sites at which test data is obtained.
A notch 72 is provided within one of the ends of
the test panel 14 for receiving one of the feet 54
extending from the inoculator 12. Two openings 74 are
formed near the opposite end of the test panel 14 for
receiving the other two feet 54 of the inoculator. The
test panel itself may include a plurality of feet 76 for
facilitating the handling thereof, positioning it within a
test instrument, and protecting the transparent tape 72.
A vent channel 78 extends between the end of the elongate
ridge 60 and an edge of the test panel.
The disk-shaped supports 32 within each cylindrical
test well 64 are preferably made from an absorbent
material such as alpha cellulose or pH neutralized glass
fiber. Alpha cellulose in the form of cotton lint paper
is particularly preferred. The thickness of the disks
should be sufficient to carry an effective amount of
reagent for reaction with the particular liquid
inoculant. In general, a thickness of from 0.2mm to
2.Omm has been found suitable where the supports have been
pieces of filter paper. In such cases, a solution of
test reagent is absorbed by the supports and dried prior
to their receiving the liquid inoculum from the inoculator
12. A support thickness of 0.5mm to 0.9mm is generally
preferred for such analyses.
The shape of the support within each test well 64
is not critical. The thickness thereof, in cooperation
with the surface area, determines the volume of liquid
required to completely wet each support. The void volume
of each support is preferably between about one
microliter and twenty-five microliters.
In operation, the liquid inoculum is introduced
into the reservoir of the carrier 18 by pouring or
- 13 -2 0~ 0920
l pipetting it into the fill area 38. A desirable feature
of the invention is that the sample volume introduced to
the inoculator can vary over a wide range, e.g. 300-1000
microliters, with no effect upon the accuracy of the
volumes deposited upon the test sites.
The liquid inoculum deposited within the carrier
generally will not have a sufficient volume to cover all
of the capillaries simultaneously upon introduction. Due
to the hydrophobic surfaces which define the reservoir,
the liquid inoculant tends to form a rolling mass covering
only about one quarter of the capillaries. Additional
liquid is not necessary, however, thereby avoiding the
wasting of inoculum. After depositing the total volume of
liquid inoculum in the fill area 38, the operator tilts
the inoculator 12 slightly towards the trap end to move
this mass of liquid sequentially over the top ends of the
capillaries, filling each as it is covered. Filling takes
place partially by gravity flow, but primarily through
capillary action. The dual trough defined by the side
walls 24 and elongate ridge 36 is narrow enough that each
capillary must be filled as the liquid passes over it. If
no ridge is employed, the distance between the side walls
should be sufficiently small such that the liquid engages
each capillary. Once all of the capillaries are filled
and the excess liquid is situated at the trap end, the
inoculator is tilted about its longitudinal axis towards
the trap 42 itself by 30-40 to flow the liquid over the
ridge 44 and into the trap sponge 48. The hydrophobic
surfaces of the carrier retain essentially no liquid in
the channels above the capillaries. The cover tape 56
helps prevent spillage.
The liquid deposited within the capillary
inoculator 12 should preferably contain a visible dye to
provide confirmation that all capillaries are completely
filled. When viewed from the top of the carrier 18, the
capillary cavities are relatively long and narrow. A
small amount of dye is accordingly easily discernible; a
- 14 - 2040320
l partially filled capillary will be visibly lighter in
color.
Alternatively, by selecting a liquid having the
same refractive index as the plastic from which the
capillaries are made, the need for using a dye may be
obviated. Instead of looking for a relatively dark
column of liquid to verify that each capillary is filled,
the bore of the capillary would appear to vanish when it
is filled.
The inoculator 12 is preferably, though not
essentially, mounted to the test panel 14 prior to
filling. The foam gasket 16 or other resilient separating
means between the inoculator and test panel should be
stiff enough to prevent contact between the projections 28
and the disk-shaped supports 32 during normal handling.
In addition, the capillaries themselves should be of
appropriate size and configuration that no liquid is
jarred loose during normal handling, including turning the
inoculator on end or upside down.
Liquid transfer from the inoculator 12 to the test
sites within the test panel 14 is preferably machine
initiated. A self-aligning plate (not shown) is pressed
down on top of the inoculator, forcing the tips of the
capillaries into contact with the supports 32, as shown in
Fig. 5. The liquid quickly and substantially
simultaneously wicks out of the capillaries upon such
contact if the supports are sufficiently absorbent. While
the same procedure could be accomplished manually, it is
important for many applications that instrument readings
be taken immediately after inoculation. Repeatable tests
could not be consistently performed through manually
initiated inoculation. As discussed above, inoculation
could also be accomplished through the use of gas pressure
above the capillaries or by sudden acceleration of the
inoculator.
Depending upon what reactive substrate is
incorporated within each support 32, the appropriate tests
may be conducted subsequent to inoculation. Reactions
- 15 - 2040920
1 between the inoculum and substrate may in some cases be
visually observed. Since the liquid does not readily
evaporate from the capillaries, it is not critical that
inoculation of the test sites be accomplished immediately
after filling. The foam gasket 16 protects the capillary
tips from any air flow while the cover tape 56 protects
the upper openings of the capillaries. Evaporation is
accordingly minimized.
Instrument readings are taken with the inoculator
in the position shown in Fig. 4. Alternatively, the
inoculator may be removed from the test panel and
discarded.
If desired, the sponge 48 or other absorbent
material placed within the inoculator may include a
reagent dried thereon. When excess inoculum is run off
into the trap, a large volume of analyte is brought into
contact with the reagent. A visible color change or
fluorescent reaction may be used to indicate a positive
result. The procedure may be used for tests which require
a relatively large volume, but not a precise volume which
is in contrast to the tests conducted at the test sites
within the test panel 14 which require small, precise
samples.
The inoculator 12 and the method of inoculation
described above provide a number of significant advantages
where small, precise quantities of liquid inoculum must be
deposited upon test sites. Since no pressure or suction
is required for inoculum transfer, errors associated with
the compressibility of air are avoided. The filling of
the inoculator in a single pipetting step or pour
operation does not require high position or volume
accuracy. The inoculum is divided into a number of
precise volumes in a single operation. The individual
volumes of inoculum are isolated from each other, thereby
precluding cross-contamination when the inoculum is
transferred to the test sites. Excess inoculum is trapped
within the inoculator, thereby preventing spillage,
accidental contact, and errors in the amount of inoculum
- 16 - 2010920
1 transferred to each test site. By using the inoculator in
the described manner, good accuracy and repeatability can
be obtained even when simultaneously inoculating test
sites with volumes of five microliters or less. The
inoculator allows one to visibly determine whether the
capillaries have been correctly filled, and insures that
evaporation does not significantly affect the liquid
within the capillaries in the normal period between
capillary filling and inoculation. Liquid is retained
within the capillaries regardless of the orientation of
the inoculator.
An alternative embodiment of the invention is shown
in Figs. 6-7. The assembly 10' shown therein is generally
the same in structure and function as that shown in Figs.
1-5, but is preferred for handling relatively small
volumes of liquid.
The assembly includes an elongated inoculator 12'
and a test device 14' matched thereto. The inoculator 12'
may be engaged to the test device 14' by means of
conventional interlocking sn~ps (not shown) formed
integrally within the respective components. A pair of
resilient buttons 16' positioned between the inoculator
12' and test device 14' on opposite ends of the assembly
10' maintain the inoculator 12' in the raised position
2~ shown in Fig. 6. Only one of the buttons is shown in Fig.
6.
The use of the buttons 16' in place of a foam
gasket 16 allows air to move through the assembly. In
order to minimize evaporation losses, the inoculator 12'
is accordingly formed with wall members 17' which fit
within a substantially oval groove 19' surrounding the
test wells 64' within the test device 14'. The relative
positions of these wall members 17' and the outer walls
21' of the test wells 64' impede the flow of air across
the bottoms of the capillaires 26', thereby reducing the
evaportion which would otherwise occur.
The inoculator 12' includes a carrier 18' including
a flat upper surface Z2' and peripheral side walls 24'
- 17 - 2040920
1 projecting from the flat upper surface. The capillaries
26' are substantially tangent to the side walls to
facilitate the entry of liquid. No ridge is provided
between the rows of capillaries. A flange 52' similar in
structure and function to the flange 52 of the embodiment
shown in Figs. 1-5 is also provided.
The test device 14' is similar in structure to the
above-referenced embodiment with certain exceptions as
noted above. In addition, the disk-shaped supports 32'
are adhered to the bottom surface of the test device
itself rather than to the tape 72 disclosed above.
Filling of the inoculator 12' and inoculation of the test
sites is accomplished in substantially the same manner
discussed above with respect to the first-described
inoculator 12. The inoculator 12' is filled while in the
position shown in Fig. 6, while inoculation takes place
when it is moved to the position shown in Fig. 7.
- Substantially all of the liquid within each capillary 26'
is dispensed as the projections 28' contact the absorbent
test sites.
Although illustrative embodiments of the present
invention have been described herein with reference to the
accompanying drawings, it is to be understood that the
invention is not limited to those precise embodiments, and
that various other changes and modifications may be
effected therein by one skilled in the art without
departing from the scope or spirit of the invention.
3o