Note: Descriptions are shown in the official language in which they were submitted.
Title: PROCESS FOR PRODUCING ART:[CLES COMPRISING ANODIZED
FILMS EXHIBITING AREAS OF DIFFERENT COLOUR AND THE
ARTICLES THUS PRODUCED 2042~0
BACKGROUND OF THE INVENTION
I. FIELD OF THE INVENTION
This invention relates to a process for producing
articles comprising anodized films having areas of visibly
different colour, hue, colour shading or colour density, etc.
(referred to hereinafter merely as different colours) forming
a pattern, picture, printing or other marking or indicia, etc.
(referred to hereinafter merely as patterns). More
particularly, the invention relates to such a process in which
the colours are generated at least in part by optical
interference effects. The invention also relates to patterned
articles and structures of this kind.
II. DESCRIPTION OF THE PRIOR ART
It is well known to provide articles made of aluminum or
anodizable aluminum alloys with a protective and/or decorative
oxide coating by anodization. This involves electrolyzing the
article as an anode in an electrolyte containing an acid in
order to form a porous oxide layer on the aluminum surface.
The oxide layer, or anodic film as it is often called,
consists of aluminum oxide having pores open at the exposed
outer surface of the film but closed adjacent to the
oxide/metal interface by a dense imperforate thin barrier
layer of aluminum oxide.
It is also well known that anodized articles produced in
this way can be coloured by electrolytically depositing a
metal (often referred to as an inorganic pigment) into the
pores of the anodic film in order to create scattering and/or
absorption of light incident on the article surface. This
colouring procedure is often referred to as the ANOLOK
(trademark of Alcan Aluminium Limited) process.
The standard ANOLOK (trademark) process has been modified
in two ways in order to produce a greater range of colours.
One such modification is disclosed in US Patent No. 4,066,816
issued on January 3, 1978 to Sheasby et al and assigned to the
same assignee as the present application. This modification
involves reducing the height of the deposits in the pores so
.
2 ~04~
that light interference effects contribute to the observed
colour. The interference effects result from the very small
distance between the semi-reflective surfaces formed by the
outer ends of the deposits and the reflective surface of the
underlying oxide/metal interface.
The second modification is disclosed in US Patent
4,310,586 issued on January 12, 1982 to Sheasby et al and also
assigned to the same assignee as the present application.
This modification is similar to the one described above but
involves an additional step of increasing the thickness of the
oxide layer between the inner ends of the deposits and the
oxide/metal interface by carrying out further anodization.
This procedure creates additional colours (which may also be
dichroic, i.e. variable with viewing angle) due to the greater
spacing between the outer ends of the deposits and the
oxide/metal interface, without increasing the lengths of the
deposits which would result in increased light absorption and
thus "muddying" of the exhibited colours.
US Patent 4,066,516 to Sato issued on January 3, 1978
relates the formation of patterns on anodized articles
coloured using an ANOLOK (trademark) type process. In the
process disclosed in this patent, the article is anodized in
the normal way and then, prior to the deposition of an organic
pigment, limited areas of the anodized surface are subjected
to anodization at high voltage in order to increase the thick-
ness of the barrier layer in such areas. An inorganic pigment
is then electro-deposited into the pores in the normal way
but, because the thickened barrier layer acts as an electrical
insulator in the treated areas, deposition of the pigment
takes place only in the untreated areas and a light on dark
pattern becomes visible at the surface of the article. While
this procedure is capable of producing visible patterns
without resorting to the use of masks and multiple treatments,
which are difficult to carry out, time consuming and expensive
on a commercial scale, it does not make use of the improved
colours of which can be obtained by the process disclosed in
US Patent 4,310,586.
3 204~1~0
OBJECTS OF THE INVENTION
An object of the invention is to provide a process for
producing articles having anodized surfaces provided with
patterns of different colours, e.g. areas of one colour
against a contrasting background.
Another object of the invention is to produce such
articles in which both patterned areas and background areas
exhibit a colour different from uncoloured anodized surfaces.
Yet another object of the invention is to produce such
articles by a technique which can be carried out simply, with
relatively few steps, economically and optionally on a
continuous basis.
A further object of the invention is to produce such
articles without resorting to the use of potentially harmful
dies, pigments, masking materials and solvents.
A still further object of the invention, in preferred
forms, is to produce patterned articles consisting of anodic
films isolated from any substrate article on which they may
have initially been formed.
SUMMARY OF THE INVENTION
According to one aspect of the invention there is
provided a process for producing an article comprising an
anodic film exhibiting areas of different colour, comprising:
anodizing a surface of a substrate made of or coated with a
metal selected from the group consisting of aluminum and
anodizable aluminum alloys to produce an anodic film on said
surface having a porous outer layer and a non-porous inner
barrier layer between said porous outer layer and said
surface; electrodeposiling a metal into pores in said porous
anodic film to form deposits in said pores which generate a
colour by effects including interference of light; and
bringing a cathodically-biased electrode into contact with or
close proximity to limited areas of said porous anodic film to
cause said barrier layer to thicken in said limited areas
only.
Optionally, either before or after, or alternatively,
both before and after, bringing the cathodically biased
4 204~
electrode into contact with or close proximity to the limited
areas, further anodizing of the surface may be carried out at
a voltage which causes the film to thicken beneath the
deposits, at least in areas of the film other than the limited
areas contacted by the electrode. However, a further
anodizing step of this kind is not absolutely required; the
thickening of the barrier layer caused by the cathodically-
biased electrode may be sufficient in itself to produce a
difference of colour between the limited areas contacted by
the electrode and the remaining areas of the film.
According to another aspect of the invention there is
provided an article having an anodic film exhibiting areas of
different colour, comprising: a reflective metal substrate; a
porous anodic film overlying a surface of said reflective
substrate; and metal deposits in pores of said film having
outer ends separated from said metal surface sufficiently to
generate a colour by effects including interference of light;
wherein said film has different areas in which spacings
between inner ends of said deposits and said metal surface are
sufficiently different from each other that said different
areas exhibit different colours.
It is to be noted that, in the present invention, the
anodic film need not be immersed in an electrolyte when the
- film is contacted by, or brought into close proximity to, the
cathodically-biased electrode because liquid carry-over from a
previous step (usually a rinse with deionized water) is
normally sufficient to act as an electrolyte. This makes it
much easier to produce the desired pattern with the electrode.
An additional electrolyte may be used, however, if this is
desired or if the film is dry.
The electrode used to thicken the film beneath the
deposits may be a wand, probe or the like of any suitable
thickness at the tip or contacting surface, or a planar
electrode such as a sheet or plate, and may be made of a
conductive metal or other conductive material (e.g. graphite).
It is unnecessary to pack a hollow electrode with a high
viscosity electrolyte as in the Sato process discussed above,
204~1~0
which again makes pattern formation much simpler, but an
electrode of the type used by Sato may be used, if desired.
While the electrode normally is normally brought into
actual contact with the surface of the anodic film, a similar
barrier layer thickening effect can be obtained if the
electrode merely closely approaches the film surface. In such
a case, the resulting pattern may have less clear lines of
separation due to the formation of an electrical field
gradient extending laterally over a substantial distance. A
transition zone is then visible between the areas of different
colour, but this may be desirable for artistic appeal. In
fact, the demarkation between the zones of different colour
may not be sharp in some cases even when the electrode is
directly contacted with the anodic film. The variables 15 affecting the sharpness of the resulting transition zone
include the distance of separation between the electrode and
the anodic film, the electrolyte conductivity, the cathode
potential of the electrode and the contact duration. These
variables can be changed in any particular case in order to
produce a required degree of sharpness of colour transition
between the areas of different colour.
BRIEF DESCRIPTION OF THE DRAWINGS
Figs. 1 to 10 are diagrammatic cross-sections of anodic
films produced during various steps of several preferred
processes according to the present invention, with Figs. 4, 5,
7, 8 and 10 representing final products.
In the drawings, no attempt has been made to show the
relative dimensions of various elements and layers to scale.
Throughout the several views, like items are indicated by
the same reference numerals, where possible.
DETAILED DESCRIPTION OF THE INVENTION AND THE PREFERRED
EMBODIMENTS
In the present invention, at least in preferred forms, an
article made of aluminum or an anodizable aluminum alloy (at
least at an exposed surface of the article) can be provided
with an anodized surface having a visible pattern formed ~y
areas of different colour by a process which involves the
6 204~1~0
following steps.
First of all, the surface of the article to be treated is
anodized in an electrolyte which produces a porous anodic
film. Suitable electrolytes include, for example, aqueous
solutions of strong inorganic acids, such as sulphuric acid or
phosphoric acid, or organic acids such as oxalic acid. The
anodization step is normally carried out at room temperature
at a voltage in the range of 5-25V for sulphuric acid, and
preferably at 10-20V, for a time sufficient to produce a
porous anodic film having a thickness preferably in the range
of 0.1 to 30 microns. Direct or alternating current
conditions can be used in this step. For other acids, these
ranges will change but can be determined by simple trial and
experimentation, if not already known.
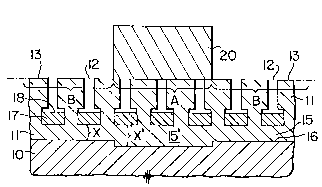
Fig. 1 of the accompanying drawings represents a cross-
section of the resulting article in which the substrate metal
10 has an overlying transparent anodic film 11 of aluminum
oxide. The film 11 has pores 12 extending inwardly from an
outer surface 13 towards the substrate metal 10. However, the
pores are closed at their inner ends 14 by an imperforate
continuous thin barrier layer 15 of anodic oxide which
separates the pores 12 from the underlying metal surface 16.
: If necessary or desired, a second step may be carried out
to enlarge the cross-sectional areas of the pores 12 at their
inner ends. The reason for this is to ensure that, after a
metal deposition step to be described below has been carried
out, metal deposits in the pores have a sufficient surface
area to create strong reflections and consequently strong
interference effects. This optional step can be carried out
by subjecting the anodized surface to further electrolytic
treatment in an electrolyte having a high dissolving power for
aluminum oxide, e.g. aqueous phosphoric acid. Direct or
alternating current conditions can also be used for this step.
Direct current voltages are generally in the range of 8 to 50
volts and alternating current voltages are generally in the
range of 5 to 40 volts at temperatures in the range up to
50C, preferably 10-35C, and phosphoric acid concentrations
7 Z04;~o
preferably in the range of 10-200 g/l, particularly 50-150
g/1. The upper limit of a dissolution treatment designed to
increase pore diameter is set by the point where the film
loses strength and becomes powdery or crumbly through
reduction of the thickness of oxide lying between adjacent
pores.
A film resulting from this pore widening step is
represented in cross-section in Fig. 2. It will be seen that
the additional electrolysis has increased the overall
thickness of the film 11 and has provided the pores 12 with
widened lower ends 12'.
The next step of the procedure is to deposit a metal
(sometimes referred to as an inorganic pigment) into the lower
ends 12' of the pores 12 in order to create a semi-reflective
layer within the film 11. This can be done by electro-
deposition in the conventional manner, e.g. as described in US
,~~v~ ~ Patents 4,066,816 and 4,310,586 (the disclosures of which are
incorporated herein by reference). The metal chosen for this
step should be one which can resist the strongly acidic
conditions to which it is exposed in subsequent steps without
significant dissolution and the preferred metals for this
purpose are noble metals, such as palladium, and acid-
resistant alloys, such as Sn-Ni and Cu-Ni alloys. However,
less acid-resistant metals, e.g. tin, nickel, cobalt, copper,
silver, cadmium, iron, lead, manganese and molybdenum, may be
used if they are displaced by or coated with an acid-resistant
metal, e.g. a noble metal such as palladium, platinum or gold,
after the less acid resistant metals have been deposited in
the pores. For example, this can be achieved by immersion of
the film containing the less acid resistant metal deposits in
a solution of a noble metal salt, or by spraying a solution of
the noble metal salt onto the film.
Fig. 3 represents the film following the metal deposition
step. The enlarged ends 12' of the pores 12 contain metal
deposits 17 which together create a discontinuous but
generally flat semi-reflective surface 18.
As represented in Fig. 4, limited areas A of the film 11
.
8 204~0
are then briefly contacted by, or brought into close proximity
to, an electrode 20 biased as a cathode whose electrical field
potential is greater than that of the general anodizing field
potential used for the formation of the film 11. This causes
the barrier layer lS to thicken (from X to X') in the areas A
beneath the electrode, but does not affect the thickness (X)
of the barrier layer in areas B not contacted by, or brought
closely adjacent to, the electrode 20. This thickening effect
in areas A comes about because the thickness of the barrier
layer 15 is proportional to the applied voltage, whereas the
thickness of the porous region of the film is proportional to
current passed. When the electrode briefly contacts, or comes
close to, the surface 13 of the anodic film 12, localized
anodizing takes place and, although the porous region of the
film grows only slightly in thickness in the contacted areas,
the barrier layer 15 thickens substantially and almost
instantaneously. It has been found that a contact time of as
little as 0.01 second can produce a visible colour difference
between regions A and B in the final product.
In general, the contact of electrode 20 is maintained for
a period of 0.01 to 10 seconds when the electrode has a
cathodic potential in the range of 10 to SOV. This generally
produces a thickened barrier film 15' having a thickness in
the range of 0.01 to 0.05 microns.
The electrode 20 may be in the form of a wand or the like
which is positioned on or stroked across the surface 13 to
produce the desired patterning effect, or it may be in the
form of a plate or roll having cut-out, contoured or etched
regions exhibiting a desired pattern, picture, printing or
other indicia, which is brought briefly into contact with the
entire surface 13. The wand, plate or roll may be solid or
may alternatively be hollow and open at contact points and may
contain a high viscosity electrolyte so that it can be used
both on wet and dry surfaces. As a further alternative, a
thickened electrolyte may be printed (e.g. by silk screening)
on the film 11 (when dry) and then overlaid by a continuous
metal plate or foil acting as the electrode 20. The barrier
g Z04~60
layer thickening then takes place only in those regions
contacted by the electrolyte since anodization is not possible
in the other (dry) regions.
The steps described so far produce areas A and B of
different colour if the potential of the electrode 20 is
suitably high, in which case no further steps need be carried
out. The film 11 appears to be coloured when illuminated with
white light because of (i) selective wavelength absorption by
the pigment deposits 17 and, to a lesser extent, by the
substrate metal 10, (ii) scattering of certain wavelengths by
the deposits 17 and (iii) interference effects caused by
reflections from the semi-reflective surface 18 and from the
oxide/metal interface 16. While the coloration produced by
effects (i) and (ii) are essentially the same in both regions
A and B of the film 11, the coloration produced by effect
(iii) is different in these two regions because of the
difference in separation between reflective surfaces 18 and 16
in these two regions which leads to different optical paths
taken by reflected light. In this type of film, coloration
produced by effect (iii) makes a dominant contribution to the
observed colour when the spacing between surfaces 18 and 16 is
"optically thin" (less than 3 microns and preferably less than
1 micron). When this is the case, regions A and B have
different overall exhibited colours if the distances X and X'
are suitably different. Consequently, if the film thickening
effect of the electrode 20 is suitably great, the resulting
interference effects produce noticeably different colours in
the areas A and B and the film 11 then appears to be
patterned. However, if necessary or desired, the colour
differences between the areas A and B can be enhanced and
different colours may be generated by carrying out the
following additional steps.
After removal of the electrode 20, further porous
anodization of the entire surface may be carried out at a
voltage lower than the cathodic voltage of the electrode 20.
This produces pore-elongation in the regions B but it has been
unexpectedly found that substantially no pore elongation takes
~O~
place in regions A having the thickened barrier layer 15'.
This may ~e because anodizing current cannot pass through the
thickened barrier layer 15' until sufficient dissolution, and
thus thinning, of the barrier layer 15' has taken place.
The film 11 following this further anodization step is
represented in Fig. 5. In the regions B, the pores 12 have
been elongated by the formation of extended lower ends 12",
but substantially no pore elongation (or in some cases a
minimal or significantly reduced pore elongation) has taken
place in region(s) A. In regions B, the overall thickness of
the anodic film 11 has been increased to Y' whereas, in
region(s) A, the overall thickness Y remains substantially the
same as before. This increases or exaggerates the difference
in separation of the surfaces 18 and 16 in the areas A and B
caused initially by the electrode 20 and this increased
difference of separation causes the regions A and B to exhibit
greater differences of colour for the reasons mentioned above.
An alternative procedure for enhancing the differences of
colour is shown in Figs. 6 and 7. In this case, the procedure
is the same as above until the structure of Fig. 3 is obtained
but then the structure is re-anodized without first contacting
limited areas of the film 11 with the cathodically-biased
electrode 20. The resulting structure is shown in Fig. 6, in
; which the metal surface 16 is essentially planar and pore
extensions 12" are formed in all regions of the film.
The resulting structure is then contacted in area(s) A by
the cathodically-biased electrode 20 having a sufficiently
high electrode potential to cause thickening of the barrier
layer 15 in the regions of the film contacted by the
electrode. As a result, as shown in Fig. 7, the separation
between the semi-reflective surface 18 and the reflective
metal surface 16 is different in the contacted area(s) A and
the non-contacted areas B of the film and different colours
are exhibited.
Preferably, a further reanodization step is then carried
out to produce the structure shown in Fig. 8. The
reanodization elongates the pores in the non-contacted areas B
204~ 0
11
to produce pore extensions 12'". In the contacted area(s) A,
however, no pore elongation (or in some cases, less pore
elongatlon) takes place. As a result, the optical path
between the semi-reflective surface 18 and the metal surface
16 is again different in the contacted and non-contacted
areas, which leads to different colours. In such a case, the
colours of the contacted area(s) A may be the same in the
structures of both Figs. 7 and 8, but the colours in the non-
contacted areas B are different.
The further reanodization step leading to the structure
of Fig. 8 is desirable because, as in the first colour
enhancing step described above, it incréases the ratio of
separation of the semi-reflective layer 18 and the metal
surface 16 in the contacted and non-contacted areas A and B.
In the case of all of the above structures produced
according to the invention, the pores 12 may, if desired, be
sealed using conventional procedures for sealing architectural
anodized surfaces, e.g. hot water sealing with or without smut
inhibitors such as nickel acetate, various cold sealing
techniques, the application of overlayers, e.g. clear lacquer,
and the like.
Moreover, prior to such pore sealing effects the film may
be contacted by a dichromate solution in order to make the
deposits 17, if made of non-noble metal, less likely to be
leached from the film either during the pore sealing step or
later when the article is in use.
If desired, for example to make a patterned packaging
sheet or the like, the anodic film 11 may be made detachable
from the substrate 10 and the substrate 10 replaced by a very
thin sputtered metal film in order to provide the required
reflective surface 16. This can be achieved, for example, by
the procedure shown in Figs. 9 and 10 which starts from the
structure shown in Fig. 5, but could be applied equally well
to the structures shown in Fig. 7 or Fig. 8.
3S The procedure employs a pore-branching step as described
in European patent application EP 0178831 published on April
23, 1986 and assigned to the same assignee as the present
2~
12
application (the dlsclosure of which is incorporated herein by
reference). The procedure requires a reduction of voltage in
a final anodization step, which causes pore branching 20 at
the bottoms of pore extensions 12' " or widened pore sections
12' which collectively introduce weakened strata into the
anodic film 11. The starting voltage used for this pore
branching step must be the same as, or higher than, the
voltage used in the previous anodization step and is
preferably between 3 and 200V, more preferably 6-80V. The
voltage is then reduced in steps or continuously until it
approaches zero.
Following the pore-branching step, an overlayer 21
(preferably a flexible transparent polymer film e.g. of
polyester) is adhered by means of an adhesive (not shown) or
by heat sealing to the surface 13 of the film to form a
structure as shown in Fig. 9.
The overlayer 21 is then used to detach (by pulling or
peeling) the film 11 from the substrate 10 and the film acts
as a support for the film 11 when detached. The exposed
surface 22 of the film is then covered with a thin reflective
metal film 23 by a vacuum deposition technique, such as
sputtering. The metal used to form the film 23 may be any
metal capable of undergoing the deposition technique and need
not be aluminum or an aluminum alloy.
The resulting structure still exhibits the areas of
contrasting colour (visible through the transparent overlayer
21) but is sufficiently thin, flexible and non-porous that it
can be used as a packaging sheet or the like.
As a final point it should be mentioned that it is
possible to form multiple (more than two) colours in the same
anodic film ll by carrying out the cathodic contact and
reanodizing process several times. This results in several
areas of different film thicknesses beneath the deposits in
different parts of the film. The result is a film having
several different colours on a background of uniform colour.
One colour is derived from the shortest separation of the
surfaces 18 and 16, the second colour from the next largest
13 xc~ o
separation, etc., with the background colour resulting from
the largest separation between the surfaces 18 and 16.
The structures of the present invention can be used, for
example, as architectural finishes, signs, indoor decorative
5 materials for stores, picture frames, decorative packaging
films, foils and laminates.
The invention is illustrated further by the following
non-limiting Examples 1 to 11.
The substrates used in Examples 1-11 were pre-processed
10 in an identical fashion as follows. Aluminum alloy AA5252
sheet was cut into 2.5 cm by 20 cm panels, etched in 5% NaOH
at 65C for 5 minutes, and anodized in 21C 1.5M H2SO4 at 16
volts DC for a period of 30 minutes to create a porous anodic
film measuring 12 microns in thickness. The panels were
subsequently re-anodized in 21C H3PO4 at 15 volts DC for 2
minutes, rinsed well, and transferred to a room temperature
solution containing 25g/l NiSo4~7H2o/ 20g/l MgSO4.7H2O, 25g/l
H3BO4, and 15g/l (NH4)2SO4 at pH 5.5. A voltage of 11 volts
peak AC was applied between the anodized panel and a graphite
electrode for a period of 20 seconds. The deposit was then
stabilized by immersing for 2 minutes in a 350 ppm Pd (as
PdSO4.2H2O) solution of pH 2. At this stage in the process the
panel appeared medium bronze in colour.
EXAMPLE 1
Following deposit stabilization, the panel was immersed
in the sulphuric acid solution and once again connected to the
positive terminal of a DC power supply. 15 V was applied for
a period of 40 seconds (the panel colour was yellow) the panel
was then rinsed and with it still being connected to positive
a 20 volt DC cathodically biased graphite brush wetted in the
pH 2 rinse water was brought into contact with the surface of
the panel and stroked on localized areas as an artist would
apply paint to canvas. The panel was then re-immersed in the
anodizing electrolyte and anodized for an additional 30
seconds at 15 volts DC (the background colour was blue with
yellow brush stroked areas). The barrier modification
procedure was repeated and the panel was anodized as before
for 20 more seconds. The final result was a pink panel with a
14 Z~ 60
yellow and blue brush stroked pattern. Violet fringes to the
yellow areas and green fringes to the blue areas were the
result of a delayed current recovery where the barrier layer
had not been thickened to the same extent as those areas where
direct contact was made and residence time was sufficient.
Processing was completed by sealing the anodic film in boiling
water for 30 minutes.
EXAMPLE 2
Following deposit stabilization, the panel was immersed
in the sulphuric acid solution and once again connected to the
positive terminal of a DC power supply. 15 V was applied for
a period of 40 seconds (the panel colour was yellow). While
still immersed, a 3mm diameter aluminum wire (electrically
shielded with tape everywhere except its tip) was brought into
contact with the panel. The wire was then pulsed for a period
of 1 second with a 20 volt cathodically biased DC voltage.
The probe was removed and normal 15 volt DC anodizing resumed
for an additional 30 seconds. The panel was then rinsed and
sealed in boiling water for 30 minutes. The result was a blue
panel with a 3mm diameter yellow spot. Around the perimeter
of the spot one could see a very thin pink line.
EXAMPLE 3
Following deposit stabilization, the panel was immersed
in the sulphuric acid solution and once again connected to the
positive terminal of a DC power supply. 15 V was applied for
a period of 40 seconds (the panel colour was yellow). While
still immersed, a 3mm diameter aluminum wire (electrically
shielded with tape everywhere except its tip) was brought into
contact with the panel. The wire was then pulsed for a period
of 0.1 second with a 20 volt cathodically biased DC voltage.
The probe was removed and normal 15 volt DC anodizing resumed
for an additional 30 seconds. The panel was then rinsed and
sealed in boiling water for 30 minutes. The result was a blue
panel with a 3mm crescent shaped pink spot. It was obvious
that either the pulse duration or the 5 volt differential
between the pulse and normal anodizing voltages was not
sufficient to preclude a delayed current recovery hence a pink
2C~16C~
rather than yellow spot of odd shape.
EXAMPLE 4
Following deposit stabilization, the panel was immersed
in the sulphuric acid solution and once again connected to the
positive terminal of a DC power supply. 15 V was applied for
a period of 40 seconds (the panel colour was yellow). While
still immersed, a 3mm diameter aluminum wire (electrically
shielded with tape everywhere except its tip) was brought into
contact with the panel. The wire was then pulsed for a period
of 0.1 second with a 25 volt cathodically biased DC voltage.
The probe was removed and normal 15 volt DC anodizing resumed
for an additional 30 seconds. The panel was then rinsed and
sealed in boiling water for 30 minutes. The result was a blue
panel with a 3mm diameter yellow spot. Around the perimeter
of the spot one could see a very thin pink line.
EXAMPLE 5
Following deposit stabilization, the panel was immersed
in the sulphuric acid solution and once again connected to the
positive terminal of a DC power supply. 15 V was applied for
a period of 40 seconds (the panel colour was yellow). While
still immersed, a 3mm diameter aluminum wire (electrically
shielded with tape everywhere except its tip) was brought into
contact with the panel. The wire was then pulsed for a period
: of 0.01 second (10 milliseconds) with a 25 volt cathodically
biased DC voltage. The probe was removed and normal 15 volt
DC anodizing resumed for an additional 30 seconds. The panel
was then rinsed and sealed in boiling water for 30 minutes.
The result was a blue panel with a 3mm crescent shaped pink
spot. It was obvious that either the pulse duration or the 10
volt differential between the pulse and normal anodizing
voltages was not sufficient to preclude a delayed current
recovery hence a pink rather than yellow spot of odd shape.
EXAMPLE 6
Following deposit stabilization, the panel was immersed
in the sulphuric acid solution and once again connected to the
positive terminal of a DC power supply. 15 V was applied for
a period of 40 seconds (the panel colour was yellow). While
16 2 ~ 4~
still immersed, a 3mm diameter aluminum wire (electrically
shielded with tape everywhere except its tip) was brought into
contact with the panel. The wire was then pulsed for a period
of 0.01 second with a 30 volt cathodically biased DC voltage.
The probe was removed and normal 15 volt DC anodizing resumed
for an additional 30 seconds. The panel was then rinsed and
sealed in boiling water for 30 minutes. The result was a blue
panel with a 3mm diameter yellow spot. Around the perimeter
of the spot one could see a very thin pink line.
EXAMPLE 7
Following deposit stabilization, the panel was immersed
in the sulphuric acid solution and once again connected to the
positive terminal of a ~C power supply. 15 V was applied for
a period of 40 seconds (the panel colour was yellow). While
still immersed, a 3mm diameter aluminum wire (electrically
shielded with tape everywhere except its tip) was brought into
contact with the panel. The wire was then pulsed for a period
of 1 second with a 30 volt cathodically biased DC voltage.
The probe was removed and normal 15 volt DC anodizing resumed
for an additional 30 seconds. The panel was then rinsed and
sealed in boiling water for 30 minutes. The result was a blue
panel with a 3mm diameter yellow spot. Around the perimeter
of the spot one could see a pink line measuring 0.5-1 mm in
width.
EXAMPLE 8
Following deposit stabilization, the panel was immersed
in the sulphuric acid solution and once again connected to the
positive terminal of a DC power supply. 15 V was applied for
a period of 40 seconds (the panel colour was yellow). While
still immersed, a 3mm diameter aluminum wire (electrically
shielded with tape everywhere except its tip) was brought
within about 2mm of the panel surface. The wire was then
pulsed for a period of 1 second with a 30 volt cathodically
biased DC voltage. The probe was removed and normal 15 volt
DC anodizing resumed for an additional 30 seconds. The panel
was then rinsed and sealed in boiling water for 30 minutes.
The result was a blue panel with a lOmm diameter yellow spot.
17 20~2~0
Around the perimeter of the spot was a pink zone measuring 5mm
in width.
EXAMPLE 9
Following deposit stabilization, the panel was immersed
in the sulphuric acid solution. While immersed, a 3mm
diameter aluminum wire (electrically shielded with tape
everywhere except its tip) was brought into contact with the
panel. The wire was then pulsed for a period of 1 second with
a 25 volt cathodically biased DC voltage. The probe was
removed and 40 seconds of normal 15 volt DC anodizing was
initiated. The panel was then rinsed and sealed in boiling
water for 30 minutes. The result was a yellow panel with a
3mm diameter bronze spot.
EXAMPLE 10
Following deposit stabilization, the panel was immersed
in the sulphuric acid solution and once again connected to the
positive terminal of a DC power supply. 15 V was applied for
a period of 40 seconds (the panel colour was yellow). It was
then removed and rinsed in water at pH 2. A fine stainless
steel mesh grid was wrapped around a 3cm diameter rubber
roller and connected via a graphite brush to a DC power
supply. 20 Volts was applied to the cathodically biased roll
grid and the anodically biased panel (still wet with rinse
water) was passed under the roller at a speed of about
lOm/min. The panel was re-immersed in the anodizing solution.
30 seconds of normal 15 volt DC anodizing was initiated. The
panel was then rinsed and sealed in boiling water for 30
minutes. The result was a blue panel with a distinctive
yellow grid pattern.
EXAMPLE 11
An aluminum foil/polyester laminate was cut to 6cm by
lScm and anodized in 21~C 1.5M H2SO4 at 15 volts DC for a
period of 1 minute to create a porous anodic film measuring
less than 0.5 microns in thickness. The laminate was
subsequently re-anodized in 30~C H3PO4 at 15 volts DC for 2
minutes, rinsed well, and transferred to a room temperature
solution containing 25g/1 NiSo4.7H2o, 20g/1 MgSO4.7H2O, 25g/1
18 Z04~60
~3BO~, and 15g/1 (NH4)2SO4 at pH 5.5. 11 volts peak AC was
applied between the anodized panel and a graphite electrode
for a period of 20 seconds. The deposit was then stabilized
by immersing for 30 seconds in a 350 ppm Pd (as PdS04.2H2O),
pH 2. Following deposit stabilization, the panel was immersed
in the sulphuric acid solution and once again connected to the
positive terminal of a DC power supply. 15 V was applied for
a period of 40 seconds (the panel colour was yellow) the
laminate was then rinsed and with it still being connected to
positive a 20 volt DC cathodically biased graphite brush
wetted in the pH 2 rinse water was brought into contact with
the surface of the panel and stroked on localized areas as an
artist would apply paint to canvas. The panel was then re-
immersed in the anodizing electrolyte and anodized for an
additional 30 seconds at 15 volts DC (background colour was
blue with yellow brush stroked areas). The laminate was then
transferred to the phosphoric acid anodizing bath and anodized
at 20 volts DC for 30 seconds. The voltage was subsequently
step-wise reduced at a constant rate until after 200 seconds,
0 volts was being applied. The laminate was allowed to soak
undisturbed for 60 seconds. It was then withdrawn, rinsed,
and air dried. The surface appeared green with pink brush
strokes. The pattern was no longer yellow because the applied
- 20 volts in the final anodizing bath caused the film to grow
uniformly throughout, then thin uniformly as the voltage was
being reduced. The patterned surface of the laminate was then
heat seal laminated to plastic and this plastic layer was
delaminated from the surface pulling with it the porous oxide
film. At this point the pattern was lost since the aluminum
reflecting layer was removed. This reflecting layer was
replaced with 0.1 microns of sputter deposited gold, and with
it the pattern and colours returned. The result was a
coloured pattern transferred to plastic.