Note: Descriptions are shown in the official language in which they were submitted.
; I Ref. 34s5
204232~ Dr.Eu/Ll0808
~ubstituted 3-aminosydnone imines,lerocesses for the~r_Drepara-
tion a~d their use
The invention relates to pharmacologically active sub-
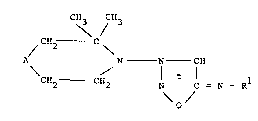
~tltuted 3-aminosydnone imines of the general formula I
C\3/CH3
~CH2 C~ :
CHz--CH N - C=N R 1 (I)
~ -`. ` O
- :
- and their pharmacologically acceptable acid~addition salts, in -~
which
A denotes the radlcal -S0~
R denotes the radical -C0-R2 or hydrogen,
R2 denotes (Cl to C~)alkyl, (Cl to C4)alkoxy-(Cl to C~)alkyl, (Cl ~o
C~)alkoxy, (Cs to C,)cycloalkyl, phenyl, or a phenyl radical which
is mono-, di-~or trisubstituted by 1 to 3 halogen atoms and/or 1
to 3 alkyl radicals having I to 4 C atoms andior 1 to 3 alkoxy
radicals having 1 to 4 C atoms.
The invention furthermore relates to processes for the
preparation of the compounds according to the invention and to
their use. ; ~ -
Alkyl radicals, lkoxyalkyl radicals and alkoxy radicals
may be straight-chain or branchsd. This also applles if they
occur as substituents of phenyl. ~ ;
(Cl to C~)alkyl radicals may be: methyl, ethyl, n-propyl,
i-propyl, n-butyl, i-butyl, tert.-butyl and sec.-butyl. Examples
of (Cl to C~)alkoxy radicals may be: methoxy, ethoxy, n-propoxy,
i-propoxy, n-butoxy, i-butoxy, sec.-butoxy and tert.-butoxy.
(Cl to C~)alkoxy-(Cl to C~)alkyl radicals may be, for
examples methoxymethyl, ethoxymethyl, propoxymethyl, butoxy-
methyl, i-butoxymethyl, 2-methoxyethyl, 3-methoxypropyl, 4-
methoxybutyl, 2-ethoxyethyl, 3-propoxypropyl, 4-propoxybutyl, 2-
butoxyethyl, 3-butoxypropyl, 4-butoxybutyl, 3-i-propoxypropyl,
-- 1 -- ~; ~
.
, .: . ~
., ~., .. , .~
., ~ ~ . , ,
, -
. . . .. . .
:
2C1 4Z324-i-propoxybutyl, 2-ethoxyethyl, 2-propoxyethyl, 2-i-propoxy-
~ ethyl, 2-butoxyethyl, 2-i-butoxyethyl, l-methoxyethyl, 1-ethoxy-
ethyl, l-propoxyethyl, 2-methoxypropyl, 2-ethoxypropyl, 2-
propoxypropyl, 2-i-propoxypropyl, 3-methoxybutyl, 3-ethoxybutyl,
3-propoxybutyl, 3-i-propoxybutyl, 3-butoxybutyl, 3-i-butoxybutyl,
3-tert.-butoxybutyl, 2-methoxybutyl, 2-ethoxybutyl, 2-propoxy-
butyl, 2-i-propoxybutyl, 2-butoxybutyl and 2-i-butoxybutyl.
Of the ( C5 to C7 ) cycloalkyl radicals, cyclopentyl and
cyclohexyl are preferred.
Possible halogen atoms for the substituted phenyl radical
are fluorine, chlorine, bromine and/or iodine, of which bromine
and chlorine are preferred.
R2 preferably denotes (C1 to C4)alkyl, (C1 to C4)alkoxy,
phenyl, or phenyl which i8 monosubstituted by chlorine, methyl or
methoxy, monosubstituted phenyl preferably being substituted in
the 4- or 2-position.
Hydrogen is preferred for Rl. Preferred compounds are: N-
p-anisoyl-3-(3,3-dimethylperhydro-1-oxo-1,4-thiazin-4-yl)sydnone
imine, N-ethoxycarbonyl-3-(3,3-dimethylperhydro-1-oxo-
1,4-thiazin-4-yl)sydnone imine and N-pivaloyl-3-(3,3-dimethyl-
perhydro-1-oxo-1,4-thiazin-4-yl)sydnone imine and the pharma-
cologically acceptable acid addition salts of the abovementioned
compounds, in particular their hydrochlorides, and the pharmaco-
logically acceptable acid addition salts, in particular the
hydrochloride, of the compound: 3-(3,3-dimethylperhydro-
1-oxo-1,4-thiazin-4-yl)sydnone imine.
A compound of the general formula I can be prepared by
cyclising a compound of the general formula II
~3~CH3
2 C~
A ~N--IN--CH2-cN (II)
CH2 ('H2 NO
in which A has the meaning already mentioned, to give a compound
of the general formula Ia
20423Z~
3/ 3
/CH2 c\ (Ia)
A~ ~N I--C H
C H C H N~ ~C=N H
o
and isolating the compound obtained as the acid addition salt, or
in the case in which it is intended to prepare a compound of the
formula I where Rl = -COR2, acylating the compound Ia or an acid
addition salt thereof with an acylating agent which introduces
the radical -COR2, and isolating the compound thus obtained or, if
desired, converting it into a pharmacologically acceptable acid
addition salt, and isolating this.
The cyclisation of the compounds II to give the compounds
Ia can be carried out in a suitable organic or inorganic solvent,
dispersant or diluent with the addition of a cyclising agent,
normally at temperatures from -10 to 40C, in particular 0 to -
40C, preferably at 0 to 20C.
Suitable cyclising agents are those which establish a pH
below 3 in aqueous solution, that is to say, for example, mineral
acids, such as sulphuric, nitric or phosphoric acid, preferably
hydrogen chloride, but also strong organic acids, such as
trifluoroacetic acid. The cyclisation is normally carried out
with ice-cooling.
0.1 to 10 mol, preferably 1 to 5 mol, of the cyclising
agents are used, for example, relative to 1 mol of the compound
of the formula II. The cyclising agent is normally employed in
excess. The use of hydrogen chloride as a cyclising agent is
particularly convenient, and it is normally passed into the
reaction mixture until it is saturated. The corresponding acid
addition salt of the compound Ia is normally obtained in the
cyclisation.
Suitable solvents, dispersants or diluents are, for
examples alcohols, for example those having 1 to 8 C atoms, in
particular those having 1 to 6 C atoms, preferably those having 1
to 4 C atoms, such as, for example, methanol, ethanol, i- and n-
propanol, i-, sec- and tert-butanol, n-, i-, sec- and
-- 3 --
:.
.
2Q9~:32~
tert-pentanol, n-hexanol, 2-ethylbutanol, 2-ethylhexanol, iso-
octyl alcohol, cyclopentanol, cyclohexanol, methylcyclohexanol
(mixture) and benzyl alcohol; ethers, in particular those having
2 to 8 C atoms in the molecule, such as, for example, diethyl
ether, methyl ethyl ether, di-n-propyl ether, di-isopropyl ether,
methyl n-butyl ether, methyl tert-butyl ether, ethyl propyl
ether, di-butyl ether, tetrahydrofuran, 1,4-dioxane, 1,2-dimeth-
oxyethane and bis-~-methoxyethyl ether; oligoethylene glycol
dimethyl ethers, such as, for example, tetraglyme or pentaglyme;
alkyl carboxylates, in particular those having 2 to 10 C atoms in
the molecule, such as, for example, methyl, ethyl, butyl or
isobutyl formate, methyl, ethyl, propyl, isopropyl, butyl, iso-
butyl or sec-butyl, amyl, isoamyl, hexyl, cyclohexyl or benzyl
acetate or methyl, ethyl or butyl propionate; ketones, in parti-
cular those having 3 to 10 C atoms in the molecule, such as, forexample, acetone, methyl ethyl ketone, methyl n-propyl ketone,
diethyl ketone, 2-hexanone, 3-hexanone, di-n-propyl ketone, di-
iso-propyl ketone, di-iso-butyl ketone, cyclopentanone, cyclo- ~
hexanone, methylcyclohexanone, dimethylcyclohexanone, benzophen-
one and acetophenone; aliphatic hydrocarbons, such as, for ex-
ample, hexane and heptane, low- and high-boiling petroleum
ethers, petroleum spirits and white spirit; cycloaliphatic hydro-
carbons, such as, for example, cyclopentane, cyclohexane, methyl-
cyclohexane, tetralin and decalin; aromatic hydrocarbons, such
as, for example, benzene, toluene, o-, m- and p-xylene, and
ethylbenzene; halogenated aliphatic or aromatic hydrocarbons,
such as, for example, methylene chloride, chloroform, carbon
tetrachloride, 1,2-dichloroethane, chlorobenzene and dichloro-
benzene; hexamethylphosphoramide; sulphoxides, such as, for
example, dimethyl sulphoxide; tetramethylene sulphone; and water.
Mixtures of different solvents or dispersants may also be used,
for example water-methanol or, preferably, ethyl acetate-
methanol.
The acylation of the compound of the formula ra, which
may also be present in the form of an acid addition salt, in
order to introduce the radical R1 = -COR2 can be carried out in a
manner known per se using a suitable acylating agent of the
-- 4 --
204232~
formula III
x-ll R2 (III)
in which X represents a radical which can be removed nucleophili-
cally.
In the formula III, X, for example, in particular denotes
halogen, preferably -Cl or -Br; -OH; -O-alkyl, in particular
having 1 to 5 C atoms; -O-aryl, the aryl radical in particular
being a phenyl radical which may also be mono- or polysubstituted
by alkyl, in particular methyl, and/or nitro, and is, for ex-
ample, a tolyl, dinitrophenyl or nitrophenyl radical; -O-CO-R2;
-O-CO-O-alkyl, in particular having 1 to 5 C atoms in the alkyl
radical, or the radical of an azole or benzazole which has at
least 2 N atoms in the quasi-aromatic 5-membered ring and is
bonded via an N atom.
The acylation i8 expediently carried out in a liquid or
liquid disperse phase in the presence of an inert solvent, dis--
persant or diluent or in an excess of the acylating agent, ex-
pediently with stirring.
In the acylation, the molar ratio between the compound of
the formula Ia and the acylating agent of the formula III is
theoretically 1 : 1. However, the acylating agent can also be
employed in excess or in a sub-equivalent amount. The acylating
agent of the formula III is expediently employed in excess.
Excesses of up to 30 mol% are usually sufficient, i.e. the molar
ratio between the compound of the formula Ia and the acylating
agent of the formula III is normally 1 : (1 to 1.3), preferably 1
: (1 co 1.2). If an acid is eliminated in the acylation reaction,
the addition of an acid scavenger, such as, for example, an
alkali metal hydroxide, such as, for example, sodium hydroxide,
potassium hydroxide or lithium hydroxide, a tertiary organic
amine, such as, for example, pyridine or triethylamine, an alkali
metal carbonate or alkali metal bicarbonate, such as, for
example, sodium carbonate or sodium bicarbonate, or an alkali
metal salt of a weak organic acid, such as, for example, sodium
acetate, is expedient. Suitable catalysts, such as, for example,
-- .
2CI14232~
4-dimethylaminopyridine, may also be added during the acylation
reaction.
The acylation may in principle be carried out at
temperatures between -10C and the boiling point of the solvent,
dispersant or diluent used. In many cases, the reaction i5 car-
ried out at O to 50~C, in particular at O to 30C and preferably
at room temperature.
The compounds of the formula III are acylating agents and
thus represent, for example: for X = halogen: acid halides or
haloformic acid esters, of which acid chlorides and chloroformic
acid esters are preferred; for -OH: carboxylic acids; for
-O-alkyl and -O-aryl: esters, of which the tolyl, 2,4-dinitro- or
4-nitrophenyl esters are preferred; for -O-CO-R: anhydrides; for
-O-CO-O-alkyl; mixed carboxylic acid/carbonic acid anhydrides; or
heterocyclic amides or azolides, in particular of N,N'-carbonyl-
diazoles, such as, for example, N,N~-carbonyldiimidazole, 2,2~-
carbonyl-1,2,3-ditriazole, 1,1'-carbonyl-1,2,4-ditriazole, N,N~--
carbonyldipyrazole and 2,2'-carbonylditriazole (compare, for
example, H.A. Staab, M. L~cking and F.H. DUrr, Chem. 8er. 95,
~0 (1962), 1275 et seq., H.A. Staab and A. Mannschreck, Chem. Ber.
95, (1962), 1284 et seq.; H.A. Staab and W. Rohr, "Synthesen mit
heterocyclischen Amiden (Azoliden)~ tSyntheses with heterocyclic
amides (azolides)] in ~Neuere Methoden der Praparativen
Organischen ChemieH [Newer methods of preparative organic
chemistry], volume V, Verlag Chemie, 1967, p. 53 et seq., in
particuiar pp. 65 to 69). The acylating agents of the formula III
can be prepared by processes which are known per se.
When using a carboxylic acid as the acylating agent, the
addition of an activating agent which has the ob~ect of increas-
ing or of activating the acylating potential of the carboxylic
acid or of converting the carboxylic acid into a reactive car-
boxylic acid derivative of the formula III in situ or preferably
shortiy before the reaction with the compound of the formula Ia
is expedient. Suitable activating agents of this type are, for
example: N,N~-disubstituted carbodiimides, in particular if they
contain at least one secondary or tertiary alkyl radical, such
as, for example, diisopropyl, dicyclohexyl or N-methyl-N'-tert.-
- 6 -
2C~4232~
butylcarbodiimide (compare Methodicum Chimicum, Verlag G. Thieme,
- Stuttgart, Vol. 6, (1974), pp. 682/683, and Houben-Weyl, Methoden
der Org. Chemie [Methods of organic chemistry], Vol. 8, (1952),
pp. 521/522); carbonic acid derivatives, such as, for example,
S phosgene, chloroformic acid esters, in particular having 1 to 5 C
atoms in the alkyl radical (compare, for example, Tetrahedron
Letters 24 (1983), 3365 to 3368); carbonic acid esters, such as,
for example, N,N~-disuccinimidyl carbonate, diphthalimidyl car-
bonate, l,l'-(carbonyldioxy)dibenzotriazole or di-2-pyridyl
carbonate (compare, for example, Tetrahedron Letters, Vol. 25,
No. 43, 4943-4946), if desired in the presence of suitable cata-
lysts, such as, for example, 4-dimethylaminopyridine. In addi-
tion, N,N~-carbonyldiazoles, such as, for example, N,N~-
carbonyldiimidazole, 2,2'-carbonyl-1,2,3-ditriazole, l,l'-car-
bonyl-1,2,4-ditriazole, N,N~-carbonyldipyrazole, 2,2~-carbonyl-
ditetrazole, N,N'-carbonylbenzimidazole or N,N'-carbonylbenzotri-
azole are suitable as activating agents (compare, for example,
H.A. Staab, M. LUcking and F.H. DUrr, loc. ciL; H.A. Staab and A.
Mannschreck loc. cit.; H.A. Staab and W. Rohr loc. cit). The
N,N~-carbonyldiazole used is frequently the commercially avail-
able N,N'-carbonyldiimidazole. However, the other N,N'-carbonyl-
azoles are also easily accessible from the respective azole and
phosgene.
In addition, suitable activating agents for carboxylic
acids are: derivatives of oxalic acid, such as, for example,
oxalyl chloride (compare, for example, GB Patent Specification
2,139,225) or N,N'-oxalyldiazoles, such as, for example, 1,1'-
oxalyldiimidazole, 1,1'-oxalyldi-1,2,4-triazole and l,l'-oxalyl-
di-1,2,3,4-tetrazole (compare, for example, Shizuaka Murata,
Bull. Chem. Soc. Jap. 57, 3597-3598 (1984)); methylethylphos-
phinic anhydride (compare, for example, German Offenlegungs-
schrift 3,101,427); diphosphorus tetraiodide (Chem. Lett. 1983,
449); dialkyl disulphite (Indian J. Chem. 21, 259 (1982)); or
other reactive agents.
Suitable solvents, dispersants or diluents are, for
example, those which have been mentioned for carrying out the
cyclisation, and moreover also, for example, pyridine and amides,
- 7 -
2~4232~
such as, for example, dimethylformamide. In addition to water,
polar organic solvents, such as dimethylformamide, dimethyl
sulphoxide or pyridine, are preferred for the acylation. Solvent
mixtures, such as, for example, a mixture of water and methylene
chloride, are also suitable.
The substituted 3-aminosydnone imines of the general
formula I can form acid addition salts with inorganic or organic
acids. Inorganic or organic acids are suitable for the formation
of acid addition salts of this type. For the formation of pharma-
cologically acceptable acid addition salts, suitable acids are,
for example: hydrogen chloride, hydrogen bromide, naphthalene-
disulphonic acids, in particular 1,5-naphthalenedisulphonic acid,
phosphoric, nitric, sulphuric, oxalic, lactic, tartaric, acetic,
salicylic, benzoic, formic, propionic, pivalic, diethylacetic,
malonic, succinic, pimelic, fumaric, maleic, malic, sulphamic,
phenylpropionic, gluconic, ascorbic, isonicotinic, methane-
sulphonic, p-toluenesulphonic, citric or adipic acid. The acid~
addition salts may be prepared in a customary manner by combining
the components, expediently in a suitable solvent or diluent.
The compounds of the formula Ia are unstable in the free
form, but are isolated in the form of their acid addition salts.
In the synthesis of the compounds of the formula Ia, the acid
addition salts are normally obtained.
The starting compounds of the general formula II required
may be prepared in a manner known per se by Strecker~s amino-
nitrile synthesis from compounds of the general formula IV or
their acid addition salts
3/ 3
/cH2 c\ (IV)
A ~N--N H 2
CH CH
in which A has the meaning already mentioned, by reaction with
formaldehyde and hydrocyanic acid or sodium cyanide in a suitable
solvent or dispersant or solvent or dispersant mixture, for
example in water, a compound of the general formula V
-- 8 --
.: :
.` '
20423Z~
C~3~C H 3
2 \ (V)
A ~N--NH- CH2 - CN
CH CH
first being formed, which is converted by nitrosylation into the
compound II. The nitrosylation i8 carried out in a known manner
in a suitable solvent or dispersant or solvent or dispersant mix-
S ture, for example in water, for example at temperatures of -10 to
10C. The nitrous acid i8 in this case normally generated from an
alkali metal nitrite, for example sodium nitrite, and hydro-
chloric acid. It is expedient to adjust the aqueous solution of
the compound V to a pH of 1 to 3 with hydrochloric acid and to
add the alkali metal nitrite dropwise in the form of an aqueous
solution to the stirred and cooled solution. The solution of the
compound II obtained here can be sub~ected directly to the -
cyclisation reaction. However, normally it is appropriate to take
up the nitroso compound II in a suitable organic solvent first
and to carry out the cyclisation to give the compound of the
formula Ia in it, if desired after addition of a further solvent.
The compounds of the general formula IV can be prepared,
starting from compounds of the general formula VI
C~ 3 C H 3 ( VI ),
A N--H
CH2 --CH
by either
a) nitrosylating a compound of the formula VI to qive the N-
nitroso compound VII and then reducing, expediently with lithium
aluminium hydride~
204232~
( V l I C H 2--C H 2 C H Z--C H z
(VII ) ~ IV)
or, in a manner known per se
b) converting a compound of the formula VI into the urea deriva-
tive VIII with potassium cyanate in acidic medium, and then
converting by means of oxidation with sodium hypochlorite by the
Hoffmann degradation into the compound IV:
A ; N--H A~ N--CO-NHz
( V I ) ( V I I I )
~3~CH3 C~3/CH3
A\N--C O - N H _ N a O C 1 ~C H C
CH CH CH2 CH2
(VIII I ( IV)
Compounds of the formula I according to the invention can
also be prepared by reacting a compound of the formula I where A
= -S- or an acid addition salt of such a compound, with an
oxygen-donating ~ubstance and converting the compound obtained,
if desired, into an acid addition salt and isolating it, or in
the case in which a compound of the formula Ia has been obtained
and it is intended to prepare a compound of the formula I where
A = -S0- and R2 = -COR2, acylating the compound Ia or an acid
addition salt thereof with an acylating agent which introduces
the radical -COR2 and isolating the compound thus obtained or, if
-- 10 --
.
.
-
.
20423Z~
desired, converting it into a pharmacologically acceptable acid
addition salt and isolating this.
Examples of suitable oxygen-donating substances which may
be mentioned are: hydrogen peroxide, peroxo acids, such as, for
example, performic, peracetic, perbenzoic, m-chloroperbenzoic and
perphthalic acid, and also diacyl peroxides. Mixtures of various
oxygen-donating substances may also be used. The oxygen-donating
substance is normally employed in the stoichiometrically
necessary amount or in a slight excess to this stoichLometrically
necessary amount. Larger excesses are avoided as otherwise the
sulphone, i.e. a compond of the formula I where A = -SO2-, is
formed. The reaction may expediently be carried out in a suitable
solvent or dispersant or solvent or dispersant mixture, for
example, at temperatures from 0C, preferably from room tempera-
ture, up to the boiling point of the solvent or dispersant or
solvent or dispersant mixture. Suitable solvents or dispersants
are, for example, the ethers already mentioned, aliphatic, cyclo-
aliphatic and aromatic hydrocarbons, halogenated aliphatic and
aromatic hydrocarbons, water, but in particular lower carboxylic
acids, such as formic or acetic acid.
An acylation with an acylating agent introducing the
radical -COR2 to be carried out, if desired, after the reaction
with the oxygen-donating agent is carried out in the manner
already mentioned using an acylating agent of the formula III.
The conversion to be carried out, if desired, into the
acid addition salt is carried out, as already mentioned above, in
a manner known per se.
The compounds of the formula I where A = -S- can be
prepared analogously to the compounds of the formula I where
A = -SO- from compounds of the formula II where A = -S-.
The compounds of the general formula I according to the
invention and their pharmacologically acceptable acid addition
salts have useful pharmacological properties, for example haemo-
dynamic, thrombocyte function-inhibiting and antithrombotic, in
particular coronary antithrombotic, properties. Their action on
the cardiova~cular system is particularly pronounced. Compared
with known sydnone imine compounds, for example those of French
-- 11 --
204232~
Patent Specification 1,496,056 and the compounds described in the
- examples of German Offenlegungsschrift 3,820,210, they unex-
pectedly have a surprisingly longer duration of action. For
example, they lower the blood pressure as well as the pulmonary
artery pressure and the left ventricular end-diastolic pressure
and thus contribute to a relief of the load on the heart in the
sense of an antianginal action, without provoking reflex tachy-
cardia at the same time.
The compounds of the formula I according to the invention
and their pharmacologically acceptable acid addition salts can
therefore be administered to humans as medicaments by themselves,
in mixtures with one another or in the form of pharmaceutical
preparations which permit enteral or parenteral use and which
contain as the active component an effective dose of at least one
compound of the formula I according to the invention or an acid
addition salt thereof, in addition to one or more customary
pharmaceutically acceptable excipients or diluents and, if de-~
sired, one or more additives.
The medicaments cah be administered orally, for example
in the form of tablets, film tablets, coated tablets, hard and
soft gelatin capsules, microcapsules, granules, powders, pellets,
solutions, syrups, emulsions, suspensions, aerosols, foams, pills
or pastilles. Administration may, however, also be carried out
rectally, for example in the form of suppositories, or parenter-
ally, for example in the form of in~ection solutions, or per-
cutaneously, for example in the form of ointments, creams, gels,
pastes, aerosols, foams, powders, tinctures, liniments or so-
called transdermal therapeutic systems (TTS).
The pharmaceutical preparations can be prepared in a
manner known per se using pharmaceutically inert inorganic or
organic auxiliaries, excipients, fillers or diluents. For the
preparation of pills, tablets, film tablets, coated tablets and
the pellet or granule fillings of hard gelatin capsules, for
example calcium phosphate, lactose, sorbitol, mannitol, starches,
prepared starches, chemically modified starches, starch hydroly-
sates, cellulose, cellulose derivatives, synthetic polymers, talc
etc., can be used. Excipients or diluents for soft gelatin
- 12 -
~-
. . ~.
, . .
2~4232~
capsules and suppositories are, for example, fats, waxes, semi-
-solid and liquid polyols, natural or hardened oils etc. Suitable
excipients or diluents for the preparation of solutions and
syrups are, for example, water, polyols, solutions of sucrose,
dextrose, glucose etc. Suitable excipients for the preparation of
in~ection solutions are, for example, water, alcohols, glycerol,
polyols or vegetable oils. Suitable excipients or diluents for
ointments, creams and pastes are, for example, natural petroleum
~elly, synthetic petroleum ~elly, viscous and mobile paraffins,
fats, natural or hardened vegetable and animal oils, neutral
oils, waxeæ, wax alcohols, polyethylene glycols, polyacrylic
acid, silicone gels etc.
The pharmaceutical preparations may also contain, in a
manner known per se, in addition to the active compounds and
diluents, fillers or excipients, one or more additives or auxili-
aries, such as, for example, disintegrants, binders, glidants,
lubricants, mould release agents, wetting agents, stabilisers,-
emulsifiers, preservatives, sweeteners, colourings, flavourings,
aromatisers, buffer substances, and in addition solvents or
solubilisers, solution accelerators, antifoams, salt-forming
agents, gelling agents, thickeners, flow regulators, absorbents,
agents for achieving a depot effect or agents, in particular
salts, for changing the osmotic pressure, coating agents or
antioxidants, etc. They may alæo contain two or more compounds of
the formula I or their pharmacologically acceptable acid addition
salts and also one or more other therapeutically active sub-
stances.
Examples of other therapeutically active substances of
this type may be: ~-receptor blockers, such as, for example,
propranolol, pindolol, metoprolol; vasodilators, such as, for
example, carbochromen; tranquilisers, such as, for example,
barbituric acid derivatlves, 1,4-benzodlazepines and meprobamate;
diuretics, such as, for example, chlorothiazide; cardiotonic
agents, such as, for example, digitalis preparations; hypotensive
agents, such as, for example, hydralazine, dihydralazine, prazo-
sine, clonidine, Rauwolfia alkaloids; agents which lower the
fatty acid level in the blood, such as, for example, bezafibrate,
- 13 -
2~4232~
fenofibrate; and agents for thrombosis prophylaxis, such as, for
- example, phenprocoumon.
The content of the active compound or the active com-
pounds of the formula I in the pharmaceutical preparations can
vary within wide limits and is, for example, 0.05 to 50% by
weight, preferably 0.05 to 20% by weight. In solid administration
forms, such as coated tablets, tablets etc., the content of one
or more active compounds of the formula I is in many cases 2 to
20% by weight. Liquid administration forms, such as drops, emul-
sions and in~ection solutions frequently contain 0.05 to 2% byweight, preferably 0.05 to l~ by weight, of one or more active
compolmds of the formula I. The content of one or more active
compounds of the formula I in the pharmaceutical preparations may
be partially replaced, if desired, for example up to 50% by
weight, preferably up to 5 to 40% by weight, by one or more other
therapeutically active substances.
The compounds of the formula I according to the inven--
tion, their pharmacologically acceptable acid addition salts and
pharmaceutical preparations which contain the compounds of the
formula I according to the invention or their pha Dacologically
acceptable acid addition salts as active compounds can be used in
humans for combating or preventing disorders of the cardio-
vascular system, for example as antihypertensive medicaments in
the various forms of high blood pressure, and in combating or
prevention of angina pectoris etc. The dosage may vary within
wide limits and is to be suited to the individual requirements in
each individual case. In general, a daily dose of about 0.5 to
100 mg, preferably l to 20 mg, per human individual is suitable
for oral administration. For other administration forms, the
daily dose, owing to the good absorption of the active compounds,
is in similar amount ranges, i.e. in general also O.S to lO0 mg/
human. The daily dose is normally divided into several, for
example 2 to 4, part administrations.
The pharmacological action of the compounds of the
formula I according to the invention was determined by a
modified method of Godfraind and Kaba (Arch. Int. Pharmacodyn.
Ther. 196, (Suppl) 35 to 49, 1972) and of Schuman et al.
- 14 -
.
.. .. . .
2~4;~Z~
~Naunyn-Schmiedeberg's Arch. Pharmacol. 289, 409 to 418, 1975).
In this connection, spiral strips of the pulmonary artery of the
guinea-pig are depolarised with 40 mmol/l of potassium after
equilibration in calcium-free Tyrode solution. An addition of
0.5 mmol~l of CaCl2 then induces a contraction.
The relaxing action of the test substance is determined
by cumulative addition in 1/2 log 10 stepped concentrations. From
the concentration-effect curve (abscissa: -log mol/l of test
substance, ordinate: ~ inhibition of the maximum contraction,
average value of 4 to 6 vessel strips), the concentration of the
test substance is determined which inhibits the contraction by
50~ (= IC50, mol/l). The duration of action of the test substance
is shown by the time which i8 needed after the addition of the
test substance until the starting value is obtained again. The
values thus obtained are indicated in the following table.
IC50 values and duration of action of sydnone imines of the
formula R R
C H C
A~ N N -- C H
CHz--C/ N\ /1=NHHC1
Compound A R IC50Duration of action
No. in mol/lin min
~ --- ---
1 OS CH3 3~x 10-6270
2 O H 1 x 10-6 80
3 ~ CH3 1 x 10-6120
Compound No. 1 of the table is the compound according to
the invention 3-(3,3-dimethylperhydro-1-oxo-1,4-thiazin-4-yl)-
sydnone imine hydrochloride.
Compound No. 2 of the table is the compound 3-morpholino-
sydnone imine hydrochloride known from Example 2 of French Patent
-- 15 --
` Specification 1,406,056. 2Q4232~
- Compound No. 3 of the table is the compound 3-(3,3-di-
methylmorpholin-4-yl)sydnone imine hydrochloride of Example No. 1
of German Offenlegungsschrift 3,820,210.
As can be seen from the above table, the compounds
according to the invention have a substantially longer duration
of action at a comparable IC53 value than the two comparison
compounds.
If not indicated otherwise in the examples which follow,
percentage data are percentages by weight.
Example 1
3-~3.3-Dimethylperhydro-l-oxo-1.4-thiazin-4-yl)sydnone imine
hydrochloride
a) 2.2-Dimethylaziridine
100 g of 2-amino-2-methylpropan-1-ol are dissolved in
200 ml of water. A cold solution of 110 g of conc. sulphuric acid
in 200 ml of water is then added dropwise with stirring and the
water i8 then distilled off at normal pressure until an internai
temperature of 115-C is attained. Distillation is continued in a
water-~et vacuum up to a bath temperature of 180C and the flask
contents are kept under these conditions until they are solid.
The mixture is then additionally heated in a water-~et vacuum and
at 180C for 1 hour more.
The product i8 treated with a solution of 100 g of NaOH
in 200 ml of water, pulverised and allowed to stand overnight.
Water is distilled off from the suspension under normal pressure.
50 g of ROH are added to the residue with stirring and the mix-
ture is stirred for 3 hours. After allowing to stand, the upper
layer is separated off, treated with 20 g of KOH and allowed to
stand overnight. The oil is poured off, treated with 10 g of ROH
and distilled under normal pressure.
Yield: 60.3 g
b~ 2-Hydroxyethyl 2-amino-2-methylDropyl ~ulphide
234 g of 2-mercaptoethanol are dissolved in 90 ml of
dimethoxyethane. 63.3 g of 2,2-dimethylaziridine are added drop-
wise to this solution and it is stirred at 70C for 4 hours,
concentrated and distilled in a water-~et vacuum.
2~4232~.
~ B.p.: 141-142C (at 20 mbar) M.p.: 49-51C
c) 3 3-Dimethylperhydro-1.4-thiazine
Hydrogen chloride is introduced with stirring into a
solution of 14.9 g of 2-hydroxyethyl 2-amino-2-methylpropyl
sulphide (step b~ in 100 ml of dimethoxyethane until it is
saturated and then 17.9 g of thionyl chloride are added dropwise
and the mixture is subsequently stirred at room temperature for
1 hour and then heated to reflux for 1 hour. The solution is then
concentrated in a water-~et vacuum. The residue of 2-chloroethyl
2-amino-2-methylpropyl sulphide hydrochloride crystallises out.
The hydrochloride obtained is dissolved in water, the
solution is rendered strongly alkaline using potassium carbonate,
the free base is taken up in 100 ml of toluene and the aqueous
phase is extracted again by shaking with 2 x 30 ml of toluene.
The organic phases are combined, dried over potassium carbonate,
concentrated and distilled in a water-~et vacuum.
Yield: 8.1 g B.p.: 73-75C (at 20 mbar)
d) 4-Nitroso-3 3-dimethylperhydro-1.4-thiazine
A mixture of 15.7 g of 3,3-dimethylperhydro-1,4-thiazine
(step c) and 60 ml of ice-water are treated with cooling with
12 ml of 10 N HCl. A solution of 12.4 g of sodium nitrite in
40 ml of water is then added dropwise at 4 to 6C. The mixture is
subsequently stirred at room temperature for 4 hours, rendered
alkaline with pota~sium carbonate and extracted by shaking with
ethyl acetate. The organic phase is dried and concentrated in a
water-~et vacuum.
Yield: 17.5 g ~ M.p.: Yellow oil
e) 4-Amino-3 3-dimethyl~rhydro-1,4-thiazine hydrochloride
A solution of 17.2 g of 4-nitro~o-3,3-dimethylperhydro-
1,4-thiazine (step d) in 150 ml of tetrahydrofuran is treated
with 4.4 g of lithium aluminium hydride in portions at 60-65-C
and the mixture i~ then heated to reflux for 2 hours. The suspen-
sion is cooled and a solution of 5 ml of water in 50 ml of tetra-
hydrofuran i~ added dropwise. After 20 minutes, 10 ml of 27%
strength by weight sodium hydroxide solution are added dropwise
and the mixture is allowed to stand for a few hours. The precipi-
tate i~ then filtered off and the hydrochloride is precipitated
- 17 -
2al4232~
in the ice-cooled filtxate.
Yield: 12.2 g
f) 4-(2-Cyanoethyl-amino~-3.3-dimethylperhydro-1 4-thiazine
A solution of 6.1 g of XCN in 30 ml of water i8 added
dropwise to a solution, cooled to -3 to -5C, of 12.1 g of
4-amino-3,3-dimethylperhydro-1,4-thiazine hydrochloride (step e).
The pH of the solution i8 then adjusted to 7.2 with hydrochloric
acid and 16.3 g of 39% strength by weight aqueous formaldehyde
are then added, the pH being kept at 6 to 7. The mixture is sub-
sequently stirred overnight at a temperature rising to roomtemperature. The suspension is extracted by shaking with ethyl
acetate and the organic phase is dried and concentrated.
Yield: 9.4 g M.p.: oil
) ~-(3 3-Dimethylperhydro-1 4-thiazin-4-yl)sydnone imine
hydrochloride
9.4 g of 4-(2-cyanoethylamino)-3,3-dimethylperhydro-
1,4-thiazine (step f), 40 ml of ethyl acetate, 40 g of ice-water
and 5 ml of 10 N hydrochloric acid are cooled to -5C. 5.3 g of
sodium nitrite are introduced into this mixture and it is subse-
quently stirred at room temperature for 3 hours. The organicphase is separated off and dried, the solid is filtered off and
20 ml of a saturated solution of HCl in isopropanol are added
dropwise with cooling. The mixture i8 subsequently stirred over-
night at room temperature. The precipitate is separated off and
recrystallised from isopropanol.
Yield: 5.9 g M.p.: 172C (decomposition)
h) 3-(3 3-Dimethylperhydro-l-oxo-1 4-thiazin-4-yl)sydnone imine
hydrochloride
The solution obtained from 3 g of 3-(3,3-dimethyl-
- 30 perhydro-1,4-thiazin-4-yl)sydnone imine hydrochloride (step g) is
treated at room temperature with stirring with 1.2 g of a 35%
strength aqueous hydrogen peroxide solution, the temperature
rising somewhat. The mixture is stirred for 2 hours and then
concentrated in a water-~et vacuum, and the residue is recrystal-
lised from ethyl acetate.
Yield: 2.5 g M.p. 192-C (dec.)
- 18 -
.
, .
Example 2 2~4232~
-- N-p-Anisoyl-3-(3,3-dimethylperhydro-1-oxo-1,4-thiazin-4-yl)-
sydnone imine
3 g of 3-(3,3-dimethylperhydro-1-oxo-1,4-thiazin-4-yl)-
sydnone imine hydrochloride (Example 1 h) are dissolved in 20 ml
of ice-water and 2 g of NaHCO3 are added. 2.1 g of p-anisoyl
chloride, dissolved in 30 ml of ethyl acetate, are added drop-
wise. The mixture is subsequently stirred for 3 hours, the
temperature rising to room temperature. The phases are then
separated, the aqueous phase is extracted by shaking with ethyl
acetate, and the organic phases are combined, dried and concen-
trated. The crystalline product obtained is recrystallised from
isopropanol.
Yield: 1.8 g M.p. 183 to 185C (decomposition)
Example 3
N-Ethoxycarbonyl-3-(3,3-dimethyl~erhydro-1-oxo-1 4-thiazin-
4-yl)sydnone imine
The compound is obtained analogously to Example 2 using~
ethyl chloroformate (1.8 g) instead of p-anisoyl chloride and is
recrystallised from isopropyl acetate.
Yield: 1.4 g M.p. 138C (decomposition)
ExamDle 4
N-Pivaloyl-3-(3,3-dimethylperhydro-1-oxo-1,4-thiazin-4-yl)sydnone
imine
The compound is obtained analogously to Example 2 using
2.1 g of pivaloyl chloride instead of p-anisoyl chloride and is
recrystallised from diisopropyl'ether.
Yield: 1.3 g M.p.: 110C (decomposition)
Pharmaceutical preparations are described in the follow-
ing examples A to H.
Example A
Soft gelatin capsules, containing 5 mg of active compound per
capsule:
Per capsule
Active compound 5 mg
Triglyceride mixture fractionated
from coconut oil 150 mg
-- 19 --
Capsule contents 155 mg 2~4232~
Example B
In~ection solution, containing 1 mg of active compound per ml:
per ml
Active compound 1.0 mg
Polyethylene glycol 400 0.3 ml
Sodium chloride 2.7 mg
Water for in~ection purposes to 1 ml
Example C
10 Emulsion, containing 3 mg of active compound per 5 ml
per 100 ml of emulsion
Active compound 0.06 g
Neutral oil q.s.
Sodium carboxymethylcellulose 0.6 g
Polyoxyethylene stearate q.s.
Glycerol, pure 0.2 to 2.0 g
Flavouring q.s.
Water (demineralised or distilled) to 100 ml
Example D
20 Rectal medicament form, containing 4 mg of active compound per
suppository
Der su~pository
Active compound 4 mg
Suppository ba~e to 2 g
Example E
Tablets, containing 2 mg of active compound per tablet
- Der tablet
Active compound 2 mg
Lactose 60 mg
30 Cornflour 30 mg
Soluble starch 4 mg
Magnesium stearate 4 mg
100 mg
Example F
35 Coated tablets, containing 1 mg of active compound per coated
tablet
- 20 -
204232~
per coated tablet
Active compound 1 mg
Cornflour 100 mg
Lactoæe 60 mg
sec Calcium phosphate 30 mg
Soluble starch 3 mg
Magnesium stearate 2 mg
Colloidal silicic acid 4 m~
200 mg
Example G
The following recipes are suitable for the preparation of the
contents of hard gelatin capsules:
a) Active compound 10 mg
Cornflour 190 m~
200 mg
b) Active compound 40 mg
Lactose 80 mg
Cornflour _80 mg
200 mg
Example H
Drops can be prepared according to the following recipe (20 mg of
active compound in 1 ml = 20 drops):
Active compound 2.00 g
Methyl benzoate 0.07 g
Ethyl benzoate 0.03 g
Ethanol, 96% strength 4 ml
Demineralised water to 100 ml
- 21 -