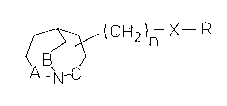Note: Descriptions are shown in the official language in which they were submitted.
-- 1 --
56651.19
Bicyclic 1-aza-cYcloalkanes
The present invention relates to novel bicyclic 1-
aza-cycloalkanes of general fo:rmula I, to processes for
their preparation and to their use as pharmaceutical
compositions.
According to the present invention we provide
compounds of general formula I
0 ~ "(CH2)n X--R
A h-C
wherein
R represents a Cl6-alkyl group, a C36-alkenyl group or
a C36-alkynyl group, the alkyl, alkenyl or alkynyl
group being optionally substituted by a substituted
or unsubstituted phenyl group, a substituted or
unsubstituted biphenyl group, a substituted or
unsubstituted oxetan ring or a substituted or
unsubstituted 5-, 6- or 7-membered heterocyclic
group; or
R represents a substituted or unsubstituted phenyl
group, a substituted or unsubstituted biphenyl
group or a substituted or unsubstituted 5-, 6- or
7-membered heterocyclic group;
X represents oxygen or sulphur;
A, B and C independently of one another represent CH2 or
a single bond;
n represents 0, 1 or 2;
. ' ' , '
., . ;
2 ~
2 --
and all racemic and tautomeric forms, enantiomers,
diastereomers and mixtures thereof and acid addition
salts thereof and additionally the quaternary salts
thereof; with the proviso that i) for a compound of
formula I wherein the bicyclic ring system is
quinuclidine, R may not represent an unsubstituted
alkyl, alkenyl or alkynyl group; ii) for a compound of
formula I wherein the bicyclic ring system is 1-
azabicyclo~3,2,1]octane, the group -(CH2)n-X-R may not
represent a propoxy group; and iii) for a compound of
formula I wherein the bicyclic ring system is 1-
azabicyclo[2,2,2]octane, the group -(CH2~n-X-R may not
represent a 3-(2-pyridinyloxy)- group.
Examples of alkyl groups for the purposes of this
invention are branched or unbranched C16-alkyl groups
such as methyl, ethyl, propyl, butyl, pentyl and hexyl
groups as well as the branched isomers thereof such as
isopropyl, isobutyl, tert.-butyl, sec.-butyl etc.; the
C36-alkenyl groups are branched or unbranched C36-alkenyl
groups - such as propenyl, butenyl, pentenyl and hexenyl
groups - having a single double bond; C36-alkynyl groups
are branched or unbranched C36-alkynyl groups - such as
propynyl, butynyl and pentynyl - having a single triple
bond. Alkenyl or alkynyl groups in which a double or
triple bond respectively is in a terminal position are
preferred.
The term "substituted phenyl" indicates, unless
otherwise stated, phenyl groups which are mono-, di- or
trisubstituted by a halogen atom, or by a hydroxy, a
branched or unbranched C15-alkyl, a substituted or
unsubstituted C36-cycloalkyl, a C15-hydroxyalkyl, an
amino or a mono- or disubstituted-C~4-alkylamino group.
The terms "substituted cycloalkyl" and "substituted
biphenyl" indicate cycloalkyl and biphenyl groups in
which the cycloalkyl ring and either of the phenyl rings
in a biphenyl group may be substituted as specified
~2~
-- 3 --
above for a substituted phenyl group. Examples of
substituted phenyl groups include:
2-chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl,
3-fluorophenyl, 2,3-dichlorophenyl, 4-hydroxyphenyl,
2-methylphenyl, 4-methylphenyl, 3-ethylphenyl,
4-propylphenyl, 4-isopropylphenyl, 4-butylphenyl,
4-tert.-butylphenyl, 4-pentylphenyl, 2,4-dimethylphenyl,
2-trifluoromethylphenyl, 3-trifluoromethylphenyl,
2-methoxyph~nyl, 4-methoxyphenyl, 3-ethoxyphenyl,
2-propoxyphenyl, 4-butoxyphenyl, 2,4-dimethoxyphenyl,
3,4,5-trimethoxyphenyl.
The term "heterocyclic group" indicates a 5- to 7-
membered ring which contains one or more heteroatoms
selected from oxygen, sulphur and nitrogen and onto
which another aromatic ring, preferably a phenyl group,
may optionally be fused.
Examples of saturated heterocyclic 6-membered rings
include: 1,4- and 1,3-dioxan, morp~oline,
thiomorpholine, piperidine, piperazine, 4-C14-
alkylpiperazine, N-hydroxy-Cl4-alkylpiperazine, 2,5-
diketopiperazine and tetrahydropyran. Examples of
saturated heterocyclic 5-membered rings include:
tetrahydropyrrole, tetrahydrofuran, proline,
tetrahydropyrazole, imidazolidine, hydroxyproline,
pyrrolidine, pyrazolidine, pyrrolidone, thiolan,
butyrolactone and 1,2-oxathiolan.
Examples of 5-, 6--and 7-membered mono- and
polyunsaturated heterocyclic rings include:
pyrrole, imidazole, imidazoline, 1,2,4- and 1~2,3-
triazole, tetrazole, isothiazole, furan, dihydrofuran,
thiophene, pyridine, pyrimidine, pyran, 2,5-
dihydropyrrole, thiazole, thiadiazine, azepine, 1,2-
oxathiepan, pyrazoline, dimethylpyrrole, 2-acylfuran and
dihydrothiophene. Examples of other heteroaryl groups
include pyrazinyl, quinolyl, isoquinolyl, quinazolyl,
-- a, --
quinoxalyl, thia~olyl, be~zothiazolyl, isothiazolyl,
oxazolyl, b~nzoxazolyl, isoxazolyl, imidazolyl,
benzimidazolyl, pyrazolyl, and indolyl. A pre~erred
heterocyclic group is the pyridine group which may be
mono- to tetrasubstituted, preferably mono- to
disubstituted, with identical or different substituents,
selecte~ from hydroxy, Cl4-alkyl, C14-alkoxy, NH2, mono-
or di-(C~4)-alkylamino, halogen, CF3, CN or nitro.
Examples of 5, 6 or 7-membered heterocyclic rings
bound via a nitrogen atom also include:
phenanthridin-6-one, quinolin-2-one, isoquinolin-1,3-
dione, benz[c,d]indole, 1,4-benzoxazin-3-onP, indol-2,3-
dione, indol-2-one, 1,2,4-triazolo[4,3-a]pyridin-3-one,
1,2-benzisothia201-3-one, lH-indazole, lH-benzimidazole,
lH-benztriazole, benzothiazin-3-one, isoindol-1,3-dione,
benz[d,e]isoquinolin-1,3-dione, 4-quinazolinone,
isoindol-l-one, pyrrolo[l,2-c]imidazol-1,3-dione, 1,3-
dihydro-2H-indol-2-one, tetrahydro-lH-isoindol-1,3-
dione, 3,7-dihydro-lH-purin-2,6-dione, indole, indazole,
benzimidazole, benzimidazol-2-one-,-1,4-benzothiazin-3-
one and lH-isoindol-1,3-dione.
The heterocyclic group may be mono- or
polysubstituted by a halogen atom, or a hydroxy, a
branched or unbranched C14-alkyl, a Cl4-hydroxyalkyl or a
C14-alkoxy group. The heterocyclic group may
additionally contain a keto-function as in e.g.
dihydrotetrafuranone.
Preferred compounds according to the present
invention are compounds of general formula I as defined
above wherein X represents oxygen; n represents 0 or 1;
the substituent -(CHz)n-X-R is in the ~- or ~-position
relative to the carbocyclic bridgehead; and R represents
an optionally substituted C13-alkyl, C34-alkenyl, or C3 4-
alkynyl group, a substituted or unsubstituted phenyl
group or a substituted or unsubstituted 5- or 6-membered
aromatic heterocyclic group.
Particularly preferred compounds according to the
6 ~
- 5 -
present invention are quinuclidine compounds of general
formula I as defined above wherein n represents o or l;
X represents oxygen; R represents a substituted or
unsubstituted pyridine, thiophene, furan, pyrimidine,
imidazole, pyrazole, 1,2,4-triazole, oxetan,
tetrahydrofuran, pyrrolidine or oxolan group, a
substituted or unsubstituted phenyl group, or a
substituted or unsubstituted benzyl group; the side
chain -(CH2)n-X-R being in the 3-position of the
quinuclidine system.
Further particularly preferred compounds according
to the present invention are l-azabicyclo[2,2,1]heptanes
of general formula I as defined above wherein X
represents oxygen; n represents 0 or 1; R represents an
optionally substituted C13-alkyl, C34-alkenyl or C3 4-
alkynyl group, a substituted or unsubstituted pyridine,
thiophene, furan, pyrimidine, imidazole, pyrazole,
1,2,4-triazole, oxetan, tetrahydrofuran, pyrrolidine or
oxolan group, a substituted or unsubstituted phenyl
group or a substituted or unsubstituted benzyl group;
the side chain -(CH2)n-X-R being in the 3-position of the
bicyclic system.
Further particularly preferred compounds according
to the present invention are 1-aza-bicyclo[3,2,1]octanes
of general formula I as defined above wherein X
represents oxygen; n represents 0 or 1; R represents an
optionally substituted C13-alkyl, C34-alkenyl group or
~ 4-alkynyl group, a substituted or unsubstituted
pyridine, thiophene, furan, pyrimidine, imidazole,
pyrazole, 1,2,4-triazole, oxetan, tetrahydrofuran,
pyrrolidine or oxolan group, a substituted or
unsubstituted phenyl group, a substituted or
unsubstituted benzyl group; the side chain -(CHz)n-X-R
being in the 3- or 6-position of the bicyclic group.
Further particularly preferred compounds according
to the present invention are l-aza-bicyclo[3,3,13nonanes
of general formula I as defined above wherein X
-- 6
represents oxyger.; n represents 0 or 1; R represents an
optionally substituted C13-alkyl, C34-alXenyl or C34-
alkynyl group, a substituted or unsubstituted pyridine,
thiophen~, furan, pyrimidine, imidazole, pyrazole,
1,2,4-triazole, oxetan, tetrahydrofuran, pyrrolidine or
oxol~n group, a substituted or unsubstituted phenyl
group or a substituted or unsubstituted benzyl group;
the side chain -(CH2)n-X-R being in the 3-position of the
bicyclic group.
Especially preferred compounds according to the
present invention are compounds of general formula
~ ~CH2 )n - R
J
N
wherein n represents o or l; R represents a group of
formula
-CH2~ I Rl)k -~ RlJk
-CH2 ~ (R1lk ~ (~1)k
-CH2 ~I R2)l _~( R2)
-CH2~ IR2 ), ~ (R2 )I - .
-CH2 ~ R2JI ~1 R2),
- 7 - 2~
,~,CH2 ,~,
R`--N_~,N R --~ N
Q~N J~
-CH2/ 1 ~
R3 R3
-C~2 ~ ~F~2)
15-CH2 ~ (R2)l ~ (R2
20-CH2 ~ (R2~ 2)
R3 R3
-CH2 ~ ~ ( R 2 )
R3 R3
in which
30 Rl represents a hydrogen atom, a C14-alkyl group,
preferably methyl, a C14-alkoxy group, preferably
methoxy, an amino, C14-alkylamino, C14-dialkylamino,
hydroxy, or C36-cycloalkyl group, a substituted or
unsubstituted phenyl group or a keto-function;5 k represents 1, 2 or 3, whilst if k is greater-than 1
the R1 groups may be identical or different,
R2 represents a hydrogen or halogen atom, a C14-alkyl
- : - .
.
2~2~
-- 8
group, preferably methyl, a C14-alkoxy yroup,
preferably methoxy or a keto-function;
1 represents 1 or 2, preferably 1, whilst when 1
represents 2 the groups R2 may be identical or
different;
R3 represents a hydrogen atom or a C~ 4 alkyl group,
preferably methyl;
and all racemic and tauto:meric forms, enantiomers,
diastereomers and mixtures thereof and acid
addition salts thereof and additionally the
quaternary salts thereof.
Further especially preferred compounds according to
the present invention are those of general formula
(C~2 )n- O - R
N ~ ( CH2)n- 0 - R
~ ~CH2)n 0- R
N
wherein
n represents 0 or 1, preferably zero;
R represents a C3-alkynyl group, a C3-alkenyl group, or a
group of formula
2 $ ~
_ 9 _
-CU ~-(Rl)k ~(Rl)k
-CH ~ ~ I R ~ ~ k --~ ~ R ~ ~ k
-CH2 ~(R2~1 ` ~(R2Jt
-CH2 ~ (R2)l ~ (R2
2 0 - CH2 ~ ( R 2 ) I N~ N
in which R1, R2, k and l are as hereinbefore defined; and
all racsmic and tautomeric forms, enantiomers,
diastereomers and mixtures thereof and acid addition
salts thereof and additionally the quaternary salts
thereof.
Further especially preferred compounds according to
the present invention are those of general formula
~J--(CH2)n- O- R
-- 10 --
wherein
n represents O or l;
R represents a C3-alkynyl group, a C3-alkenyl group, or a
methyl, ethyl or propyl group, or a group of formula
-cH2~(R1~k ~Rl~k
-CH2 ~ ~ R ~ ~ < ` ~ ( R 1 J k
-C~12 ~( R2~l ~(
~; N N~ N
-CH ~( R2~l ~ ~( R2
2t~ ~ (R2 )l
wherein R1, ~, k and 1 are as hereinbefore defined; and
30 all racemic and tautomeric forms, enantiomers,
diastereomers and mixtures thereof and the acid addition
salts thereof and additionally the quaternary salts
thereof.
Certain compounds analogous to compounds of general
35 formula I are described in the prior art. Thus, for
example, EP 370415 discloses compounds of formula Ia
-- 1 --
~(CHz1",- X- R (Ia)
wherein Rl represents an unsubstituted C16-alkyl, C36-
alkenyl or C36-alkynyl group; X' represents an oxygen or
sulphur atom; and m represents 0, 1 or 2.
UK Patent No. 2208385 discloses compounds of general
formula Ib
~
N (Ib)
~ - -
wherein R" represents a 3-(2-pyridinyloxy)- group.
However these compounds are described as 5-HT-
antagonists. European Patent No. 257741 discloses
compounds of formula Ic
R " '
~ (Ic)
wherein R"' represents a propyloxy group.
However certain of the optically active compounds
of formulae Ia, Ib and Ic are not specifically mentioned
by name in EP 370415, GB 2208385 and EP 257741.
.
.. . . .
,
- 12 -
~ lence according to a further feature of the present
invention there are pro~ided compounds of formulae Ia,
Ib and Ic as defined above in the form of individual
enantiomers and acid addition salts thereof and
additionally the quaternary salts thereof, collectively
termed the compounds of formula (Id); with the proviso
that in the compounds of formula Ia when X' represents
oxygen and m represents 0, R' cannot represent a C3-
alkynyl group or an ethyl group.
A most especially preferred compound according to
the present invention is (+)-(propargyloxymethyl)-l-
azabicyclo[2,2,2]octane and acid addition salts thereof
and additionally the quaternary salts thereof.
When in the compounds of general formula I, R
represents an aliphatic group such compounds may be
prepared analogously to the methods described by L.
Stotter et al. in Heterocycles, Vol. 25 (1987), page 25.
Hence, according to a further feature of the
present invention we provide a process ~or the
preparation of a compound of formula I as defined above
wherein R represents an aliphatic group, characterised
in that a corresponding optionally N-protected
derivative of general formula I (in which R represents H
and the nitrogen atom of the bicyclic group is bloc~ed
by a protecting group Z, e.g. BH3) is deprotonated, for
example with a strong base, and is subsequently reacted
with an alkylating reagent of general formula Y-R,
wherein R is as defined hereinbefore and Y represents a
readily removable leaving group such as halogen or p~
toluenesulphonate. The reaction is carried out in polar
inert organic solvents such as dimethylformamide,
tetrahydrofuran, dioxan.
Whereas the deprotonation and conversion of the
hydroxy compound into a metal salt is pre~erably carried
out at ambient or slightly elevated temperature, the
subsequent alkylation is carried out whilst cooling with
ice. A~ter the reaction has ended the protecting group
- 13 -
is cleaved and the compounds are optionall~ con~erted
into their acid addition salts or quaternary compounds
using known reaction conditions; the pre~erred
quaternary compounds are the methoiodides and
methobromides.
Preferred reagents for the deprotonation step are
sodium hydride, sodium amide and alkali metal alkoxides
such as potassium tert.-butoxide.
Preferred acid addition salts are physiologically
acceptable acid addition salts and include, for example
salts with hydrochloric, hydrobromic, sulphuric,
phosphoric, methanesulphonic, ethanesulphonic,
toluenesulphonic, benzenesulphonic, lactic, malonic,
succinic, maleic, fumaric, malic, tartaric, citric and
benzoic acid.
The starting compounds required for the synthesis,
namely the corresponding hydroxyazabicycloalkanes, are
Xnown from the prior art, being of formula
1CH2~nH~ C }~CH2~
IIa II b IV
Thus, for example, for those wherein n represents O from
JACS 74, 2215 (1952) (IIa), J. Org. Chem. 33, 4376
(1968) (IIb), European Patent Application 307 140 (III)
and J. Pharm. Science 52, 331 (1954) (IV). Starting
com~ounds (R = H) wherein n represents 1 or 2 can be
obtained by reduction of the corresponding alkylesters
(X-R = COOalkyl). Starting from the corresponding
ketones the thiols of general formulae II and III may be
prepared analogously to the method described in J. Chem.
43, 1965.
~2~
When the group R in the compounds of general
formula I is an aromatic or heteroaromatic group, the
compounds of general formula I may be obtained using the
Mitsunobu reaction (O. Mitsunobu in Synthesis, 1, 1981,
Georg Thieme Verlag, Stuttgart; J. C. S. Perkin I, page
462, 1975).
Hence, according to a yet further feature of the
present invention is provided a process for the
preparation of compounds of formula I as defined above
wherein R represents an aromatic or a heterocyclic
group, characterised in that a compound of the formula
~Sy(CH2Jn X--H
A -~ ~C
I
z
wherein A, B, C, n and X are as hereinbefore defined, is
reacted with a compound of formula HOR (in which R
represents an aromatic or heterocyclic group as
hereinbefore defined) in the presence of triphenyl-
phosphine and alkylazodicarboxylate. The reaction is
generally carried out in inert organic solvents at
ambient temperature.
The novel individual enantiomers of formula Id may
be prepared by a separation process, as is now described
for the first time in Example 3, starting from the
corresponding racemic compounds using optically active
salts e.g. (+)- or (-)- tartaric acid, by methods known
E~_ se. This process comprises a further feature of the
present invention. The corresponding racemate may be
prepared by a process analogous to that for the
preparation of the compounds of formula I as
hereinbefore described using appropriate starting
materials.
The novel compounds of general formulae I and Id
2 ~ a
- 15 -
according to the present invention have valuable
pharmacological properties as do the known compounds of
formulae Ia, Ib and Ic. Thus, in bonding studies, the
compounds showed affinities for muscarinic receptors and
muscarin-agonistic GTP ~hifts (GTP = guanosine
triphosphate). (Birdsall, N.I.M., E.C. Hulme and I.M.
Stockton 1984 in T.I.P.S. Supp:Lement, Proc. Internat.
Symposium on Subtypes of Muscarinic Receptors, Ed.
Hirschowitz, Hammer, Giacchetti, Klirns, Levine;
Elsevier p. 4 - 8).
The receptor binding stud:ies were carried out in
accordance with the following literary reference [A.
Closs~, H. Bittiger, D. Langenegger and A. Wahner;
Naunyn-Schmiedeberg's Arch. Pharmacol. 335, 372 - 377
(1987)].
Table A: Receptor bindinq studies
Radioliq~a~: L(~)cis-[2-methyl-3H~-N,N,N-trimethyl-1,3-
dioxolan-4-methanammonium-iodide-NET-647, Messrs. NEN
(New England Nuclear DU PONT).
orqan: Cerebral cortex (rat)
Table A:
Example R* Ki [nMol/l]
1 ~ ~ 120
4 -O-CH2-phenyl 229
9 -O-C6~5 89
As muscarinic agonists (cholinomimetics) the
compounds of formulae I, Id, Ib and Ic are therefore
therapeutically useful for the treatment of diseases
caused by the reduced function of the cholinergic
system.
In the light of the studies on binding to the
^
,
- 16 -
muscarine subtypes M1, M2 and M3 (rat) and ~he
stimulation of the phosphatidyl inositol turnover (CHO
cell cultures with human M1 receptor subtype), ~he
compound (+)-(propargyloxymethyl)-l-aza-
bicyclo[2.2.2]octane was found to be more effective thanthe racemate and the (-)-enantiomer in vitro and this
(+)-enantiomer is thus particularly preferred for use in
therapy. The cholinomimetic in-vivo efficacy o~ (+)-
(propargyloxymethyl)-l-aza-bicyclo-[2.2.2]octane was
demonstrated in the rabbit by EEG (theta wave increase).
Surprisingly the (~)-enantiomer has less than half the
toxicity of the racemate.
In the light of the pharmacological findings the
compounds of general formulae I, Id, Ib and Ic as
defined above are therefore useful for treatment of, for
example, the following diseases: Alzheimer's disease,
senile dementia and cognitive disorders; the compounds
may also be used to improve memory performance.
Quaternary compounds of general formulae I, Id, Ib
and Ic are particularly suitakle ~or peripheral
application, e.g. for treating glaucoma.
According to a yet further feature of the present
invention there are provided pharmaceutical compositions
comprising as active ingredient at least one compound of
formula I or Id, as defined above, or a physiologically
acceptable acid addition salt thereof in association
with one or more pharmaceutically acceptable carriers,
diluents or excipients.
The compounds of general formula I or Id may be
used on their own or in conjunction with other active
substances according to the invention, possibly in
conjunction with other pharmacologically active
substances, e.g. cerebroactivators and/or a peripheral
cholinergic blocker. Suitable preparations include, for
example, tablets, capsules, suppositories, solutionsr
syrups, emulsions or dispersible powders.
Corresponding tablets may be made for example by
.
2 ~
- 17 -
mixing the active substance or substances with known
excipients, e.g. inert diluents such as calcium
carbonate, calcium phosphate or lactose, disinkegrants
such as corn starch or alginic acid, binders such as
starch or gelatine, lubricants such as magnesium
stearate or talc and/or agents for achieving delayed
release such as carboxymethylcellulose, cellulose
acetate phthalate or polyvinylacetate. The tablets may
also consist of several layers.
A yet further feature of the present invention is a
method of treatment of diseases or conditions in a
subject which arise from the reduced function of the
cholinergic system which comprises administering to said
subject an ~ffective amount of a compound of formula I,
Id, Ib or Ic as hereinbefore defined above or a
physiologically acceptable acid addition salt thereof or
a quaternary salt thereof, in particular wherein the
disease or condition is Alzheimers disease, senile
dementia, a cognitive disorder, glaucoma or reduced
memory performance.
Coated tablets may be produced in the same way by
coating cores made analogously to the tablets with
agents conventionally used in tablet coating, such as
collidone or shellac, gum arabic, talc, titanium dioxide
or sugar. In order to achieve delayed release or avoid
incompatibilities the core may also consist of several
layers. Similarly, the tablet coating may be made of
several layers in order to achieve delayed release,
using the excipients mentioned for the tablets above.
Syrups of the active substances or combinations of
active substances according to the invention may
additionally contain a sweetener such as saccharin,
cyclamate, glycerol or sugar and a flavour enhancer,
e.g. a flavouring such as vanillin or orange extract.
They also contain suspension adjuvants or thickeners
such as sodium carboxymethylcellulose, wetting agents,
e.g. condensation products of fatty alcohols with
2~6~
- 18 -
ethylene oxide or preservatives such as p-hydroxy-
benzoates.
Injectable solutions are produced in the usual way,
e.g. by adding preservatives such as p-hydroxybenzoates
or stabilisers such as alkali metal salts of
ethylenediaminetetraacetic acid and trans~erring them
into in~ection vials or ampoules. The capsules
containing one or more active substances or combinations
thereof may be produced for example by mixing the active
substances with inert carriers such as lactose or
sorbitol and encapsulating the mixture in gelatine
capsules.
Suitable suppositories can be produced for example
by mixing with carriers provided for this purpose such
as neutral fats or polyethyleneglycol or derivatives
thereof.
The therapeutically effective single dose is in the
range from 1 to 100 mg.
The following non-limiting Examples serve to
illustrate the present invention: -
~ ~ ~ 2 $ ~ ~!
- 19 -
Examples of Pharmaceutical Formulations
A) Tablets Per tablet
Active substance 80 mg
Lactose 1~0 mg
Corn starch 240 mg
Polyvinylpyrrolidone15 mg
Magnesium stearate__~_~g
480 mg
The finely ground active substance, lactose and
some of the corn starch are mixed together. The mixture
is screened, then moistened with a solution of
polyvinylpyrrolidone in water, kneaded, moist-granulated
and dried. The granules, the remaining corn starch and
magnesium stearate are screened and mixed together. The
mixture is compressed to form tablets of suitable shape
and size.
B) Tablets per tablet
Active substance 60 mg
Corn starch 190 mg
Lactose 55 mg
Microcrystalline cellulose 35 mg
Polyvinylpyrrolidone15 mg
Sodium carboxymethyl starch 23 mg
Magnesium stearate 2 ma
380 mg
The finely ground active substance, some of the
corn starch, lactose, microcrystalline cellulose and
polyvinylpyrrolidone are mixed together, the mixture is
screened and processed with the remainder of the corn
starch and water to form granules which are dried and
screened. The sodium carboxymethyl starch and the
(s ~ ~
- 20 -
magnesium stearate are added thereto and mixed and the
mixture is compressed to form tablets of a suitable
size.
C) Ampoules
Active substance 20 mg
Sodium chloride 10 mg
Twice distilled water q.s. to 1.0 ml
Preparation:
The active substance and sodium chloride are
dissolved in twice distilled water and the solution is
transferred under sterile conditions into ampoules.
D) Drops
Active substance 5.0 g
Methyl p-hydroxybenzoate0.1 g
Propyl p-hydroxybenzoate0.1 g
Demineralised water q.s. to lO0.0 ml
Preparation:
The active substance and preservatives are
dissolved in demineralised water and the solution is
filtered and transferred into 100 ml vials.
- 21 -
Example 1
.
3-Proparqyloxy-l-azabicyclo~2~2~1]heptane
5.6 g (0.05 mol) of 1 azabicyclot2,2,1]heptan-3-ol
are dissolved in 150 ml of absolute tetrahydrofuran
under a nitrogen atmosphere and at 0C 50 ml of lM
boran-THF-complex are added. After it has all been
added the resulting mixture is stirred for one hour at
ambient temperature, evaporated to dryness, the residue
is taken up in saturated saline solution and extracted
with dichloromethane. The combined organic phases are
dried and concentrated by evaporation, ~he residue is
dissolved in 120 ml of absolute THF and 2.08 g
(0.052 mol) of sodium hydride are added in batches under
a nitrogen atmosphere. After one hour the mixture is
cooled to 0C and at this temperature 17.55 g of
propargyl bromide are added dropwise as a 50% solution
in THF. The mixture is stirred for 12 hours at ambient
temperature, then the excess hydride is decomposed with
ethanol, the mixture is evaporated down, the residue is
taken up in saturated saline solution and extracted with
dichloromethane. After drying and evaporation of the
combined organic phases, an oil is obtained which is
distilled under a high vacuum (BP1 ~ar = 45-46C). The
fumarate is prepared with one equivalent of fumaric
acid, recrystallised from ethanol/ether and dried in
vacuo. 2.1 g of colourless crystals of the title
compound are obtained, m.p. 121-123C.
1H-NMR(250 MHz, CD30D, TMS): ~ = 6.68 (2H, s, fumaric
acid); 4.47 (lH, m, H-3); 4.21 (2H, m, CH2-8); 3.73-2.86
(7H, m, CH2-2, 6, 7; H-4); 2.95 (lH, t, J=3Hz, H-9);
2.30; 1.96 (2H, m, CH2-5).
2 ~
- 22 -
Example 2:
3-Phenoxy-l-azabicyclo[2 t 2~2]octane
3.82 g (0.03 mol) of 3-hydroxyquinuclidine, 2.82 g
t0.03 mol) of phenol and 7.96 g (0.03 mol) of
triphenylphosphine and 5.22 g (0.03 mol) of diethylazo-
dicarboxylate are dissolved in 150 ml of absolute THF
and stirred for 2 days at ambient temperature. The
mixture is evaporated to dryness, the residue is taken
up in 20 ml of 6N HCl and 50 ml of H20 and extracted with
ether. The aqueous phase is made alkaline and extracted
with ethyl acetate, the combined ethyl acetate phases
are dried and concentrated by evaporation. After
distillation, 3.6 g of colourless oil are obtained
(Bpo 1 ~ar = 104-105C~
,~0 C6H5
1H-NMR (250 MHz, CDCl3, TMS): ~=7.26; 6.87 (5H, ~,
aryl-H); 4.36 (lH, m, H-3); 3.34-2.63 (6H, m, CH2-2, 6,
7); 2.19-1.27 (5H, m, CH-4); CH2 5, 8).
The base is converted in an ethanolic solution into
the fumarate, which is precipitated with ether and
recrystallised from acetonitrile.
4.1 g of colourless crystals are obtained, m.p.
122-124C.
~ac2~
- 23 -
Example 3:
(+)- and (-)-3-(ProparavloxYmethyl)-1-azabicyclo-
r 2,2,2loctane
+-(3-quinuclidinylmethyl)-acetate
Racemic 3-quinuclidinylmethanol is prepared by
methods known from the literature, e.g. using
cyanohydrin synthesis from quinuclidin-3-one according
to Helv. Chim. Acta 37, 1695 (1954) and acylated with
acetylchloride and triethylamine in chloroform as
solvent at ambient temperature, the racemic (3-
quinuclidinylmethyl)-acetate being obtained in an 82%
yield and with a boiling point of 130 to 134 at
30 mbar.
(-)-3-quinuclidinylmethanol
26.5 g of (3-quinuclidinylmethyl)-+-acetate and
21.7 g of L-(+)-tartaric acid are heated to boiling in
275 ml of 95% ethanol; during the subsequent slow
cooling, crystalline tartaric acid salt is obtained
which is recrystallised from ~5% ethanol about 5 times
until the angle of rotation is constant. The 13.5 g of
salt thus obtained, with a rotational value [~]20=
-16.93 (c = 2, H2O) are stirred in 80 ml of 2N sodium
hydroxide solution for 2 hours at ambient temperature,
then 80 g of potash are added to the solution and the
mixture is worked up extractively with chloroform. In
this way, with simultaneous ester saponification, 5.6 g
of (-)-3-quinuclidinylmethanol are liberated as a light
coloured oil with [~]20= -66 (c = 1, 2, in lN HCl).
Analytical checking of the enantiomeric purity was
carried out after derivatisation with phenylisocyanate
using HPLC on a chiracel-OD column and yielded an
enantiomer distribution of 98.6% (-) : 1O4% (+)-
- 2~ -
enantiomer.
~ 3-quinuclidinylmethanol
The accumulated mother liquors obtained during the
production of (-)-3-quinuclidinylmethanol are evaporated
down in vacuo. The residue is taken up in a little
water, made alkaline with potash and extracted with
chloroform. The resulting 19.5 g of optically enriched
3-quinuclidinylmethyl acetate are converted into the
diastereomeric salt with 16.0 g of D-(+)-tartaric acid
in 200 ml of 95% ethanol. After 5 recrystallisations
from 95% ethanol, 12.7 g of salt are obtained, with a
rotary value [~]2~ = 16.8 (c = 2, H20), from which 4.7 g
of (+)-3-quinuclidinylmethanol are liberated as a light
coloured oil with [~]20= +66.5 (c = 1, in lN HCl),
analogously to the (-)-enantiomer, using 75 ml of 2N
sodium hydroxide solution.
After derivatisation with phenylisocyanate, the
enantiomeric purity was determined by HPLC on a
chiracel-OD column with 97.4% (+)- : 2.6% (-)-
enantiomer.
3a: (+)-3-(propargyloxymethyl)-l-azabicyclo[2~2~2]
octane
5.6 g of (+)-3-quinuclidinylmethanolhydrochloride,
1.32 g of sodium borohydride and 100 ml of
tetrahydrofuran are stirred overnight. The solvent is
removed from the filtrate, the residue is taken up in
ethyl acetate and the resulting solution is washed with
saturated saline solution. After drying and removal of
the solvent, 3.4 g of (+)-3-quinuclidinylmethanol-boran
complex remain in the form of a light coloured oil.
3.4 g of the boran complex are stirred in 100 ml of
tetrahydrofuran for 30 minutes with 2.63 g of 60% sodium
hydride at an)bient temperature, then reacted with 4.89 g
of 80% propargyl bromide and stirred for a further 6
~12~
- 25 -
hours. The reaction mixture is carefully decomposed
with alcohol, the solvent is eliminatecl, the residue is
taken up in 150 ml of ethylacetate, the solution is
washed with saturated saline solution and the solvent is
removed once more. In order to destroy the boran
protecting group the residue is taken up in 50 ml of
acetone and stirred overnight with 20 ml of 3N HCl.
After the acetone has evaporated off the aqueous phase
is washed with ethylacetate, made alkaline with potash
and extracted with ethylacetate. The extraction residue
is flash-chromatographed on silica gel using
ethylacetate:methanol:NH3 = 85:15:1 as eluant and yields
4.3 g of propargylether, which is converted in alcohol,
with the calculated quantity of fumaric acid, into the
fumaric salt which is re-precipitated from alcohol-ether
and is obtained in a 3.9 g yield with a melting point of
132-133C and [~]20= + 28.47 (c = 1, methanol).
3b: (-)-3-(Propargyloxymethyl)-1-azabicyclo[2,2,2]octane
Analogously to 3a, the boran protecting group is
introduced into (-)-3-quinuclidinylmethanol,
etherification is carried out with propargyl bromide and
after the boran protecting group has been split off the
free base is converted into the fumarate, m.p. 132-133C
and [~]Z= -28.42 (c = 1, methanol).
.
,
6~,~3 I'~f. 2~
The following compounds of general formula I may be
prepared by analogous methods of synthesis to those
described in the Examples above.
TABLE I R
Compounds of formula ~ ~
Example R* M.P. C
I - 0~-2HC1 236 tdecomp.)
N
-O~N 164 - 16
Fumarate
3 -0 ~ 160 - 161
N Fumarate
4 -0-CH2 ~ 135-136
Fumarate
4a (+)Enantiomer -0-CH2 ~ ~ 27,5
tC = 1, H20)
.
- 27 -
Example R* M.~. oc
4b (-)Enantiomer [a]D =
- 25.6
--O-CH2~g) (C= 1, H20)
- O-CH ~CI lo (Decomp.)
6 - 0-cH2 ~ 110-112
Fumarate
7 --C~2 ~ 112-114
Fumarate
8 ~=~ 108-110
-0-CH2 ~ S Fumarate
9 ~ 122-124
- 0 ~ Fumarate
2 ~
- 28 -
Example R* M.P. C
/~\
9a (+)-Enantiomer~ -O ~ + 22.2
(c = 2, H20)
9b (-)-Enantiomer~ -O ~ [~]D =
- 22.9 (c = 2,
H20 )
/==~ 147 - 149
~ 3 Fumarate
lOa - o ~ CH2 -CH ~CH3) 2
/~ /=\
lOb -O ~ \\ ~ )
10C - --/~{>
~ ~ ~ C~3 126 - 129
Fumarate
The reaction takes place with inversion.
' :
.
,
2 o ~ ~
- 29 -
Exam~le R* M.~. C
lla ,, CH3 (+) -Enantiomer
-O~CH~
llb_O ~ ~ (-)-Enantiomer
.
12 ~ 125 - 126
C~ Fumarate
Cl
13- O ~ 147 - 149
Cl Fumarate
14 0 ~ 128-131
~ Fumarate
OCH3
5 -O~)
OCH3
16 -S~3
.' ' ~ .
. . .
. .
.
-- 30 --
Example R* M. p. C
.
17 -O~\S
CH3
8 -o~3
N 197-198
o ~ 3 Fumarate
N
N~
--0~/ ~CH3
N
~N
-S~ ~
N
22 /~N
--O--CH2--N~
23 . N
C~2~_~
CH3
24 C~2~N3
CH3
--O ¢~,N
N
CH3
, ~ ' ' ' : .:
. , . . ~: . .
- ,
.. , . `
'- :` .' '
- 3 1 ~
Example R* M . p . C
-
26 0-CH2 ~0
27 --O--CH2~
H I
CH3
CH3
28 --O--CH2~
Lo
29 --O--CH2 ~\o
~ 0--CH
N
31----CH2~'N ~N
CH3
32-CH:2-- ~3 117 - 18
33 -CH2--Y/ =~ 189 - 191
Fumarate
- ' , - , -
:
- . . .
2~6~
-- 32 --
Example R* a~
33a -CH2-O ~7 158 - 160
Fumarate
.
2 l3 ~
TABLE II
Compounds of formula ~ R
Example R* M.p. C
34 121-127
- O - CH2 - C - CH Fumarate
-0-cH2-cH-cH2
N
36 -O ~
37 ~O ~ 114 - 116
Oxalate
38 - 0 - CH2 ~
N=\
39 -0 ~\ ~
- S ~ \)
',~ S~
TABLE III
Compounds of formula ( ~J
Example R*
41 endo -O-CH2- C--CH 121-124
Oxal ate
exo -O-CH2- C--CH
42 Oil
Oxalate
43 - O -CH2- C-CH 93~95
2 oxalate
44 -0
~0 -CH2 -~
46 -0~3
N
47 ~~\S
CH3
N~
48 -o4N ~)
,
.
- 35 -
TABLE IV
Compounds o~ formula ~ ) R~
Example R* M.p. C
49 endo -O-CH2- C-CH 148-149
Fumarate
49a 147 - 148
exo -O-CH2- C-CH Fumarate
99-101
exo -o-cH2-cH=cH2 Fumarate
50a endo -o-cH2-cH=cH2 113-114
Fumarate
51 exo -0-CH2-CH3 147 148
Fumarate
51a endo 0-CH2-CH3 115-117
Fumarate
52 -0-C6Hs
53
-O-CH2- C6Hs
J ~
-- 36 --
ExampleR* M . p . C
N=\
5~ -0~\ ~
-o~3
N
,
-- 37 --
TABLE V ,
Compounds o~ formula ( ~) R~
~N
Exp. R* M.p. C
56 -0-CH2-GH= CH~ :
57 - O - CH2-C-CH
58 ~ O- C6H5
59 -0
N
- O ~\ ~
61 - O-C~- C6H5
62 0-CH2-cH3