Note: Descriptions are shown in the official language in which they were submitted.
20429'9
- 1 - C3371
FABRIC TREATMEZJT COMPOSITIONS
The present invention relates to fabric treatment
compositions in aqueous medium and containing a relatively
high proportion of fabric conditioner. In particular, the
present invention relates to fabric treatment compositions
which comprise as conditioners one or more
fabric-softening materials and one or more emulsion
forming components to result in a structure of a
dispersion of an emulsion and a dispersion of lamellar
droplets in a continuous aqueous phase.
Lamellar droplets are a particular class of
surfactant structures which, inter alia, are already known
from a variety of references, e.g. H.A.Barnes,
'Detergents', Ch.2. in K.Walters (Ed), 'Rheometry:
Industrial Applications', J.Wiley & Sons, Letchworth 1980.
Lamellar fabric-softening compositions are for
example known from EP 303 473 (Albright and Wilson). This
patent application describes fabric-softening compositions
comprising an aqueous base, a cationic fabric softener
2~4~9~9
- 2 - C3371
having two long alkyl or alkenyl groups and dissolved
electrolyte to form an optically anisotropic spherulitic
composition.
The presence of lamellar droplets in a
fabric-softening product may be detected my means known to
those skilled in the art, for example optical techniques,
various rheometrical measurements, X-ray or neutron
diffraction, and electron microscopy.
The droplets consist of an onion-like configuration
of concentric bi-layers of molecules of fabric-softening
material, between which is trapped water or electrolyte
solution (aqueous phase). Systems in which such droplets
are nearly or fully close-packed provide a very desirable
combination of physical stability and useful flow
properties.
It is desirable to add other components to fabric
softening compositions in the form of emulsions in the
aqueous phase, to provide added benefits such as crease
reduction and ease of ironing as well as improving
softening. For example hydrocarbons such as mineral oils
which give added softening and lubricating effects when
applied to textile fibres and fabrics, perfume emulsions
and solutions of perfumes in carrier emulsions.
In the past it has been recognised that certain
stability problems arise when hydrocarbons are added to a
dispersion of fabric softening material. For example in
GB 1 601 360 (Procter and Gamble Co/Goffinet) certain
textile treatment compositions are disclosed comprising a
water insoluble cationic fabric softener, a hydrocarbon
and a relatively high proportion of a cationic surfactant
which is water soluble. It is believed that such water
2042979
- 3 - C3371
soluble surfactants are not lamellar phase forming and are
present to solubilise the hydrocarbon. Such compositions
can still suffer from high viscosity. In EP 13 780
(Procter and Gamble/Verbruggen) low levels of non-cyclic
hydrocarbons of fatty acids are suggested as viscosity
control aids in compositions comprising up to 20% of
certain imidazolinium salts. There is no disclosure of
how to incorporate higher levels of the hydrocarbon or
fatty acid without encountering viscosity problems.
It is believed that the presence of the lamellar
dispersion of fabric softening material can flocculate the
emulsion component by a mechanism of depletion. This
phenomenon is well known in mixed disperse systems and in
systems containing either a structured surfactant phase or
a non-adsorbed polymer for example D.Fairhurst, M.Aronson,
M.Gun and E.Goddard Colloids Surf. 1983, 7, 153. This
depletion flocculation leads to increases in the viscosity
of the fabric treatment composition due to reduction of
the inter-particle spacings.
There are two main factors determining the viscosity
and stability of the fabric softening composition, the
combined volume fraction of the dispersed lamellar phase
and the emulsion and their state of aggregation.
Generally speaking, the higher the volume fraction of the
dispersed lamellar phase (droplets) and emulsion phase
(particles), the higher the viscosity which in the limit
can result in an unpourable or gelled product. When the
volume fraction is around 0.6, or higher, the droplets are
just touching (space-filling). This allows reasonable
stability with an acceptable viscosity (say no more than
2.5 Pas, preferably no more than 1 Pas most preferably no
more than 0.5Pas at a shear rate of 21s 1). However,
flocculation of the particles can also occur. As
2~429~~
- 4 - C3371
previously explained, the lamellar dispersion can cause
depletion flocculation of the emulsion component.
Flocculation of either the lamellar dispersion or the
emulsion can lead to instability because reduction of the
inter-particle inter-droplet spacings will make their
packing more efficient. Consequently, more lamellar
droplets or emulsion will be required for stabilisation
which will again lead to a further increase of the
viscosity.
The volume fraction of the droplets is increased by
increasing the softener concentration, and may be reduced
by increasing the electrolyte level. However, the
stability of the emulsion component is very sensitive to
electrolyte levels. When electrolyte is added to an
emulsion it reduces the effects of depletion but the
levels required to prevent depletion are sufficient to
cause flocculation of the emulsion by an electrostatic
mechanism and thus the problem is not solved.
Thus, in practice, there are limits to the amounts of
fabric softening material, emulsion component and
optionally electrolyte which can be incorporated whilst
still having an acceptable product. In principle, higher
levels of fabric softening materials are desired for
convenience and for reduction of costs, the presence of
emulsion components are desired for providing added
benefits such as fabric lubrication and perfume delivery
and certain levels of electrolyte are desired to give, in
certain circumstances, better delivery and anionic
carry-over protection.
We have now found that the dependency of stability
and/or viscosity upon the volume fraction of softening
material and the volume fraction of the emulsion component
2~42~~~
- 5 - C3371
can be favourably influenced by incorporating into the
compositions a deflocculating polymer comprising a
hydrophilic backbone and one or more hydrophobic side
chains.
Accordingly, the present invention relates to a
fabric treatment composition comprising an aqueous base,
one or more fabric-softening materials, and an emulsion
component, said composition having a structure of lamellar
droplets of the fabric-softening material in combination
with an emulsion, said composition also comprising a
deflocculating polymer comprising a hydrophilic backbone
and one or more hydrophobic side chains.
The deflocculating polymer allows, if desired, the
incorporation of greater amounts of softening materials
and/or emulsion components than would otherwise be
compatible with the need for a stable, easily dispersable
product of acceptable viscosity. It also allows (if
desired) incorporation of greater amounts of certain other
ingredients to which lamellar dispersions and emulsions
have been highly stability-sensitive.
The present invention allows formulation of stable,
pourable products wherein the volume fraction of the
lamellar droplets and the emulsion is 0.5 or higher.
The volume fraction of the lamellar droplet phase and
emulsion component may be determined by the following
method. The composition is centrifuged, say at 40,000 G
for 12 hours, to separate the composition into a clear
(continuous aqueous) layer, a turbid active-rich
(lamellar/emulsion) layer and (if solids or liquids are
suspended) a third layer. The conductivity of the
continuous aqueous phase, the lamellar phase and of the
i
~~4~~~~
- 6 - C3371
total composition before centrifugation are measured.
From these, the volume fraction of the lamellar phase and
emulsion component is calculated or estimated, using the
Bruggeman equation, as disclosed in American Physics, 24,
636 (1935). The volume fraction of the emulsion component
can be calculated if desired, provided the density is
known and the volume fraction of the lamellar phase
calculated.
Preferably, the viscosity of the aqueous continuous
phase is less than 25mPas, most preferably less than
lSmPas, especially less than lOmPas, these viscosities
being measured using a capillary viscometer, for example
an Ostwald viscometer.
In practical terms, i.e. as determining product
properties, the term 'deflocculating' in respect of the
polymer means that the equivalent composition, minus the
polymer, has a significantly higher viscosity and/or
becomes unstable. It is not intended to embrace the use
of polymers which would increase the viscosity but not
enhance the stability of the composition. It is also not
intended to embrace polymers which would lower the
viscosity simply by a dilution effect, i.e. only by adding
to the volume of the continuous phase. Although within
the ambit of the present invention, relatively high levels
of the deflocculating polymers can be used in those
systems where a viscosity reduction is brought about;
typically levels as low as from about 0.01 by weight to
about 5.0~ by weight can be capable of reducing the
viscosity at 21 s 1 by up to 2 orders of magnitude.
Especially preferred embodiments of the present
invention exhibit less phase separation on storage and
n 1
f
2~429~"~
- 7 - C3371
have a lower viscosity than an equivalent composition
without any of the deflocculating polymer.
In the context of the present invention, stability
for these systems can be defined in terms of the maximum
separation compatible with most manufacturing and retail
requirements. That is, the 'stable' compositions will
yield no more than 2~ by volume phase separation as
evidenced by appearance of 2 or more separate phases when
stored at 25°C for 21 days from the time of preparation.
In the case of the compositions where the combined
lamellar/emulsion phase volume fraction is 0.5 or greater,
it is not always easy to apply this definition. In the
case of the present invention, such systems may be stable
or unstable, according to whether or not the droplets or
particles are flocculated. For those that are unstable,
i.e. flocculated, the degree of phase separation may be
relatively small, e.g. as for the unstable non-flocculated
systems with the lower volume fraction. However, in this
case the phase separation will often not manifest itself
by the appearance of a distinct layer of continuous phase
but will appear distributed as 'cracks' throughout the
product. The onset of these cracks appearing and the
volume of the material they contain are almost impossible
to measure to a very high degree of accuracy. However,
those skilled in the art will be able to ascertain
instability because the presence of a distributed separate
phase greater than 2~ by volume of the total composition
will readily be visually identifiable by such persons.
Thus, in formal terms, the above-mentioned definition of
'stable' is also applicable in these situations, but
disregarding the requirement for the phase separation to
appear as separate layers.
CA 02042979 2000-08-08
- 8 - C3371
Especially preferred embodiments of the present
invention yield less than 0.1% by volume visible phase
separation after storage at 25°C for 21 days from the time
of preparation.
However, it is usually possible to obtain a figure
which, whilst approximate, is still sufficient to indicate
the effect of the deflocculating polymer in the
compositions according to the present invention. Where
this difficulty arises in the compositions exemplified
hereinbelow, it is indicated accordingly.
The compositions according to the invention may
contain only one, or a mixture of deflocculating polymer
types. The term 'polymer types' is used because, in
practice, nearly all polymer samples will have a spectrum
of structures and molecular weights and often impurities.
Thus, any structure of deflocculation polymers described
in this specification refers to polymers which are
believed to be effective for deflocculation purposes as
defined hereabove. In practice these effective polymers
may constitute only part of the polymer sample, provided
that the amount of deflocculation polymer in total is
sufficient to effect the desired deflocculation effects.
Furthermore, any structure described herein for an
individual polymer type, refers to the structure of the
predominating deflocculating polymer species and the
molecular weight specified is the weight average molecular
weight of the deflocculation polymers.
Suitable deflocculating polymer types for use in
compositions of the invention are for instance described
in Canadian Patent No. 1,336,385 and in PCT publications WO 91/06622 and
WO 91 /06623.
CA 02042979 2000-08-08
- 9 - C3371
A preferred class of polymers are biodegradeable
polymers having a hydrophilic backbone and at least one
hydrophobic side chain. The basic structure of polymers
having a hydrophilic backbone and one or more hydrophobic
side chains is described in Canadian Patent No. 1,336,385.
The hydrophilic backbone of the polymer generally is
a linear, branched or cross-linked molecular composition
containing one or more types of relatively hydrophilic
monomer units, possibly in combination with minor amounts
of relatively hydrophobic units. The only limitations to
the structure of the hydrophilic backbone are that the
polymer must be suitable for incorporation in an
active-structured aqueous liquid softener composition and
the hydrophilic backbone is relatively soluble in water in
that the solubility in water of 20°C at a pH of 7.0 is
preferably more than 1 g/1, more preferably more than
g/1, most preferably more than 10 g/1.
Preferably the hydrophilic backbone is predominantly
linear in that the main chain of the backbone constitutes
at least 50% by weight, preferably more than 75%, most
preferably more than 90% by weight of the backbone.
The hydrophilic backbone is constituted by monomer
units, which can be selected from a variety of units
available for the preparation of polymers. The polymers
can be linked by any possible chemical link, although the
following types of linkages are preferred:
O Q
-O-, -C-O, -C-C-, -C-, -C-N-, -N-
~U4~J'~9
- 10 - C3371
Water-soluble monomers suitably employed to form the
hydrophilic backbone are for example those which are
sufficiently water-soluble to form at least a one weight
percent solution when dissolved in water and readily
undergo polymerisation to form polymers which are
water-soluble at ambient temperature and at a pH of 3.0 to
12.5, preferably more than 1 gram per litre, more
preferably more than 5 grams per litre, most preferably
more than 10 grams per litre. Exemplary water-soluble
monomers include ethylenically unsaturated amides such as
acrylamide, methacrylamide and fumaramide and their
N-substituted derivatives such as 2-acrylamido-2-
methylpropane sulphonic acid, N-(dimethylaminomethyl)
acrylamide as well as N-(trimethylammoniummethyl)
acrylamide chloride and N-(trimethylammoniumpropyl)
methacrylamide chloride; ethylenically unsaturated
carboxylic acids or dicarboxylic acids such as acrylic
acid, malefic acid, methacrylic acid, itaconic acid,
fumaric acid, crotonic acid, aconitic acid and citroconic
acid; and other ethylenically unsaturated quaternary
ammonium compounds such as vinylbenzyl trimethyl ammonium
chloride; sulphoalkyl esters of unsaturated carboxylic
acids such as 2 sulphoethyl methacrylate; aminoalkyl
esters of unsaturated carboxylic acids such as
2-aminoethyl methacrylate, dimethyl aminoethyl
(meth)acrylate, diethyl aminoethyl (meth)acrylate,
dimethyl aminomethyl (meth)acrylate, diethyl aminomethyl
(meth)acrylate), and their quaternary ammonium salts;
vinyl or allyl amines such as vinyl pyridine and vinyl
morpholine or allylamine; dially amines and diallyl
ammonium compounds such as diallyl methyl ammonium
chloride; vinyl heterocyclic amides such as vinyl
pyrrolidone; vinyl aryl sulphonates such as vinylbenzyl
sulphonate; vinyl alcohol obtained by the hydrolysis of
vinyl acetate; acrolein; allyl alcohol; vinyl acetic acid;
:. ~ 20429'~~
- 11 - C3371
sodium vinyl sulphonate; sodium ally sulphonate, as well
as the salts of the foregoing monomers. These monomers
may be used singly or as mixtures thereof.
Optionally, the hydrophilic backbone may contain
small amounts of relatively hydrophobic units, e.g. those
derived from polymers having a solubility of less than
1 g/1 in water, provided that the overall solubility of
the hydrophilic polymer backbone still satisfies the
solubility requirements as specified here above. Examples
( of relatively water-insoluble polymers are polyvinyl
acetate, polymethyl methacrylate, polyethyl acrylate,
polyethylene, polypropylene, polystyrene, polybutylene
oxide, polypropylene oxide, polyhydroxypropyl acrylate.
Suitable hydrophobic monomers for forming the side
chains generally include those which are (1)
water-insoluble, i.e. less than 0.2 weight part of the
hydrophobic monomer will dissolve in 100 weight parts
water and (2) ethylenically unsaturated compounds having
hydrophobic moieties. The hydrophobic moieties (when
isolated from their polymerisable linkage) are relatively
water-insoluble, preferably less than 1 g/1, more
preferably less than 0.5 g/1, most preferably less than
0.1 g/1 at ambient temperature and a pH of 3.0 to 12.5.
The hydrophobic moieties preferably have at least 3
carbon atoms and are most preferably pendant organic
groups having hydrophobicities comparable to one of the
following:~aliphatic hydrogen groups having at least
three carbons such as C3 to C50 alkyls and cycloalkyls;
polynuclear aromatic hydrocarbon groups such as napthyls;
alkylaryls wherein the alkyl groups has one or more
carbons; haloalkyls of 3 or more carbons, preferably
perfluoroalkyls; polyalkyleneoxy groups wherein alkylene
2p429~~
- 12 -
is propylene or high alkylene and there is at least one
alkyleneoxy unit per hydrophobic moiety; and siloxane
moieties. Exemplaryhydrophobic monomers include butyl
acrylate, isobutyl acrylate, 2-ethylhexyl acrylate and
the corresponding methacrylates, the higher alkyl esters
of alpha, beta-ethylenically unsaturated carboxylic acids
such as dodecyl acrylate, dodecyl methacrylate, tridecyl
acrylate, tridecyl, methacrylate, tetradeculacrylate,
octadecyl acrylate, octadecyl methacrylate, octyl half
ester of malefic anhydride, doictyl diethyl maleate, and
other alkyl esters and half esters derived from the
reactions of alkanols having from 3 to 50 carbon atoms
with ethylenically unsaturated carboxylic acids such as
acrylic acid, methacrylic acid, malefic anhydride, fumaric
acid, itaconic acid and aconitic acid; alkylaryl esters
of ethylenically unsaturated carboxylic acid such as
nonyl- -phenyl acrylate, nonyl- -phenyl methacrylate,
dodecyl- -phenyl acrylate and dodecyl- -phenyl
methacrylate; N-alkyl, ethylenically unsaturated amides
such as N-octadecyl acrylamide; N-octadecyl
methacrylamide, N,N-dioctyl acrylamide and similar
derivatives thereof, -olefins such as octene-1, decene-1,
dodecene-1 and hexadecene-1; vinyl alkylates wherein
alkyl has at least 4 carbon atoms such as vinyl laurate
and vinyl stearate; vinyl alkyl ethers such as dodecyl
vinyl ether and hexadecyl vinyl ether; N-vinyl amides
such as N-vinyl lauramide and N-vinyl stearamide; and
alkylstyrenes such as t-butyl styrene. The hydrophobic
monomer may be used singly or mixtures thereof may be
employed. The ratio of hydrophilic to hydrophobic
monomers may vary from about 500:1 to 5:1. The weight
average molecular weights (Mw.) of the resultant polymers
vary from 500 to 500,000 or above when measured by gel
permeation chromatography using a polyacrylate standard,
or by specific viscosity (SV) measurements using a
polyacrylate standard.
v 2~4297~
- 13 - C3371
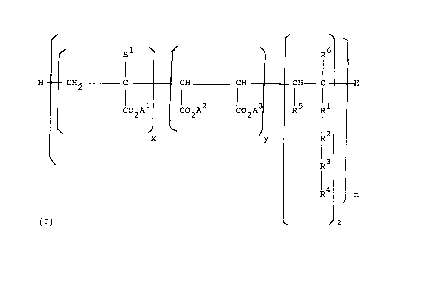
Products of the invention preferably comprise
polymers of the general formula:
B1 R6
H CH2 C CH CH CH--- C
1 ~ 2 ~ 15 I1
C02A C02A C02A R R
i2
x y R
1 3
R
~4
R n
(z) ~ J z
wherein z is 1; (x+y):z is from 4:1 to 1,000:1; preferably
from 6:1 to 250:1 in which the monomer units may be in
random order; y being from 0 up to a maximum equal to the
value of x; and n is at least 1;
R1 represents -CO-O, -O-, -O-CO-, -CH2-, -CO-NH- OR
is absent;
R2 represents from 1 to 50 independently selected
alkyleneoxy groups, preferably ethylene oxide or propylene
oxide groups, or is absent, provided that when R3 is
absent and R4 represents hydrogen, then R2 must contain an
alkyleneoxy group with at least 3 carbon atoms;
R3 represents a phenylene linkage, or is absent;
R4 represents hydrogen or a 01_24 alkyl or C2_24
alkenyl group, with the provisos that:
20429°~~
- 14 - C3371
a) when R2 is absent, R4 is not hydrogen and when R3
is also absent then R4 must contain at least 5 carbon
atoms;
R5 represents hydrogen or a group of formula -COOA4;
R6 represents hydrogen or C1-4 alkyl; and
A1, A2, A3 and A4 are independently selected from
hydrogen, alkali metals, alkaline earth metals, ammonium
and amine bases and C1-4, or (C2H40)tH wherein t is from
1-50, and wherein the monomer units may be in random
order.
Each B1 is independently selected from -CH20H, -OH or
-H.
Another class of polymers in accordance with the
present invention comprises those of formula II:
R8 R7
1 2
H CH2 C CH2 Q Q H
I10 9 ~ r v
q P
(II) n
wherein:
Q2 is a molecular entity of formula IIa:
~, 20429'9
- 15 - C3371
R6
CH2 CH H CH CH C
1 2 ~ 3 15 I1
C02A x C02A C02A ~ R R
'2
R
1 3
R
14
R
z
(IIa) '
wherein z and R1 6 are as defined for formula (I);
Al 4 are as defined for formula (I);
Q1 is a multifunctional monomer, allowing the
branching of the polymer, wherein the monomers of the
polymer may be connected to Q1 in any direction, in any
order, therewith possibly resulting in a branched polymer.
Preferably Q1 is trimethyl propane triacrylate (TMPTA),
methylene bisacrylamide or divinyl glycol;
n and z are as defined above; v is 1; and (x + y + p
+ q + r):z is from 5:1 to 500:1; in which the monomer
units may be in random order; and preferably either p and
q are zero, or r is zero;
R7 and R8 represent -CH3 or -H;
R9 and R10 represent substituent groups such as
amino, amine, amide, sulphonate, sulphate, phosphonate,
phosphate, hydroxyl, carboxyl and oxide groups, or
~~429'~9
- 16 - C3371
(C2H40)tH, wherein t is from 1-50, and wherein the monomer
units may be in random order. Preferably they are
selected from -S03Na, -CO-O-C2H4, -OS03Na,
-CO-NH-C(CH3)2-CH2-S03Na, -CO-NH2, -O-CO-CH3, -OH. In any
particular sample of polymer material in which polymers of
formulae I and II are in the form of a salt, usually some
polymers will be full salts (A1-Ay4 all other than
hydrogen), some will be full acids (A1-A4 all hydrogen)
and some will be part-salts (one or more A1-A4 hydrogen
and one or more other than hydrogen).
The salts of the polymers of formulae I and II may be
formed with any organic or inorganic cation defined for
A1-A4 and which is capable of forming a water-soluble salt
with a low molecular weight carboxylic acid. Preferred
are the alkali metal salts, especially of sodium or
potassium.
Another class of polymers in accordance with the
present invention comprises those of formula III:
20429'~~
- 17 - C3371
R6 R6
3 4
H ' Q ~ CH2 C CH2 C Q H
I II
~a d
R1 R1
(CH2)w ~ (CH2)w
11 It 11
R N --R N
11 ~ 1
R11 B1 R R
b c n
(III)
wherein Q3 is derived from a monomeric unit IIIa
comprising:
R11
11*
CH2 CH2 R
N+ B2
11*
CH2 C CH2 R
~11
R
(IIIa)
Q4 is derived from the molecular entity IIIb:
2~D42~'~9
- 18 - C3371
R6 R6 R6
..H- C Q CH2 C CH2 C
.5 f
R1 R1 R1
'2
R (CH2)w ~ (CH2)w
~+
R3 R12 - - R1 ~ N
i~
14 12 3 12 ~ 1
R R B ~ R R
a g h
(IIIb)
and Q5 is derived from a monomeric unit IIIc:
R11
CH2 C CH2 R12
N+ B4
12
CH2 C CH2 R
'll
R
(IIIc)
R1-R6 are defined as in formula I;
' '
- 19 - C3371
(a + b + c): Q4 is from 5:1 to 500:1, in which the
monomer units may be in random order, a, b, c, d, e, f, g,
h may be an integer or zero, n is at least 1;
B1, B2, B3, B4 are organic or inorganic anions;
w is zero to 4;
R11 is independently selected from hydrogen or C1-C4
alkyl; and
R12 is independently selected from C5 to C24 alkyl or
alkenyl, aryl cycloalkyl, hydroxyalkyl or alkoxyalkyl.
The anions represented by B1, B2, B3, B4 are
exemplified by the halide ions, sulphate, sulphonate,
phosphate, hydroxide, borate, cyanide, carbonate,
bicarbonate, thiocyanate, sulphide, cyanate, acetate and
the other common inorganic and organic ions. Preferred
anions are chloride and methosulphate.
Another class of polymers in accordance with the
present invention comprise those of formula IV
2~14297~
- 20 - C3371
R6 RE
H CH2 C CH c H
j
R1 R5 RI
13 2
R R
14 1 3
R R
15 4
R
IV
where R1-R6 are defined as in formula I, z is 1 and j:z is
from 5:1 to 500:1, in which the monomer units may be in
random order, and n is at least 1;
R13 represents -CH2-, -C2H4-, -C3H6- or is absent.
R14 represents from 1 to 50 independently selected
alkyleneoxy groups, preferably ethylene oxide groups, or
is absent.
R15 represents -OH or hydrogen.
Other preferred polymers are hydrophobically modified
polysaccharides. Possible sugar units for use in those
polymers include glucosides and fructosides for example
maltoses, fructoses, lactoses, glucoses and galactoses.
Also mixtures of sugar groups may be used. The sugar
groups may be connected to each other via any suitable
linkage, although 1-4 linkages and/or 1-6 linkages are
2~429'~~
- 21 - C3371
preferred. The polysaccharides are preferably
predominantly linear, but also branched polymers may be
used. An example of a preferred polysaccharide has the
following formula:
CH2R7
H O
7'
R CH CH O CH2
CH ----CH CH O
R R CH CH O CH2
R CH CH CH O
~7 17 ~ 7'
R R v CH CH R
7~
R CH CH
7' ~y I
R i
~w
Wherein:
Each R7 is R7 or -R1-R2-R3-R4;
R7 is independently selected from -OH, -NH-CO-CH3, -S03A1,
-OS03A1, -NHS03A1, -COOA1; R7 is preferably -OH
n is the total number of -R1-R2-R3-R4 groups per molecule;
n is at least 1;
~p429'~9
- 22 - C3371
m is the total number of R7 and R7 groups that are not
_R1-R2-R3-R4
i
the ratio m:n is from 12:1 to 3,000:1, preferably from
18:1 to 750:1; wherein the monomer units may be in random
order. v and w are determined by the molecular weight of
the polymer.
It is believed that on the basis of this formula, the
skilled person will be able to derive similar formulas for
other polysaccharide polymers for use in compositions of
the invention.
R1 is as defined above for formula I, or can be -NHCO;
-OCH2CONH; or -O-CH2-CO-O-;
R2 4 are as defined for formula I;
A1 is as defined for formula I.
Other preferred polymers are of the formula:
2fl4~9'~9
- 23 - C3371
R6
s
s
H CH2 CH CH2 CH C H
CH
~ y
~ ~
I 8 i 9 1 5 I
R i; ~ R R1
R
~
I
OH ~ OS ~ R2
I
~
f
x
y
R3
i
I R4
t
(,VI z
) n
Wherein:
z z
and is
n from
are 4:1
as
defined
for
formula
I;
(x+y):
to preferably
1,000:1,
preferably
from
6:1
to
250:1;
y
being value
from of
zero x;
up
to
a
maximum
equal
to
the
wherein rder.
the
monomer
units
may
be
in
random
o
R1
6
are
as
defined
for
formula
I;
R$
and
R9
represent
-CH2-
or
are
absent;
S OOA1,
is
selected
fro
-CO(CH2i2CO0A1,
-CO(
H)
C
i
i
2
-COCH2C(OH)
(CODA
)CH
COOA
,
-COCH
COOA
,
2 CH(CH
2 )COOA1
-CO(CH(OH))2COOA1,
-COCH2CH(OH)COOA1,
-COCH
2 3
and
-COCH2C(=CH2)COOA1;
A1
is
as
defined
for
formula
I;
Other
preferred
polymers
as
of
the
formula:
20429'9
- 24 - C3371
4
D A B -~-- D
i
I
VII ~ ~n
Wherein:
D is -H or -OH; n is at least 1;
(Q1 (Q2 ~
A is O----CH-----CH or O CH H
1 ~ 1 ~ 2
CODA COOA COOA CO
OR
Wherein:
Each A2 is A1 or R10~
Q1:Q2 is from 4:1 to 1,000:1, preferably from 6:1 to
250:1;
R10 represents a C5-24 alk(en)yl group;
B is ---~O CO-~tll-~CO
R11 represents -CH2-, -C2H4-, C3H6-, or an aryl link said
aryl link optionally being substituted with one or more
-COOA1 groups, or a benzophenone link;
A1 is as defined in formula I.
For the polymers of formula I, II and IV and their
salts, it is preferred to have a weigth average molecular
weight in the region of from 500 to 500,000, preferably
CA 02042979 2000-08-08
- 25 - C3371
from 1000 to 200,000 more preferably from 1500 to 50,000,
even more preferably from 3,000 to 6,000 when measured by
GPC using polyacrylate standards. For the purposes of
this definition, the molecular weights of the standards
are measured by the absolute intrinsic viscosity method
described by Noda, Tsoge and Nagasawa in Journal of
Physical Chemistry, Volume 74, (1970), pages 710-719.
It is difficult to determine accurately the molecular
weight distribution of polymers of Formula III, because of
( the highly cationic nature of these polymers and
subsequently adsorption on the GPC columns. Instead, a
measure of molecular weight can be made by measuring a
standard viscosity (S.V.), determined at 15.0% solids,
23°C in a 1.0 molar sodium chloride solution using a
Brookfield Synchro-lectric(R) viscometer, Model LVT with
an LCP adaptor, at a speed of 60 RPM. It is preferred to
have a polymer with an S.V. from 1 to 100 mPas, more
preferably from 2-50 mPas, most preferably 3-25 mPas.
Polymers according to formulas V-VII preferably have
a molecular weight of 500-250,000, more preferably from
2,000 to 50,000, even more preferably from 3,000 to 6,000.
Generally, the deflocculating polymer will be used at
from 0.01% to 5.0% by weight in the composition,
preferably from 0.02 to 2.0%, most preferably from 0.03 to
1%.
Compositions of the present invention preferably
comprise from 1 to 80%, by weight of fabric-softening
- 26 - C3371
materials, more preferably from 2 to 70o by weight, most
preferably from 5 to 50°s by weight of the composition.
The fabric softening materials may be selected from
cationic, nonionic, amphoteric or anionic fabric softening
material.
Suitable amphoteric fabric-conditioning materials for
use in a composition according to the invention are
fabric-substantive amphoteric materials forming a
particulate dispersion at a concentration of less than
1 g/1 at at least one temperture between 0 and 100°C,
preferably at least one temperature between 10 and 90°C,
more preferably between 20 and 80°C. For the purpose of
this invention a fabric-substantive amphoteric material is
preferably an amphoteric or zwitterionic tertiary or
quaternary ammonium compound having either one single long
hydrocarbyl side chain or two long hydrocarbyl chains.
From these compounds the use of amphoteric or zwitterionic
ammonium compounds having two long hydrocarbyl chains is
particularly preferred for many reasons including costs,
ease of processing and better stability and performance.
Suitable amphoteric materials are for example disclosed in
EP 236 213.
In this specification the expression hydrocarbyl
chain refers to linear or branched alkyl or alkenyl chains
optionally substituted or interrupted by functional groups
such as -OH, -O-, -CONH-, -COO-, etc.
Preferably the amphoteric fabric-substantive
materials are water insoluble and have a solubility in
water at pH 2.5 at 20°C of less than 10 g/1. The HLB of
the amphoteric fabric-substantive material is preferably
less than 10Ø
2042979
- 27 - C3371
Suitable cationic fabric-softener materials for use
in a composition according to the present invention are
cationic materials which are water-insoluble in that the
material has a solubility in water at pH 2.5 and 20°C of
less than 10 g/1. Highly preferred materials are cationic
quaternary ammonium salts having two C12-C24 hydrocarbyl
chains.
Well-known species of substantially water-insoluble
quaternary ammonium compounds have the formula:
Rl ~ R3 +
N X
R2 R4
wherein R1 and R2 represent hydrocarbyl groups from about
12 to about 24 carbon atoms; R3 and R4 represent
hydrocarbyl groups containing from 1 to about 4 carbon
atoms; and X is an anion, preferably selected from halide,
methosulphate and ethyl sulphate radicals.
Representative examples of these quaternary softeners
include ditallow dimethyl ammonium chloride; ditallow
dimethyl ammonium methyl sulphate; dihexadecyl dimethyl
ammonium chloride; di(hydrogenated tallow) dimethyl
ammonium methyl sulphate; dihexadecyl diethyl ammonium
chloride; di(coconut) dimethyl ammonium chloride.
Ditallow dimethyl ammonium chloride, di(hydrogenated
tallow) dimethyl ammonium chloride, di(coconut) dimethyl
ammonium chloride and di(coconut) dimethyl ammonium
methosulphate are preferred.
- 28 - C3371
Suitable materials also include dialkyl ethoxyl
methyl ammonium methosulphate based on soft fatty acid,
dialkyl ethoxyl methyl ammonium methosulphate based on
hard fatty acid, and a material in which R3 and R4
represent methyl, R1 is C13-15' R2 is CH2CH20COR, where R
is stearyl, and X is methosulphate. Ditallow dimethyl
ammonium chloride, di(hydrogenated tallow alkyl) dimethyl
ammonium chloride, di(coconut alkyl) dimethyl ammonium
chloride and di(coconut alkyl) dimethyl ammonium
methosulfate are preferred.
Other preferred cationic compounds include those
materials as disclosed in EP 239 910 (P&G),
Other preferred materials are the materials of
formula
O
R5- C O CH2-CH2 CH2-CH2-OH
N+
O CH3S04_
R5 - C - O - CH2-CH2 CH3
R5 being tallow, which is available from Stepan under the
trademark stepantex vRH 90,
and
~~,N
20429'~~
- 29 - C3371
R6COOCH2~
CH-CH2N+R8R9R10X_
R7C00
where R8, R9 and R10 are each alkyl or hydroxyalkyl groups
containing from 1 to 4 carbon atoms, or a benzyl group.
R6 and R7 are each an alkyl or alkenyl chain containing
from 11 to 23 carbon atoms, and X is a water-soluble
anion. These materials and their method of preparation
are described in US 4 137 180 (LEVER BROTHERS).
Another class of preferred water-insoluble cationic
materials are the hydrocarbylimidazolinium salts believed
to have the formula:
CH2 CH2
N +N . C2H4 N - C --~tli A-
C R12
R13
R14
wherein R13 is a hydrocarbyl group containing from 1 to 4,
preferably 1 or 2 carbon atoms, R11 is a hydrocarbyl group
containing from 8 to 25 carbon atoms, R14 is an
hydrocarbyl group containing from 8 to 25 carbon atoms and
R12 is hydrogen or an hydrocarbyl containing from 1 to 4
carbon atoms and A is an anion, preferably a halide,
methosulphate or ethosulphate.
CA 02042979 2000-08-08
- 30 - C3371
Preferred imidazolinium salts include 1-methyl-1-
(tallowylamido-) ethyl -2-tallowyl- 4,5-dihydro
imidazolinium methosulphate and 1-methyl-1-
(palmitoylamido) ethyl -2-octadecyl-4,5- dihydro-
imidazolinium chloride. Other useful imidazolinium
materials are 2-heptadecul-1-methyl-1- (2-stearylamido)-
ethyl-imidazolinium chloride and 2-lauryl-1-hydroxyethyl
-1-oleyl-imidazolinium chloride. Also suitable herein are
the imidazolinium fabric-softening components of US patent
No. 4 127 489.
Representative commercially available materials of
the above classes are the quaternary ammonium compounds
*Arquad 2HT (Ex Ax2o) *Noramium M2SH (ex cECA) ; Aliquat-2HT
(Trade Mark of General Mills Inc), Stepantex Q185 (ex
Stepan); Stepantex VP85 (ex Stepan); Stepantex VRH90 (ex
Stepan); *Synprolam FS (ex ICI) and the imidazolinium
compounds Varisoft 475 (Trade Mark of Sherex Company,
Columbus Ohio) and Rewoquat W7500 (Trade Mark of REWO).
The compositions according to the invention may also
- contain, possibly in addition to the above mentioned
softening agents, one or more amine softening materials.
The term "amine" as used herein can refer to
(i) amines of formula:
R15
R16 N ~ (I)
R17
wherein R15, R16 and R17 are defined as below;
*denotes trade mark
CA 02042979 2000-08-08
- 31 - C3371
(ii) amines of formula:
Ri8 R2
R19 N ( CH2 ) n N 1
(II) _.
wherein R18, Ri9, R20 and R21, m and d are defined as
below.
(iii) imidazolines of formula:
CH2 CH2
O
N N C2H4 N-C-Ril
C R12
R14
wherein R11, R12 and R14 are defined as above.
(iv) condensation products formed from the reaction of
fatty acids with a polyamine selected from the group
consisting of hydroxy alkylalkylenediamines and
dialkylenetriamines and mixtures thereof. Suitable
materials are disclosed in European Patent Application
199 382 (Procter and Gamble).
When the amine is of the formula I above, R15 is a C6
to C24, hydrocarbyl group, R16 is a C1 to C24 hydrocarbyl
group and R17 is a C1 to C1~ hydrocarbyl group. Suitable
amines include those materials from which the quaternary
CA 02042979 2000-08-08
- 32 - C3371
ammonium compounds disclosed above are derived, in which
R15 is R1, R16 is R2 and R17 is R3. Preferably, the amine
is such that both R15 and R16 are C6-C20 alkyl with
C16-Ci$ being most preferred and with R17 as C1-3 alkyl,
or R15 is an alkyl or alkenyl group with at least 22
carbon atoms and R16 and R12 are C1-3 alkyl. Preferably
these amines are protonated with hydrochloric acid,
orthophosphoric acid (OPA), C1-5 carboxylic acids or any
other similar acids, for use in the fabric-conditioning
compositions of the invention.
When the amine is of formula II above, Ri$ is a C6 to
C24 hydrocarbyl group, R19 is an alkoxylated group of
formula -(CH2CH20)yH, where y is within the range from 0
to 6, R20 is an alkoxylated group of formula -(CH2CH20)ZH
where z is within the range from 0 to 6 and m is an
integer within the range from 0 to 6, and is preferably 3.
When m is 0, it is preferred that Ri8 is a Cl6 to C22
alkyl and that the sum total of z and y is within the
range from 1 to 6, more preferably 1 to 3. When m is 1,
it is preferred that R1$ is a C16 to C22 alkyl and that
the sum total of x and y and z is within the range from 3
to 10.
Representative commercially available materials of
this class include *Ethomeen (ex Armour) and *Ethoduomeen
(ex Armour).
Preferably the amines of type (ii) or (iii) are also
protonated for use in the fabric-conditioning compositions
of the invention.
When the amine is of type (iv) given above, a
particularly preferred material is:
* denotes trade mark
~s~;r.
CA 02042979 2000-08-08
- 33 - C3371
H R220H
N~R23 N
O O
R24 C__R24
where R22 and R23 are divalent alkenyl chains having from
1 to 3 carbon atoms, and R24 is an acyclic aliphatic
hydrocarbon chain having from 15 to 21 carbon atoms. A
commercially available material of this class is~Ceranine
HC39 (ex Sandoz).
The compositions also contain an emulsion component
such as hydrocarbons, perfumes, natural fats and oils,
fatty acids and/or esters thereof, fatty alcohols and
silicones.
Highly preferred hydrocarbons are paraffins and
olefines but alkynes and halogenated paraffins such as
myristyl chloride are not excluded. Materials known
generally as paraffin oil, soft paraffin wax, petroleum
and petroleum jelly are especially suitable. Examples of
specific materials are tetradecane, hexadecane, octadecane
and octodecene.
Preferred perfumes are *Portia 40 ex IFF Ltd, *LFU 384 ex Quest and
*Coccoon SN3000 ex Givaudan.
Examples of natural oils are coconut, corn, olive and
sunflower as well as naturally occuring waxy solids such
as lanolin. Examples of animal fats are butter, tallow
and sardine.
* denotes trade mark
CA 02042979 2000-08-08
- 34 - C3371
Preferred fatty acids and esters are lauric,
myristic, palmitic and stearic acids, methyl laurate,
ethyl myristate, ethyl stearate, methyl palmitate and
ethylene glycol monostearate. Examples of fatty alcohols
include decanol, dodecanol, tetradecanol, pentadecanol,
hexadecanol and lauryl and palmityl alcohols. Examples
of estols include isopropylmyristate.
Examples of silicones and aminosilicones suitable
for use in the invention are disclosed in DE 2 631 419
(Procter and Gamble/Dumbrell) and those supplied
commercially as *Vp 1487E ex Wacker and the *Magnasoft
range ex Union Carbide.
We have found that deposition of the emulsion
component is improved when the particles are positively
charged. When a cationic fabric softener is used it is
convenient to make the fabric treatment composition in a
one-stage process where the fabric softener acts as
emulsifier. When this is not possible it may be
necessary to make the emulsion separately in which case a
separate emulsifier may be required. Suitable
emulsifiers are single or di-alkyl ammonium salts, the
esters of sorbitan, glycerol or polyethylene glycol and
ethoxylated alcohols.
The levels of emulsion component in the composition
is typically from 1 to 80%, by weight preferably from 2
to 70% and most preferably from 5 to 50%. The
compositions according to the invention may optionally
contain electrolyte. The level of dissolved electrolyte
is typically from 0% to 0.2%, preferably 0.05 to 0.2%.
Compositions according to the present invention
preferably have a pH of less than 6.0, more preferred
less than 5.0, especially from 1.5 to 4.5, most preferred
from 2.0 to 4Ø
* denotes trade mark
~~4~~79
- 35 - C3371
The compositions can also contain one or more
optional ingredients selected from non-aqueous solvents
such as C1-C4 alkanols and polyhydric alcohols,
pH-buffering agents such as weak acids, e.g. phosphoric,
benzoic or citric acids, re-wetting agents, viscosity
modifiers, aluminium chlorohydrate, antigelling agents,
fluorescers, colourants, hydrotropes, antifoaming agents,
antiredeposition agents, enzymes, optical brightening
agents, opacifiers, stabilisers such as guar gum and
polyethylene glycol, anti-shrinking agents, antioxidants,
anti-corrosion agnets, preservatives such as Bronopol
(Trade Mark), a commercially available form of 2-bromo-
2-nitropropane-1,3-diol, to preserve the fabric treatment
composition, dyes, bleaches and bleach precursors,
drape-imparting agents, antistatic agents and ironing
aids.
These optional ingredients, if added, are each
present at levels up to 5~ by weight of the composition.
The invention will be further illustrated by means of
the following examples.
Examples
In Examples I-II the following polymers were used.
Each polymer was obtained from National Starch as an
aqueous solution of from 30-60~ by weight solids level.
All percentages for the polymer refer to 100 active
polymers.
Basic Structures of Polymers: General Formula II
wherein R8 = H, r = O, v = 1
wherein Q2; x = y = O, R1 = COO, R3 absent, R5 = H,
CA 02042979 2000-08-08
- 36 - C3371
R6 = CH3.
Polymer R10 q R7 R9 p R4 SV(cps)
Ref
435/173 COOC2H40H 25 - - O C12H25 3~5
435/174 COOC2H40H 25 - - O C12H25 4~3
448/20 COOC2H40H 10 - - O C12H25
Basic Structure of Polymers~ General Formula III
wherein b = c = O, R4 - -C12H25' R6 - CH3, d = 1. In
IIIa, R11 - 11, R11* = CH , B2 = C1. In IIIb, a = 1,
3
f = g = h = i = O, R1 - COO, R3 is absent, R5 - H,
B3 - C1.
Polymer a d R4 R6 SV(cps)
Ref
433/20 10 1 C18H37 CH3 5.5
442/25 10 1 C12H25 CH3 4.6
442/44 10 1 C12H25 CH3 6.3
442/60 10 1 C8H17 H -
Example 1
Fabric softening compositions were made by adding the
deflocculating polymer under sturring, to a preheated
(70°C) mixture of the emulsion component and fabric
softening material in aqueous dispersion. The fabric
softening material was Arquad 2HT (a dimethyl ditallow
ammonium chloride) ex Atlas. The emulsion component was
a combination of *Sirius M85 a mineral oil, ex Dalton & Co., *Silkolene 910 ex
Dalton & Co., a petroleum jelly and optionally lanolin.
The following compositions were obtained:
* denotes trade mark
v 20429'9
- 37 - C3371
Component A B C D E F
by weight
Arquad 2HT 4.25 4.25 4.25 4.25 9 9
Sirius M85 10.70 10.70 10.70 10.70 24 24
Silkolene 910 5.30 5.30 5.30 5.30 12 12
Lanolin 2.0 2.0 3.0 3.0 - -
Polymer 433/20- 0.13 - 0.09 - 0.1
Water - - - - balance - - - - - - - - -
- - - -
In all compositions B, D and F a significant reduction in
viscosity was observed when compared to that of the
corresponding composition without polymer. In
compositions E and F a reduction in viscosity from 0.7 Pas
to 0.3 Pas was measured at a shear rate of 21s 1.
Example II
Fabric softening compositions were made according to
the method of Example I. The fabric softening material
was either Arquad 2HT as used in Example I or Arquad 2T ex
Atlas. In Arquad 2T the tallow is not hardened.
2fl429'~9
- 38 - C3371
Component A B C D
by weight
Arquad 2HT 7 7 - -
Arquad 2T - - 7 7
Sirius M85 18.7 18.7 18.7 18.7
Silkolene 910 9.3 9.3 9.3 9.3
Polymer 435/174 - 0.27 - 0.3
Water - - - - - balance - - - - - - -
-
Viscosity mPas 2952 338 729 182
110s 1
21s 1 14721 709 3458 404
Example III
Fabric softening compositions were made according
to
the method of Example 1 excepting that
the electrolyte
(when present) was added along with the polymer.
Component A B C D E
Arquad 2HT 7 7 7 7 7
Sirius M85 18.7 18.7 18.7 18.7 18.7
Silkolene 910 9.3 9.3 9.3 9.3 9.3
Polymer 435/173 0.3 0.3 0.3 0.3 0.3
NaCl - 0.05 0.1 0.15 0.2
Water - - - - - - - balance - - - - - - -
- -
Viscosity mPas
110s 1 210 65 125 160 295
21s 1 658 473 431 616 879
From comparison with Example IIA it can be
seen
that
a reduction in viscosity from 14.7 Pas o 0.6 occurs
t Pas
~0429'~9
- 39 - C3371
on addition of the deflocculating polymer. The addition
of electrolyte (Example IIIB) then reduces this viscosity
further. Continued addition of electrolyte however leads
eventually to a viscosity increase.
Example IV
A typical formulation for use as a rinse conditioner
comprises:
by weight
Arquad 2HT 7
Sirius M85 18.7
Silkolene 910 9.3
Lanolin 0.5
Polymer 442/25 0.04
Water to balance
This composition has a viscosity at 21s 1 of 0.44
Pas.
2042979
- 40 - C3371
Example V
Fabric softening compositions were made according to the
method of Example I. The fabric softening material was
Rewoquat W75 (a 1-methyl-1 (tallowylamido-)
ethyl-2-tallowyl-4,5-dihydroimidazolinium methosulphate).
Component Composition
A B C D E F
Rewoquat W75 16 16 16 16 16 16
Sirius M85 1 2 2 1 2 2
CaCl2 0.01 0.01 0.01 0.01 0.01 0.01
Polymer 442/44 - - - - 0.43 -
Polymer 442/60 - - 0.36 0.5 - -
Polymer 448/20 - - - - - 0.4
Water -----------balance-----------
Viscositv mPas
21s 1 86 756 79 40 122 253
110s 1 45 122 43 26 49 121
In compositions C, D, E and F a significant reduction in
viscosity was observed when compared to that of the
corresponding composition without polymer. For example
composition A compared to composition D or composition B
compared to compositions C, E or F.
20429'9
- 41 - C3371
Example VI
Fabric softening compositions were made according to the
method of Example I.
Component Compositions
A B C D
Rewoquat W75 16 16 16 16
Sirius M85 3 3 3 3
CaCl2 0.01 0.01 0.01 0.01
Polymer 442/44 - 0.36 - -
Polymer 442/60 - - 0.47 -
Polymer 448/20 - - - 0.40
Water -------balance-------
Viscositv mPas
21s 1 907 160 80 289
110s 1 282 60 37 125
In compositions B, C and D a significant reduction in
viscosity was observed when compared to that of the
corresponding composition without polymer (A).
2~4297g
- 42 - C3371
Example VII
Fabric softening compositions were made according to the
method of Example I.
Component Composition
A B C
Rewoquat W75 16 16 16
Steric Acid 2 2 2
CaCl2 0.025 0.025 0.025
Polymer 442/44 - 0.39 -
Polymer 442/60 - - 0.36
Water ------balance------
Viscosity mPas
21s 1 12$3 325 215
110s 1 475 129 101
In compositions B and C a significant reduction in
viscosity was observed when compared to that of the
corresponding compositions without polymer (A).
~Q,~29? 9
- 43 - C3371
Example VIII
Fabric softening compositions were made according to the
method of Example I.
Component Composition
A B _C D
Rewoquat W75 15 15 15 15
Octadecane 12 12 12 12
Polymer 442/44 - p,g9 - -
Polymer 442/60 - - 0.36 -
Polymer 448/20 - - - 0.40
Water ------balance-------
Viscositv mPas
21s 1 5853 1760 1614 1891
110s 1 1016 453 482 496
In compositions B, C and D a significant reduction
in viscosity was observed when compared to that of the
corresponding composition without polymer (A).