Note: Descriptions are shown in the official language in which they were submitted.
~d~438'~2
_ , ._
METHOD OF NITRIDING STEEL
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention relates to a method of
nitriding steel for nitrogen case-hardening of steel
which comprises subjecting a steel work to a special
pretreatment that is conducive to a deep and uniform
nitride layer or case,
Brief Description of the Prior Art
For the purpose of improving the wear resistance,
corrosion resistance and mechanical properties such as
fatigue strength etc. of steel, it is common practice
to form a nitride layer or case on the surface of
steel. Typical of this technique is the nitriding (gas
nitriding, gas soft nitriding) process employing
ammonia gas alone or a mixed gas composed of ammonia
and a carbon source-containing gas (RX gas). Methods
of this kind have problems with process stability in
that when an alloy steel work or a steel work with an
intricate configuration is treated, the resulting _
nitride case tends to be uneven.
While steel works are generally nitrided at
temperatures not below 500°C, the adsorption and
diffusion of nitrogen on and into the surface layer of
2o~~~~z
- 2 -
steel requires not only the absence of organic and
inorganic stains but also the absence of an oxide film.
Furthermore, the steel surface itself must be high in
activity, too. Actually, however, it is impossible to
prevent formation of an oxide film or obtain complete
activation of the steel surface in such nitriding
processes. Taking an austenitic stainless steel work
as an example, it is generally cleaned with hydro-
fluoric acid-nitric acid to remove the passivation film
from the surface prior to charge into the nitriding
furnace but it is difficult to completely remove the
passivation fi Ln and impossible to completely activate
the surface layer of the steel. Therefore, it is near
to impossibility to form a satisfactory nitride case.
Moreover, the removal of organic and inorganic stains
prior to nitriding is generally carried out by alkali
degreasing or organic cleaning with, for example,
trichloroethylene but the recent antipollution regula-
tions (control against destruction of the ozonosphere)
frustrate the practice of organic cleaning which is the
most effective cleaning method so far available and
this f actor is also a major obstacle to the formation
of a satisfactory nitride case.
Under the circumstances, the inventors of the
present invention previously found that when a steel
CA 02043872 2000-OS-26
work prior to nitriding is first fluorided in heated
condition under a fluorine-containing gas blanket such
as NF3 and, then, nitrided, both the cleaning (removal
of organic and inorganic stains and removal of the
oxide film) and activation of the steel surface can be
accomplished to give a satisfactory nitrogen case and a
patent on the technology is pending (Japanese laid-open
Patent Application No. ~-44 457 published on February 26, 1991)
In this mehtod, the steel work is first heated and contacted with
a gas, such as NF3, in a furnace for pretreatrrent. As a result, the
organic and inorganic stain components adhering to the
steel surface are destroyed by the activated fluorine
atoms to leave a clean steel surf ace and, at the same
time, the passivation film, inclusive of the oxide
film, on the steel surf ace is converted to a fluoride
film to cover and protect the steel surface. The steel
work is then nitrided. In this nitriding process, the
above fluoride film is destroyed and removed by intro-
ducing a mixed gas composed of a nitrogen source-con-
twining nitriding gas (e. g. NH3 gas) and H2 gas into
the furnace under heating. More specifically, the
destruction and removal of said fluoride film leaves a
clean and activated steel surface and the N atoms in
the nitriding gas rapidly penerate and diffuse into
this cleaned, activated steel to form a uniform and
CA 02043872 2000-OS-26
- 4 -
deep nitrate case. However, despite the above-mentioned
desirable performance characteristic of NF3 gas, it has the
disadvantage of high cost. Moreover, a fairly high
temperature (280-500°C) is required for adequate fluoriding
and this means a significant thermal energy consumption,
thus adding to the cost of treatment.
OBJECT AND SUMMARY OF THE INVENTION
Having been developed under the above
circumstances, the present invention has as its object to
provide a method of nitriding steel which is capable of
forming a uniform and deep nitride case at low cost.
More specifically, the invention provides a
method of nitriding steel comprising the steps of
fluoridizing a steel work at a temperature of 150 to 500°C
in an atmosphere of a mixed gas composed of 0.05-20% by
volume of fluorine gas and inert gas, thereby forming a
fluoride film onto said steel work; removing the film
formed during the fluoridizing step; and nitriding the
steel work in heated condition in an atmosphere of
nitriding gas.
The invention also provides a method of nitriding
steel comprising the step of . fluoridizing a steel work at
a temperature of 200 to 500°C in an atmosphere of a mixed
gas composed of 1-5% by volume of fluorine gas, 1-20% by
volume of nitrogen trifluoride gas and inert gas, thereby
forming a fluoride film onto said film work; removing the
fluoride film formed during the fluoridizing step; and
nitriding the steel work in heated condition in an
atmosphere of nitriding gas.
CA 02043872 2000-OS-26
- 4a -
DETAILED DESCRIPTION OF THE INVENTION
Thus the present invention is directed, in a
first aspect, to a method of nitriding steel characterized
by fluoriding a steel work in heated condition under a
blanket of a fluorine gas-inert gas mixture and, then,
nitriding the same work in heated condition under a blanket
of nitriding gas and, in a second aspect, to a method of
nitriding steel characterized by fluoriding a steel work in
heated condition under a blanket of a fluorine gas-nitrogen
trifluoride gas-inert gas mixture and, then nitriding the
same work in heated condition under a blanket of nitriding
gas.
The inventors of the present invention performed
CA 02043872 2000-OS-26
- 5 -
series of investigations for the cost reduction of a
nitriding process using NF3 as a fluoriding gas and
found that fluorine gas iF2) which was not formerly
considered to be suited for fluoriding at the stage of
development of the above-mentioned basic invention
employing NF3 as the fluoriding gas actually has
excellent fluoriding activity and that fluorine gas
achieves fluoriding at a considerably lower temperature
than NF3. The present invention is based. on the above
finding.
More specifically, the first aspect of the invention is
directed to a fluoriding process employing a mixture of
F2 and an inert gas such as N2. By this technique,
substantial fluoriding can be accomplished at a com-
paratively low temperature in the range of about 150°C
to about 300°C, preferably about 200°C to about 250°C.
The second aspect of the invention is concerned with a fluoriding
process employing a mixed gas composed of N2, F2 and
NF3. In this latter process, fluoriding can be accom-
plished at a temperature in the range of about 200°C to
about 400°C, preferably about 250°C to about 300°C,
which is lower than the temperature required for the
prior process using NF3 as the fluoriding gas, although
this temperature is slightly higher than that required
for the first-mentioned process employing a mixed gas
~44387~
- 6 -
canposed of N, and F, as the fluoriding gas, It was, thus, found that
there is a temperature difference of as mush as 100°C to 150°C
between
the fluoriding temperature in the case of using F, alone (F, + N,) and
the fluoriding temperature in the case of using NF, alone (NF, + N,),
It should be understood that, in the present invention, fluoriding can
be performed at a temperature beyond the above-mentioned range, for
example about 500°C at the maximum, if desired. As the F, gas
(fluorine gas), not only a general F, gas which is formed by a melting
electrolytic method and the like, but also F, gas which is formed by
thermal-cracking by introducing a F-containing composed such as BF,,
CF, , HF, SFs , C, Fs , WFe , (~', , SiF4 into a thermal-cracking apparatus
may be used. F, used in this invention includes such F, formed by
thermal-cracking. .
The present invention is now. described in further detail.
In accordance with the preseW invention, either (1) a mixed gas of
N, + F, or ( 2 ) a mixed gas of N, + F,, + NF, is employed for f luoriding
as mentioned above.
In the case of using (1) a binary mixture of Ns + F,, the concentration
of F, is set at 0.05 to 20$ (by volume; the same applies hereinafter). The
drawback of F, is that since it is highly reactive, control of fluoridirxl
is difficult at a high concentrarion. Thus, though F, is rather easy to
control at a concentration not exceeding 1$, prolonged trearment is required
for sufficient case hardening of steel. Therefore, the preferred F,
c~centration is 3 to 10$. In the case of using (2) a mixed gas of F, + NF,
+ N, , the preferred c~centration of F, is 1 to 5~ and that of NF, is 1 to
2043~~2
200. In the case of using the ternary mixture of F2 +
NF3 + N2, the proportions of F2 and NF3 depend on the
scheduled fluoriding time and temperature. Thus, since
a longer fluoriding time means a longer working time,
the ratio of F2 to NF3 in the ternary gaseous mixture
is determined in consideration of this disadvantage and
the cost of the fluoriding gas.
The substrate steel for the present invention
includes a variety of steels such as carbon steel,
stainless steel and sa on. These steels~are not
limited in shape or the like and may be in the form of
plate or coil or even in the processed shape of a screw
or the like. The substrate steel for the present
invention is not limited to said steels, either, but
includes alloys of said steels and alloys based on said
steels and supplemented with other metals.
In accordance with the present invention, the
substrate steel is either treated using (A) a first
heat treating furnace for fluoriding and a second
treating furnace for nitriding or (B) in a single heat
treating apparatus having both a fluoriding chamber and
a nitriding chamber.
In the case of treating the substrate steel using
(A) a heat treating furnace for fluoriding and a heat
treating furnace for nitriding, the process may for
- a - 20438'72
example comprise the following steps. First, fluoriding is
performed in said heat treating furnace for fluoriding in the
following manner. Thus, the steel work to be case-hardened is
placed in the first heat treating furnace for fluoriding and heated
to a temperature of 150-300°C , preferably 200'-250°C . Then, in
the
same condition, fluorine gas (F, + N,) is introduced into the
heating furnace and the steel work is maintained at the same
temperature as above in an atmosphere of said fluorine gas for
about 10 to 120 minutes, preferably for about 20 to 90 minutes, and
for still better results for about 30 to 60 minutes. In the case of
using F, formed by cracking a compound such as BF,, a cracking
apparatus is disposed in front of the heat furnace or in the
vicinity of the heat furnace. After thermal-cracking the above-
mentioned compound, formed F, is mixed with N, and the mixture is
introduced into the heat furnace. By this procedure, the
passivation film (mainly composed of oxide) an the steel surface is
converted to a fluoride film. This reaction proceeds for example
in accordance with the following reaction formulas.
Fe0 + F, -~ FeF, + 1 / 20,
Cr, 0, + 2F, -~ 2CrF, + 3/20,
The above treatments are each carried out using a heat treating
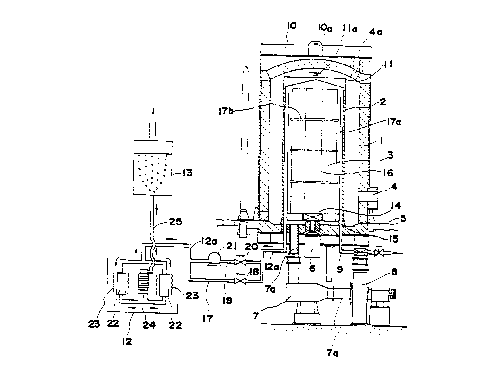
furnace such as, for example, the one illustrated in Fig. 1.
Referring to the accompanying drawings, the reference numeral 1
indicates a bell-shaped outer cover and 2 indicates a cylindrical inner
cover which is covered with said outer cover. Integrally disposed on
2~~3~~~
_ g _
top of said outer cover 1 is a frame structure 10
having an engaging means l0a for engaging the hook of a
crane or the like. Integrally disposed on top of said
inner cover 2 is a cover structure 11 having an engaging
means lla for engaging the hook of a crane or the like.
Formed within said inner cover 2 is a fluoriding
chamber and the space between the two covers 1 and 2
constitutes a heating chamber. The reference numeral 3
indicates steel works which are charged into and taken
out from said inner cover 2. The steel works 3 are
mounted on a platforml5 having a center hole 14 and
staged up in the space between a first cylindrical
wire-mesh member 16 extending upwards from said center
hole 14 and a second cylindrical wire-mesh member 17a
extending upwards from the periphery of said platform
15 through interposed porous dividers 17b each having a
center hole. The reference numeral 4 indicates a port
for installation of a burner as formed in the peripheral
wall in the lower part of said outer cover 1, and 4a
indicates an exhaust port formed in the top wall of the
outer cover 1. The reference numeral 5 indicates a
base and 6 indicates a fan for circulation of the
furnace atmosphere. This fan 6 faces the center hole
14 of the platform 15 and circulates the furnace
atmosphere via the center hole 14 and the cylindrical
T
2~4~~'~2
-lo-
wire-mesh member 16 extending upwards therefrom. The
reference numeral 7 indicates a heat exchanger which is
disposed in a p;..pe 7a extending downwardly from the
base of said inner cover 2. The reference numeral 8
indicates a circulation blower far forced cooling which
is installed in the pipe 7a downstreams of said heat
exchanger 7, while a pipe for introducing fluorine gas '
into the inner cover 2 is indicated at 9. Indicated at
12a is an exhaust gas pipe for withdrawal of spent gas,
from the inner cover 2, which is bifurcated in an
intermediate position, with one of branch pipes 17
being equipped with a valve 18 and the other branch
pipe 19 being equipped with a valve 20 and a vacuum
pump 21. When the spent.gas pressure in the inner
cover 2 is high, the route of branch pipe 17 is used,
while the route of branch pipe 19 is used
for vacuum evacuation by the suction force of the
vacuum pump 21 when the spent gas pressure is low. The
reference numeral 12 indicates an antipollution device
which is connected to the terminal end of said exhaust
gas pipe 12a. This antipollution device 12 comprises a
transverse pair of activated carbon columns 22, a
heater coil 23 wound round the periphery of each
column, and a fin-type heat exchanger 24 and functions
in such a manner that the spent gas introduced into the
2o4Js~z
- 11 -
activated carbon column 22 is converted to harmless CF4
by thermal reaction of residual F2 etc. with the
activated carbon and fed to the fin-type heat exchanger
24 for cooling. Indicated at 13 is~a scrubber disposed
in a pipe 25 extending from said heat exchanger 24.
This scrubber 13 contains water and functions to
thoroughly treat the spent gas harmless for release
into the atmosphere by reducing the spent gas from the
pipe 25 into bubbles so as to dissolve the HF fraction
(which is by-produced by reaction of F2 with H20 and H2
in inner cover 2) of the spent gas in the water.
Using this heat treating furnace, fluoriding is
performed as follows. Thus, the hook of a crane or the
like (not shown) is engaged with the engaging means l0a
and lla of said outer cover 1 and inner cover 2 to
suspend the outer cover 1 and inner cover 2 with the
crane or the like. In this condition, the substrate
steel 3 is set up on the platform 15 as illustrated and
the outer cover 1 and inner cover 2 are lowered to the
original positions (the condition shown in Fig. 1).
Then, the heat of the flame is radiated from a burner
(not shown) set in the burner hole 4 into the heating
chamber formed between the outer cover 1 and inner
cover 2, whereby the steel work 3 in the inner cover 2
is heated. Then, a fluorine-containing gas such as NF3
- ,2 - 2~~~872
is introduced into the inner cover 2 from its bottom
through a pipe 9 for fluoriding. The duration of this
fluoriding is generally about 30 to 60 minutes as ...
mentioned hereinbefore.
Then, nitriding is performed as follows. Thus, '
since the steel work 3 after the above fluoriding
treatment is covered with a fluoride film, it remains
intact without surface oxidation even if it is exposed
to the atmosphere such as air. The steel work in this
condition is either stored or immediately subjected to
nitriding in said second heating furnace for nitriding.
This second heating furnace for nitriding is similar in
construction with the first heating furnace described
above. Thus, the inner cover 2 and outer cover 1 of
this second heating furnace A' are suspended up, the
steel work 3 is then stacked, and the inner cover 2 and
outer cover 1 are lowered into the original positions.
Then, the heat of a flame is radiated from a burner
into the space between the inner cover 2 and outer
cover 1 to heat the steel work in the inner cover 2 at
a nitriding temperature of 480-700°C. In this condition,
NH3 gas or a mixed gas composed of NH3 and a carbon
source-containing gas is introduced into the furnace
from the bottom of the heating furnace through a pipe 9
and the steel work is maintained in this condition for
-m - 2~43~72
.bout 120 minutes or more. In this process, said fluoride film
is reduced or destroyed by H2 or a small amount of
water (by-produced in the course of nitriding reaction),
for example in accordance with the following _reaction.
formulas, to give rise to an active steel surface.
CrF4 + 2HZ -~ Cr + 4HF
2FeF3 + 3HZ -~ 2Fe + 6HF
Referring to the above removal of the fluoride
film, the film may be destroyed by introducing a mixed
gas of N2 and H2 or HZ gas prior to introduction of the
nitriding gas. Rather, this practice is preferred in
that the trouble due to by-production of ammonium
fluoride can be avoided.
On the active steel surface thus formed, the
active nitrogen derived from the nitriding gas acts to
penetrate and diffuse into the steel work. As a
result, towards the inside of the steel work from its
surface, an ultrahard compound layer (nitride layer)
containing nitrides such as CrN, Fe2N, Fe3N and Fe4N is
formed uniformly and to a sufficient depth, followed by
formulation of a hard diffusion layer of N atoms, and
the above-mentioned compound layer and diffusion layer
constitute the entire nitride case.
In the case of perfarming both fluoriding and
nitriding in a single heat treating furnace (B), a
20~3~'~2
- 14 -
furnace of the structure illustrated in Fig.. 2, for
instance, is employed. In the view, 1' indicates a
furnace and 2' a metal basket which is loaded with
steel work (not shown). The reference numeral 3'
indicates a heater, 5' an exhaust gas pipe, 6' a
diabetic wall, 7' a door, 8' a fan, 10' a post, 12' a
vacuum pump, and 13' a spent gas treating unit.
Indicated at 21' is a furnace body having an adiabatic
wall, which is internally divided into compartments 23'
and 24' by a partitioning wall or shutter 22' which can
be freely opened and closed. The shutter 22' is
adapted to keep the two compartments 23',24° gas-tight
and insulated against heat and free to open and close
by sliding vertically as shown. The reference numeral
23' indicates a fluoriding chamber, while a nitriding
chamber is indicated at 24'. Each of the fluoriding
chamber 23' and nitriding chamber 24' is formed with a
base 25' which accepts the metal basket 2'. The base
25' consists of a pair of rails and it is so arranged
that the metal basket 2' may slide on the rails selec-
tively into the fluoriding chamber 23' or the nitriding
chamber 24'. The reference numeral 26' indicates a gas
inlet pipe for introduction of fluoriding gas into the
fluoriding chamber 23', while a temperature sensor
probe is indicated at 27'. The front opening of the
243872
- 15 -
fluoriding chamber 23' is releasably covered with a
laterally-driven cover 7'. The reference numeral 28'
indicates a nitriding gas pipe for introduction of the
nitriding gas into the nitriding chamber 24'.
In the above heating furnace, nitriding is per-
formed as follows. First, the basket 2' containing the
steel work is set in the fluoriding chamber 23' and, in
this condition, the internal temperature of the fluo-
riding chamber 23' is increased to heat the steel work
to 150-300°C. Then, in this condition, the fluorine-
containing gas (F2+N2) is introduced into the chamber for
fluoriding for 30 to 60 minutes. Upon completion of
fluoriding, the fluoriding chamber 23' is vented to
exhaust the gas.
Then, nitriding is performed as follows. The
shutter 22' mentioned above is opened to transfer the
steel work and the metal basket 2', as a
unit, to the nitriding chamber 24' and the shutter 22'
is then closed. In this condition, the internal
temperature of the nitriding chamber 24' is increased
to heat the steel work to 480-600°C and H2
gas is introduced into the nitriding chamber 24'
to hold the condition for 1 hour, whereby
the fluoride film covering the steel surface is
destroyed to expose the substrate surface of the work.
20438'2
,~
Then, nitriding is conducted at that temperature for 4-5
hours introducing a nitriding gas, i.e. a mixed gas com-
posed of NH3, N2, H2, Co and C02 into the nitriding
chamber 24'. Thereafter, the internal temperature is de-
creased to 350-450°C and, in this condition, cleaning is
conducted for 1 hour by introducing a mixed gas composed of
H2 and N2 or a mixed gas composed of N2, H2 and C02. There-
after, the spent gas within the nitriding chamber 24 is exhausted.
out and the shutter 22' is opened. Then, the steel
work and the metal basket 2' are transferred, as a
unit, to the fluoriding chamber 23' and the shutter
wall 22' is closed, followed by cooling in that condi-
tion. This cooling is effected by introducing nitrogen
gas from the gas inlet pipe 26'into the fluoriding
chamber 23'. The thus-treated steel work has a deep
and uniform nitride case. In this connection, the
heating of steel work for fluoriding may be carried out
in the nitriding chamber 24' by heating the same. That
is, the steel work is placed directly in the nitrid-
ing chamber 24' and heated therein. Then, the shutter
22' is opened and the work is transferred to the
fluoriding chamber 23' for fluoriding. The steel work
is then placed in the nitriding chamber 24' again for
nitriding. In this case, preheating of the nitriding
chamber 24' can be effected by utilizing the heat for
2~43~'~2
-m-
fluoriding of steel work.
Thus, in accordance with the present invention,
the steel surface exposed upon destruction of the
fluoride film has been highly activated and the nitro-
gen atoms act on this activated steel surface to form
an ultrahard nitride layer of great depth and uniform-
ity. Moreover, the gas used for fluoriding is a mixed
gas based on F2 and compared with the use of NF3, it is
not only inexpensive but permits the use of a lower
fluoriding temperature, thus helping reduce the cost of
treatment in a substantial measure.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a cross-section view showing an example
of the heat treating furnace used in the present
invention, and
Fig. 2 is an elementary view of another heat
treating furnace.
Examples of the invention are give below.
EXAMPLES
First, an example of using a couple of heating
furnaces is described.
Example 1
Fluoridin
A plurality of austenitic stainless steel screws
!samples) were manufactured and cleaned with trichloro-
CA 02043872 2000-OS-26
- 18 -
ethylene vapor. The screws were charged into a first
heating furnace (Fig. 1), in which they were suffi-
ciently baked at 200°Cas mentioned hereinbef ore. Then,
in this condition, a mixed gas composed of 10~ by vohurie of F2
and the balance of N2 was introduced into the furnace
at a rate equal to 5 times the internal volume of the
furnace per unit time and the work was maintained for
60 minutes. Thereafter, some of the samples were taken
out and the surface layer of each sample was examined.
It was conffirmed that a fluoride film had been formed
all over the surface.
Nitriding
The samples subjected to the above fluoriding
treatment were transferred to a second heating furnace
A' and NH3 + 50~ by volume of RX gas was introduced into the furnance
for nitriding at 530°C for 6 hours. After completion of this
treatment, the samples were air-cooled and taken out
from the furnace. The above procedure provided nitro-
gen case-hardened austenitic stainless steel screws.
Comparative Example 1.
The procedure described in Example 1 was repeated
except that the fluoriding gas was replaced with a
mixed gas of N2 + NF3 (concentration 1%) and the
fluoriding temperature was replaced wits X10°C to
provide nitrogen case-hardened austenitic stainless steel screws.
-19 _ ~o43a~2
The hardness, condition and thickness of the
nitride case of the product of Example 1 were compared
with those of the product of Comparative Example 1. As
a result, both products were found to be equivalent in
quality. In contrast, the cost of the product of
Example 1 was one-third of the cost of the product of
Comparative Example 1.
Example 2
Fluoridin~c~
A plurality of automotive engine suction valves
(samples) were manufactured and glaced directly in a
heating furnace A to raise their temperature at 280°C. In
this condition, a mixed gas composed of N2 + 10~ F2 + 8$ NF3
was introduced at a rate equal to 10 ti~-nes the internal volume
of the furnace per unit time and the work was held for
30 minutes. Thereafter, some of the samples were taken
out and the surface layer of each sample was examined.
As a result, it was confirmed that a fluoride film had
been formed throughout the surface.
Nitriding
The samples subjected to the above fluoriding
treatment was transferred to a second heat treating
furnace A' and heated to 570°C. In this condition, a
nitriding gas of NH3 + 50°s RX was introduced far X20
minutes. Thereafter, the samples were air-cooled and
20436'~~
- 2O -
taken out from the furnace.
Comparative Example 2
Fluoriding was carried out at 380°C using a
blanket gas of NF3~gas (1%) + N2 under otherwise the
same conditions as Example 2 to provide samples of an
engine valve.
The product of Example 2 was equivalent in quality
to the product of Comparative Example 2. The
proportion of the cost of fluoriding gas in the cost of
the product engine valve in Example 2 was lower by 40%
as compared with the product of Comparative Example 2
obtained using NF3. Moreover, the heating and cooling
time in the fluoriding step could be reduced by 75
minutes.
Some examples using a single heat treating furnace
(B) are given below.
Example 3
Fluoriding and nitriding were performed using a
heat treating furnace having a fluoriding chamber and a
nitriding chamber as shown in Fig. 2. The respective treatments were
carried out as previously described in the text of this specification
and the conditions in each treatment were the say as in Example 1. The
same result was obtained as that of Example 1.
Example 4
Fluoriding and nitriding were performed using a
_ ~1 _ 20~3~'~2
heat treating furnace having a fluoriding chamber and a nitriding
chamber as shown in Fig. 2. The respective treatments were carried
out as previously described in the text of the specification and
conditions in each treatment were the same'as in Example 2, The
same result was obtained as that of Example 2.
As mentioned hereinbefore, the method of the
present invention employing a mixed gas based on
inexpensive fluorine gas for fluoriding permits a
drastic reduction of treatment cost. Furthermore,
since fluoriding can be accomplished at a temperature
lower by 100-150°C than that of fluoriding with NF3,
the thermal energy requirements are reduced and this
also contributes remarkably to cost reduction. Par-
ticularly because fluoriding can be accomplished at
such a comparatively low temperature, the cooling time
following fluoriding can also be curtailed so that the
whole process can be expedited. Furthermore, because
fluorine gas has an intense odor, it is more amenable
to leak detection than NF3 and the pollution problem
associated with harmful F2 can be prevented with
greater assurance. Furthermore, this lower temperature
for fluoriding brings forth further advantages
design-wise in the case of a heat treating furnace
icontinuous furnace) having both a fluoriding chamber
and a nitriding chamber. For example, there is the
2043872
- 22 -
advantage that the serviceable life of the seal packing
for the shutter between the nitriding chamber and the
fluoriding chamber is prolonged. Thus, since the
fluorine gas used for fluoriding is highly corrosive,
the aging of characteristics of the seal packing is
less pronounced when the temperature of the fluoriding
chamber is low, so that a longer packing life can be
realized. Among other advantages are the simplifica-
tion and longer lives of reinforcing and other members
of the structure.