Note: Descriptions are shown in the official language in which they were submitted.
~044~~ ~
SPECIFICATION
CORROSION-RESISTANT RARE EARTH METAL-TRANSITION
METAL SERIES MAGNETS AND METHOD OF PRODUCING THE SAME
TECHNICAL FIELD
This invention relates to rare earth metal-
transition metal series magnets having not only
excellent magnetic properties but also improved
corrosion resistance and temperature-dependent
properties and a method of producing the same.
BACKGROUND ART
As a typical permanent magnet manufactured at
the present, there are mentioned Alnico magnets, ferrite
magnets, rare earth metal magnets and the like.
The Alnico magnets are manufactured from the old time,
but their demand is lowering in accordance with the
development of cheap ferrite magnets and rare earth
metal magnets having higher magnetic properties. On the
other hand, the ferrite magnets are chemically stable
and low in the cost because oxides are used as a main
starting material, so that they are the main current as
a magnet material even at the present, but they have a
drawback that maximum energy product is small.
Recently, Sm-Co series magnets having a combina-
tion of magnetic isotropy inherent to rare earth metal
ion and magnetic moment inherent to transition metal
element have been developed, whereby the conventional
-2-
204417
value of maximum energy product is largely increased.
However, the Sm-Co series magnet is mainly composed of
resourceless Sm and Co, so that it is obliged to become
expensive.
Now, it has been attempted to develop cheap
magnet alloys containing no expensive Sm and Co and
having high magnetic properties, and consequently Egawa
et al developed stable ternary alloys by sintering
process (Japanese Patent Application Publication
to No. 61-34242 and Japanese Patent laid open
No. 59-132104) and J. J. Groat et al developed alloys
having a high coercive force by liquid quenching process
(Japanese Patent laid open No. 59-64739). These magnets
are composed of Nd, Fe and B, and their maximum energy
product exceeds that of Sm-Co series magnet.
However, Nd-Fe-B series magnets contain greater
amounts of a light rare earth element such as Nd having
very high activity or the like and corrosive Fe as a
main component, so that the corrosion resistance is poor
2o and hence the magnetic properties are degraded to damage
the reliability as an industrial material.
Therefore, in order to improve the corrosion
resistance, there are taken countermeasures such as
surface plating (Japanese Patent laid open
No. 63-77103), coating treatment (Japanese Patent laid
open No. 60-63901) and the like on the sintered magnets,
~''~ 64881-387
-3-
204 417'
and surface treatment on resin bonded type magnets
before kneading magnet powder with a resin and the like,
but they can not be said to be an effective rustproof
treatment over a long period of time, and the cost
becomes higher due to such a treatment and further there
are caused problems such as magnetic flux loss due to
the presence of protective film and the like.
As a solution to the above problems, the
inventors have previously proposed rare earth metal-
1o transition metal-boron series magnet alloys in which Fe
in the Nd-Fe-B series magnet is replaced with high
concentrations of Co and Ni (Japanese Patent laid open
No. 2-4939).
Such magnets are excellent in the corrosion
resistance and high in the Curie point, so that the
reliability as a magnet material is largely increased.
The invention is concerned with rare earth
metal-transition metal series magnets of two phase
structure further developed from the above magnet.
2o Moreover, magnets having excellent magnetic
properties through two alloying process in which rare
earth rich phase and rare earth poor phase are mixed and
sintered in liquid phase state have previously been
proposed as Nd aeries magnet of two phase structure
(Japanese Patent laid open No. 63-93841 and
No. 63-164403). In this case, the magnetic properties
64881-387
_4_ 2 04 4 1 7 ~
are improved, but there is still remaining a problem on
the corrosion resistance.
DISCLOSURE OF INVENTION
The invention is to advantageously solve the
aforementioned problems and to propose rare earth metal-
transition metal series magnets of two phase structure
being excellent in not only the magnetic properties but
also the corrosion resistance and a method of
advantageously producing the same.
1o At first, details of elucidating the invention
will be described.
The inventors have made various metallographical
studies on the above magnet using high resolution
electron microscope or the like, and confirmed that this
magnet contains Nd2(Fe, Co, Ni)14B phase having a large
saturated magnetic flux density, and intergranular
phases surrounding crystal grains of the above phase and
developing a strong coercive force such as Nd2(Fe, Co,
Ni)1~, Nd(Fe, Co, Ni)5, Nd2(Fe, Co, Ni)7, Nd(Fe, Co,
20 Ni)4H and Nd(Fe, Co, Ni)12B6 and further Ndl_~TM~ of CrB
structure (TM is mainly Ni) and the like.
Furthermore, it has been found that better
corrosion resistance is exhibited as the amount of Nd
phase being a point of causing corrosion is less and the
concentration of Ni or Co in the above intergranular
phase becomes high.
64881-387
204 4171
Now, the inventors have made further studies
with respect to this point and found that the above
intergranular phase hardly appears in a range of Nd-Fe-B
ternary phase diagram other than Nd2(Fe, Co, Ni)1~ and
is rather a phase appearing only in the range of Nd-Co-B
system.
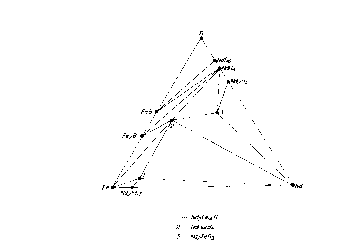
For the reference, Nd-Fe-B ternary phase diagram
is shown in Fig. 1 (N. F. Chaban, Yu. B. Kuzma, N. S.
Bilonizhko, O. O. Kachmar and N. U. Petrov, Akad Nauk,
1o SSSR, SetA, Fiz.-Mat. Tekh, Nauki No. 10 (1979) 873),
and Nd-Co-B ternary phase diagram is shown in Fig. 2 (N.
S. Bilonizhko and Yu. H. Kuzma, Izv. Akad. Nauk SSSR
Neorg. Mater, 19 (1983) 487) (In the original report,
Nd2Fel4H phase and Nd2Co14H phase are misinterpreted as
Nd2Fe9B phase and Nd2Co9B phase, so that they are
corrected in Figs. 1 and 2).
In Fig. 1, a phase of number 1 is Nd2Fe14B phase,
and NdFe4B4 phase (phase of number 2), Nd phase, Nd2Fel~
phase and Fe phase appear as a composition near thereto.
2o In Fig. 2, however, Nd2Col~ phase, NdCoS phase, Nd2Co~
phase, NaCo4B phase (phase of number 2) and NdCol2BS Phase
(phase of number 7) appear in a magnet prepared from a
composition close to Nd2Co14B phase of number 1, and Nd
phase does not naturally appear at an equilibrium state.
As previously mentioned, Nd phase is not only a
point of causing rust but also a magnetically useless
64881-387
64881-387
Z04 417
- 6 -
phase, so that it should be eliminated.
It is, therefore, an object of the invention to
provide permanent magnets having excellent magnetic properties
and corrosion resistance by using magnetically useful two
phases, i.e. REZTM14B phase having a high residual magnetic flux
density and a low melting point RE-TM phase or RE-TM-B phase
enhancing the sinterability and possessing a cleaning action
against grain boundary of main phase and further forming an
electrochemically nobel composition as a starting material to
prepare a two phase magnet.
That is, the invention provides a corrosion-resistant
rare earth metal-transition metal series permanent magnet
consisting essentially of RE: not less than 10 at% but not more
than 25 at% (where RE: one or more Y, Sc and lanthanide), B:
not less than 2 at% but not more than 20 at% and the remainder
being substantially TM (TM is one or more of Fe, Co and Ni),
wherein the magnet has a texture comprised of (i) a phase of
REZTM14B (TM is the same as mentioned above) having Nd2Fe14B
structure and (ii) a phase of at least one member selected from
the group consisting of an RE-TM series intermetallic compound
(TM is Ni or a mixture of Ni and Fe or Co), RE-TM series
eutectic structure (TM is Ni or a mixture of Ni and Fe or Co)
and RE-TM-B series intermetallic compound (TM is Ni or a
mixture of Ni and Fe or Co) each having a melting point lower
than that of the RE2TM14B phase.
Furthermore, the invention provides a method of
producing such a corrosion-resistant rare earth metal-
transition metal series magnet, which comprises subjecting a
'~.
r
204 4171
_ 7 _
mixture of powder composed mainly of RE2TM14B series
intermetallic compound phase (TM is one or more of Fe, Co and
Ni) and powder having a melting point lower than that of the
above powder and composed of mainly at least one member
selected from the group consisting of an RE-TM series
intermetallic compound phase (TM is Ni or a mixture of Ni and
Fe or Co), RE-TM series eutectic structure (TM is Ni or a
mixture of Ni and Fe or Co) and RE-TM-B series intermetallic
compound (TM is the same as mentioned above) to a compression
molding to form a molded body and then sintering the molded
body.
In the invention, in order to further improve the
corrosion resistance, it is effective to make the
intergranular phase electrochemically more nobel than the main
phase, so that it is preferable that the content of Ni and/or
Co in TM of the low melting point RE-TM and RE-TM-B series
phase is made higher than that in RE2TM14B phase.
Particularly, the increase of Ni content is effective to
improve the corrosion resistance and to reduce the cost.
In the invention, it is favourable that a ratio of
RE2TM14B intermetallic compound phase to RE-TM, RE-TM-B series
intermetallic compound phase is about 95:5 to 40:60 as a
formula unit. Because, when this ratio is
64881-387
2~441~y
outside the above range, there is caused a disadvantage
of bringing about considerable degradation of coercive
force and saturated magnetic flux density. The term
"formula unit" used herein corresponds to a case that
Nd2FelqB is considered as one molecule (this is called
as formula in case of solid). The particle size of each
of the above powders to be mixed is desirable to be about
0.5-5 ~m for handling easiness and homogeneous mixing.
A typical composition of RE-TM series
intermetallic compound phase (inclusive of eutectic
structure, same as above) and RE-TM-B series
intermetallic compound phase having a melting point
lower than that of RE2TMIqB intermetallic compound phase
is as follows.
~RE-TM series
RE2TM17, RETMS, RE2TM7, RETM3, RETM2, RE1TM1_x, RE7TM3,
REgTM and RE-TM eutectic structure
~RE-TM-B series
RETMqB, RE3TM11Bq, RE2TM5B2, RE2TM7B3, RE2TM5B3, RETM12B6r
RETM2B2, RETMgBq, RE2TMB3
Moreover, powder composed mainly of the above
RE2TMIqB, RE-TM series and RE-TM-B series intermetallic
compound phases can be obtained as follows.
That is, constitutional elements are weighed so
as to have a given composition and shaped into an ingot
by arc melting or high frequency melting under vacuum or
_ 9 _ 20441y fi
in an inert gas atmosphere. Then, the ingot is held at
a temperature of 600-1000°C under vacuum or in an inert
gas atmosphere for 1-30 days to form a single phase of
intermetallic compound. In general, the intermetallic
compound phase has frequently a solid solution range to
a certain extent (~20~), so that the starting composition
is allowed to have a composition width in accordance
therewith.
The single phase of the intermetallic compound
is roughly ground by means of a hammer mill and then
finely divided into a particle size of 0.5-5 ~m by using
a jet mill or an attritor . Moreover, when the hardness
is low and the pulverization is difficult in the low
melting point RE-TM and RE-TM-B phases, hydrogen
brittleness is previously carried out within a
temperature range of room temperature to about 350°C for
several hours before the grinding with a hammer mill,
whereby the subsequent pulverization is made easy.
According to the invention, powder composed
mainly of the previously prepared intermetallic compound
having a composition of RE2TM14B is mixed with at least
one powder composed mainly of the previously prepared
RE-TM series intermetallic compound and RE-TM-B series
intermetallic compound phases having a melting point
lower than that of the above powder, pressed and
sintered, whereby high magnetic properties and high
2044171
corrosion resistance can simultaneously be provided.
This is considered to be due to the fact that
the powder having a melting point lower than that of the
powder composed mainly of RE2TM14B intermetallic
compound phase promotes the sintering and forms an
intergranular phase between crystal grains of REZTM14B
to improve coercive force.
In RE2TM14B phase, Nd and Pr are desirable as RE
from viewpoints of magnitude of magnetic moment and
1o magnetic coupling with TM atm as well as the cost, but
it is needless to say that the other RE or a combination
of Nd, Pr therewith may be used.
As to TM, one or more of Fe, Co and Ni is
sufficient, and particularly it is preferable to
increase the content of Ni from a viewpoint of high
corrosion resistance of the magnet. Further, REZTMIaB
phase bears the saturated magnetic flux density of the
magnet, so that the contents of Fe, Co and Ni in TM are
desirable to be not less than 10 at% but less than 73
20 at% in Fe, not less than 7 at% but not more than 50 at%
in Co and not less than 5 at% but not more than 30 at%
in Ni. Even when the main phase is RE2TM14H phase in
which Fe as TM is 100%, the corrosion resistance of the
permanent magnet according to the invention is superior
to that of the conventional RE-TM-H magnet, so that the
above phase can naturally be used as a main phase in
64881-387
X0441
-11-
accordance with the use purpose of the magnet.
As RE in the low melting point phase of RE-TM
system and RE-TM-B system, light rare earth element such
as La, Ce. Pr, Nd or the like is advantageously adaptable
importantly considering the cost, and middle to heavy
rare earth elements from Sm to Lu and Y, Sc and the like
are adaptable for more enhancing the corrosion
resistance.
As to TM, the presence of Ni and/or Co,
particularly Ni is effective to improve the corrosion
resistance, so that according to the invention Ni is
necessarily contained as TM. In this case, the content
in TM is preferable to be not less than about 8~.
The addition effect of Ni is as follows.
i) The melting point of RE-TM system and RE-TM-B system
is lowered, and the wetting of liquid phase in the
liquid phase sintering is promoted to increase the
sintering density and enhance the residual magnetic flux
density.
ii) The effect of cleaning grain boundary in liquid
phase is enhanced in the liquid phase sintering to more
increase the coercive force by the same reason as in the
above item i).
iii) It is effective to the improvement of corrosion
resistance and cheap as compared with Co.
Furthermore, when the ratio of Ni and/or Co in
-12 - 20441' 4
the low melting point phase is made higher than that of
REzTMI4B phase, the corrosion resistance can be more
improved because the phases of these powders tend to
preferentially corrode in the grain boundary as compared
with RE2TM14B phase in the sintered body if the
structure of TM is same and is advantageously acted by
previously making electrochemically noble. Furthermore,
the magnetically useless Nd phase can be eliminated, so
that the residual magnetic flux density increases and
hence the maximum energy product (BH)max also increases.
In this connection, even when an alloy having an
average composition as a whole magnet is melted from the
first as in the conventional technique, pulverized,
pressed and sintered so as to approach to an equilibrium
state, the Nd phase is not obtained. For this purpose,
it is necessary to conduct the heating at a high
temperature for long time, during which abnormal growth
of crystal grain is undesirably caused to considerably
degrade the coercive force.
Moreover, it is not necessary that the same
element is used in RE of the main phase and RE of the
low melting point phase. And also, in the magnet
consisting of the above two phases, the effect of the
invention is not lost even when a part of RE and TM is
replaced with at least one of Mg, A1, Si, Ti, V, Cr, Mn,
Cu, Ag, Au, Cd, Rh, Pd, Ir, Pt, Zn, Ga, Ge, Zr, Nb, Mo,
- 20441'
In, Sn, Hf, Ta and W in an amount up to 8 ate of a full
magnet.
As to the production method, there may be
carried out a method wherein a mixture of powder of
RE2TM14B composition and powder composed mainly of low
melting point RE-TM series and/or RE-TM-B series
intermetallic compound phases is placed in an iron pipe
under vacuum and then sintered while hot rolling as a
method of producing large size magnets in addition to
the method in which the above powder mixture is
subjected to compression molding and then sintered.
BRIEF DISCLOSURE OF DRAWINGS
Fig. 1 is a Nd-Fe-B three component phase
diagram; and
Fig. 2 is a Nd-Co-B three component phase
diagram.
BEST MODE FOR CARRYING OUT THE INVENTION
Example 1
An alloy button was prepared by arc melting
neodymium, transition metal and boron at an atomic ratio
of 2:14:1, which was subjected to a normalizing
treatment in a vacuum furnace at 950°C for 7 days and
further to rough grinding and fine pulverization,
whereby fine powder having a particle size of few
microns was obtained. In this case, the ratios of Fe,
Co, Ni in the transition metal were varied to produce a
20 .441w' ~
plurality of alloy powders.
Similarly, powder having a ratio of neodymium or
(neodymium + dysprosium) to nickel of 1:1 was prepared.
In this case, the normalizing treatment conditions were
680°C and 5 days.
Then, powders selected from the above two groups
were mixed at a mixing ratio shown in Table 1, pressed
while applying a magnetic field of 15 kOe, sintered at
1000°C under vacuum for 2 hours and then quenched to
room temperature.
The magnetic properties and corrosion property
of the thus obtained samples were measured to obtain
results shown in Table 1. Moreover, the corrosion
property was evaluated by exposing the sample to an
environment at a temperature of 70°C and a humidity of
95~ for 48 hours and measuring a rusted area ratio on
the surface of the sample.
For the comparison, the measured results of a
sample produced by the conventional method in which a
full composition for the sintered magnet was melted at
once and subjected to rough grinding - fine
pulverization - pressing in magnetic field - sintering
steps are also shown in Table 1.
- 20~~1'~1
~ ' ~ ~ v ~ ~ ~ ~ ' O ~
rl ri N N M M er V' I!1 ttf l0 10
--i -'~ ~ '~ ~
' .,~ ' -,a' -..1' -..1' -..1
x ~ +~ ~ +~ ~ a~ 'o .u ~ .u - ~
N a~ d d d 0~ d N d v tV tv
w '~ ro '~ ~ '~ ro ~ ro '~ ~o ~ ro
.-~ ~ ,~ ,~ ~ ~ .-~ .a ,~ .a ,~ ,~
ro " ~ ~' N " ~ ~'-'a " w " w
a a a n. n, a c~, a, a a a c~
~~ b ~~ ~d a~ rd a~ ~ ~~ ~o a~ ro
a ~ ~ ~ ~ ~
d ' w ' 0.10' a ' a. ' a, ' a.
~d b 10 ~u 1a 1o ro 1o 10 ~o b
x ~ ~ ~ E ~ E ~ ~ x ~ ~ ~
x x x x x x x x x x x
V O V O V O V O V O V O
W W W W W W W W W W W W
U '~ U '~ U '~ U ~ U '~ U
b
d
N C1 O O N rl N M 1~
41 ri M
l.~
~
lSs
x~
td tf1 N 00 O O d' tD O tf7 O O r
~
~ N ~ 01 00 N vC O~ N ri t1f O ri
('~
M M N N M N N N M M M M
U N h 00 O N O O f~ 1~ In ri O
4J
x ~ ~ ~ ; ~ o o ; 0 o a
o
~ ~ o r o 0 0
O M CO tf1 t11 O 10 O d' 00 M tD
ya
U
W N M ri N rl N rl N ri N r-IN
x
ri rl rl ri r~ r~ ri ri ri ri r-1ri
cc r ~ c~
M ~
N _ M _ _ _ N _ M
t11 u1 ~ ~ N ~f1
~O 01 10 01
10 a ~ O
-rN M N M
w x _ _ ~ _ ~ _ - _
C r r
o i 1 _ N _ ~ _ i i
U
N N
w o~ c o~ a
~ ~
O ~ 10 M 1D M
W M _ O _ h _ ~ _ M _ ~ _
o r m ' ~' r m
o o
A i 1 i i i i i ~ _ _
H o 0 0
01 N ~ ~ d' 00
o ,dr ~ r o
v x ~ _ ~ _ ~ _ ~ _ ~ _ ~ _
b
w
w -.~ -,~ -wn -mn .,~ .,,
v o o o o
-
o~" x x x x x x
~ .. .. .. .. .. .
.. .. .. .. ..
0 .
0 ..
y o O o O O O
-~ o 1n o o o o
~
U U U U U U
N M M N
u~ .. .. .. .. .. .
.. .. .. .. ..
p p p p p . p
C ..
O O O O
~ O
d -'1 y -'i d -'i~ -'iy y G~ .
O ~ 1n ~ u1 ~ 1n ~ o O
G4 W G4 w Gu w
r-~ r .., t0 .~,10 .,.,ri .,~ OD .,,
tA N N tA N V1
O O 1f1O O O In O O O tf1O
o O tf1 O 111 O In
~'
1n n, u, o. 1n a, a a 1n a wn CL
1n c u, 1n 1n v~
.u 0 0 0 0
0 0
y U U U U ~ U ~ U
- -
v a~ d m x a~ x
trW w w w w .-i w ~ w
.-1 rt ~-1 ~ ~ ~-1
p,~ 00 00 00 00 00 00
-.~ ~c .c ~c .c o .c o .o
_~,
~ tT tr~ ~T O~ O~ tr~
p 3 3 3 3 3 3
- O O ~ ~ , O , O
E ~ -..1r-1-..1.~ -~1.-i .a o -..io -.~
~ tv y N N .-~ tv .-id
y PO .u C4 a~ CG +~ C1 .u a7 r..1
a~ > er > ~ > > A > A
~ -i 1
O O O
. .- v .-ac .-i v ~
d ri N ri m -1 N i O -I O ' O
~ ri a .-IW r-t ~ -I t7 ~ v
N ~
. r - r '-id
Tj ~ ~ ~ ~ ~ E r ~ ~ E ~ .Lt
~ -.1 1d -.ictf -.i c0 ~ cd ~ 10 ~ ~
-.a of
3 E H E E E E
y, x x x x p
O N U N U N U N U N U N U
O rl N ~--IN r-1 ~ r-i d ' Ol 'o d
w 2s >a o N u w o w 2s ~ b a
w o .a b .c ro .o ~o .c .~ -c
xx ~y xx a~ xx ~~ xx aw xx ~x.~xx a~
p ~ N M a m 1o r ao o~ '"1N
x
204~1'~1
-16-
As seen from the above table, the rare earth
metal-transition metal series magnets of two phase
structure according to the invention considerably
improve not only the magnetic properties but also
corrosion resistance as compared with those obtained by
melting the full composition from the first as in the
conventional technique.
Example 2
An alloy button was prepared by arc melting
neodymium, transition metal and boron at an atomic ratio
of 2:14:1, which was subjected to a normalizing
treatment in a vacuum furnace at 950°C for 7 days and
further to rough grinding and fine pulverization,
whereby fine powder having a particle size of few
microns was obtained. In this case, the ratios of Fe,
Co, Ni in the transition metal were varied to produce a
plurality of alloy powders.
Similarly, powder having a ratio of neodymium
and/or dysprosium or praseodymium to nickel or (nickel
+ cobalt) of 3:1 was prepared. In this case, the
normalizing treatment conditions were 485°C and 5 days.
The magnetic properties and corrosion property
of the thus obtained samples were measured to obtain
results shown in Table 2.
For the comparison, the measured results on the
properties of a magnet produced by the technique
-17-
20441'1
disclosed in Japanese Patent laid open N0. 63-164403 are
also shown in Table 2.
- ~U~41'~1
dr >r oao >COU01>01 00 >O dr-ISriNN 'N NM 'M dC' jct'1r10
ra r~ r~ ra ri r-i ri ra ri ri ri .i ri i'.,
ri ri ri r~ ri
.~G ~ r.l1'7 ~ ~ +1 'n t~ .D y l.7y,r ~ t~ ~ .u O
d y d N G7 d d f .-I
a ~ t0 b c0 at 0 t0 ~ I ~ 10 ~ Id ~ tc ~ -.~
.-I.-1~ .-I.-m.-1 G7 G7 G7 C7 G7 G7 d G7 d c~ ~
1 ~ ~ u a u -I r~ -1 1 -1 +r a
a --1 -a a -1
~s ,. ~ w ~ ,. .. . >a . . . r + r r . E
o. a a n, a. . a , >a w >~ . >a c~ >~ a
a a a a a c> a a a
~ c a
E ~ ro ~ is ~ 1~ ~ . ,~ , ,~ , ,~ . ,~ ,~
E E E E E E d E d , a~ E . E . , a~
G! a~ d E E E E d GI E
E E
a~ ro a id a ~ a a, w a a o. x
U ro U is U io U id U td U ft) U id U ~ >
id itt ed t~ id
GL x E x E x E E E E E E w
U x U x U x U x U X U x U x U x C
x x x x x
w O w O w O '~ O ~ O '~ O ~ O EC O
d w '~ w ~ w w w w w w w w w w w
' U U U U U U U U U
b
d
td
IffO O O O If7 M O N ~ M ~ M O M 00
N lf1 n-I N N N N n-1
s.y.
O
x~
O N N CO O N O tD O M rI lO 00 0 N t11117
'
~ 1D Ifl ~ N N ~C'N M N if W O 00 r ~O tf1
f
.'T. M M M M M M M M M M M M M M ri ri M
~.
U O N O ~ if1p u1 ,...Itft N ~ M M r~ It1 M O
O
N ri If1 O ill O N O CO ttft0
r ~ p 01 t0 O
r~ rl rl rl ,-~ ~ ,..~rl N ri rl
l0 r-IO 00 lt1M 10 M 00 lf7N OD ri M O p M
N M N N rl N r-IN r~ N N N N M ~ ~ N
yr ri ri ri r-1r~ r~ r~ ri r~ ri r~ ra ri ri ri
N N N N ~ V' N M N
N _ N _ N _ N _ ~ _ M _ N _ 01 _ N
N ~ d'
..a .-1 r '"1- N M oo c
.U -.1pp _ 00 _ a! _ ~ _ M _ O _ N _ O _ 1
x
b N N ~D N N rl O d'
N N ri tf1 N tD O M
C~ 01 ~O M ~ C~ W M
I 1 ~ - ~ - ~ - ~ - ~ - ~
O N N rl ri ri N rl N
t0 01 iD ri 00 N in 10 Lff r
E d O r-I U1 d' M 00 ri N lt1
fV4"IW M ri r 00 d' 01 r-I in N
o r in a~ u i m w n
O
C
O ~ 1 1 1 I I 1 I I ~ _ I 1 1 I 1 1 I
ra
E-I.r.,
O A I I I I I I N - I I i I 1 1 1
~ p
E ri
V ao 0 o c m c o r o
.d 00 O O 10 1 1 If1 O p O
x ao ' oo ' ao ' ao - r ' a~ ' ' 00
0
1
.r.,
da
~' -~ -.~ -wn -.~ -~ -.10 .~ .~ -~
In o o o o o o o
o
x x x x x x x x x
.. .. .. .. .. .. .. .. ..
. . . . . . . . .
dw
w o0 00 00 00 oin o0 00 00 00
E
O U UM UM UN U'-I UN UM UN UM
v
O C >~ G >~ >~ G C C
-'~
O
p .. O .. ~ .. O .. O .. O .. O .. O .. O ..
E . . . . . . . . .
-'"i
-.-i
O
.~ .,.., .,., .r., .,., .,a .,.., .,..,
0
~ JJ .LJ ~1 .IJ iJ JJ J
1
G7 .,1N .,iN - .,i!y .,id -
O O to tn O - O - - O
i
w H W ~ GfIN w Gy w N W u G ' W
r-1 r lO p CO 00 00
O O J 4 N
a a
o , ct, a a, c~ n. o.
-~ in E in E in E in E o E o E in E in E in
in in in ,n o o in in in
10 O 1D O 1D O 10 O r O r O ~D O it) O 1D
M M M M M M M er M
U U U U U U U U
ri
4.~ N N N ~~ N d GJ ty
o w w w x w w ~-iu..l~-Iw w .-a
~. rl .-i ,-1 M r-, .-I .-a rl .-,
~
O
P
d O O O _ O O O O O
O O O O O O O O
-'i 3 M o 3
~
o
d~ tT tri b~ C~ O tT tr~ tr~O
x-~-~ 3 3 3 3 3 3
c a a a a o a o a a d
-''i
U
~
.-i,-rr-1 -.,,.-1-.1 .-~-..i,~ .i .-~1U.i r.~U.r .-1 -.ar-Ifs
E ~ to to o d O d d G7
E m ~
~
pq > a7 r t~ ~ 00 .u W y C7 ~ to ~ t0 ~..>pct
w ' > > >v > '~ > 00 > .I > > ~
N
C .-Ic .-ac r-I aTGI.-I~r r-1c~ r-1 er .-1~r ~ er
o o o O o O ' 0 o
d ~ ai .~ d .-ral ~--ia~ ~-axa~ ~,~d ~~,a~ ~ am ~-10
w ,--i.o ~-i ~ .-1~ r .o It ~ ~ ~-I
,o - E ~ E ~ E ~ E E E ~ E ~ E ~ E ~
V ~ id -~ e0 -~ 10 o~ id o irix 10 x aS -~ is1U
Hx Hx Hx H E
H.~ H'. Hx H...
p
NM U NM U NM U N U NN U NM U NM U NM U NM
d d N d N Gf d d
'O l.,'O H 21 a 'o s.aH 1-~'O >a 'd a 'O N 'Cf
'O x 'O .r~Tf .C ~ .C S-~ .>~'O .>~ 'b .C >, .r~'O
xx ~~ xx ~x~xx ay x~.a~ ww a~ xx a~ xx ~~ xa ~~ xx
o M a w ~a r ao c~ o ~ N M v i wo r o~ o~
x rl ri ri rl rl rl e1 N N N N N N N N N N
i~:f~~~1'~1
As seen from the above table, the rare earth
metal-transition metal series magnets of two phase
structure according to the invention are excellent in
the magnetic properties and corrosion resistance.
Furthermore, when Acceptable Example 8 is compared with
Acceptable Example 13, it is apparent that the corrosion
resistance is improved as the Ni ratio in RE3(Ni, Co)1
becomes particularly higher. Moreover, in the
conventional example, the magnetic properties are good,
but the corrosion resistance is poor because Ni is not
contained.
Example 3
A fine alloy powder of REZTMIqB composition was
prepared by the same manner as in Example 1, while a
fine alloy powder in which ratios of Ni and Co in TM
were made higher than those of RE2TM14B powder was
prepared as a starting powder. After these powders were
mixed, a sintered magnet was produced by the same manner
as in Example 1.
The properties of the thus obtained sintered
magnet are shown in Table 3 together with those of the
sintered magnet produced by the conventional method.
~(~~41'~1
-20-
N v a~ ~ d p
u~ ~n ~o ~c r r
r-I ~ r-i ~ r-I ~
ri .~ .~ r-1 rl r-1
.n
~ '
a ~-I ~ -i ~ ~O
~ b ~ -I ~
' . r o. , r ,
a ~ o. ~' n, ~'
c~. a a a, a
n ~
v ~ v ~ v ~
e ro ro w ro ro
d
oW ~W oW ~W oW
U U U
.d
47
10
N O M O M O M
~
CP
O
~,
x~
is u~ ao m ~-~Ir
~ rl ri 01 O N ri
Vr
M M N M M M
O~
U N rl 1!1 00 O N
d
xo
~; r vrf M oo vo
a N
t9
f~ ~ N rl N ri
.x'
'. r-I .1 ri r-I .1 .1
a o 0
C1 _ M _ M
r ao ao
a~ o~ o~
w o, o0 0
x ~ _ ~ _ ~ _
a~ p r
E U r I ri _ ri
N N N
W 111 O O
O d t0 M M
p w ri _ r _ r _
p c a' a'
.,.,
I I 1 I I I
O o1 ~ d'
~ yD OD N
o x N _ N _ ~ _
U r1 ~~ rl
O
O N 00 t0
.i .i O O
~ u'1
t0 O N O CO O
-ri M ri lff r-1 M ri
O
N
i
. .. .. .. .. .. ..
.. .. ..
..
l-i tf1 O
r-I
~
~J O O
Id O
~
.u U u1 0 0 0o O
M 0
rl
W y0 M M M 111 M
CJ M
.. .. .. ..
.. ..
..
O . ..
O .
If1 N
+~ N O
~ p'
1I1 N ~l1 O O O O
t11 r
Q; w lf1 r 10 rl 10
10 ri M
N
O O O
O O O O
dp
O W N ~ tf1 -ri
O ~ tff ~ ~
' '
~O tt7 tt7
d aT d
~ G7 G7 N
aJ v! N (~
a .p ,p .O
ld O O O
H JJ y y!
~ ~
W W W
x O O O
i O O O
C U U U
~ O~ O~ tr~
U G7 07 N
C C p
.-i .a .-1
a a7 ~ Ca +~ Ca ~
a .C .C x
O ~! .-1 V' .-1 Q' .-I
rl 3 rl 3 ri 3
~ a
3 a~ ~ ~ E ~ E
a ~ ai N ~ N
.
~ NE U NE U NH U
W O O O
'Cf sr 'O H b a
'C3 "p 'C1 .C7 'C1 .t7
xx a xx ~ xx
to ~
O O ri N M W t1
x M M M M M M
- 2a~~~~1
As seen from the above table, when using the
fine alloy powder in which the ratios of Ni and Co in TM
are higher than those of RE2TMIqB powder as a powder to
be mixed, the more improvement of the corrosion
resistance is attained.
INDUSTRIAL APPLICABILITY
According to the invention, the rare earth
metal-transition metal series magnets having improved
corrosion resistance and magnetic properties can be
produced as compared with the conventional production
method. Particularly, the corrosion resistance is
improved, so that the considerable improvement of
reliability as an industrial material is realized.