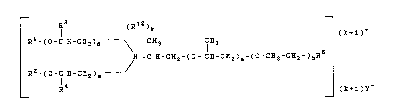Note: Descriptions are shown in the official language in which they were submitted.
204423~
The invention relates to poly(oxyalkylene)-
aminoalkanolamine esters of the general formula
r l3 (Rl2)1~ --¦
R -~o-C ~-C}~2~ CH3 C1~3
~ -C ~-CH2-~0- C~-CH2~a-~0-C~2-C~2-)bR (1)
R2 _ ( o -C ~ -Cl~z ) "--
0 L R~ _ (~1 )Y
Y~r~ R5 nay ba a
(R~3) R7
C~ 3_ 1 ~ ( C~2- CX-O ~ -R9
_(o-c~z-c ~ c ~ CH2-CI~-o-~g
RD
group or -OH or -o-R6,
R6 may be an alkyl group having from 1 to 5
carbon atoms, or a -C-R11 group,
o
and where R1, R2, R9 and R10 may be,
independently of one another, hydrogen, an alkyl group
having from 1 to 5 carbon atoms, and preferably CH3, or
a C- R11 group,
11
R11 may be a hydrocarbon group having from 6 to
21, and preferably from 7 to 17, carbon atoms which is
optionally branched and optionally contains multiple
bonds or hydroxyl groups, with the proviso that at least
one of the groups R1, R2, R9 and R10 must be the group
2510:CHS2510
2044234
-C-Rll, and
R3, R4, R7 and R8 may be, independently of one
another, H or CH3, and
R12 and R13 may be, independently of one
another, H, CH3, C2Hs or a benzyl group,
k and l are from 0 to 1,
k + l is from 0 to 2,
a + c is from 1 to 40, and preferably from 1 to
15,
b is from 0 to 40, and preferably from 0 to 15,
d + e + f + g is from 2 to 12, and
y~ is a mono- or polyvalent anion, and
preferably a lactate, methosulfate, sulfate or chloride
with a total of k + 1 negative charges.
The invention also relates to a process for
producing these compounds and their use in the production
of emulsifiers, detergents and disinfectants.
The following amino compounds of formulas (2)
and (3) may be used as starting compounds to produce the
ester amines:
CIH3 l~3
H2~-CH-CH2-~PO~a-(EO)b-(P0)C-cH2- CH ~H2 (2)
c~3
~2N-C~-c~2-(Po)~ o)b-(~o)c-o R
2510:CMS2510
2~4234
CH
1 3
where Po is -(O-CH2-CH) and EO is -(O-CH2-CH2)-,
a is 0 to 20,
c is 0 to 20, and
a, c, b and R6 have the meanings given above,
R6 being more particularly an alkyl group having from 1
to 5 carbon atoms, and c being 0.
These products are produced commercially and
are generally obtained by known processes by reacting
polyoxyalkylene alcohols with ammonia under pressure.
Polyoxyalkylene alcohols are typically prepared
by the addition of an alkylene oxide, especially
propylene oxide, ethylene oxide or a mixture of the two,
by a conventional method to a compound which contains one
or more active hydrogen atoms, or by polymerization of
alkylene oxides.
Illustrative of compounds containing one or
more active hydrogen atoms are monohydric alcohols such
as ethanol, isopropanol, butanol, lauryl alcohol, stearyl
alcohol, and particularly methanol, or glycols such as
ethylene glycol, propylene glycol, diethylene glycol,
glycerol, trimethylolpropane, pentaerythritol, sorbitol,
polyglycerol, and polyvinyl alcohols.
The polyoxyalkylene alcohols have molecular
weights ranging from about 100 to 10,000, and preferably
from about 150 to 5,000, and most preferably from about
150 to 2,000.
The further reaction to give amines is carried
out typically by conventional methods through aminolysis
of the free hydroxyl groups or their esters, particularly
the sulfates. In the case of higher alcohols, the -OH
group is exchanged for the amino group by means of
homogeneous catalysis, and especially of heterogeneous
2510:CMs2510
204423~
catalysis on solid catalysts. For this purpose, at least
two methods in particular are available. One employs
dehydrating catalysts, and the other, catalysts having
hydrogenating/dehydrogenating action.
Alumina with up to about 20% of chromic oxide,
stannous oxide, zinc oxide or ferric oxide is used as
catalyst.
Alumina deposited on silica gel or activated
charcoal is suitable for the conversion at atmospheric
pressure of higher fatty alcohols to fatty amines, for
example. (See British patent 384,714 and U. S. patents
2,017,051 and 2,078,922.) Acids of aluminum phosphate,
or phosphoric acids, either as such or on supports such
as silica gel or diatomaceous earth (U. S. patent
2,073,671), are frequently used in production at standard
or elevated pressure (U.S. patent 2,043,965). The
operating temperatures range from 200 to 600C, depending
on the catalyst. The literature is replete with data on
the effect of temperature and pressure, on an excess of
ammonium, and on the necessary residence times. (See,
for example, Houben-Weyl, Methoden der organischen
Chemie, Georg Thieme Verlag, Stuttgart 1957, vol. II/1,
p. 108 et seq.)
In accordance with the invention, the following
compounds are preferred:
Of formula (2),
a + c = 2 - 6 (I)
b = 0
or
a + c = 2 - 3 (II)
b = 6 - 9
2510:CMS2510
20~4234
and of formula (3),
a + c = 6 - 10 ~III)
b = 1 - 2
R6 = -CH3
The compounds of formulas (2) and t3) are then
alkoxylated, and preferably ethoxylated or propoxylated,
typically by conventional methods. The usual procedure
is to react the amines in a pressurized reactor at
between 120 and 160 C, optionally in the presence of
basic, and more particularly alkaline, catalysts at from
1 to 4 bars with a quantity of alkylene oxide correspond-
ing to the desired degree of alkoxylation, ethylene oxide
and propylene oxide or mixtures thereof being preferred
in accordance with the invention.
What is obtained are compounds of the general
formulas
H-tO-CH-CH2)d \ ~ CH2CH-O)f H
~-cH-c~2-A-c~2-c~-N (4
~_(O-C~~c~{z)e -- (CH2CH~O)g~H
R4 R3
and
CI~3
~-(o-c~-cHz~a ~~
~-C~-CH2-A-O-R6 ( 5
H- ( O -C~-CH2 ~ e
I
R~
2510:CMs2510
2044234
where A is ~(Po)a-(Eo)b-(po)c~ and where a, b and c, and
R6, EO and PO, all have the same meaning as above and
d + e + f ~ g = 2-12, and
R3 R4 R7 and R8 = H
and, independently of each other, may be H or CH3.
Preferred compounds of formula (4) are
compounds where
d + e + f + g = 4-6, and (IV)
R3, R4, R7 and R8 = H
and preferred compounds of formula (5) are compounds
where
d + e = 2-3 (V)
R3 and R4 = H
The subsequent esterification of the compounds
(4) or (5) with carbonic acids or derivatives thereof
gives compounds of the general formulas
R3 R7
R -(O-C~I-CH2~d \ I 1 3 ~CII2-CH-O)~--R9
N-CX-CH2--A-C~2- C~-N~ ( 6
( C~2-C~-0 )g-Rl
R2- ( O -CH-CE., ), 1 8
and
7 CH3 (
Rl-~O-Cx-c~2)~ \N_CH_c}I2-A-o-R
1~. --(o--c~-cx2)~
l4
2510:C~IS2510
204~234
here a, b, c, d, e, f and g, R3, R4 R7 R8 and R6
A have the same meaning as above and R1, R2, R9 and R10
are, independently of one another, hydrogen, an alkyl
group having from 1 to 5 carbon atoms, or a -C-R11 group,
S 11
R11 being a hydrocarbon group, optionally branched, and
optionally containing multiple bonds or hydroxyl groups,
having from 6 to 21, and preferably from 7 to 17, carbon
atoms, with the proviso that at least one of the groups
Rl, R2, R9 and R10 must be a -C-R11 group.
15 Fatty acids which are suitable for
esterification or transesterification are the monobasic
synthetic fatty acids known and commonly used in this
field, and especially the fatty acids based on natural
plant and animal oils having from 6 to 22, and more
particularly from 8 to 18, carbon atoms, such as those
derived from coconut oil, palm oil, tallow, and castor
oil. These may be used as glycerides, as esters with
short-chain alcohols, or as free acids.
Their esterification or transesterification is
carried out by the known method.
The alkanolamines are thus reacted at from 160
to 240C with a quantity of fatty acid or fatty acid
ester corresponding to the desired degree of
esterification, optionally in the presence of a catalyst,
and the water of reaction or the alcohol is continuously
distilled off, a vacuum being applied if necessary to
complete the reaction.
Preferred compounds of formula (6) are
substances where
2510:CMs2510
2~44234
R1, R , R , R , = 2 x H
2 X -C-R11 (VI)
o
R11 being C17H35, and preferred compounds of formula (7),
are where
R1 = H
R2 = C-R11 (VII)
1 0 0
R11 being C17H35.
The quaternization or the preparation of the
salts of the compounds of formulas (6) and (7) typically
are carried out by methods known in this field and yields
the inventive ester amine quaternary ammonium or ester
amine salts of the general formula (1), where R12 and R13
have the meanings indicated, k and 1 are greater than
zero, and k + 1 preferably has values between 0.5 and 2.
As a general rule, the salts are prepared by
adding the acids, optionally as aqueous or alcoholic
solutions, in a quantity corresponding to the desired
degree of salt formation at 20-80C with vigorous
stirring, and optionally with cooling, in portions to the
poly(oxyalkylene)alkanolamine esters introduced as
initial charge. The quaternization is carried out by one
of the generally known methods, the poly(oxyalkylene)-
alkanolamine esters being heated to 40-80C, optionally
with the concurrent use of a solvent, and reacted in
portions with the quaternizing agent in a quantity corre-
sponding to the desired degree of quaternization.
2510:C~IS2510
2044234
Suitable anions which are preferred thus are:
o o
Il 11
CH--O --S --O~ CE~3CHz --O --S --O . HCOO . CH3COO
O o
C~ 3 --C~ --C OO ~ , O ~C~ 2COO ~ , ~ OO C --C ~ --C ~ --C O O ~
OH OH OH OH
OOC --CH--CH2 --COO . OOCCH2 --C--CH2 --COO , C6H5C
0~ COo
OOC --(CIIz~n --COO , Cl, Br, J, 5o42, PO93 and ~O3,
here n = O - 10.
Particularly preferred anions in accordance with the
2 0 invention are the anions
o
Cl-, S042-, C~9 --O --S --O~ and~r C~{3 --C~ --COO-
O OK
Several anions may be present adjacent to one another.
The inventive compounds of the general formulas
(6) and (7) may be used as components in the production
of polyesters, polyester amides, polyurethanes or
polyepoxies, which find use as flexible coatings, molded
articles, sealing compounds and fiber-reinforced
composite materials.
2510:CMS2510
20~4234
They are also of advantaqe in the formulation
of emulsifiers for use in agriculture, metalworking, and
to some extent in cosmetics.
The inventive compounds of the general formula
(1) with k + 1 > 0 find use especially as antimicrobial
agents. They are suitable for use as preservatives or
disinfectants for all kinds of commercial products used
in crop protection, agriculture and cosmetics.
They further lend themselves to the
preservation of glues, surface coatings or paints based
on organic vehicles. They can also be used as wood
preservatives.
By combining the compounds of the general
formula (1) with surface-active substances, washing and
cleaning agents having antibacterial action are obtained
which can be used as antimicrobial textile finishes.
The analytical methods used in the examples
which follow are those commonly employed in this field.
1 Total amine value and tertiary amine value
.
The total amine value gives the number of
milligrams of potassium hydroxide equivalent to the total
amine basicity of 1 gram of the amino compound (mg
KOH/g). The tertiary amine value gives the number of
milligrams of potassium hydroxide equivalent to the
tertiary amine basicity of 1 gram of the amino compound.
The values are determined by A.O.C.S. Official
Nethod Tf 2a - 64.
2. Sa~onification value
The saponification value is a measure of the
free and combined acids in fats and commercial fatty
acids. It gives the number of milligrams of potassium
hydroxide required for the complete saponification of 1
gram of fat or commercial fatty acid.
2510:CMS2510
204~234
The values are determined by the unit methods
of Deutsche Gesellschaft fur Fettchemie (DGF) (German
Society for Fat Chemistry), DGF C-V3.
3. Hydroxyl value
The hydroxy value is used to determine the
hydroxyl-group content and gives the number of milligrams
of potassium hydroxide required to neutralize the acetic
acid consumed by 1 gram of fat in acetylation (mg KOHtg).
The values are determined by DGF unit method
C-V17a.
4. Acid value
The acid value is a measure of the free-acid
content of a fat or of commercial fatty acids and gives
the milligrams of potassium hydroxide required to
neutralize 1 gram of substance.
The values are determined by DGF unit method
C-V4.
5. Content of cation-active substance. Cat S03
This method is used to determine the content of
cation-active substances. Cation-active substances here
are short- and long-chained compounds containing primary,
secondary and tertiary amino groups or ammonium groups.
The content is expressed as S03 per 100 g of the substance
being tested.
The content is determined by two-phase
titration in conformity with IS0 standards 2871-1 and
2871-2 (1988 edition).
2sl0~ s2510
2044234
EXAMPLES
(I) Preparation of hYdroxYlamines
of the qeneral formulas ~4) and (5)
Example 1
912 g (2 mols) of an amine of the general
formula ~2) with
a + c = 6.6
b = 0
was mixed in an autoclave at 145-160 C in portions with
352 g (8 mols) of ethylene oxide so that the pressure was
maintained at between 1 and 3 bars. After the amount of
ethylene oxide added had been reacted, 1,264 g of a clear
liquid of the general formula (4), with
a + c = 6.6
b = 0
a + e + f + d = 4 and
R3, R4, R7 R8 = H
was obtained.
This compound had a total amine value of 179 mg
KOH/g, a tertiary amine value of 175 mg KOHlg, and a
hydroxyl value of 348 mg KOH/g.
Exam~le 2
912 g (2 mols) of an amine of the general
formula ~2) with
a + c = 6.6
b = 0
was alkylated in an autoclave at 145-160 C in portions
with 352 g (8 mols) of ethylene oxide so that the
pressure was maintained at between 1 and 3 bars. After
the ethylene oxide added had been reacted, 0.2 g of KOH
was added to the reaction mixture, and then another 352
g (8 mols) of ethylene oxide was added as described above
2510:CM52510
2044234
14
and reacted. 1,616 g of a clear liquid of the general
formula (4), with
a + c = 6.6
b = 0
5d + e + f + g = 8 and
R3, R4, R7 R8 = H
was obtained.
The analytical values of this compound were:
Total amine value: 141 mg KOH/g
Tertiary amine value: 141 mg KOH/g
Hydroxyl value: 296 mg KOH/g
- Example 3
237 g (1 mol) of an amine of the general
formula (2),
with
a + c = 2.8
b = 0,
was alkylated as described in Example 1 with 176 g (4
mols) of ethylene oxide. 413 g of a clear liquid of the
general formula (4), with
a + c = 2.8
b = 0
d + e + f + g = 4 and
R3, R4, R7 R8 = H
was obtained.
This compound had the following analytical
values:
Total amine value: 275 mg KOH/g
Tertiary amine value: 268 mg KOH/g
Hydroxyl value: 567 mg KOH/g
2510:1:Ms2510
2044234
Example 4
237 g (1 mol) of an amine of the general
formula (2), with
a + c = 2.8
b = 0
was alkylated as described in Example 1 with 352 g (8
mols) of ethylene oxide. 589 g of a clear liquid of the
general formula (4), with
a + c = 2.8
b = o
d + e + f + g = 8 and
R3, R4, R7 R3 = H
was obtained.
This compound had the following analytical
values:
Total amine value: 197 mg KOH/g
Tertiary amine value: 197 mg KOH/g
Hydroxyl value: 439 mg KOH/g
Example 5
615 g (1 mol) of the amine of the general
formula (2), with
a + c = 2.5
b = 9,
was alkylated with 176 g (4 mols) of ethylene oxide, as
described in Example 1. 791 g of a clear liquid of the
general formula (4), with
a + c = 2.5
b = 9
d + e + f + g = 4 and
R3, R4, R7, R8 = H,
was obtained.
This compound had the following analytical
values:
2510:C~IS2510
2044234
16
Total amine value: 144 mg KOH/g
Tertiary amine value: 142 mg KOH/g
. Hydroxyl value: 291 mg KOH/g
Exam~le 6
550 g (1 mol) of an amine of the general
formula (3), with
a = 7.7
b = 1
R6 = CH3,
was alkylated with 132 g (3 mols) of ethylene oxide, as
described in Example 1. 682 g of a clear liquid of the
general formula (5), with
a = 77
b = 1
R6 = CH3
d + e = 3 and
R3 R4 = H
was obtained.
This compound had the followig analytical
values:
Total amine value: 84 mg KOH/g
Tertiary amine value: 84 mg KOH/g
Hydroxyl value: 180 mg KOH/g
Exam~le 7
912 g (2 mols) of an amine of the general
formula ~2), with
a + c = 6.6
b = 0,
was alkoxylated with 464 g (8 mols) of propylene oxide.
1,376 g of a clear liquid of the general formula (4),
with
2510:CMS2510
2044234
a + c = 6.6
b = 0
d + e + f + g = 4
R3, R4, R7, Rs = CH3
Thecompound had the following analytical
values:
Total amine value: 163 mg KOH/g
Tertiary amine value: 162 mg KOH/g
Hydroxyl value: 323 mg KOH/g
(II) Pre~aration of ester amines
of the qeneral formulas (6) and (7)
Exam~le 8
625 g (1 mol) of the amine ethoxylate from
Example 1 was mixed with 570 g (2 mols) of tallow methyl
ester (C17H3SCOOCH3), 1.5 g of solid, powdered NaOH and 3
g of sodium hypophosphite and, under a nitrogen
atmosphere, stirred and heated to 180 C. The methanol
forming during the reaction was distilled off. After
approximately 90% of the theoretical amount of methanol
had been removed, a vacuum of about 20 mbars was applied
and the transesterification completed. After
approximately 7 hours, 1,135 g of a yellow liquid of the
general formula (6) with
a + c = 6.6
b = 0
d + e + f + g = 4
R3, R4, R7 R3 = H
R1, R2, R9, R10 = 2 X H, 2 X -C-C17H35
O
This compound had the following analytical
values:
Total amine value: 98 mg KOH/g
Tertiary amine value: 97 mg KOH/g
2510:CMs2510
204~23~
18
Hydroxyl value: 93 mg KOH/g
Saponification value: 106 mg KOH/g
Exam~le 9
625 g (1 mol) of the amine ethoxylate from
Example 1 was transesterified with 855 g t3 mols) of
tallow methyl ester, 2 g of NaOH and 4 g of sodium
hypophosphite, as described in Example 8. 1,390 g of a
yellow liquid of the general formula (6) with
a + c = 6.6
b = o
d + e + f + g = 4
R3, R4, R7 R3 = H
Rl, R2, R9, R10 = 1 X H, 3 X -C-Cl7H35
o
was obtained.
This compound had the following analytical
values:
Total amine value: 78 mg KOH/g
Tertiary amine value: 74 mg XOH/g
Hydroxyl value: 57 mg KOH/g
Saponification value: 117 mg KOH/g
Exam~le 10
799 g (1 mol) of the amine ethoxylate from
Example 2 was transesterified with 570 g (2 mols) of
tallow methyl ester, 2 g of NaOH and 4 g of sodium
hypophosphite, as described in Example 8. 1,311 g of a0 . yellow liquid of the general formula (6) with
a + c = 6.6
b = 0
d + e + f + g = 8
2510:CHs2510
20~4234
R3, R4, R7 R8 = H
Rl, R2, R9, Rl0 = 2 X H, 2 X -C-Cl7H35
was obtained.
This compound had the following analytical
values:
Total amine value: 89 mg KOH/g
Tertiary amine value: 88 mg KOH/g
Hydroxyl value: 88 mg KOH/g
Saponification value: 82 mg KOH/g
Example 11
775 g (1 mol) of the amine ethoxylate from
Example 5 was transesterified with S70 g (2 mols) of
tallow methyl ester, 1.5 g of NaOH and 3 g of scdium
hypophosphite, as described in Example 8. 1,285 g of a
yellow liquid of the general formula (6) with
a + c = 2.5
b = 9
d + e + f + g = 4
R3, R4, R7 Rs = H
R1, R2, R9, R10 = 2 X H, 2 X -C~C17H3s
O
This compound had the following analytical
values:
Total amine value: 86 mg KOH/g
Tertiary amine value: 85 mg KOH/g
Hydroxyl value: 86 mg KOH/g
Saponification value: 91 mg KOH/g
Example 12
407 g (1 mol) of the amine ethoxylate from
Example 3 was transesterified with 570 g (2 mols) of
2510:CMS2510
20~423~
tallow methyl ester, 1.1 g of NaOH and 2.3 g of sodium
hypophosphite, as described in Example 8. 916 g of a
yellow liquid of the general formula (6)
with
5a + c = 2.8
b = 0
d + e + f + g = 4
R3, R4, R7 R3 = H
R1, R2, R9, Rl0 = 2 X H, 2 X -C-C17H35
1 0 0
was obtained.
This compound had the following analytical
values:
Total amine value: 121 mg XO~/g
Tertiary amine value: 121 mg KOH/g
Hydroxyl value: 118 mg ROH/g
Saponification value: 127 mg KOH/g
Example 13
625 g (1 mol) of the amine ethoxylate from
Example 1 was mixed with 539 g (2 mols) tallow fatty acid
and 1.2 g of hypcphosphoric acid under a nitrogen
atmosphere with stirring and heated to 18Q C. The water
forming during the reaction was distilled off. After an
acid value of about 10 had been obtained, a vacuum of
about 20 mbars was applied and the reaction was continued
until the acid value was less than 2. 1,129 g of a clear
liquid of the general formula (6) with
a + c = 6.6
b = 0
d + e + f + g = 4
2510:CHS2510
2044234
R3, R4, R7 R3 = H
R1, R2, R9, R10 = 2 X H, 2 X -C~C17H3s
was obtained.
This compound had the following analytical
values:
Total amine value: 93 mg KOH/g
Tertiary amine value: 93 mg KOH/g
Hydroxyl value: 95 mg KOH/g
Saponification value: 98 mg KOH/g
Acid value: 1.3 mg KOH/g
Example 14
1,669 g ((2.5 mols) of the amino alcohol from
Example 6 was transesterified with 1,059 g (3.75 mols) of
tallow methyl ester, 2.8 g of NaOH and 5.8 g of sodium
hypophosphite, as described in Example 8. 2,626 g of a
clear yellow liquid of the general formula (7) with
a = 7.7
b = 1
R6 = CH3
d + e = 4
R3 R4 = H
R1, R2, = 0.5 X H, 1.5 X -C-C17H35
was obtained.
This compound had the following analytical
values:
Total amine value: 55 mg KOH/g
Tertiary amine value: 55 mg KOH/g
Hydroxyl value: 41 mg KOH/g
Saponification value: 76 mg KOH/g
2510:CHS2510
2044234
Example 15
625 g (l mol) of the amine ethoxylate from
Example 1 was reacted with 1,140 g (4 mols) of tallow
methyl ester, 2 g NaOH and 3 g of sodium hypophosphite,
as described in Example 8. 1,642 g of a yellow liquid of
the general formula (6) with
a + c = 6.6
b = 0
d + e + f + g = 4
R3, R4, R7 R8 = H
R1, R2, R9, R10 = 4 X -C-Cl7H35
was obtained.
The reaction product had the following
analytical values:
Total amine value: 68 mg XOH/g
Tertiary amine value: 68 mg KOH/g
Hydroxyl value: 1.5 mg KOH/g
Saponification value: 139 mg KOH/g
Exam~le 16
625 g (1 mol) of the amine ethoxylate from
Example 1 was reacted with 285 g (1 mol) of tallow methyl
ester, 1.5 g of NaOH and 3.0 g of sodium hypophosphite,
as described in Example 8. 882 g of a yellow liquid of
the general formula (6) with
a + c = 2 - 6
b = 0
d + e + f + g = 4
R3, R4, R7, R8 = H
R1, R2, R9, R10 = 3 X H; 1 X -C-C17H35
O
2510:c~s2510
2044234
was obtained.
The reaction product had the following
analytical values:
Total amine value: 128 mg KOH/g
Tertiary amine value: 128 mg KOH/g
Hydroxyl value: 64 mg KOH/g
Saponification value: 191 mg KOH/g
(II) PreParation of quaternarv ammonium compounds
or of amine salts
Exam~le 17
1,149 g (1 mol) of the ester from Example 8 was
mixed at 60 C with 252 g (2 mols) of dimethyl sulfate in
portions, with stirring, so that the temperature of the
reaction mixture could be maintained at between 6C and
70 C. 1,397 g of a yellow liquid of the general formula
(1) with
a + c = 6.6
b = 0
d + e + f + g = 4
R3, R4, R7 R8 = H
Rl R2, R9, R10 = 2 X H, 2 X -!IC-Cl7H35
O
R13 = -CH3
o
Il
y~ = 2 x ~ -OS-OCH3
o
was obtained.
This compound had the following analytical
3S values:
Cat SO3 acidic: 9.6 g SO3/100 g
Total amine value: 2.3 mg KOH/g
2510:CM52510
2044234
Example 18
1,149 g (1 mol) of the ester from Example 8 was
neutralized at 60O c with 200 g (2 mols) of a 90~ aqueous
solution of lactic acid with stirring. 1,349 g of a
clear yellow liquid of the general formula (1) with
a + c = 6.6
b = o
d + e + f + g = 4
R3, R4, R7 R3 = H
R1, R2, R9, R10 = 2 X H, 2 X -C-C17H35
Rl2, R = H
y~ = 2 X CH3 - CH - COO~
ll
OH
was obtained.
This compound had the following analytical
values:
Cat SO3 acidic: 10.0 g SO3/100 g
pH (5% isopropane/water 1:1): 5.2
Exam~le 19
1,149 g (1 mol) of the ester from Example 8 was
mixed at 60 C with 126 g (1 mol) of dimethyl sulfate in
portions, with stirring, so that the temperature of the
reaction mixture could be maintained at between 60 and
70 C. Then 100 g (1 mol) of a 90% aqueous solution of
lactic acid was added for neutralization of the mixture.
1,373 g of a clear yellow liquid of the general formula
(l) with
a - c = 6.6
b = 0
d + e + f + g = 4
2510:~1s2510
204~23~
R3, R4, R7 R8 = H
R1, R2, R9, R10 = 2 X H, 2 X -C-C17H35
5R12, R13 = 1 x H , 1 x CH3
y~ = 1 x ~ -OS-OCH3, 1 x CH3-CH-COO-
10 O OH
was obtained.
This compound had the following analytical
value:
15Cat SO3 acidic: 10.1 g SO3/100 g
Exam~le 20
1,305 g (1 mol) of the ester from Example 11
was quaternized with 252 g (2 mols) of dimethyl sulfate,
20as described in Example 17. 1,557 g of a clear yellow
liquid of the general formula (1) with
a + c = 2.5
b = 9
d + e + f + g = 4
25R3, R4, R7 R8 = H
Rl, R2, R9, R10 = 2 x H, 2 x -C-Cl7H35
R12 Rl3 = -CH3
o
y~ = 2 x ~ -OS-OCH3
O
was obtained.
This compound had the following analytical
values:
2510:CY52510
204423~
26
Cat SO3 acidic: 9.0 g S03tl00 g
Total amine value: 2.2 mg KOH/g
Exam~le 21
679 g (0.65 mol) of the ester from Example 14
was quaternized with 41 g (0.325 mol) of dimethyl
sulfate, as described in Example 19, and the reaction
mixture was then neutralized with 37 g of 90% lactic
acid. 7S7 g of a clear yellow liquid of the general
formula (1) with
a + c = 7.7
b = 1
R6 = -CH3
d + e = 4
R3, R4 = H
R1, R2 = 0-5 x H, 1.5 x -C-C17H35
o
R12 = 0-5 x CH3, 0.5 x -H
y = 0.5 x -OS-OCH3 , 0.5 x CH3-CH-COO-
o OH
was obtained.
This compound had the following analytical
value:
Cat SO3 acidic: 6.4 g SO3/100 g
Example 22
1,149 g (1 mol~ of the ester from Example 8 was
mixed at 60 C in portions, with vigorous stirring, with
196 g (2 mols) of a 36% aqueous HCl solution.
2510:CIIS2510
2044234
27
1,345 g of a pale-yellow liquid of the general
formula (1) with
a + c = 6.6
b = 0
d + e + f + g = 4
R3, R4, R7 R8 = H
R1, R2, R9, R10 = 2 x H, 2 x -C-C17H35
R12, R13 = H
y~ = 2 x Cl
was obtained.
This compound had the following analytical
~alues:
Cat SO3 acidic: 10.3 g SO3/100 g
pH (5% isopropanol/water 1:1): 4.2
2510:CMS2510