Note: Descriptions are shown in the official language in which they were submitted.
Z044454
This invention relates to a high-purity ultrafine
spherical powder of nickel suitable for making a conductive
paste filler for use in electronic parts or the like. The
invention also relates to a novel method of making the new
nickel powder.
Ultrafine metallic powders according to this invention
consist of spherical particles having limited dispersion of
particle size, i.e. having an average particle size in the
range of about 0.1 to several microns. The expression
"particle size", as used herein, is intended to mean the
average diameter determined by the specific surface area of
the particles. Ultrafine metallic powders according to this
invention have improved paste properties and, when used to
form conductors in an electronic circuit, enable formation of
fine conductor patterns and also enable reduction in the
thicknesses of conductor layers. Such powders are therefore
in much demand.
BACKGROUND OF THE INVENTION
Laminated ceramic capacitors used as electronic circuit
components are generally manufactured in such a manner that
layers of ceramic dielectric are alternately layered with
internal electrodes and the resulting layered structure is
pressed and fired so as to be integrally combined. In this
case it is necessary to use, as an internal electrode
material, a precious metal such as Pt or Pd which does not
melt at the temperature at which the dielectric ceramic is
sintered, and which is not oxidized by firing in an atmosphere
having a high oxygen partial pressure which does not decompose
~L
2 ~
Z044454
or reduce the dielectric ceramic. Where such an expensive
material is used, it is difficult to manufacture a
large-capacity low-price capacitor.
In an effort to solve this problem a ceramic has been
developed which enables use of a base metal as the internal
electrodes, which is not changed into a semiconductor by
firing in an atmosphere of low oxygen partial pressure or in
a reducing atmosphere to maintain metallic state of the base
metal, and which has excellent dielectric characteristics and
a specific resistance sufficient for use as a dielectric for
capacitors.
With recent progress of the development of small large-
capacity electronic parts, a need for reduction of thickness
and of resistance of the internal electrodes has arisen.
The thickness of the internal electrode is limited by the
particle size of the filler used in the paste. This thickness
cannot be smaller than the particle size itself. Accordingly,
a filler powder having a smaller particle size may be used to
afford reduction of thickness. However, there is a practical
limit to the available extent of reduction of particle size,
because the filling properties of the filler deteriorate if
the particles are too small.
A method of manufacturing an ultrafine nickel powder is
disclosed in Japanese Patent Publication No.59-7765. Nuclei
2S of the metal generated in interface unstable regions are grown
to form ultrafine particles by controlling differences between
the flow rates of a metal halide gas and a reducing gas and
by utilizing the difference between the specific gravities of
ZOA4454
the gases to form an ultrafine nickel powder. In this case
particles having a crystal habit such as a cubic shape (noted
in Table 1 of the reference) are formed into a nickel powder.
Such particles, while less expensive than precious metal
powders, cause a problem of filling when the powder is used
as a paste filler.
A similar method of obtaining an ultrafine nickel powder
utilizes a vapor phase hydrogen reduction reaction of nickel
chloride. Such method is disclosed in the thesis "Manufacture
of ultrafine particles of nickel, cobalt or iron by vapor
phase hydrogen reduction of chloride" (Nihon Kagaku Kaishi,
1984, (6), pp 869 to 878) authored by Kenichi Ohtsuka et al.
In this method, reaction is effected at a temperature of 750
to 950C and a chloride vapor density of 0.02 or lower to
obtain an ultrafine powder having a particle size of 0.1 ~m
or smaller. This method also entails a serious problem
because of formation of particles having crystal habits.
Japanese Patent Publication No.2-49364 discloses a method
in which a reducing agent such as sodium boron hydride is
added to an aqueous solution containing nickel ions to reduce
and precipitate nickel. This method entails problems
including a need for various reducing agents, complicated
manufacturing conditions, and a need to use an expensive high-
purity reducing agent for obtaining a high-purity product.
This reducing precipitation method uses a batch type process
which is difficult to practice as a continuous process.
The so-called carbonyl method is known among other
methods for manufacturing very fine powders of nickel and
X044454
iron. However, this method cannot satisfy demands for finer
or thinner conductor patterns because the particle size
attained by this method is too large.
Japanese Patent Laid-Open Nos.62-63604 and 62-188709
disclose methods for manufacturing powders of copper and
silver. According to these methods a metal halide is
vaporized, the vapor of the metal halide is supplied to a
reaction section by its vapor pressure or by an inert gas
carrier, and the metal halide vapor and a reducing gas (such
as hydrogen gas) are brought into contact and mixed with each
other in the reaction section. Particles of the metal are
thereby immediately reduced and separated out in the gas and
is discharged through an outlet together with the gas. It
is thus possible to continuously supply the raw-material
metal halide and to continuously collect the formed powder.
In comparison with the copper powder in Japanese Patent
Laid Open No. 62-63604 and the silver powder in Japanese
Patent Laid Open No. 62-188709, nickel powders formed by
conventional methods include particles having cubic,
octahedral and other crystal habits, which crystal habits
create a major problem in terms of filling when the powder is
used as a paste filler.
Accordingly, it has been a serious drawback that fine
nickel powders manufactured by conventional methods include
particles having undesirable crystal habits when the particle
size is reduced to about 1 ~m or smaller. The filling
properties and performance of the resulting fillers at the
time of internal electrode paste printing have been found to
2~445~
be unsatlsfactory. Serlous problems of low flller denslty,
large amounts of volds formed by flrlng, and lncrease ln
electrlcal reslstance have accordlngly been encountered.
There ls also an lncreased posslblllty of delamlnatlon of the
resultlng layered structure at the tlme of flrlng. No nlckel
powder has heretofore been provlded whlch has a partlcle slze
of 3 ~m or smaller and has a satlsfactorlly hlgh purlty.
Known nlckel powder flllers used as components of electronlc
parts cannot be lmproved to provlde a reductlon ln the
reslstance of the electrodes or by preventlng undeslrable
lnfluences on the dielectrlc.
SUMMARY OF THE INVENTION
In vlew of these problems, an ob~ect of the present
inventlon ls to provlde a method of manufacturlng, at a low
cost, an ultraflne hlgh-purlty nlckel powder conslstlng of
spherlcal partlcles havlng a deslred partlcle slze, such as,
from 0.05 to 3 ~m, havlng a hlgh flller denslty, and useful
as a flller. Thus, one aspect of the present lnventlon
provldes the method of manufacturlng ultraflne nlckel powder
conslstlng of substantlally spherlcal nlckel partlcles havlng
an average dlameter of from about 0.05 to about 3 ~m and a
nlckel content of from about 99.5% to about 100% by welght,
whlch method comprlses: [A] brlnglng (a) nlckel chlorlde
vapor dlluted wlth an lnert gas wlth (b) hydrogen at a
maxlmum ratlo of hydrogen to the total of the nlckel chlorlde
vapor and the lnert gas of 1/2.11 under chemlcal vapor
reactlon condltlons, thereby reactlng nlckel chlorlde wlth
hydrogen and formlng the spherlcal nlckel partlcles, whereln:
-- 6
73461-26
.
2~44~5~
the nlckel chlorlde vapor is controlled so that, durlng the
chemlcal vapor reaction, a partlal pressure of the nlckel
chloride vapor based on supplied gas excluding hydrogen is
from about 0.05 to about 0.3, and the chemical vapor reaction
is conducted at a temperature of from about 1,004C to
1,453C, and [B] cooling and collecting the spherical nickel
partlcles.
Preferably, the partial pressure of the nickel
chlorlde vapor based on the total of the nlckel chlorlde
vapor and the lnert gas ls from about 0.06 to about 0.015.
Preferably, the chemlcal vapor reaction ls
conducted at a temperature of from about 1,010C to about
1,100C.
Preferably, (a) the nlckel chlorlde vapor dlluted
wlth the lnert gas and (b) hydrogen are brought together at a
ratlo of hydrogen to the total of the nlckel chlorlde vapor
and the lnert gas of from l/Z.ll to 1/2.86.
Preferably, the chemlcal vapor reactlon is
conducted in a cylindrlcal reactor disposed in a
substantially horizontal posltlon, the cylindrical reactor
comprising an inlet for the lnert gas at one end, a nozzle
for supplylng hydrogen into a reactlon sectlon of the
cylindrlcal reactor and a vaporlzation section for vaporizlng
nlckel chlorlde between the inert gas inlet and the reaction
section.
Another ob~ect is to provide a novel ultraflne
high-purlty nlckel powder overcomlng the problems of the
prior art and hlghly useful as a filler for electronic parts.
- 6a -
,-
- 73461-26
2U~4454
Thus, another aspect of the present lnvention provldes an
ultraflne nickel powder formed of substantlally spherlcal
partlcles whereln the particles range ln slze from about 0.1
to 3 ~m and whereln the powder has a nlckel content of about
99.5% to about 100% by welght.
A thlrd aspect of the present lnventlon provldes a
use of the novel ultraflne hlgh purlty nlckel powder as a
flller for electronlc parts.
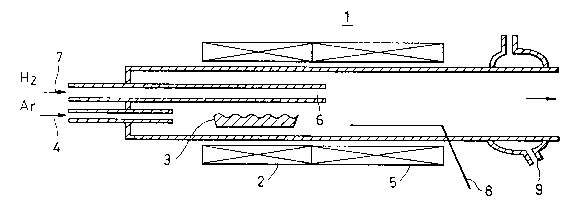
BRIEF DESCRIPTION OF THE DRAWINGS
Flgure 1 ls a schematlc dlagram of a reactor
sultably used to carry out the method accordlng to thls
lnventlon;
Flgure 2 ls a mlcroscoplc photograph of the
structure of partlcles of a flne nlckel powder manufactured
by a method of the present inventlon in accordance wlth
Example l;
Flgure 3 ls a mlcroscoplc photograph of the
structure of partlcles of a flne nickel powder manufactured
by a method
- 6b -
. 73461-26
2044454
of the present invention in accordance with Example 2;
Fig. 4 is a microscopic photograph of the structure of
particles of a fine nickel powder manufactured in accordance
with Comparative Example l; and
Fig. 5 is a graph of the relationship between nickel
chloride vapor density and reaction temperature in reactions
forming nickel powder.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention relates to a method of
manufacturing an ultrafine spherical nickel powder containing
99.5% or more by weight of nickel (about 99.5~-100%) in which
nickel chloride vapor and hydrogen are reacted while
controlling the nickel chloride vapor density in the range of
about 0.05 to 0.3 and the reaction temperature in the range
of about 1,004C (1,277K) to 1,453C (1,726K). This invention
further relates to an ultrafine spherical nickel powder formed
of substantially spherical particles having a particle size
of about 0.1 to 3 ~m and containing about 99.5% or more by
weight of nickel.
The present invention further relates to an ultrafine
spherical nickel powder formed by chemical vapor phase
reaction of nickel chloride with hydrogen and to a method of
manufacturing this powder.
To manufacture an ultrafine nickel powder by chemical
vapor phase reaction, nickel chloride vapor which is diluted
with inert gas, such as argon, is brought into contact with
and mixed with hydrogen and is reacted. Ultrafine nickel
powder thereby formed passes through a cooling section
Z044454
together with the resulting gas and is thereafter collected.
A remarkable phenomenon is believed to take place in
nickel particles in this process. It is believed that when
the nickel chloride and the reducing gas are brought into
reactive contact with each other, atoms of the resulting
nickel or clusters of a monomer are generated, and that
ultrafine nickel particles are formed by collision and
coalescence of the monomer. The nickel particles are believed
to be grown by further collision and coalescence.
Ordinarily, ultrafine powders of copper or silver are not
to be compared to nickel because they normally consist of
spherical particles. In contrast, nickel powders generally
consist of polyhedral particles. With respect to the
comparatively large particle sizes, the proportion of surface
energy to internal energy is reduced so that the powder tends
to develop and possess undesirable crystal habits. In
particular, in the case of nickel, particles having distinct
cubic or octahedral crystal habits strongly tend to be formed
if the particle size is greater than about 0.1 ~m. Therefore,
it is surprising that the method of this invention is capable
of producing a finely divided nickel powder having
substantially completely spherical particles even when the
particle size is substantially greater than 0.1 ~m.
After having fully examined the reaction and generation
of fine nickel powders, it has been found that a substantially
completely spherical powder can be obtained by reacting the
nickel chloride with hydrogen if the nickel chloride vapor
density (partial pressure in the supplied gas except for
2044454
hydrogen) is in the range of about 0.05 to 0.3, and if the
reaction/powder generation temperature is within a range from
about 0.74 time as high as the melting point of nickel
(1,726K) in terms of absolute temperature to the nickel
melting point, i.e., a range of about 1,004C (1,277K) to
1,453C (1,726K). The present invention has been achieved
based on this finding.
An important reason for the limitation of the nickel
chloride vapor density in the supplied gas to about 0.05 to
0.3 is as described below.
It has been found by experiment that if the nickel
chloride vapor density in the supplied gas such as argon is
lower than about 0.05, crystal habits are developed and it is
not possible to obtain a spherical powder. This may be
because the particles grow at a comparatively low speed. If
the nickel chloride vapor density exceeds about 0.3, the
nickel particles are excessively large and it is not possible
to obtain a powder having a desired particle size. Also, if
the particle size is excessive crystal habits readily occur.
Most preferably, the nickel chloride vapor density in the
supplied gas is about 0.06 to 0.15.
The reason for the limitation of the reaction temperature
to about 1,004C to 1,453C is as described below.
If the reaction temperature is lower than about 1,004C,
crystal habit particles are mixed and the reaction rate is
reduced. The upper limit of the reaction temperature is,
preferably, equal to or lower than about the melting point of
nickel, i.e., 1,453C (1,726K). If the reaction temperature
Z0444 ~4
is substantially higher than the melting point, generated
particles exist in a liquid state, so that the probability of
particles growing to a very large size is high, the particle
size distribution is extended, and the amount of nickel
attached to the reactor wall is increased.
Most preferably, the reaction temperature is about
1,010C to about 1,100C.
It is believed that this temperature dependency of the
particle shape relates to the influence of the temperature
upon the reaction rate, i.e., the rate of generation of atoms
or monomer clusters, that is, the particle growth speed
influences the particle shape. It is explained that if the
reaction temperature is higher, the anisotropy of the particle
growth is reduced so that the particles tend to grow into
spherical bodies. It is considered that the density
dependency of the particle shape relates to the influence of
the density upon the homogeneous nuclei formation speed. In
this case, it is also believed that the particle shape depends
upon the particle growth speed as in the case of the
temperature dependency.
In a case where the reaction is carried out in a reaction
tube heated in an electric furnace, since this reaction is an
exothermic reaction, spherical nickel particles can be
attained even if the set temperature of the electric furnace
is lower than the predetermined temperature mentioned above,
provided that the set temperature is high enough to be
supported by the exothermic reaction. That is because it is
important to control the temperature at which the nickel
2044454
particles grow by formation, collision and coalescence of
metallic monomers during reaction.
Further, according to the present invention, the nickel
content in the nickel powder is controlled to about 99.5% or
more by weight, the lower limit of the particle size
thereof is about 0.05 ~m, the upper limit of the same is
smaller than about 3 ~m, and the shape of the particles is
substantially limited to a spherical shape.
If the nickel content is less than about 99.5% by weight,
the desired resistance of electrodes or the desired
reliability of electronic parts cannot be achieved owing to
undesirable influence upon dielectric characteristics. The
nickel content is therefore about 99.5% to about 100% by
weight.
Particles having a particle size smaller than about 0.05
~m tend to agglomerate easily. If such particles are used as
a paste to be printed as internal electrodes of a laminated
ceramic capacitor or the like, the filling performance of the
filler is very poor so that the electrode layers after being
fired are porous, have a high electrical resistance and are
reduced in strength of bonding to the dielectric layer,
resulting in delamination. In the case of particles having
a particle size greater than about 3 ~m, it is impossible as
a practical matter to reduce the thickness of the electrode
layers for physical reasons.
If the particles are spherical the resulting structure
achieves a degree of filling close to optimum density filling
when printing internal electrodes, and high-quality electrodes
Z044454
can be obtained by firing which are uniform, in which the
amount of voids is small and which electrodes have low
resistance. It is also possible to limit the shrinkage of the
electrode layers at the time of firing and, hence, to prevent
occurrence of cracks in the dielectric layer and
delamination.
EXAMPLES
The following examples are intended to be illustrative
and not to define or to limit the scope of the invention,
which is defined in the appended claims.
Example 1
A reactor 1 such as that shown in Fig. 1 was used and 10
g of raw-material nickel chloride was placed in a quartz boat
3 in a vaporization section and was vaporized in argon gas 4
supplied at 2 liter/min so that the density (partial pressure)
of the vaporized nickel chloride was 5.0 x 10-2. The raw-
material gas thereby formed was introduced into a reaction
section 5 controlled at 1,050C (0.77 time as high as the
melting point of nickel in terms of absolute temperature), and
was brought into contact and mixed with hydrogen supplied at
a rate of 1 liter/min. through a central reaction nozzle 6.
The temperature measured by a thermocouple 8 protected with
a quartz tube was increased up to 1,090C (0.79 time as high
as the melting point of nickel). The nickel powder produced
was collected by a cylindrical filter paper after passing
through a cooling section 9 together with the gas. The
specific surface area of the produced powder was 3.5 m2/g
which was a spherical powder having a particle size of 0.2 ~m
2044454
as observed with an electron microscope.
Fig. 2 shows an electron microscope photograph of the
nickel powder obtained in accordance with this example. As
can be seen in this photograph, the shape of nickel powder
particles obtained is nearly perfectly spherical.
Example 2
A nickel powder was manufactured under the same
conditions as Example 1 except that the reaction temperature
was 960C (0.714 time as high as the melting point of nickel
in terms of absolute temperature). The temperature measured
by thermocouple 8 was increased up to 1,004C (0.74 time as
high as the melting point of nickel). The specific surface
area of the produced powder was 3.7 m2/g and the product was
a spherical powder having a particle size of 0.18 ~m as
observed with an electron microscope.
Fig. 3 shows an electron microscopic photograph of the
nickel powder obtained in accordance with this example.
Example 3
A nickel powder was manufactured under the same
conditions as Example 1 except that the reaction temperature
was 960C (0.714 time as high as the melting point of nickel
in terms of absolute temperature), and that the density
(partial pressure) was set to 8.0 x lO . The temperature
measured by thermocouple 8 was increased up to l,006C (0.74
time as high as the melting point of nickel). The specific
surface area of the produced powder was 3.0 m2/g and the
product was a spherical powder having a particle size of 0.22
~m as observed with an electron microscope.
Z044454
Example 4
A nickel powder was manufactured under the same
conditions as Example 1 except that the reaction temperature
was controlled at 1,000C (0.74 time as high as the melting
point of nickel in terms of absolute temperature), and that
the density (partial pressure) was controlled at 8.5 x 10-2.
The temperature measured by thermocouple 8 was increased up
to 1,053C (0.77 time as high as the melting point of nickel).
The specific surface area of the produced powder was 2.9 m2/g
and the product was a spherical powder having a particle size
of 0.23 ~m as observed with an electron microscope.
Example 5
A nickel powder was manufactured under the same
conditions as Example 1 except that the reaction temperature
was controlled at 1,050C (0.767 time as high as the melting
point of nickel in terms of absolute temperature), and that
the density (partial pressure) was set to 3.0 x 10-. The
temperature measured by thermocouple 8 was increased up to
1,120C (0.81 time as high as the melting point of nickel).
The specific surface area of the produced powder was 0.9 m2/g
and the product was a spherical powder having a particle size
of 0.8 ~m as observed with an electron microscope.
Comparative Example 1
A nickel powder was manufactured under the same
conditions as Example 1 except that the reaction temperature
was set at 950C (0.71 time as high as the melting point of
nickel in terms of absolute temperature), and that the density
(partial pressure) was 4.5 x 10-2. The temperature measured
20~'154
by thermocouple 8 was increased up to 993C (0.73 time as high
as the melting point of nickel). The specific surface area
of the produced powder was 3.6 m2/g and the powder was
observed with an electron microscope as having a particle size
of 0.2 ~m and having cubic, octahedral and other crystal
habits as shown in Fig. 4.
Comparative Example 2
A nickel powder was manufactured under the same
conditions as Example 1 except that the reaction temperature
was 950C (0.71 time as high as the melting point of nickel
in terms of absolute temperature), and that the density
(partial pressure) was 8.0 x 10-2. The temperature
measured by thermocouple 8 was increased up to 998C (0.73
time as high as the melting point of nickel). The specific
surface area of the produced powder was 3.4 m2/g and the
product was observed with an electron microscope as a powder
having a particle size of 0.2 ~m and having cubic, octahedral
and other crystal habits.
Comparative Example 3
A nickel powder was manufactured under the same
conditions as Example 1 except that the reaction temperature
was 1,000C (0.74 time as high as the melting point of nickel
in terms of absolute temperature), and that the density
(partial pressure) was 4.5 x 10-2. The temperature measured
by thermocouple 8 was increased up to 1,042C (0.76 time as
high as the melting point of nickel). The specific surface
area of the produced powder was 3.4 m /g and the product was
observed with an electron microscope as a powder having a
20~4S~
particle slze of 0.2 ym and havlng cublc, octahedral and
other crystal hablts.
Comparatlve Example 4
A nlckel powder was manufactured under the same
condltlons as Example 1 except that the reactlon temperature
was l,100C (0.795 tlme as hlgh as the meltlng polnt of
nlckel ln terms of absolute temperature), and that the
denslty (partlal pressure) was 3.6 x 10-1. The temperature
measured by thermocouple 8 was lncreased up to 1,160C (0.93
tlme as hlgh as the meltlng polnt of nickel). The speclflc
surface area of the produced powder was 1.0 m2/g and the
product was observed wlth an electron mlcroscope as a powder
havlng a partlcle slze of 0.8 ym and havlng cubic, octahedral
and other crystal hablts.
Flg. 5 collectlvely shows the relationshlps between
varlous nlckel chlorlde vapor densltles and reactlon
temperatures wlth respect to each of Examples and Comparatlve
Examples descrlbed above.
Table 1 below shows the relatlonshlp of the nlckel
chlorlde vapor denslty, the argon gas supply rate, the total
supply rate of the nlckel chlorlde vapor and the argon gas,
the hydrogen supply rate and the ratlo of the hydrogen gas
supply rate to the total supply rate of the nlckel chlorlde
vapor and the argon gas. The nlckel chlorlde vapor denslty,
the argon gas supply rate and the hydrogen supply rate are
descrlbed above. The total supply rate of the nlckel
chlorlde vapor and the argon gas and the ratlo of the
hydrogen gas supply rate to the total supply rate of the
- 16 -
73461-26
2~4~5~
nickel chlorlde vapor and the argon gas were obtalned by
calculatlon uslng the values descrlbed above.
Example NiCl2 Ar gas Total H2 gas Ratio of
No. vapor supply rate supply rate supply H2/(NiC12
density (liter/min) of NiC12+Ar rate vapor+ AR2
(liter/min) (liter/ gas)
min)
1 0.05 2 2.11 1 1/2.11
2 0.05 2 2.11 1 1/2.11
3 0.08 2 2.17 1 1.2.17
4 0.085 2 2.19 1 1/2.19
0.3 2 2.86 1 1/2.86
The present lnventlon makes lt posslble to
contlnuously produce, at a low manufacturlng cost, an
ultraflne nlckel powder whlch conslsts of spherlcal particles
havlng a partlcle slze of about 0.05 to 3 ~m hlghly superlor
for use as a conductlve paste flller, and which contalns
about 99.5% or more by welght of nlckel.
Although the lnventlon has been descrlbed wlth
respect to partlcular reactors, powders and reactlon gases
and gas mlxtures, lt wlll be appreclated that many varlatlons
may be made wlthout departlng from the splrlt and scope of
the lnventlon as deflned ln the appended clalms.
7 3461-26
. . .