Note: Descriptions are shown in the official language in which they were submitted.
i
20 45356
"INTEGRATED TWO REACTION ZONE PROCESS
FOR C,4,~,r AND C,6 ISOMERIZATION"
This invention relates generally to the isomerization of hydrocarbons.
This im~ention relates more specifically to the isomerization of Cd, Cs and C6
cyclic
hydrocarbons using a solid catalyst.
High octane gasoline is required for modern gasoline engines.
Formerly it was common to accomplish octane number improvement by the use of
1o various lead-containing additives. As lead is phased out of gasoline for
environmental reasons, it has become increasingly necessary to rearrange the
structure of the hydrocarbons used in gasoline blending in order to achieve
high
octane ratings. Catalytic reforming and catalytic isomerization are two widely
used processes for this upgrading.
A gasoline blending pool normally included C4 and heavier
hydrocarbons having boiling points of less than 205oC (400°F) at
atmospheric
pressure. This range of hydrocarbon includes G-C6 paraffins and especially the
Cs and C6 normal paraffins which have relatively low octane numbers. The C4-C6
hydrocarbons have the greatest susceptibility to octane improvement by lead
2 o addition and were formerly upgraded in this manner. Octane improvement can
also be obtained by using isomerization to rearrange the structure of the
paraffinic hydrocarbons into branch-chained paraffins or reforming to convert
the
2 20 45356
C6 and heavier hydrocarbons to aromatic compounds. Normal Cs hydrocarbons
are not readily converted into aromatics, therefore, the common practice has
been
to isomeric these lighter hydrocarbons into corresponding branch-chained
isopara~ns. Although the Ca and heavier hydrocarbons can be upgraded into
aromatics through hydrocyclization, the comrersion of C6's to aromatics
creates
higher density species and increases gas yields with both effects leading to a
reduction in liquid volume yields. Therefore, it is common practice to charge
the
C6 paraffins to an isomerization unit to obtain C6 isopara~n hydrocarbons.
Consequently, octane upgrading commonly uses isomerization to convert C6 and
io lighter boiling hydrocarbons and reforming to convert C~ and higher boiling
hydrocarbons.
The isomerization of paraffins is a reversible first order reaction. The
reaction is limited by thermodynamic equilibrium. It has been generally found
that lower temperatures shift the equilibrium of Cs and C6 hydrocarbons toward
higher isoparaffin to normal paraffin ratios. These temperatures are typically
in
the range of 105-180°C (200-355°F). When isomerizing butane, its
refractory
nature demands somewhat higher temperatures usually greater than 170°C
(340°F) to obtain high equilibrium ratios of isobutane to butane.
A number of catalyst systems have been used in effecting isomerization
2 o reactions. Traditional catalyst systems are a hydrochloric acid promoted
aluminum chloride system and a supported aluminum chloride catalyst. Recently
zeolite catalysts, particularly mordenite, are also finding increased usage
due to
their decreased sensitivity to sulfur and water. A platinum group metal is
usually
incorporated into both catalysts. All of these catalyst systems are very
reactive
and can generate undesirable side reactions such as disproportionation and
cracking. These side reactions not only decrease the product yield but can
form
20
olefinic fragments that combine with the catalyst and shorten its life. One
commonly practiced method of controlling these undesired reactions has been to
carry out the reaction in the presence of hydrogen. However, high
concentrations
of hydrogen and high molecular weight species tend to inhibit the butane
isomerization reactions. Therefore, it has been difficult to isomeric butane
in
the presence of C,s-Ca hydrocarbons without sacrificing isobutane yields or
obtaining low yields of C~-C6 isoparaffins along with undesirable high gas
production and catalyst fouling.
As a result butane isomerization and the isomerization of Cs and C6
1 o hydrocarbons are typically carried out in separate reaction zones and
processes.
The use of separate processes increases the equipment and operating expenses
associated with the isomerizing of C4 through C6 hydrocarbons.
U.S. Patent 3,242,228 issued to Riordan et al. teaches an isomerization
catalyst consisting of an alumina base with 0.01 to 1.0 wt.% platinum, and 2S
to
7.0 wt.% chlorine. The catalyst is used at process conditions including a
liquid
hourly space velocity (LHS~ of from 0.5 to 2.0 hr: 1, a hydrogen to
hydrocarbon
mole ratio within the range of from 0.1:1 to 5.0:1, and a temperature of 150-
200°C
(3~-390°F) for butane isomerization, or a temperature of 120-
160°C
(250-320°F) for Cs-C6 isomerization.
2 o U.S. Patent 3,789,082 is directed to a method for practicing low
temperature isomerization using a chlorided platinum-alumina catalyst. The
process operates in the presence of a hydrogen chloride promoter in an amount
up to 0.1 to 5 wt.% of the feedstock and temperatures in the range of 100-
200°C
(210-390°F) for the isomerization of feedstreams comprising C4 and/or
Cs and/or
2 5 C6 fractions.
4
2045356
U.S. Patent 4,113,789 mentions the isomerization of G-C6
hydrocarbons at temperatures ranging from 120-180°C (250-355°F)
and butane at
temperatures ranging from 150-200°C (300-390°F) in the presence
of a chlorided
platinum alumina catalyst and hydrogen to hydrocarbon ratios in the range of
0.1:1.0 t0 1:1.
U. S. Patent 4,804,803 discloses a process for the isomerization of G-G
para~ns that uses a highly active chlorided, platinum alumina catalyst to
carry out
the process with a hydrogen to hydrocarbon ratio of 0.05 or less in the
effluent
from the isomerization zone.
io BR F DESCRIPTION OF THE INVENTION
This invention is a process for the isomerization of a G feedstock and a
Cs-G feedstock that reduces equipment and operating expenses by utilizing a
process flow scheme that provides beneficial heat integration and facilitates
the
use of common recovery zone while permitting a wide variation in the relative
ratio of the C4 to the Cs-C6 feedstock. The isomerization of the G feedstock
takes
place in a separate reaction zone which is run at a higher temperature than
the CS
and C6 hydrocarbon isomerization step. The effluent from the G isomerization
zone is heat exchanged against or mixed with the Cs-G feedstock ahead of an
additional isomerization zone that converts the Cs-C6 hydrocarbons, and if
present
2 o normal G hydrocarbons, to more highly branched hydrocarbons. Effluents
from
both isomerization zones enter a common separation section that removes light
gases from the isomerate product.
Accordingly in one embodiment, this invention is a process for
isomerizing a first feedstock comprising normal butane and a second feedstock
comprising Cs and Cs paraffinic hydrocarbons. The process combines the first
5
2p 4535' ._
feedstock with a first hydrogen stream to producx a first combined feedstream
comprising hydrogen and normal butane. The first combined feedstream is
passed to a first isomerization zone and contacted, at butane isomerization
conditions, including a higher temperature than that used in the second
isomerization step. with an isomerization catalyst. A first isomerization zone
effluent comprising isobutane and hydrogen is withdrawn from said first
isomerization zone. The first isomerization zone effluent at least indirectly
contacts the second feedstock thereby heating the second feedstock and a
second
hydrogen stream is admixed therewith to produce a second combined feedstream.
1o The second combined feedstream passes to a second isomerization zone and
- contacts isomerization catalyst at conditions, including a temperature less
than
that utilized in the first step, for the isomerization of Cs and C6
hydrocarbons. A
second isomerate effluent is withdrawn from the second isomerization zone and
passed along with the first effluent to a common separation zone. A light gas
stream containing hydrogen and at least one product stream comprising branched-
chain hydrocarbons are withdrawn from said separation zone.
In a more limited embodiment, this invention comprises a process for
-isomerizing a first feedstock comprising normal butane and a second feedstock
comprising Cs and C6 paraffinic hydrocarbons. The process includes the steps
of
2 o combining the first feedstock with a first hydrogen stream to produce a
first
combined feedstream comprising hydrogen and normal butane; passing the first
combined feedstream to the first isomerization zone and contacting the first
combined feedstream, at butane isomerization conditions, including a higher
temperature than that utilized in the second isomerization zone, with an
isomerization catalyst comprising alumina, 0.01 to 0.25 wt.% platinum and from
2-
10 wt.% of a chloride component and withdrawing the first isomerization zone
6
2 5358
effluent comprising isobutane and hydrogen; mixing at least a portion of the
first
isomerization zone effluent with the second feedstock to heat same and to
produce a second combined feedstream containing hydrogen and maintaining a
hydrogen concentration in the second combined feedstream that will produce a
hydrogen to hydrocarbon ratio in a second effluent stream from a second
isomerization zone that is less than 0.1; passing the second combined
feedstream
to the second isomerization zone and contacting the second combined feedstream
with an isomerization catalyst comprising alumina, 0.01 to 0.25 wt.% platinum
and
from 2-10 wt.% of a chloride component at conditions for the isomerization of
Cs
1 o and C6 hydrocarbons, the conditions including a temperature that is lower
than
the temperature in the first isomerization zone, and withdrawing a second
isomerization zone effluent having said hydrogen to hydrocarbon ratio of less
than
0.1 from the second isomerization zone; passing the first and second effluent
stream to a separation zone; withdrawing a first light gas stream, containing
essentially all of the hydrogen entering the separation zone from the
separation
zone and removing the light gas stream from the process; and withdrawing at
least
one product stream comprising branched-chain hydrocarbons from the separation
zone.
Additional details, objects and embodiments of this invention are
2 o disclosed in the following detailed description of this invention.
,BRIEF DESCRIPTION OF THE DRAWINGS
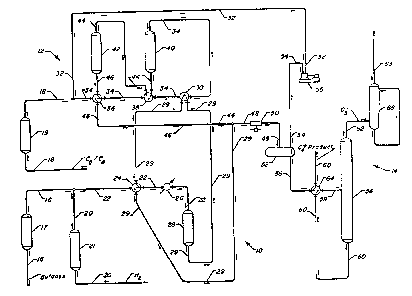
Figure 1 is a schematic diagram of an isomerization process arranged in
accordance with this invention showing indirect contact of the first effluent
with
the second feedstock and the recycle of hydrogen from the separation section.
w
20 4535fi
Figure 2 is schematic diagram of an isomerization zone awanged in
accordance with this invention showing the direct mixing of the first effluent
with
the second feedstock and the miong of a portion of the hydrogen stream with
the
second feedstock.
nFTATi FTj DESCR ON OF THE INVENTION
This invention simplifies the simultaneous isomerization of G and
Cs-C6 feedstocks. It offers significant cost and operational advantages to
newly
designed units and is beneficial in the revamp of existing isomerization units
to
either add or improve butane isomerization capabilities. For example, butane
1o isomerization capability may be incorporated into an existing Cs-C6
isomerization
unit by adding as few pieces of major equipment as a butane drier, a feed
exchanger, and one reactor. Moreover, this invention can be incorporated into
a
new or existing isomerization unit in a variety of arrangements.
Figure 1 provides a simplified flow diagram of one arrangement for the
15 process of this invention. In order to facilitate an understanding of this
invention,
additional equipment such as valves, pumps and instruments have been omitted
from Figure 1.
As Figure 1 shows, the process of this invention uses a C4 isomerization
zone 10, a Cs-C6 isomerization zone 12, and a common separation facilities 14.
2 o This process uses two feedstreams, a first feedstock that enters an
isomerization
zone operated for C4 isomerization and a second feedstock that enters an
isomerization zone operated for Cs-C6 isomerization. The first feedstock and
the
second feedstock enter the process via lines 16 and 18, respectively, while a
stream of make-up hydrogen enters the process through line 20. Both feedstocks
2 5 and the make-up hydrogen pass respectively through a drier 17, 19 and 21
before
8
2~ 45356
entering the isomerization zones. The Briers preferably contain an adsorbent
material with a type 4A molecular sieve being particularly preferred for the
hydrogen and C5-C6 feedstock and a type 13X molecular sieve being particularly
preferred for the G feedstock. However, any type of drier that will meet the
limitations for moisture as hereinafter discussed can be used for the
feedstocks
and hydrogen.
The feedstock carried by line 16 is admixed with make-up hydrogen
from line 20 to form a first combined feedstream. Line 22 carries the first
combined feedstream through an exchanger 24 to heat the incoming feed against
io the effluent of the isomerization zone 10 carried by line 29. Final heating
of the
combined feedstream takes place in a charge heater 26 that exchanges the
feedstream against medium pressure steam. After final heating, the first
combined feedstream enters a single reactor 28 that contains a hereinafter
described preferred catalyst composition. The effluent from reactor 28
comprising a G isomerate product stream is taken from the reactor 28 by line
29,
cooled in a charge heater 30 and exchanger 24 before entering separation
section
14.
Following passage through drier 19, line 18 carries the second feedstock
into admixture with a stream of recycled hydrogen carried by line 32 to form a
2 o second combined feedstream transported by line 34. Line 34 conducts the
second
combined feedstream through a series of exchangers 36, 38 and charge heater
30.
Isomerization zone 12 includes a first reactor 40 and a second reactor 42;
both
reactors 40 an 42 contain a preferred catalyst composition as hereinafter
described. From charge heater 30, line 34 delivers the second combined
2 5 feedstream to reactor 40. Reactor 40 contacts the feedstream with the
catalyst
contained therein to produce an intermediate isomerate product made up
9
2045356
primarily of isopentaaes and isohexanes. Line 44 comreys the isomerate product
through exchanger 38 and into reactor 42. Contact with the catalyst in reactor
42
further isomerizes the intermediate isomerate product stream to produce a Cs-G
isomerate producrt. The Cs-Ca isomerate product is withdrawn from reactor 42
by
a line 46 which directs the Cs-G isomerate product through exchanger 36 and
into
admixture with the G isomerate product carried by line 29 to produce a single
isomerate product from the two feedstocks that first entered the process.
The feedstocks that can be used in this invention include hydrocarbon
fractions rich in G normal paraffins and hydrocarbon fractions rich in Cs-C6
to normal paraffins. The term "rich" is defined to mean a stream having more
than
50% of the mentioned component. A suitable feedstream for the G
isomerization zone will have at least 40 mol % normal butane with at least 25%
of
any balance comprising isobutane. Preferred feedstocks are substantially pure
normal paraffin streams having over 60 mol% normal butane. Suitable C4
feedstreams are available from several sources in a refinery or as field
butane
streams.
The feedstream for the Cs-C6 isomerization zone will contain large
quantities of normal and mono-methyl branched paraffins. Preferred feedstock
are substantially pure normal hydrocarbons of roughly equal proportions of Cs
2 o and C6 paraffins. Other useful feedstocks include light natural gasoline,
light
straight run naphtha, gas oil condensate, light raffinates, light reformate,
light
hydrocarbons, and straight run distillates having distillation end points of
about
77oC (170°F) and containing substantial quantities of Cs and C6
paraffins. The
feedstream may also contain low concentrations of unsaturated hydrocarbons and
2 5 hydrocarbons having more than 6 carbon atoms. The concentration of these
materials should be limited to 10 wt.% for unsaturated compounds and 20 wt.%
io '
20 45358
for heavier hydrocarbons in order to restrict hydrogen consumption and
cracking
reactions. The feed may also contain substantial quantities of naphthenic
hydrocarbons, the concentration of these components should not normally exceed
35 mol%.
Hydrogen is admixed with each feed in an amount that will provide a
hydrogen to hydrocarbon ratio equal to or less than 1.0 at the inlet of the
isomerization zone. The hydrogen to hydrocarbon ratio of 1 or less has been
found to provide sufficient excess hydrogen for operation of the isomerization
zones. Although no net hydrogen is consumed in the isomerization reactions,
the
io isomerization zones usually have a net consumption of hydrogen often
referred to
as the stoichiometric hydrogen requirement which is associated with a number
of
side reactions that occur. These side reactions include cracking and
disproportionation. Other reactions that will also consume hydrogen include
olefin and aromatics saturation. High hydrogen concentrations tend to inhibit
the
isomerization of butanes by reducing the partial pressure of butane in the
vapor
phase and thus reducing the rate of reaction, therefore, high hydrogen to
hydrocarbon ratios in the C~ isomerizadon zone should be avoided. In general,
a
preferred hydrogen to hydrocarbon ratio is between 0.05 to 0.5.
Hydrogen may be added to the feed mixture in any manner that
2 o provides the necessary control for the addition of small hydrogen
quantities.
Metering and monitoring devices for this purpose are well known by those
skilled
in the art. As currently practiced, a control valve is used to meter the
addition of
hydrogen to the feed mixture. The hydrogen concentration in one or both of the
effluent streams or one of the outlet stream fractions can be monitored by a
2 5 hydrogen monitor and the control valve positions adjusted to maintain the
desired
hydrogen concentration.
m
20 ~535s
The hydrogen and hydrocarbon feed mixture entering either
isomerization zone is contacted in at least one reaction zone with an
isomerization catalyst. This invention can be practiced using a variety of
different
catalyst compositions and is not limited to a particular catalyst or
combination of
catalysts for either isomerization zone. A preferred isomerization catalyst
consists
of a high chloride catalyst on an alumina base containing platinum. In which
case
the alumina is preferably an anhydrous gamma-alumina with a high degree of
purity. The catalyst may also contain other platinum group metals. The term
platinum group metals refers to noble metals excluding silver and gold which
are
io selected from the group consisting of platinum, palladium, germanium,
ruthenium, rhodium, osmium, and iridium. These metals demonstrate differences
in activity and selectivity such that platinum has now been found to be the
most . '
suitable for this process. The catalyst will typically contain from about 0.1
to 0.?.5
wt.% of the platinum. Other platinum group metals may be present in a
concentration of from 0.1 to 0.25 wt.%. The platinum component may exist
within
the final catalytic composite as an oxide or halide or as an elemental metal.
The
presence of the platinum component in its reduced state has been found most
suitable for this process.
The preferred catalyst also contains a chloride component. The
2 o chloride component termed in the art "a combined chloride" is present in
an
amount from about 2 to about 10 wt.% based upon the dry support material. The
use of chloride in amounts greater than 4 wt.% have been found to be the most
beneficial for this process.
There are a variety of ways for preparing the preferred catalytic
2 5 composite and incorporating the platinum metal and the chloride therein.
The
method that has shown the best results in this invention prepares the catalyst
by
12
20 45358
impregnating the carrier material through contact with an aqueous solution of
a
water-soluble decomposable compound of the platinum group metal. For best
results, the impregnation is carried out by dipping the carrier material in a
solution of chloroplatinic acid. Additional solutions that may be used include
ammonium chloroplatinate, bromoplatinic acid or platinum dichloride. Use of
the platinum chloride compound serves the dual function of incorporating the
platinum component and at least a minor quantity of the chloride into the
catalyst.
Additional amounts of the chloride must be incorporated into the catalyst by
the
addition or formation of aluminum chloride to or on the platinum-aluminum
to catalyst base. An alternate method of increasing the halogen concentration
in the
final catalyst composite is to use an aluminum hydrosol to form the aluminum
- carrier material such that the carrier material also contains at least a
portion of
the halogen. Halogen may also be added to the carrier material by contacting
the
calcined carrier material with an aqueous solution of the halogen acid such as
hydrogen chloride, hydrogen fluoride, or hydrogen bromide.
It is generally known that high chlorided platinum-alumina catalysts of
this type are highly sensitive to sulfur and oxygen-containing compounds.
Therefore, the feedstocks and any make-up hydrogen entering the process must
be relatively free of such compounds when the preferred catalyst is used. A
sulfur
2 o concentration no greater than OS ppm is generally required. The presence
of
sulfur in the feedstock serves to temporarily deactivate the catalyst by
platinum
poisoning. Activity of the catalyst may be restored by hot hydrogen stripping
of
sulfur from the catalyst composite or by lowering the sulfur concentration in
the
incoming feed to below 0.5 ppm so that the hydrocarbon will desorb the sulfur
that has been adsorbed on the catalyst. Water can act to permanently
deactivate
the catalyst by removing high activity chloride from the catalyst and
replacing it
13
2045356
with inactive aluminum hydroxide. Therefore, water, as weU as oxygenates, in
particular Ci-CS oxygenates, that can decompose to form water, can only be
tolerated in very low concentrations. In general, this requires a limitation
of
oxygenates in the feed to about 0.1 ppm or less. The feedstock and hydrogen
stream may be treated by any method that will remove water and sulfur
compounds. Sulfur may be removed from the feedstream by hydrotreating. A
variety of commercial dryers are available to remove water from the feed
components. Adsorption processes for the removal of sulfur and water from
hydrocarbon streams are also well known to those skilled in the art.
1 o Operation of the isomerization zones with the preferred catalyst also
requires the presence of a small amount of an organic chloride promoter. The
organic chloride promoter serves to maintain a high level of active chloride
on the
catalyst as low levels are continuously stripped off the catalyst by the
hydrocarbon
feed. The concentration of promoter in the reaction zone is maintained at from
30 to 300 ppm. The preferred promoter compound is carbon tetrachloride. Other
suitable promoter compounds include oxygen-free decomposable organic
chlorides such as propyldichloride, butylchloride, methylenechloride, and
chloroform to name only a few of such compounds. The need to keep the
reactants dry is reinforced by the presence of the organic chloride compound
2 o which may convert, in part, to hydrogen chloride. As long as the process
streams
are kept dry, there will be no adverse effect from the presence of small
amounts
of hydrogen chloride.
Operating conditions within the isomerization zones are selected to
provide a good selectivity of the particular isoalkane product from the feed
components. The core of the operation of the C, isomerization zone is passage
of
the Cd feedstock through a reactor at butane isomerization-promoting
conditions
14
2p 4535 ~. _ _.. ._
including the presence of an acidic isomerization catalyst. This is normally a
relatively low pressure operation performed at a pressure of from about 700 to
4000 kPag and at an elevated temperature as required by the activity of the
catalyst. The average reactant temperature may be as high as 500' C, but is
preferably between 100 and 320' C. It is preferred that the G feedstock is
passed
vertically through one or more fixed beds of catalyst located within the
reactor at
a liquid hourly space velocity between 2.0 and 100 hr: 1, but space velocities
in the
broad range of OS to 12.0 hr: lcan be employed if desired.
The CS-C6 isomerization zone will operate at conditions to maximize
1o the isomerization of Cs and Ca hydrocarbons. Thus, temperatures within the
reaction zone will range from about 90-225°C (194-435°F). Lower
reaction
temperatures in this range favor equilibrium mixtures of Cs and Ca isoalkanes
versus normal pentane and hexane. However, higher temperatures in the range of
200-225°C (390-435°F) are preferred when large quantities of
normal butanes are
in the combined feed to the Cs-C6 isomerization zone. The higher temperatures
offer a significant increase in isobutane production with only a minimal
decrease
in the ratio of Cs and C6 isoalkanes to pentane and hexane. Of course, the
most
suitable temperature will depend on the composition of the feed. For feeds
having few isomerizable C4 hydrocarbons, temperatures of between 120-
205°C
2 0 (248-400°F) may be most advantageous. The Cs-C6 isomerization zone
may also
be maintained over a wide range of pressures. Pressure conditions in the
isomerization of Cs-C6 paraffins range from 700 to 7000 kPag. Preferred
pressures for this isomerization are in the range of from 2000 to 3~0 kPag.
The
feed rate to this reaction zone can also vary over a wide range and includes
liquid
2 5 hourly space velocities ranging from 0.5 to 10 hr.-1, however, space
velocities
between 0.5 and 4 hr: 1 are preferred.
15
20 45356
The Cs-C6 isomerization zone will usually contain multiple stages. A
typical Cs-C~ isomerization zone will have a two-reactor system comprising a
first
stage reactor and a second stage reactor. The catalyst used in a multiple
reaction
stage system is usually distributed equally between the different reaction
stages. It
is not necessary that either reaction zone be carried out in two or more
reactors
but the use of at least two reactors confers several benefits on the process.
The
use of two reactors and specialized valuing (not shown) allows partial
replacement
of the catalyst in the system without taking the subject isomerization zone
off
stream. For the short periods of time during which replacement of catalyst may
io be necessary, the entire flow of reactants may be processed through only
one
reaction vessel while catalyst is replaced in the other. 'Itvo reactors can
also be
used to maintain lower catalyst temperatures in a portion of the Cs-C6
isomerization zone. This is accomplished by having any exothermic reaction
such
as hydrogenation of unsaturates performed in a first reaction vessel with the
rest
of the reaction carried out in a final reactor stage at lower temperature
conditions. Therefore, the first reactor can operate at a somewhat higher
temperature, of about 200-225°C, (390-435°F) which favors the
isomerization of
butanes and the lower temperature of the second reactor will increase the Cs
and
C6 isoparaffin to paraffins ratios by a small amount without reversing the
2 o isobutane yield. When two reactors are used in this manner, the last
reactor in
the Cs-C6 isomerization zone can be operated at a temperature below
190°C
(375°F) and possibly as low as 150°C (302°F).
Operation of the C, isomerization zone at a relatively higher
temperature than that of the Cs-C6 isomerization zone and the operation of
reactor 40 at a relatively higher operating temperature than reactor 42 allows
the
process arrangement of this invention to take advantage of a beneficial heat
~.... . ~~ tra n_ _ . .. ...
20 45356
integration. As shown in Figure 1, the second combined feed is first
progressively
heated by indirect heat exchange with the effluent from reactors 42 and 40.
After
the initial heat exchange, the C~ isomerate product carried by line 29 has
enough
heat, in most cases, to raise the temperature of the second combined
feedstream
to the desired inlet temperature for reactor 40. Of course, heat from line 29
may
be supplemented, if necessary, by an additional charge heater if a higher
inlet
temperature is desired for reactor 40. As hereinafter described, heat from the
C,
isomerate product may be transferred to the second combined feedstream by
indirect heat exchange, direct contact, or a combination thereof.
1 o The effluents from both isomerization zones are combined following
any heat exchange. Figure 1 shows the isomerate products carried by lines 29
and
46 combined into a common product stream 48 that enters separation facilities
for
recovering the isomerization product. At minimum, the separation facilities
divide the reaction zone effluent into a product stream comprising C, and
heavier
hydrocarbons and a gas stream which is made up of lighter hydrocarbons and
hydrogen. Suitable designs for rectification columns and separator vessels are
well known to those skilled in the art. The separation section may also
include
facilities for recovery of normal alkanes. Normal alkanes recovered from the
separation facilities may be recycled to the isomerization reaction zone to
2 o increase the conversion of normal alkanes to isoalkanes. One typical
arrangement
for the separation facilities is shown in Figure 1 and includes an overhead
condensor 50 that cools the combined isomerate products and a product
separator
52 that receives the cooled effluent from condensor 50 via line 48. Product
separator 52 recovers hydrogen and other light gases in recycle stream 54 and
2 5 directs unstabilized liquid products to a stabilizer column 56 by a line
58.
Hydrogen from line 54 is compressed in a recycle compressor 55 for recycle to
the
17
20 45356
C~-C6 isomerization zone by line 32. The stabilizer 56 column is operated to
deliver a bottoms fraction 60 containing G and heavier hydrocarbons and an
overhead fraction 62 of C3 hydrocarbons and lighter boiling compounds.
Products
taken from the bottom of the column can be cooled with the combined product
stream in heat exchanger 64 before it enters the column. C3 and lighter
hydrocarbons taken overhead from stabilizer column 56 can be vooled and
separated into a gas stream and reflux that returns to the separation column.
When the preferred catalyst is used, net gas from the separator column
will ordinarily enter a scrubber section 66 that contacts the gas with a
suitable
1 o treatment solution for neutralizing and/or removing acidic components that
may
have originated with the chloride addition to the isomerization zone and may
be
present in the gas stream. T~rpically, the treatment solution will be a
caustic that
is pumped in a loop around a contacting vessel. After treatment in the
scrubber
section, the net gas is removed from the process by line 63 and usually put to
use
as a fuel.
In most isomerization processes and as depicted by Figure 1, hydrogen
is separated from the effluent in a product recovery section and recycled to
the
isomerization zone. When the hydrogen to hydrocarbon ratio of the reactor
effluent is less than 0.05, it is possible to separate light ends from an
isomerization
2 o effluent without the recovery and recycle of hydrogen to either of the
isomerizadon zones. As the quantity of hydrogen leaving the product recovery
section increases, additional amounts of C4 and other product hydrocarbons are
taken with the light ends that are separated from the process. These product
hydrocarbons are typically in the fuel gas stream from the product recovery
2 5 section. The value of the lost product or the additional expense
associated with
i8
20 45356
recovery facilities to prevent the loss of product do not normally justify
operating
the process without recycle at effluent hydrogen to hydrocarbon ratios above
0.05.
In another embodiment of this process, as illustrated by Figure 2,
isomerate product from the G isomerization zone directly contacts the
isomerate
product from the Cs-G isomerization zone and the separation facilities operate
without the recycle of hydrogen. The process arrangement of Figure 2 still
contains isomerization zones 10 and 12 and as described in conjunction with
Figure 1 as well as separation facilities. This arrangement simplifies the
process
arrangement of Figure 1 by eliminating the product separator 52, the recycle
io compressor 55 and the heater 30 as shown in Figure 1.
Looking then at Figure 2, the G feedstock again enters the process via
line 16 and after drying in drier 17 is combined with the dried hydrogen
stream
from line 20. The amount of hydrogen combined with the G feed may vary and at
a minimum will equal the minimum amount of hydrogen necessary for the
operation of the C4 isomerization zone and at a maximum will equal enough
hydrogen to supply the hydrogen requirements of both isomerization zones. The
first combined feedstream is taken by line 22 and passed through the first
isomerization zone in the manner previously stated for the description of
Figure 1.
The effluent from reactor 28 is taken by line 100 and passed through exchanger
24
2 o to heat the incoming feed.
Depending on the flow capacity of the reactors in the Cs-C6
isomerization zone, all or a portion of the isomerate product from the G
isomerization zone is mixed directly with the second combined feedstream
carried
by line 34 to provide a mixed feedstream carried by line 34'. It is preferable
to
combine all of the isomerate product from line 100 directly with the second
19
w 2p 45356
combined feedstream since this allows all of the required hydrogen for the Cs-
C6
isomerization zone to be transferred directly to the second feedstock and all
of the
available heat in line 100 to be utilized. Any flow capacity limitation will
stem
from space velocity limitations in the reactors of isomerization zone 12. If
the Cs-
C6 isomerization zone has insu~cient flow capacity for all of the effluent
carried
by line 100, a portion of the line 100 effluent is diverted into the egluent
from the
CsC6 isomerization zone by line 102. If the flow through line 102 is large, a
heater similar to heater 30 shown in Figure 1 can be provided to heat the
second
feedstock against line 102. Thus in a further embodiment of this invention the
to effluent from the C4 isomerization can be split between line 100 and line
102, and
the split regulated to control the space velocity in isomerization zone 12,
the
amount of hydrogen added to the second feedstock via line 100 and the amount
of
direct heating obtained from line 100.
When all of the hydrogen requirements for isomerization zone 12
cannot be supplied by the effluent from isomerization zone 10, a portion of
the
hydrogen for the Cs-C6 isomerization zone is supplied by diverting a portion
of
hydrogen stream from line 20 into line 104. Even when all of the effluent from
isomerization zone 10 is combined with the Cs-C6 feedstock, it may still be
desirable to supply a portion of the hydrogen requirements for isomerization
zone
2 0 12 through line 104 in order to lower the hydrogen partial pressure in
isomerization zone 10.
Figure 2 also shows the addition of a charge heater 106 for supplying
additional heat to the mixed feedstream carried by line 34'. The use of the
charge
heater provides additional flexibility to the process by making up for any
heat lost
by the diversion of the C~ isomerate through line 102. Except as otherwise
Zo 2p 45356 ~ -.
.~:
descn'bed, the reactors and effluent heat exchange for isomerization zone 12
are
essentially the same as that described in conjunction with Figure 1.
All of the effluent from both isomerization zones is eventually
combined and together enters a separation facility 108 via line 110. The
combined effluent is carried by line 110 through a condensor 112 and directly
into
a stabilizer column. Since in this embodiment both isomerization zones operate
with a minimum hydrogen to hydrocarbon ratio, there is no need for a product
separator to recover hydrogen for recycle to the isomerization zones. Apart
from
a slightly higher hydrogen concentration in the stabilizer overhead stream,
the
io operation of the stabilizer section for the embodiment of Figure 2 parallel
that of
Figure 1.