Note: Descriptions are shown in the official language in which they were submitted.
WO90/10277 PCT/US90/00999
METHOD AND APPARATUS FOR u~r~KMINING 204
A PROLIFERATION INDEX OF A CELL SAMPLE
BACKGROUND OF THE INVENTION
The invention relates to a system for performing
a biochemical assay of a cell sample to provide an
accurate quantitative analysis of a characteristic of the
cells which have been sampled. More particularly, the
invention is directed to a system which receives images of
stained cells and enhances the cell images prior to
further processing to determine the proliferation index of
the enhanced cell images.
One of the problems which faces pathologists in
their clinical practice is that of determining whether a
cell sample taken from a patient during a biopsy procedure
or the like is benign or malignant. Although a surgeon
may have a good intuition about the type of tissue mass
which he has removed, nevertheless he must confirm his
preliminary diagnosis with a histological examination of
the cell sample removed from-the patient. The
histological examination entails cell staining procedures
which allow the morphological features of the cells to be
seen relatively easily in a light microscope. A
pathologist after having ~x~ined the stained cell sample,
makes a qualitative determination of the state of the
tissue or the patient from whom the sample was removed and
reaches a conclusion as to whether the patient is normal,
has a premalignant condition which might place him at risk
of a malignancy in the future or has cancer. While this
diagnostic method has provided some degree of
predictability in the past it is somewhat lacking in
scientific rigor since it is heavily reliant on the
subjective judgement of the pathologist.
eY~mination process. In U.S. Patent No. 4,741,043 to
Bacus for Method and Apparatus for Image Analyses of
Biological Specimens, an automated method and a system for
measuring the DNA of cells are disclosed which employ
differential staining of the DNA in cell nuclei with a
Feulgen Azure A stain and image processing. While the
sys~em provides an accurate assay of the cellular DNA its
predictive power for cell replication, a key indicator of
the presence of cancer, could be improved.
It is well known that cells follow a replication
cycle. Most somatic cells of an adult human replicate at
a relatively slow rate, only rapidly enough to replace
cells shed by the body and lost to normal cellular wear
and tear. At any instant, most of those somatic cells are
in the G0 or resting phase of the replication cycle. When
they leave the resting phase they enter the Gl or first
gap phase but are not yet producing extra DNA. Upon
becoming committed to the S-phase, however, they do
produce other material such as proliferation substances
e.g. cyclin and other S-phase proteins. The cells in the
synthesis or S-phase are actively synthesizing DNA and
produce double the amount of DNA normally contained in the
cell nuclei in preparation for mitosis or division of the
cell nuclei during cell replication. A normal human
somatic cell contains 23 chromosome pairs and is in the
diploid state. The diploid state is also referred to as
the 2N state. At the time of replication the number of
chromosome pairs increases to 46, double the normal amount
in anticipation of cell division. The chromosome state
immediately before replication is referred to as the 4N
state. The cells then enter the second gap phase or G2
phase in which little or no DNA is synthesized. Following
the G2 phase is the mitosis or M-phase in which the cells
themselves divide. If the cells are actively
proliferating they may reenter the Gl phase.
WO90/10277 PCT/US90/00999
-3- 2~4~6~
Although DNA analysis may be adequate for estimating the
number or proportion of proliferating cells in normal
cells or tissue, it should be appreciated that this is not
the case with malignant cells, the very ones for which it
often is important to know the extent of proliferation.
This is because malignant cells often have increased
amounts of DNA, even in the G0 phase, due to the increased
chromosome count. Therefore, it is impossible to conclude
with certainty from a DNA analysis that a particular cell,
e.g. one having l.5 times the normal DNA content, is a
malignant cell with additional chromosomes or chromosome
parts or is a normal cell which is halfway through the
S-phase having only replicated one-half the DNA necessary
for cell division. Thus it is clear that an analysis
method independent of DNA, utilizing other markers, such
as variously produced proteins associated with S-phase
proliferation and the cell division process, has many
advantages
It should also be appreciated that quantitating
on cellular proliferation indices has previously been
performed by counting the numbers of cells in a cell
sample carrying an indicator or stain for a proliferation
substance. For instance, a well known method of
determining the proliferation index is to stain the cells
with an immunofluorescent dye which binds to cyclin and
manually count the stained cells to determine the
proportion of cells having proliferation substance.
Another method of determining the proliferation
index of cells is the grain counting method. In that
method, tritiated thymidine is added to a cell culture
growth medium. Proliferating cells take up the tritiated
thymidine and incorporate it into DNA being synthesized in
the cells. The cells are then fixed and placed in
proximity with a photographic emulsion. Decay products of
the tritium expose portions of the emulsion. The exposed
portions may be visualized as grains by photographic
WO90/10277 PCT/US90/00999
20 ~v~e~opment processes. The grains are then counted to
determine whether the cells are normal or abnormal. One
of the drawbacks of this method lies in the fact that it
is very time consuming. It is necessary that the cells be
harvested alive and kept alive long enough to take up the
tritiated thymidine. The cells must then be fixed and
held in proximity with the emulsion in order to expose
it. Since relatively low intensities of radiation may
emanate from the cells, it may take days or even weeks to
obtain a latent image on the emulsion, which must then be
developed. In the meantime, the patient's disease may be
progressing.
One of the drawbacks of the prior art methods is
that they are prone to human error due to the tedium of
counting the cells on a microscope slide under high
magnification. Often the people examining the slides only
are able to estimate the relative number of cells which
show a positive result for proliferation substance.
The prior imaging systems have also suffered from
the problem that while they usually accurately identify
the images of cell objects in an image being processed
they do not always accurately identify boundaries of the
cell objects being evaluated. This may be a problem when
an assay is being performed on the cell objects on the
basis of their image areas.
The prior art methods of guantitatively analyzing
the cell samples for proliferation substances could not be
automated simply. This is because it is necessary to
determine a baseline value for the total number of cells
examined as opposed to the number of cells which have
proliferation substance. In order to make this type of
evaluation an automatic system must be able to recognize
what constitutes a cell or a cell nucleus. In order to
solve this baseline recognition problem the instant
invention employs separate stains for the cell nuclei and
the proliferation substances. In addition, the stains are
WO90/10277 PCT/US90/00999
~5~ 20~5~L4
separated spectrally so that they can be readily
distinguished by optical filters which are compatible with
them. The optical separation of the two components to be
measured makes the subsequent analysis of the cell images
more convenient to automate.
A similar difficulty is encountered in an image
analysis based on cell object areas when cell objects
images overlap, touch or otherwise share contiguous
areas. In that case, what is actually a double or triple
object image may not be tallied properly resulting in an
inaccurate result or conclusion.
SUMMARY OF THE INVENTION
The present invention provides a rapid and
convenient method and an apparatus for practicing that
method for determining the amount of a proliferation
substance in a cell sample. The cell sample may be a
tissue sample or a cell preparation. Tissue samples are
frozen sections or paraffin sections of connected cells.
The cell preparations are made from body fluids such as
cerebrospinal fluid, blood, pleural effusions and the
like. Cell preparations may also be made from needle
aspirates of tumors, cysts or lymph nodes. Cell
preparations may also be made from touch preparations
which are made by touching a freshly microtomed surface of
a piece of tissue to a microscope slide to which the cells
cling. In particular, the apparatus and method employ a
rabbit anti-mouse immunoglobulin (IgG) based staining
system wherein antibodies for a proliferation substance
such as cyclin or the antigen for Ki-67 are complexed with
an enzyme in this embodiment horseradish peroxidase
(HRP). The cells are contacted with the HRP-proliferation
substance antibody conjugate which binds only to portions
of the cells which have epitopes identifying them as
3S proliferation substance. A stain, in this embodiment 3,
WO90/10277 ~ 4 -6- PCT/VS9OtO0999
3' diaminobenzidine tetrahydrochloride (DAB), and hydrogen
peroxide H2O2 are placed in contact with the cells
having the antibody-HRP conjugate bound to their
proliferation substance sites. The HRP catalyzes a
chromogen forming reaction only at the areas where it is
bound. The catalyzed chromogen forming reaction produces
a red-brown chromogen precipitate bound to proliferation
sites.
The cells are then stained with a counterstain,
in this instance ethyl green, which is commonly known as
methyl green. The image of the cells is magnified in a
light microscope and split into a pair separated images.
The separated images are enhanced by a pair of narrow
bandpass optical filters. One of the narrow bandpass
optical filters preferentially transmits light having a
wavelength at the transmission peak of the counterstain
thereby producing an optically enhanced proliferation
substance image which only has background and the
red-brown chromogen. The background of the proliferation
substance image is composed of the cell nuclei, cytoplasm
and the like which have substantially zero optical
density. The proliferation substance sites have a
relatively high optical density. Thus the only features
which are easily perceivable are the proliferation
substance sites.
The other narrow bandpass optical filter
preferentially transmits the red-brown transmission peak
and blocks the counterstain peak thereby enhancing optical
density differences between the cell nuclei and the
proliferation substance chromogen. The filter produces an
optically enhanced cell nuclei image which has only
background features and the cell nuclei.
The inventive apparatus senses the enhanced
proliferation substance image with a first monochrome
television camera. The enhanced cell nuclei image is
sensed by a second monochrome television camera. Analog
signals representative of the images are fed to respective
WO90/10277 PCTtUS90/00999
~7~ 2~5~
image processors. The image processors convert the analog
signals to digitized arrays of pixels which are stored in
internal frame buffers.
When a tissue section is being examined the
apparatus computes an area of the proliferation substance
image which has high optical density, yielding an area
measure for the proliferation substance in that image
field. When a cell preparation is being examined the
apparatus computes the proliferation index on the basis of
the percentage of cell nuclei having more than a threshold
amount of proliferation substance therein.
BRIEF DESCRIPTION OF THE DRAWINGS
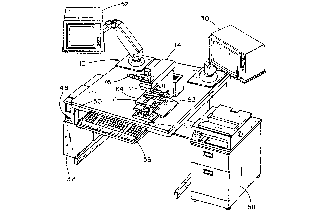
FIG. l is an isometric view of an apparatus for
determining a proliferation index of a cell sample
embodying the present invention;
FIG. 2 is a block diagram of the apparatus of
FIG. l;
FIG. 3 is an elevational view of an optical
conversion module of the apparatus of FIG. l;
FIG. 4 is a magnified view of a stained cell
sample as seen through the microscope of FIG. l without
optical filtering;
FIG. 5 is a magnified view of the stained cell
sample of FIG. 4 as seen through a 620 nanometer narrow
band optical filter which yields a cell nuclei image;
FIG. 6 is a magnified view of the stained cell
sample of FIG. 4 as seen through a 500 nanometer narrow
band optical filter which yields a proliferation substance
image;
FIG. 7 is a graph of the spectral response of a
chromogen, a counterstain and the narrow band optical
filters;
FIG. 8 is a flow chart of a sequence of steps
performed by the apparatus of FIG. l in selecting a cell
sample analysis mode;
WO90/10277 ~ ~ PCT/US90/00999
2~ ~5~ -8-
FIG. 9 is a flow chart of a sequence of steps
performed by the apparatus of FIG. 1 in determining the
proliferation index of a tissue section cell sample;
FIG. 10 is a flow chart of the steps carried by
the apparatus in determining the proliferation index of a
cell preparation cell sample;
FIG. 11 is a screen display of the tissue screen;
and
FIG. 12 is a screen display of the cell
preparation screen.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
Referring now to the drawings and especially to
FIG. 1, an apparatus embodying the present invention and
generally identified by numeral 10 is shown therein. The
apparatus 10 comprises an optical microscope 12, which may
be of any conventional type but in this embodiment is a
Reichart Diastar or Microstar. An optical conversion
module 14 is mounted on the microscope 12 to enhance
optically a magnified image of a cell sample viewed with
the microscope 12. The optical conversion module 14, as
may best be seen in FIG. 3, has a cell nuclei sensing
means comprising a cell nuclei image optical enhancement
unit 16. The cell nuclei image optical enhancement unit 16
has a 620 + 20 nanometer red narrow bandpass optical
transmission filter 18 and a television camera 20 for
receiving a filtered image from the filter 18. A
proliferation substance sensing means comprising a
proliferation substance optical enhancement module 22 has
a green 500 + 20 nanometer narrow bandpass optical
transmission filter 24 and a television camera 26 and is
also part of the optical conversion module 14. Each of
the television cameras 20 and 26 generates a standard NTSC
compatible signal representative, respectively, of an
enhanced cell nuclei image and an enhanced proliferation
substance image. An image processing system 28 is
WO90/10271 PCT/US90/00999
9 2 ~
connected to the television cameras 20 and 26 to receive
the enhanced cell nuclei image signal and the enhanced
proliferation substance image signal and to store a cell
nuclei pixel array and a proliferation substance prixel
array therein. The image processor 28 is connected to a
computer 32, in the present embodiment, an IBM personal
computer model AT for processing of the cell nuclei and
proliferation substance pixel arrays.
The computer 32 includes a system bus 34,
connected to the image processor unit 28. An 80286
microprocessor 36 is connected to the system bus 34. A
random access memory 38 and a read only memory 40 are also
connected to the system bus 34 for storage of information.
A disk controller 40 is connected by a local bus 44 to a
Winchester disk drive 46 and to a floppy disk drive 48 for
secondary information storage. A video conversion board 50
in this embodiment, an EGA board having 256K bytes of
memory, is connected to the system bus 34 to control an
instruction monitor 52 connected to the EGA board 50. A
keyboard processor 54 is connected to the system bus 34 to
interpret signals from a keyboard 56 which is connected to
the keyboard processor 54. A printer 58 is connected to
the system bus 54 for communication therewith. An X Y or
image field board 60 is connected to the system bus 34.
The X Y board 60 also is connected to a slide holder of the
microscope 12 to sense the relative position of a slide 62
with respect to a microscope objective 64 and thus identify
a field being viewed. Included is a Y position sensor 66
and an X position sensor 68. The Y position sensor 66 is
connected via a communication path 70 to the X Y board 60.
The X position sensor 68 is connected via a communication
path 72 to the X Y board 60. The microscope 12 also
includes an eyepiece 76 in optical alignment with the
objective 74 for magnification of light forming an image of
a cell sample on the slide 62.
WC90/10277 PCT/~S90/00999
20~561 ~ -lO-
The method of the instant invention is practiced
by collecting a cell sample, which may be in the form of a
tissue section made from a frozen section or a
paraffinized section and having both cell nuclei, cell
fragments and whole cells therein. Alternatively, the
cell sample may be a cell preparation of the type which
might be taken from blood, pleural effusions,
cerebrospinal fluid, or by aspirating the contents of a
cyst or a tumor. The cells of the cell sample are placed
on the slide 62 and fixed thereon. A monoclonal antibody
for a proliferation substance to be detected in the cells
is then placed in contact with them. The monoclonal
antibody may for instance be Xi-67 or may be an antibody
for, 5-bromodeoxyuridine, for cyclin or for other proteins
which indicate that cellular replication is occurring.
The monoclonal antibody selectively binds to all points on
and within the cells where the proliferation substance is
present. The monoclonal antibody also has bound thereto a
bridging antibody and a peroxidase anti-peroxidase
complex. The anti-peroxidase comprises an antibody which
specifically binds to the enzyme peroxidase. The
peroxidase enzyme is bound to the antibody and held
through the chain of antibodies to the proliferation
substance in the cells.
In order to view the proliferation substance
sites, a quantity of a mixture containing hydrogen
peroxide and 3, 3' diaminobenzidine tetrahydrochloride
(DAB) is applied to the cell sample on the slide 62. The
hydrogen peroxide and the DAB react to form a chromogen
consisting of a reddish-brown precipitate. The usual rate
of reaction however is relatively low. The peroxidase
catalyzes the chromogen-forming reaction only at the
points where the peroxidase is localized. Thus, chromogen
is precipitated only at the points in the cells where
proliferation substance is present and the cells are
preferentially stained only at the points where they
WO90/10277 PCT/US90/00999
have proliferation substance. After a period o~ Q~o~
15 minutes, the unreacted DAB and hydrogen peroxide are
removed from the cell sample. The cells are then
counterstained with methyl green (more properly known as
ethyl green) which preferentially binds with the cell
nuclei. Thus, cell nuclei are stained and the points
within the cell nuclei having proliferation substance
are stained reddish-brown.
The microscope slide 62 is then placed on a
carrying stage of the microscope 12 and the objective 64
is focused thereon. Light from the objective 64 travels
through the eyepiece 12 where it may be viewed by an
observer. In addition, the optical converter module 14
includes a beam-splitting mirror 80 which carries off
approximately 90% of the light from the objective 64 to
other portions of the converter 14. The light is fed to
a dual prism dichroic mirror 82 which reflects a portion
of the light to the red filter 18. The remaining
portion of the light is filtered by the dichroic
mirror 82 and fed to the green filter 24. The dichroic
mirror 82 selectively passes light having wavelengths
greater than 500 nanometers to the filter 18 and having
a wavelength of less than 500 nanometers to the
filter 24. Thus, the dichroic mirror 82 acts as a first
color filter before the light reaches the color
filters 18 and 24.
When the light passes through the filter 18,
the filter 18 preferentially blocks light from the green
stained cell nuclei and provides a high contrast cell
nuclei image to the camera 20. The camera 20 then
generates an NTSC cell nuclei image signal which is fed
to the image processor module 28. The image processor
module 28 has an image processor 90 and an image
processor 92. Each of the image processors 90 and 92 is
a model AT428 from the Datacube Corporation. Similarly,
the green filter 24, filter, provides a high contrast
proliferation substance image to the camera 26. The
WO90/10277 PCT/US90/00999
2 0~ lg -12-
camera 26 then feeds the proliferation substance image
signal to the image processor 92. Both of the image
processors 90 and 92 contain analog to digital
converters for converting the analog NTSC image signals
to digitized arrays of pixels which are then stored
within internal frame buffers. The internal frame
buffers may be accessed via the system bus 34 under the
control of the microprocessor 36.
The image of the cell sample viewed through the
eyepiece 12 is of the type shown in FIG. 4 wherein a
green cell nucleus 100, a green cell nucleus 102, a
reddish-brown cell nucleus 104 having proliferation
substance therein, a reddish-brown cell nucleus 106, and
a reddish-brown and green cell nucleus 108 appear in an
image field. As may best be seen in FIG. 5, the cell
nuclei are shown therein as they would appear through
the red filter 18, which causes all of the green cell
nuclei to darken and appear prominently. As may best be
seen in FIG. 6, the proliferation substance image of the
cell nuclei of FIG. 4 is shown therein with the cell
nuclei 100 and 102 being rendered substantially
transparent or invisible by the effect of the green
filter 24 which has its transmission peak at
approximately the same wavelength as the transmission
peak for the methyl green stain. The cell nuclei 104,
106 and 108 having the reddish-brown chromogen deposited
therein which is an indicator for the proliferation
substance appear clearly in high contrast.
The cell nuclei image of FIG. 5 is stored in
the internal frame buffer of the image processor 90.
The proliferation substance image of FIG. 6 stored in
the internal frame buffer of the image processor 92. It
may be appreciated that the pixel values for the images
may be sliced using standard image processing techniques
to increase the contrast between the cell nuclei and the
backgrounds. That is, the areas of high optical density
in FIG. 6 such as the cell nuclei 104, 106 and 108 may
WO90/10277 -13- PCT/U~Od~959
be shown as being very dense and stored as high optical
density pixels, while the background areas 110 may be
stored in substantially zero optical density pixels in
order to provide a clear threshold or difference between
the two areas. This is particularly helpful when
performing computations to determine the proliferation
index, since the system can differentiate more easily
between background and nuclei to be measured. This
slicing technique acts as an additional amplifying step
for the images.
Once the images have thus been acquired by the
system, the user as may best be seen in FIG. 8, is
interrogated as to whether the images are from a tissue
section or a cell preparation. More particularly, after
a starting step 120, the system 10 next displays an
initial display screen 122 on the instruction monitor 52
and then interrogates the user in a step 124 as to
whether a tissue section forms the basis for the image
being processed. If the user provides a positive
response to the system 10, control is transferred to a
step 126 wherein a tissue section screen is displayed on
the instruction monitor 52. If the response is
negative, control is transferred to a step 128 where the
user is questioned as to whether the cell sample is from
a cell preparation. If the response is positive,
control is transferred to a step 130 wherein a cell
preparation processing and result screen of the type
shown in FIG. 12 is displayed on the instruction monitor
52. In the event that neither of the selections is
made, a step 132 transfer control to a HELP screen 134.
Referring back to the step 126, it may be
appreciated that the screen of FIG. 11 is displayed
during the step 126. The screen provides a menu of
functions at the right-hand side which are of the type
well known to users of automated cell analysis
equipment. Ir. particular, the user may select a nuclear
WO90/10277 2 0 ~ S fi ~ ~ -14- PCT/US90/00999
threshold function wherein the user may specify the
threshold optical density or pixel value at which the
system l0 determines for purposes of computation that a
particular pixel value is indicative of the presence of
a portion of a cell nucleus at that point. Furthermore,
an antibody threshold may similarly be set wherein the
optical density of the image of FIG. 6 is measured and a
threshold is set indicative of the presence or absence
of antibody at a particular pixel address. In addition,
the user, once having set the thresholds, may then
display outlines or shaded areas of the cell nuclei and
the antibodies in a display nuc-anti masking function.
Once the user does this, control is transferred to a
tissue section analysis step 140 which may be seen in
more detail in FIG. 9.
A 620 nanometer cell nuclei image of the type
is received by the camera 20 in a step 150. The analogy
image signal is digitized in a step lS2 and a threshold
value for pixels indicating the presence of the cell
nuclei is selected in a step 154. Once the threshold
has been selected, pixels having a value less than the
threshold have their values set to a pre-selected
background level while the pixels having values over
leaving a high contrast pixel array for further
processing. The pixel array is transferred to the
computer system 32 where the number of pixels having
values exceeding the selected nuclear threshold value is
counted to provide a cell nuclei amount or count which
will be used as a proliferation index denominator in
later processing.
Similarly, the proliferation substance image of
the type shown in FIG. 6 is received by the camera 26 in
a step 160. The proliferation substance image is
digitized by the image processor 92 in a step 162. An
antibody threshold which has been selected by the user
reduces the background of the proliferation substance
tS 1tec'd PC~/~O O 8 M~Y t9~1
4~ PNlUS90/00i9'
-15-
image to zero and effectively isolates the pixels
representative of the proliferation substance antibody
in a step 164. The isolated pixels, that is those
pixels having a value greater than the preselected
antibody threshold, are then counted by the system 32
and a pixel count number 162 is provided in the step
166.
Thus, it may be appreciated that steps 150
through 156 effectively measure the area of the image
field of FIG. 5 wherein cell nuclei are found. The
steps 160 through 166 effectively measure the area of
the proliferation substance in the image field of
FIG. 6. The computer 32 in a step 168 then divides the
proliferation substance by the area of the cell nuclei
and generates a quotient which is equal to the
proliferation index. The proliferation index is then
displayed on the tissue section screen as a percentage
number. In addition, the total nuclear area as computed
in steps 150 through 156 is also displayed.
In the event that the user has indicated to the
system in the step 128 that a cell preparation is being
analyzed, control is transferred to step 130 which may
be seen in more detail in a step 170 as shown in FIG.
10. In a step 200, the cell nuclei image of FIG. 5 is
received by the camera 20. The cell nuclei image is
digitized in a step 202. The digitized cell nuclei
image is then analyzed in a step 204 to determine, using
neighborhood labelling, what objects are to be
considered by the system 10 to be cell nuclei and what
objects are not. The objects to be considered to be
cell nuclei are indicated by being surrounded by boxes
as displayed on the image monitor 30. In a step 206, if
two or more of the objects are in contact with each
other, the operator is given the opportunity to have the
system draw a line of demarcation between them or to
mutually separate the images himself.
8UBSm~ 1E~J.
WO90/10277 PCT/US90/00999
' 2~i6~4 -16-
In a step 210, a threshold value is then
applied to the pixel arrays in a step 208 to amplify the
differences among pixels by slicing, as was done in
steps 154 and 164 previously. Similarly, in a step 212,
the proliferation substance image of FIG. 6 is received
by the camera 26. The proliferation substance image is
digitized in the step 214 and is isolated in a step
216. The cell nuclei and proliferation substance pixel
arrays are then combined in a step 218 and displayed on
the image monitor 30. The cell nuclei are counted by
the computer 32. Likewise, the cell nuclei having
proliferation substance are also counted by the computer
32. The number of proliferation substance nuclei is
then divided by the total number of cell nuclei to
produce a proliferation index for the cell preparation
sample. The proliferation index is then displayed on
the cell preparation screen of FIG. 12.
It may thus be appreciated that the tissue
section feature of FIG. 9 allows the proliferation index
for a tissue section sample to be easily and rapidly
computed using stereological principals which are
standard in the field of microscropy. When tissue
sections are not used and stereological principals do
not apply, the cells may be counted by using the cell
principal preparation technique.
Furthermore, the system provides considerable
amplification for determination of the proliferation
index. The initial amplification takes place when the
proliferation substance is identified with the chromogen
and the cell nuclei are stained with the counterstain.
A second amplification takes place when the cell nuclei
and proliferation substance images are formed by
filtering the light through the optical filters 18 and
24. Further amplification takes place when the
threshold values for the proliferation substance and the
cell nuclei are set providing high contrast images and
high gain digital arrays for further processing.
WO90/10277 PCT/~S90/00999
-17- 2 0 ~
While there has been illustrated and described
a particular embodiment of the present invention, it
will be appreciated that numerous changes and
modifications will occur to those skilled in the art,
and it is intended in the appended claims to cover all
of those changes and modifications which fall within the
true spirit and scope of the present invention.