Note: Descriptions are shown in the official language in which they were submitted.
~58~8
HOECHST ARTIENGESELLSCHAFT HOE 90/F 192 Dr. Fi/PP
Description
2,4- and 2,5-substituted pyridine-N-oxide~, proce~ses for
their preparation and their U8e
Compounds which inhibi~ the enzymes proline hydroxylase
and lysine hydroxyla~e cau~0 ~ very selective inhibition
of collagen biosynthesis by in~luencing collagen-~pecific
hydroxylation reactions. In the course thereof, protein-
bound proline or lysine i~ hydroxylat~d by the enzymes
proline hydroxylase or lysine hydroxyla~e. If this
reaction i5 suppressed by inhibitors, a non-functional,
underhydroxylated collagen molecule i~ formed, which can
be released into the extracellular ~pace by the cell~
only to a small e~tent. In addition, th~ underhydroxy-
lated collagen cannot be inoorporated into the collagenmatrix and is ve~y easily degraded by prot~olysi3. As a
result of these effects, the amount of collagen stored
extracellularly is on the whole reduced.
Inh~bitor~ of prolyl hydroxylase are th~re~ore ~uitable
substances in the treatment of disorders in which the
storage of collagens contrlhutes decisively to the
symptoms. These include, inter alia, $i.bros~s of the
lungs, liver and skin (~cleroderma~ and atherosclerosis.
It is known that the inhibition of proline hydroxylase by
known inhibitors such a ~ dipyridyl leads to an
inhibition o~ Clq biosynthesis by macroph~es (W. ~ller
et al., FEBS Lett. 90 (1978), 218; Immunbiology 155
(1978), 47). As a result, a failure of the classical
route of complemen~ activation occur~. Inhibitors of
proline hydroxyla~e therefore also act a~ immunosuppres-
sives, for example in immune complex diseases.
It is known that the enzyme proline hydroxylase is
effectively inhibited by pyridine-2,4 and
-2,5-dicarboxylic acid (R. Ma~amaa et al.,
2~45~8
-- 2 --
Eur. J. Biochem. 138 (1984) 239-245). However, ~hese
compounds are effec~ive in cell culkure as inhibitors
only in very high concentrations (Tschank, G. et al.,
Biochem. J. 238 (1987) 625-633).
DE-~ 3,432,0g4 dascribes pyridine-2,4- and -2,5-dicar-
boxylic acid diesters haring 1-6 carbon atoms in the
ester al~yl moiety as pharmsceutical~ for the inhibition
of proline hydroxylase and lysine hydroxyla~e.
However, these lower-alkylated diestexs have the disad-
vanta~e tha~ ~hey are cleaved ~o the acid~ too rapidly in
~he organism and do not reach their ~ite of action in the
cell in sufficiently high concentration and are therefore
le ~ suitable for possibl admini~tration a8 pharmaceuti-
cals.
DE-A 3J703~959~ DE-A 3,703,962 and DE A 3,703,963 dQS-
cribe in general form mixed e~ter/amide6, higher alky-
lated diesters and diamides of pyridine-2,4- and
_2,5-dicarboxylic acid, which effsctively inhibit,
collagen biosynthesis in the animal model. ~hus,
DE-A 3,703,959, inter alia, de~cribes the synthesi~ of
N,N'-bis(2-methoxyethyl)pyridin2-2,4-d~carboxamide and
N,N'-bis(3-isopropoxypropyl)pyridine-2,4-dicarboxamide.
An improved process for the preparation of
N,N~-bis(2-methoxyethyl)pyridine-2,4-dicarbo~amide i8
propo~ed in Genman Patent Application~ P 38 26 471.4 and
P 38 28 1~0.6.
German Patent Application P 39 24 093.2 proposes ~ovel
N,N~-bistslko~yalkyl)pyridine-2,4-dicarboxamides.
German Patent ~pplication P 40 01 00~.3 describes the use
of N,N'-(nitroxyalkyl)pyridine-2,4- and -2,5-dicarbox-
amides for the preparation of pharmaceuticals inhibiting
proline hydroxyla~e and lysine hydroxylase.
Both pyridine-2,4- and -2,5-dicarboxamide
- 3 - ~ ~4~8~
(Hirakata et al., J~ pharm. Soc. Japan 77 (1957) 219 and
Haring et al., ~elv. 37 ~195~ 7, 153) and pyridine-
2,4- and -2,5-dicarboxylic acid dihydrazide ~It~i et al. r
Bl. nation. hyg. ~abor. Tokyo, 74 ~1956) 115, 117 and
Shinohara et al., Chem. High Polymer~ Japan, 15 (1958)
839) are already known as antituberculosi~ agent~.
JP 53/28175 (78/28175) de~cribes N,N'-bis~2-nitrooxy-
ethyl)pyridine~2,4- and -2,5-dicarbox~mides as 6ubstance~
having vasodilatory action.
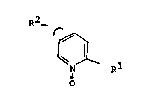
Surprisingly, i~ ha~ now been found that 2,4- and 2,5-
substituted pyridine-N oxides of the general formula I
indicated below and the phy~iolo~ically tolerable ~alts
effectively inhibit ly~ine hydroxylase and p:roline
hydroxylase in the animal model.
The invention accordingly relates to 2,4 and 2,5-~ub-
stituted pyridine N-oxides of the general formula I
R2_
o
in which
R1 is -C(o)-X-R3, where
X is O or -N(R3)~ and
R3 is hydrogen, Cl-C12-alkyl, C2-Cl2-alkenyl, C2-Cl2-
alkynyl, non-benzo-fu~ed or benzo-fused C5-C7-
cycloalkyl, aryl or heteroaryl, where the6e
radicals mentioned for R3 are unsub9tituted or
~5 are sub~tituted by one or more identical or
different radicals R4, where
R4 is halogen, hydroxyl, cyano, nitro,
_ 4 _ 2~
nitroxy, amino, carboxyl, C,-C4-alkoxy,
C~-C4-alkoxycarbonyl, Cl~C~-al~yl- or
-dialkylamino, indolyl or phenyl, where
the indolyl or phenyl radical i8 unsub-
~tituted or monosubstituted t dlsub~tituted
or tri~ub~tituted by halogen, nitro, C1--C4-
alkyl or C~-C4-alkoxy~ ~here, in the case
of poly~ubsti~ution, the radicals are
idenkical or different
or
R3, if X is -~(R3), i8 a radical -N(R5) (R6)~ in
which
Rs and R6 are identical or dif ferent and are
hydrogen, C,-C4-alkyl, C1-C3-alkylcarbonyl
or phenyl
and
R3 has the meaning of R3, where the radicals R3 and
R3 are identical or diffarent ox
R3 and R3, together with the nitrogen atom to which
~0 they are bonded, are a radical of the
formula II
r~
_ N ~ (II)
(CH2)n
in which
n is l to 3 and
A is 0, S, CHa or -N~R')-, where
R7 i~ hydrogen, phenyl, Cl-C6-alkyl, C2 CB-alkenyl
2~8~
or C2-C6~al~ynyl, whera these mentioned radical3
are uns~bstituted or substituted by
phenyl which, for it~ part, i8 un~ubstltuted,
or monosub~ uted or poly~ubsti~ted by
one or more identical or differen~ ~ub-
~tituent~ ~elect0d from the group com-
prisings halogen, nitro, cyano, carbo~yl,
hydro~yl, methyl, ethy:L, methoxy, ~thoxy
and trifluor~ethyl
or
-N~R~)z, where
R8 is hydragen or C~-C3-alkyl
or
-COOR~
or
-CON~R~)2 or CoNHR7, where
R9 has ~he meaning of ~8 or where (R9) 2
is a C4~C6~alkylene chain in which no
CH2 ~roup or a CH2 group which is not
directly ad~acent to the ni~rogen
atom is xeplaced by O, S or N-R8
or where
R7 is Cl-C4-alkoxycarbonyl or C3-C,-cycloalkyl
and in which
R2 has the meaning of R1, where the radicals Rl and R2
2~86~
are identical or diffsrent
or R2 i8 vnly present in the 4-position, and one of the
radicals R3 or R4 is in the 5-po~ition
and the physiologically tolerable salts, where the
compound~ of the general formula I are excluded in which
Rl and R2 are identical or different and are carboxyl, it~
methyl or ethyl e~ters and its diethylamidas.
The invention furthermore relates ~o the use of compound~
of the ~eneral formula I and the physiologically
tolerable salts for ~he production of a pharmaceutical
inhibiting proline hydroxylase and ly~ine hydroxylase.
Finally, the invPntion relate~ to the compounds of the
general formu~a I for use as pharmaceuticals.
The invention relates in particular to the campounds of
the formula I for use as fibrosuppressives and immuno-
suppressives and also for the inhibition of proline
hydro~ylase and ly~ine hydroxylase and for influencing
the metab~lism of collagPn and collagen-like sub~tances
or the biosyntha6is of Clq.
All said alkyl radicals having more than 2 carbon atoms
can be either straight-chain or branched.
The invention furthermore relate~ to a process for the
preparation of compound~ of the general for~ula I.
The compounds according to the invention are mo~t 6imply
prepared by adding oxidants such as, for example, hydro-
gen peroxide or p~racids such as peracetic ~cid, per-
fluoroacetic acid, perbenzoic acid or metachloroper-
benzoic acid in ~olvents such as chlorinated hydro-
carbons, such as, for example, methylene chloride,
chloroform, tri- or tetrachloroethylene, benzene or
toluene, to the pyridine compounds to be oxidized, which
2l~8~
~ 7 --
can likewise be dissolved in the abovementioned ~olvents,
and stirring at a temperature betw~en -30 and +40C,
praferably between 0 and ~5C, ~or betwean 30 minutes
and 3 days. Completion of the reaction can ~e determined,
for example, by means of thin layer chromatography. The
compound~ according to ~he invention can pre~erably be
prepared by employing the pyridine deriYative and the
oxidant in equimolar amoun~s or up to an about 5-fold
excess of oxidant.
If appropriate, an exceRs of peracid ean al80 be elimin-
ated by introducing, for example, gaseou~ ~mmonia into
the reaction solution and separating the resulting
pr~cipitate from the reac$ion sslutiQn by filtration.
I~ appropriate, the products can be worked up, for
example, by extraction or by chromatography, for ex,ample
by means of silica gel. The isolated product can be
recrystallized.
A general procedure for this oxidation method i8 also
deRcribed, Por example, in "E. ~ingsberg, Pyridine and
its Derivatives, Interscience Publi6hers, Mew York, 1961,
Par~ 2, 93".
Oxidation with hydrogen peroxide i~ described, for
example, in "E. Ochiai, J. Org. Chem. 18, 534 (1953)".
The preparation of the dif~erent pyridine derivati~es
necessary for the oxidation described is set out in the
Patent Applications already cited as prior art. ~hose
which may be mentioned are German Patent Applications
P 38 26 471.4, 38 28 140.6, 39 24 093.2, 40 01 002.3 and
DE-A-3,703,959, 3,703,962 and 3,703,963.
The compounds of the formula I according to the invention
have useful pharmacological properties and in particular
show activity as inhibitors of proline hydroxylase and
lysine hydroxylase, as a fibro~uppressive,
- 8 - 2~g~
immunosuppres~ive and antiatherosclerotic.
The antifibrotic action can be detarmined in the ~arbon
tetrachloride-induced liver fibrosis model. For this
purpose, rats ~re treated twice weakly with CCl4 ~1 ~l/kg)
- di solved in olive oil. The te~t su~stance i~ ~dminl~-
tered daily, if appropriate even ~wi~e daily, orally or
intraperitoneally - dissolved in a 3ui~ab1e tolerable
solvent. The extent of liver fibro~ 8 i~ determined
histologically and the proportion of collagen in the
li~er i8 analyzed by hydroxyproline det~rmination a~
described in ~ivirikko et al. (~nal. Biochem. 19, 249 et
seq. tl967)). The fibrogenesis activity can be determined
by radioLmmunological determination of collagen fragmants
and procollagen peptides in the ~erum~ The co~pounds
according to the invention are effective in this model in
concen~rations of 1 - 100 mg/kg.
The fibro~enesis activity can be determined by radio-
immunological determination of the N-terminal propeptide
of type III collagen or of the N- or C-terminal cross-
linking domain of type IV ~ollagen (7s colla~en or typeIV collagen-NCl) in the serum.
For thi~ purpose, the hydroxyproline, procollagen III
peptide, 7s~collagen and type IV collaqen-NCl concentra-
tions in the 1 tver of
a) untreated rats ~control~
b) rats to which carbon ~etrachloride was admini~tered
~CCl4 control)
c) rats to which first CCl4 and then a compound accor-
ding to the invention was administered
were measured ~this test method is described by Rouiller,
C., experime~tal toxic in~ury o~ the liver; in The ~iver,
C. Rouiller, ~ol. ~, pp. 335-476, New York, Academic
Pressr 1964).
Another model for the evaluation of antifibrotic action
is bleomycin-induced lung fibrosis as described in
9 2~8~
Xelley et al. (J. ~ab. Clin~ Med. 96, 954, (1980)). The
cotton pellet granuloma model, as described in ~eier et
al. J Experientia 6, 469 (1~50) can be u~ed to evaluate
the action of the compounds according to the invention in
the granulation tissue.
The compounds of the formula ~ can be used a8 mediCamentB
in the form of pharmaceu~ical preparations which contain
them, if appropriate together with tolerable pharmaceuti-
cal carriers. The compounds can be u~0d as medic~ments,
for example in thP form of pharmaceutical preparation ,
which contain these compounds in a mixture with a pharma-
ceu~ical organic or inorganic carrier suitable for
enteral, percutaneous or parenteral administrationg such
as, for example, water, gum ~rabic, gelatin, lactose,
starch, magnesium stearate, talc, vegetable oils, poly-
alkylene glycols, petroleum ~elly etc.
For this purpose, they can be administered orally in
doses cf 0.1 - 25 mg/kg/day, preferably 1 - 5 mg/kg/day
or parenterally in doses of O.Dl - 5 mg/kg~day, pre-
ferably 0.01 - 2.S mg/kg/day, in particular 0.5
1.0 mg/kg/day. ~n severe ca~e~, the do6l~ge can also be
increased. In many caaes, however, lower dose~ are also
sufficient. This information relate~ to an adult weighing
about 75 kg.
The invention furthermore includes the use of the com-
pounds according to the invention in the production of
pharmaceuticals which are employed ~or the treatment and
prophyla~is of the abovementioned metabolic disorders.
The invention further relates to pharmaceutical~ which
contain one or more compound~ of the formula ~ according
to the invention snd/or their physiologically tolerable
~alts.
The pharmaceuticals axe prepared by processes which are
known per se and which are familiar to the person skilled
20~58~
in the art. As pharmacautical~, ~he pharmacologically
active compounds according to the invention are employed
either as such or preferably in combination with suitable
pharmaceutical auxiliarias or exoipient~ in the fo~m of
tablets, coated tablets, capsule6, ~uppositorie~, emul-
sions, su~pension~ or solu~ions, ~e active compound
content being up to about 95~, advantageously b~tween 10
and 75~.
In addition to solvent~ gel-forming agen~s, ~uppoaitory
bases, tablet auxiliaries and o~her active compound
carriers, ~uitable auxiliaries or excipients for the
desired pharmaceutical formulation are al60, for example,
antioxidants, dispersants, emulsifiers, antifoams, flavor
correctants, preservatiYes, solubilizers or colorantsO
The active compounds can be administered orally, paren-
terally or r~ctally.
~he active compounds are mixed with the additives
suitable for this purpose, such a~ excipients, stabi-
lizers or inert diluents and brought into suitable
adminis~ration form6~ ~uch as ~ablet~, coated tablets,
hard gelatin capsules, agueous alcoholic or oily ~uspen-
sions or aqueous or oily &olutions, by the customary
methods.
Inert excipient~ which can be u~ed are, for example, gum
arabic, magnesiaf magnesium carbonate, potassium phos-
phate, lactose, glucose or ~tarch, in particular corn
starch. In thi~ case, preparation can be carried out both
as dry and as moist granules- Possible oily excipient~ or
~olvents are, for example, vegetable or animal oils, such
as sunflower oil or cod liver oil.
For ~ubcutaneous or intravenous administration, the
active compounds are brought into solution, fiu~pension or
emulsion, if desired usin~ the substances suitable for
this such as solubilizers, emulsifier6 or other
8 6 ~
auxiliarie~. Suitable ~olvents are, for examp}e, phy~io-
logical ~aline solution or alcohol , for example ethanol,
propanol or glycerol, and in a~dition al~o ~ugar ~olu-
tion~ such as gluco~e or mannitol 801ution~, or, altarna-
tively, a mixture of the variou~ solvent~ mentioned.
The invention i8 illu~trated in more detail below by
Exzmples.
Genaral procedure for ~he preparatiorl of the compound~
1 equivalent of p~ridine derivative tfor preparation see
description~ is initially introduced in met~yl2ne
chloride and 1 equivalent of metachloroperbenzoic acid
(~CPBA), dissolved in methylene chloxide~ dded
dropwise at room temperature. The mixture i8 s~irred at
room temperature. ~fter completion of the reaction,
gaseous ammonia is blown into the solution with ice-
: cooling until a precipitate is no longer formed. The
precipitate is filtered off, and the filtrate i~ dried
with magnesium sulfate and concentrated.
~he crude product i8 recrystallized or p~ri~ied by means
of thin layer chromatography.
The compounds mentioned in the fol~owing Examples areprepared according to ~his general procedure.
~Emple 1
N,N'~Di-(2-methoxyethyl)pyridine-2,4-dicarbo~amide
N-oxide
From 1 g of N,N'-di-~2-methoxyethyl)pyridine-2,4-dicar-
boxamide and 0~62 g o~ ~CPBA.
Yield: 620 mg (chromatography. ethyl acetate/methanol
5:1)
M.p.: 10~C
1~ 2`~8~8
E~ample 2
N~N'-Di-(3-methoxypropyl~pyridine-2,4-dicarboxamide
N-oxide
From 1 g of N,N~-di-(3-methoxypropyl)pyridine-2,4-dicarb-
oxamide and 1.2 g of ~CPBA.
Yield: 0.58 g (recrystallization: ethanol~
M.p.: 90C
~ample 3
Pyridine-2,4-dic~rboxamide N-oxide
From 1 g of pyridine-2,4-dicarboxamide and 1.2 g of
MCPBAO
Yield: 0.8 g (recrystallization: ethanol)
M.p.: 260C
~æample 4
15 N,N'-Di-(2-dimethoxyethyl)pyridine-2,4-dicaxboxamide
N-oxide
From 1 g of N,N'-di-(2 dimethoxyethyl)pyridine-2,4-dicar-
boxamide and l.l g of MCP~A.
Yield: 0.5 g (chromatography: ethyl acetate/methanol 5:1)
~.p.: 8~~
- 13 ~ ~0~58~
~;e~ple S
N, N ' -Di- ( 3 -e~hoxypropyl ) pyridine -2, 4-dicarboxamide
N-oxide
From 1 g of N, N ' -di- ( 3-ethoxypropyl ) pyridine~2, 4 -dicarb-
oxamide and 1. 5 g of NCP33A.
Yield: O . 34 g (chromatography~ ethyl acetate/methanol
5 s 1 )
M.p.: ~1C
Example 6
N, N ' -Di- ( 2-methoxyethyl ) pyridine-2, 5-dicarboxamide
N-oxide
From 1 g of ~, N ' -di- ( 2-methoxyethyl ) pyridine-2, 4 -dicarb-
oxamide and 1. 3 g of MCPBA.
Yield: 0.4 g (recrys allization: ethanol)
M.p.: 137~C
~ample 7
Di-(2-methoxyethyl) pyridine-2,4-dicarboxylate N-oxide
From 1 g of di-(2-methoxye~hyl) pyridine-2,~-dicarboxy-
late and 1.3 g of MCPBA.
2Q Yield: O.2 g ~chromatography: ethyl acetate)
M.p.: oil
2~5868
- 14 -
~ample 8
N, N '-Diethylpyridine-2~5-dicarboxamide ~-oxide
From 1 g of N,N~-diethylpyridine-2,5-dicarboxamide and
1.8 g of ~CPB~.
Yield: 0.4 g (recrystallization: e~hanol~
.p.: 1~8C
E~a~pl~ 9
N,N'-Di-(3-methoxypropyl)pyridine-2,5-dicarbo~amide ~-
oxide
From 1 g of N,N' di-(3-methoxypropyl3~yri~ine-2,5-dicar
boxamide and 1.2 g of ~CPBA.
Yield: O.3 g (recrystallization: diethyl ether/methanol)
M.p.: 123C
~ample 10
2,4-Di-[(morpholin-l yl)carbonyl]pyridine N oxide
Fro~ 1 g of 2,4-di-[(morpholin-1-yl)carbonyl~pyridine ~nd
1,2 g of ~CP~A.
Yield: 0.5 g ~chromatography: ethyl acetate/methanol 5/1
M.p.: oil
4 ~ 8
13xample 11
N,N'-Di-(4-hydroxybutyl)pyridine-2,4-dicarboxamide
N-oxide
From 1 g of N,N~-di-(4~hydro~ybutyl) pyridine-2,4-dicarb-
oxamide and 0.8 g of ~CP~A.
Yield: 0.82 g (ethanol)
M.p.: ~8C
~xam~le 12
N,N'-Dicyclohe~ylpyridine-2,4-dicarboxamide N-oxide
From 1 g of N,N~-dicyclohexylpyridine-2,4-dicarbo~nide
and 0 g of MCPBA.
Yield: 0.59 g (ethanol)
M.p.: 153C
~am~le 13
N,N'-Di-(3-chlorobenzyl)pyridine-2,4-dicarboxamide
N-oxide
From 1 g of N,N'-di-(3-chlorobenzyl)pyridine-2,4-dicarb-
oxamide and 0.65 g of MCPBA.
Yield: 0.76 g (toluene~
M.p.: 112C
2~ 8
- 16 -
E~ample 14
N t N~-Di-(4-methylbenzyl)pyridine-2~ 4-dicar~oxamide
N-oxide
From 1 g o N,N'-di-~4-methylbenzyl)p~ridine-2,4-dicarb-
S oxamide and ~.2 g of MCP~.
Yield: 0.7~ g (toluene)
M.p.: 153C
~xample 15
Di-(4-chlorobutyl) pyridine-2,4-dicarboxylate N-oxide
From 1 g of di- ( 4-chlorobutyl ) pyridine-2, 4-dicarboxy~ ate
and 0.75 g of MCPBA.
Yield: 0.83 g (ethanol)
~,p.: 98C
~ample 16
Dicyclohexyl pyridine 2,4-dicarboxylate N-oxide
From 1 g of dicyclohexyl pyridine-2,4-dicarboxylate and
0.75 g of ~CPBA.
Yield: 0.87 g
Oil, MS - 348 (M~H) molec~lar weight 347
2 ~
- 17
~xample 17
Di-(methoxycarbonylmethyl) pyridine-2,4-dicarboxylate
N-oxide
From 1 ~ of di-(methoxycarbonylmethyl~ pyridine-2,4-di-
S carboxylate and 1.1 g of ~CP~.
Yield: 0.81 g
Oil, MS = 328 (M~H~ molecular wei~ht 327
Example 18
Pharmacological activity
In order to show the efficient inhibit:ion of proline
hydroxylase and ly~ine hydroxylase by the ~ompounds
according to the inven~ion, the concentrations of bili-
rubin, bile acids and gamma GT in the serum of
a) untreated rats ~control),
b) rats treated with CCl4,
c) rats to which first CCl4 and then a compound accor-
ding to the invention have been given,
are measured. (The method i5 described by Roailler, C.~
Experimental toxic in~ury of the liver; in The Liver,
C. Rouiller, Vol. 2, pages 335-476, New York, Academic
Press 1964).
The result~ are summarized in Table 1.
2 0 ~ 8
Table 1:
Action of prolyl hydroxylase inhibitor~3 on CCl4-induced
liver fibxosi3 in rats
~rreatment Dose~ R~lirubin Bile ~CidB G~na
n~ N ~n _ U/L
51.76 ~ 0.27 26 ~ 6.8 2 0
CCl4 - 224.98 + 1.06 Bl ~ 8.7 5.3 + 1.4
Exs~le 1 20 12 6.30 + 5.497 ~ 76 4.3 ~ 3.1
(0) (0) ~27)
Ea~ple 2 20 11 2.90 t 0.94*71 + 42 3.3 + 2.2*
(~5) (18) ~5~)
The re~ults are me~n value~ ~ 6tandard deviation,
*p ~0 . 05 fox CCl4 treatment,
values in brackets are the percentage improve~nent c:om-
pared to an exclusive CCl4 treatment.
a: tutal daily oral àose.