Note: Descriptions are shown in the official language in which they were submitted.
20~8211
,
A-18184/A
ortho-HydroxyphenYlacetamides
The present invention relates to compositions comprising an organic material which is
subject to thermal, oxidative and/or actinic degradadon and at least one derivative of
ortho-hydroxyphenylacetamide and to novel derivatives of ortho-hydroxyphenylacetamide
and their use for stabilising organic material.
ortho-Hydroxyphenylacetamide has been known since 1900 [R. Stoermer, Annalen 313,
79 (1900)]. Since that time, it has been used together with its derivatives for various
purposes in natural products research [for example A.G. Hayes et al., Life Sci. 34, 1241
(1984); H. Schmidhammer et al., Helv. Chim Acta 66, 2437 (1983); M.J. Calverley et al.,
Tetrahedron Lett. 22, 1635 (1981); K. Nagajaran et al., Proc. Indian Acad. Sci. Sect. A
86A, 25 (1977)], for organic syntheses [for example A. Tsuji et al., Chem. Pharm. Bull.
20, 2528 (1972); O. Yonemitsu et al., Chem. Pharm. Bull. 19, 1158 (1971); J. Lange et al.,
Diss. Pharm. Pharmacol. _, 607 (1968); J. Derkosch et al., Monatsh. 92, 542 (1961)] and
has been suggested for pharmaceutical applicadons (for example DE-A- 1 959 898;
DE-A-2 540 552; US-A-3,331,874).
Furthermore, it is customary to stabilise organic materials with so-called "hindered
phenols", i.e. phenols having sterically demanding subsdtuents in the ortho posidons
relatdve to the OH group. The relevant literature is known to one skilled in the art (cf., for
example, Ullmanns Enzyklopadie der technischen Chemie (Ullmann's Encyclopaedia of
Industrial Chemistry), 4th edition, Weinheim 1974, volume 8, p. 19 ff.).
Surprisingly, it has now been found that derivadves of ortho-hydroxyphenylacetamide are
also highly suitable as stabilisers for organic materials.
Accordingly, the present invention relates to composidons comprising an organic material
which is subject to thermal, oxidative and/or acdnic degradation and at least one
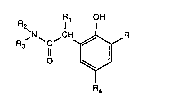
compound of the formula I
.
2048211
- 2 -
R~ OH
2`N~ ~1H~R
in which R is hydrogen, Cl 24alkyl, Cs l2cycloalkyl, phenyl, phenyl-CI4alkyl or a radical
of the formula II
OH R~
--Y~,CH ,N'
R4
in which Y is -CHRs-, sulfur or a direct bond, Rlis hydrogen, Cl4aLlcyl, phenyl or nitro-,
chlorine-, fluorine-, Cl.8alkyl- or/and methoxy-substituted phenyl, R5is hydrogen,
Cl 8alkyl or phenyl, R2 and R3, independently of one another, are hydrogen, Cl 20alkyl,
Cs 12cycloaL~yl, benzyl, phenyl, Cl 18alkyl-substituted phenyl, C24hydroxyalkyl,H3C CH3
naphthyl, radicals of the formulae (CH2),~-COR6, ~7<NR7 or
H3C CH3
R15 R15
~1H-CH2-O~ CH CH20R6
or together with the N atom to which they are bound are a 5-7-membered heterocyclic
ring, or R2 is -NHR,4 and R3is as defimed above, Rl4is hydrogen, Cl 12alkyl, phenyl or
Cl4alkyl-substituted phenyl, Rl5is hydrogen, Cl4alkyl or phenyl and d is an integer from
1-18, R6is hydrogen or Cl 2~alkyl and x is an integer from 1 to 5, R7 is hydrogen,
Cl 8alkyl, Cl 12alkoxy or CO-R9, R9is phenyl or Cl 8alkyl, or, if R3is hydrogen and R is
not a group of the formula II, R2 is also a radical -D-E, in which -D- has the formula
2048211
- 3 -
CH2~ NH~ CyH2y-NH-~
R,5 R15 R6
tCH2-CH2-NH tor
2 ~CH-CH2-0~ CH-CH2-~-
and -E has the formula
R, fH
, CH~ R , Rl 0
(IIa) ~ , -CbH2b-C-OR6 or ll (IIb)
R8
and the radicals E are bound to the NH groups of the radicals -D-, b is an integer from O to
2 and y is an integer from O to 12, R8 is hydrogen, Cl 12alkyl or C5 l2cycloaL~cyl, Rlo is
Cl 24alkyl or phenyl, R4 is hydrogen, Cl 20allcyl, Cs 12cycloaLlcyl, benzyl, phenyl,
Cl4aLlcyl-substituted phenyl, C14alkoxy, ~CnH2nC02RII or
R12 C R,3
R" ¢" ~g~N'R2 (nl)
OH R~
in which Rl, is hydrogen, Cl l8aL~yl, -CzH2z-A or -CH2-C(CH2A)3,
R~ N,R2
/ ~C~ ~R3
A iS -O-C-CnH2n~ OH o (IIIa)
R
and z is an integer from 2 to 12, n adopts the value 0, 1 or 2, R12 and Rl3, independently of
one another, are hydrogen, Cl 8aLlcyl, phenyl or Cs 12cycloaL~cyl, or, if Rl2 is methyl, Rl3 is
~048211
also tcH2~c-o-(cH2)q-o-L or tCH2~C--OR6
O O
L is a radical of the forrnula
OH R1 ,R2
R~h~CH`C~N~R
~
-C~CH2~ C - CH3 (IIIb)
11
R~CH ~N~
R3
OH R1
p is 1 or 2 and q is an integer from 2 to 12, with the proviso that R is a radical of the
formula II only if R4 is not a group of the formula III and the molecule does not contain a
radical of the formula IIIa.
ALkyl radicals R, Rl, R2, R3, R4, R5, R6, R7, R8, Rg, Rlo, R~2, Rl3, Rl4 and Rl5 in the
above formulae are branched or unbranched alkyl. The range of numbers mentioned in the
index of the symbol C refers to the number of possible C atoms. Thus, R and R1o as
Cl 24alkyl are, for example, methyl, ethyl, propyl, isopropyl, n-butyl, isobutyl, t-butyl,
pentyl, isopentyl, hexyl, heptyl, 3-heptyl, octyl, 2-ethylhexyl, nonyl, decyl, undecyl,
dodecyl, tridecyl, tetradecyl, pentadecyl, hexadecyl, heptadecyl, octadecyl, 2-ethylbutyl,
I-methylpentyl, 1,3-dimethylbutyl, 1,1,3,3-tetramethylbutyl, l-methylhexyl, isoheptyl,
I-methylheptyl, 1,1,3-trimethylhexyl, l-methylundecyl, eicosyl, hemicosyl or docosyl.
Alkyl radicals having 1-18 C atoms are preferred, while for Rlo those having 1-12 C
atoms are particularly preferred.
Examples of Rl and Rls as Cl 4alkyl are methyl, ethyl, propyl, isopropyl, butyl, isobutyl
or tert-butyl.
20~8211
- 5 -
By way of example, R2 and R3 as Cl 18alkyl can adopt the meanings mentioned for R and
Rlo, with the exception of eicosyl, hemicosyl and docosyl.
The examples given for R and Rlo may also be mentioned for R4 and R6 as Cl 20alkyl,
with the exception of docosyl and hemicosyl. Of these, the radicals having 1-12 C atoms
are preferred, while those having 1-4 C atoms are particularly preferred.
Examples of Rs,R7,R9,R,2 and R,3 as Cl 8alkyl are methyl, ethyl, propyl, isopropyl,
n-butyl, isobutyl, tert-butyl, pentyl, isopentyl, hexyl, heptyl, octyl, 2-ethylhexyl,
2-ethylbutyl, isoamyl, 1-methylpentyl, 1,3-dimethylbutyl, 1,1,3,3-tetramethylbutyl,
1-methylhexyl, isoheptyl or 1-methylheptyl.
R7,RI2 and R,3 are preferably Cl4alkyl.
Examples of R8 as Cl 12aL~cyl are the ones mentioned for Rs,R7,Rg,RI2 and R,3, with the
addition of nonyl, decyl, undecyl, dodecyl, 1-methylundecyl, 2,2,4,4-tetramethylpentyl,
1, 1,3-trimethylhexyl.
Examples of R,RI,R2,R3,R4,R8,Rlo, Rl2 and R,3 as Cs 12cycloa~yl includecyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, cyclodecyl and cyclododecyl.
Cyclopentyl, cyclohexyl and cycloheptyl are preferred, while cyclohexyl is particularly
preferred.
Examples of R and R4 as phenyl-CI4alkyl are benzyl, phenethyl, 3-phenylpropyl,
a-methylbenzyl and a,a-dimethylbenzyl. Benzyl is preferred.
Examples of R2 and R3 as Cl l8aLkyl-substituted phenyl are: methylphenyl,
dimethylphenyl, trimethylphenyl, ethylphenyl, diethylphenyl, isopropylphenyl,
t-butylphenyl, di-t-butylphenyl, methyl-di-t-butylphenyl, tert-octylphenyl and
di-tert-octylphenyl. The number of aLlcyl groups is in particular 1-3, for example 1 or 2.
The total number of C atoms of all alkyl substituents is preferably 1-18, in particular 1-12,
for example 1-6.
Examples of R2 and R3 as C24hydroxyalkyl are: 2-hydroxyethyl, 1,2-dihydroxyethyl,
2-hydroxypropyl, 2,3-dihydroxypropyl, 1,3-dihydroxypropyl, 3-hydroxypropyl,
4-hydroxybutyl, and fuIther alkyl radicals substituted with one or more hydroxyl groups
20~821~
- 6-
and having 2-4 C atoms. 2-Hydroxyethyl is preferred.
Where R2 and R3 together form a 5-7-membered heterocyclic ring, such a ring can
contain, for example, apart from the N atom, another N or O atom as further hetero ring
atom. The rings are preferably saturated and have in particular 6 ring members. Examples
of these are piperidino, morpholino, piperazino, 4-methylpiperazino and
hexamethyleneimino.
Examples of R4 and R7 as Cl4alkoxy are methoxy, ethoxy, propoxy, isopropoxy, butoxy
and isobutoxy.
R and R4 are preferably Cl4alkyl, in particular t-butyl.
Preference is given to compositions according to the invention comprising at least one
compound of the formula I in which R is Cl.l8alkyl, cyclohexyl, phenyl, benzyl or a
radical of the formula II, Rl is hydrogen, methyl, ethyl or phenyl, Y is a direct bond,
methylene or sulfur, R2 and R3, independently of one another, are hydrogen, Cl l8aL~cyl,
cyclohexyl, benzyl, phenyl, Cl4alkyl-substituted phenyl, naphthyl, -(CH2),~CO-R6,
H3C CH3
~<
CH2CH20H or a radical of the formula ~7<NR7
H3C CH3
or, if R2 is H, R3 is also a radical -D-E, in which E is a group of the formula IIa or IIb, R in
the radical of the formula IIa, III, IIIa and IIIb being Cl l8alkyl or cyclohexyl and R4 in the
radical of the formula IIa being C~ ~2alkyl or cyclohexyl, y is O to 6, R6 is hydrogen or
Cl4alkyl, R7 is hydrogen, Cl4alkyl or CO-CI4alkyl, R4 is hydrogen, Cl l2alkyl,
cyclohexyl, phenyl, ~CnH2nCO2RIl or
I
Rl2-C-R~3
R ~CH' ~N ~ R2
OH R1
2048211
-7 -
in which Rl2 and R,3, independently of one another, are hydrogen, Cl4alkyl, phenyl or
cyclohexyl or, if Rl2 is methyl, R,3iS also tCH2~ C-O-( CH2 )q -O-L or
tCH2~ C--OR6
O in which R6' is hydrogen or Cl 18alkyl and q is an integer
from 2-6.
Particular preference is given to compositions comprising at least one compound of the
formula I, in which R is Cl l8alkyl or a radical of the formula II, Rlis hydrogen or phenyl
and Y is a direct bond, R2 is hydrogen, Cl 20alkyl, benzyl, phenyl, amino or a radical of
R1 OH
H I ¦ H3C CH3
,~ ,CH~e~ (mC) or~r~lH
R4 H3C CH3
R7is hydrogen, Cl4alkyl or CO-CI4alkyl, R4is hydrogen, Cl 6alkyl, or a group of the
formula III, in which Rl2 and R13, independently of one another, are hydrogen orCl4alkyl, and, if R12 is methyl, R13 is also -(CH2)2COOCH3, R3is hydrogen and y is
2-12.
Very particular preference is given to compositions comprising at least one compound of
the formula I, in which R is Cl 6alkyl, Rl is hydrogen or phenyl, R2 is hydrogen,
Cl 20alkyl, MH2 or a radical of the formula
~CH3
NH or IIIc, in which y is 2-lO, R7is hydrogen, CH3 or -COCH3, R4 and R8
H3C CH3
are hydrogen or Cl 6aL~yl and R3is hydrogen.
~ 2o~82~
The organic materials present in the compositions according to the invention may degrade
more or less easily upon exposure to heat, light or radiation, mechanical stress (in
particular caused by shearing forces) and chemical reagents (in particular atmospheric
oxygen).
To provide protection against such influences is the function of the compounds of the
formula I, which should advantageously be present in the compositions according to the
invention in an amount of 0.01 to 10, for example 0.05 to 5, preferably 0.05 to 3, but in
particular 0.1 to 2, % by weight. One or more of these compounds can be present, and the
percentages given refer to the entire amount of these compounds. The basis for calculation
is the total weight of the organic material without the compounds of the formula I.
The materials present in the compositions according to the invention are those which are
sensitive to oxidative, thermal or/and actinic degradation. The compounds of the formula I
are particularly useful as stabilisers against oxidadve and in particular thermal
degradation. Accordingly, they can be used advantageously as processing (thermal)
stabilisers for thermoplastics.
Accordingly, the invendon also relates to the use of the compounds of the formula I for
stabilising organic material against oxidative, thermal and/or actinic degradation and to a
process for stabilising organic material which comprises adding or applying to this
material compounds of the formula I as stabilisers.
Suitable examp}es of organic materials which can be stabilised according to the invention
with the aid of the compounds of the formula I are:
1. Polymers of monoolefins and diolefins, for example polypropylene, polyisobutylene,
polybut- 1-ene, polymethylpent- 1-ene, polyisoprene or polybutadiene, as well as polymers
of cycloolefins, for example of cyclopentene or norbornene; as well as polyethylene
(which, if desired, can be crosslinked), for example high-density polyethylene (HDPE),
low-density polyethylene (LDPE) and linear low-density polyethylene (LLDPE).
2. Mixtures of the polymers mentioned under 1), for example mixtures of polypropylene
with polyisobutylene, polypropylene with polyethylene (for example PP/HDPE,
PP/LDPE) and mixtures of different types of polyethylene (for example LDPE/HDPE).
'
. : ,
~ ~ .
20 ~2~
3. Copolymers of monoolefins and diolefins with each other or with other vinyl
monomers, for example ethylene/propylene copolymers, linear low-density polyethylene
(LLDPE) and its mixtures with low-density polyethylene (LDPE), propylene/but- 1-ene
copolymers, propylene/isobutylene copolymers, ethylene/but- 1-ene copolymers,
ethylene/hexene copolymers, ethylene/methylpentene copolymers, ethylene~eptene
copolymers, ethylene/octene copolymers, propylene/butadiene copolymers,
isobutylene/isoprene copolymers, ethylene/alkyl acrylate copolymers, ethylene/alkyl
methacrylate copolymers, ethylene/vinyl acetate copolymers or ethylene/acrylic acid
copolymers and their salts (ionomers) and terpolymers of ethylene with propylene and a
diene, such as hexadiene, dicyclopentadiene or ethylidenenorbornene; as well as mixtures
of such copolymers with each other and with polymers mentioned in 1), for example
polypropylene/ethylene-propylene copolymers, LDPE/ethylene-vinyl acetate copolymers,
LDPE/ethylene-acrylic acid copolymers, LLDPE/ethylene-vinyl acetate copolymers and
LLDPE/ethylene-acrylic acid copolymers.
3a. Hydrocarbon resins (for example C5-Cg) and hydrogenated modifications thereof (for
example tackifying resins).
4. Polystyrene, poly(p-methylstyrene), poly(a-methylstyrene).
5. Copolymers of styrene or a-methylstyrene with dienes or acrylic derivatives, for
example styrene/butadiene, styrene/acrylonitrile, styrene/alkyl methacrylate,
styrene/butadiene/alkyl acrylate, styrene/maleic anhydride, styrene/acrylonitrile-methyl
acrylate; mixtures of high impact strength from copolymers of styrene and another
polymer, for example from a polyacrylate, a diene polymer or an ethylene/propylene-diene
terpolymer; and block copolymers of styrene, for example styrene/butadiene-styrene,
styrene~lsoprene-styrene, styrene/ethylene-butylene/styrene or
styrene/ethylene-propylene/styrene.
6. Graft copolymers of styrene or a-methylstyrene, for example styrene on polybutadiene;
styrene on copolymers of polybutadiene/styrene or polybutadiene/acrylonitrile; styrene
and acrylonitrile (or methacrylonitrile) on polybutadiene; styrene, acrylonitrile and
methyl methacrylate on polybutadiene; styrene and maleic anhydride on polybutadiene;
styrene, acrylonitrile and maleic anhydride or maleimide on polybutadiene; styrene and
maleimide on polybutadiene; styrene and alkyl acrylates or alkyl methacrylates on
2048211
- 10-
polybutadiene; styrene and acrylonitrile on ethylene/propylene/diene terpolymers; styrene
and acrylonitrile on polyalkylacrylates or polyalkylmethacrylates; styrene and acrylonitrile
on acrylate/butadiene copolymers, as well as mixtures thereof with the copolymers listed
under 5), for example those known as so-called ABS, MBS, ASA and AES polymers.
7. Halogen-containing polymers, such as polychloroprene, chlorinated rubber, chlorinated
or sulfochlorinated polyethylene, copolymers of ethylene and chlorinated ethylene,
epichlorohydrin homo- and copolymers, in particular polymers from halogen-containing
vinyl compounds, for example polyvinyl chloride, polyvinylidene chloride, polyvinyl
fluoride, polyvinylidene fluoride, as well as copolymers thereof, such as vinyl
chloride/vinylidene chloride, vinyl chloride/vinyl acetate or vinylidene chloride/vinyl
acetate.
8. Polymers which are derived from a,~-unsaturated acids and derivatives thereof, such as
polyacrylates and polymethacrylates, polyacr"lamides and polyacrylonitriles.
9. Copolymers of the monomers mentioned under 8) with each other or with other
unsaturated monomers, for example acrylonitrile/butadiene copolymers, acrylonitrile/alkyl
acrylate copolymers, acrylonitrile/alkoxyalkyl acrylate copolymers, acrylonitrile/vinyl
halide copolymers or acrylonitrile/alkyl methacrylate/butadiene terpolymers.
10. Polymers which are derived from unsaturated alcohols and amines, or acyl derivatives
thereof or acetals thereof, such as polyvinyl alcohol, polyvinyl acetate, polyvinyl stearate,
polyvinyl benzoate, polyvinyl maleate, polyvinyl butyral, polyallyl phthalate orpolyallylmelamine; as well as their copolymers with olefins mentioned in 1).
11. Homopolymers and copolymers of cyclic ethers, such as polyalkylene glycols,
polyethylene oxide, polypropylene oxide or copolymers thereof with bis(glycidyl) ethers.
12. Polyacetals, such as polyoxymethylene and polyoxymethylenes which contain
comonomers, ethylene oxide for example; polyacetals modified with thermoplastic
polyurethanes, acrylates or MBS.
13. Polyphenylene oxides and sulfides, and mixtures thereof with styrene polymers or
polyamides.
11 20~8211
14. Polyurethanes which are derived from polyethers, polyesters and polybutadienes with
terminal hydroxyl groups on the one hand and aliphatic or aromatic polyisocyanates on the
other hand, as well as precursors thereof.
15. Polyamides and copolyamides which are derived from diamines and dicarboxylic acids
and/or from aminocarboxylic acids or the corresponding lactams, such as nylon 4, nylon 6,
nylon 6/6, 6/10, 6/9, 6/12 and 4/6, nylon 11, nylon 12, aromatic polyamides obtained
starting from m-xylene, diamine and adipic acid; polyamides prepared from
hexamethylenediamine and isophthalic and/or terephthalic acid and, if desired, an
elastomer as modifier, for example poly-2,4,4-trimethylhexamethyleneterephthalamide or
poly-m-phenyleneisophthalamide. Block copolymers of the aforementioned polyamides
with polyolefins, olefin copolymers, ionomers or chemically bonded or grafted elastomers;
or with polyethers, for example with polyethylene glycol, polypropylene glycol or
polytetramethylene glycol. In addition, polyamides or copolyamides modified with EPDM
or ABS; as well as polyamides condensed during processing (RIM-polyamide systems).
16. Polyureas, polyimides, polyamidoimides and polybenzimidazoles.
17. Polyesters which are derived from dicarboxylic acids and dialcohols and/or from
hydroxycarboxylic acids or the corresponding lactones, such as polyethylene
terephthalate, polybutylene terephthalate, poly- 1,4-dimethylolcyclohexane terephthalate,
polyhydroxybenzoate, as well as block polyether-esters derived from polyethers having
hydroxyl end groups; in addidon polyesters modified with polycarbonates or MBS.
18. Polycarbonates and polyester carbonates.
19. Polysulfones, polyether sulfones and polyether ketones.
20. Crosslinked polymers which are derived from aldehydes on the one hand and phenols,
urea or melamine on the other hand, such as phenoVformaldehyde resins,
urea/formaldehyde resins and melamine/formaldehyde resins.
21. Drying and non-drying alkyd resins.
22. Unsaturated polyester resins which are derived from copolyesters of saturated and
unsaturated dicarboxylic acids with polyhydric alcohols and vinyl compounds as
20~8211
- 12-
crosslinking agents, and also halogen-containing modificadons thereof of low
flammability.
23. Crosslinkable acrylic resins derived from subsdtuted acrylic esters, for example epoxy
acrylates, urethane acrylates or polyester acrylates.
24. Alkyd resins, polyester resins or acrylate resins which are crosslinked with melamine
resins, urea resins, polyisocyanates or epoxy resins.
25. Crosslinked epoxy resins which are derived from polyepoxides, for example from
bisglycidyl ethers or from cycloaliphatic diepoxides.
26. Natural polymers, such as cellulose, rubber, geladn and derivadves thereof which are
chemically modified in a polymer-homologous manner, such as cellulose acetates,
cellulose propionates and cellulose butyrates, or cellulose ethers, such as methylcellulose;
and rosin resins and derivatives.
27. Mixtures (polyblends) of the polymers mendoned above, for example PP/EPDM,
polyamide/EPDM or ABS, PVC/EVA, PVC/ABS, PVC/MBS, PC/ABS, PBTP/ABS,
PC/ASA, PC/PBT, PVC/CPE, PVC/acrylates, POM/thermoplasdc PUR, PC/thermoplastic
PUR, POM/acrylate, POM/MBS, PPO/HIPS, PPO/nylon 6/6 and copolymers, PA/HDPE,
PA/PP, PA/PPO.
28. Naturally occurring and synthedc organic materials which are pure monomeric
compounds or mixtures of such compounds, for example mineral oils, animal and
vegetable fats, oils and waxes, or oi!s, waxes and fats based on synthedc esters (e.g.
phthalates, adipates, phosphates or trimellitates) and also mixtures of synthetic esters with
mineral oils in any weight rado, which mixtures may be used as spinning preparations, as
well as aqueous emulsions thereof.
29. Aqueous emulsions of natural or synthedc rubbers, for example natural latex or
ladces of carboxylated styrene/butadiene copolymers.
As organic material, the composidons according to the invention preferably contain
natural, semisynthetic or synthedc polymers, a lubricant, a metal-working fluid or a
hydraulic fluid. Compositions which contain a synthedc polymer, in particular a
.
~ . ;, ~.
20~821~
- 13-
thermoplastic or an elastomer, are preferred. Compositions which, as organic material,
comprise a polyolefin may be mentioned in particular. Examples of such polymers are to
be taken from the above enumeration of suitable materials.
Compositions which contain a lubricant, a metal-working fluid or a hydraulic fluid, in
particular a lubricant, are also particularly preferred.
Suitable lubricants are based, for example, on mineral or synthetic oils or mixtures
thereof. The lubricants are familiar to the person skilled in the art and are described in the
relevant specialist literature, for example in Dieter Klamann, "Schmierstoffe und
verwandte Produkte" [Lubricants and Related Products] (Verlag Chemie, Weinheim,
1982), in Schewe-Kobek, "Das Schmiermittel-Taschenbuch" [The Lubricant Handbook](Dr. Alfred Huthig-Verlag, Heidelberg, 1974) and in "Ullmanns Enzyklopadie der
technischen Chemie" [Ullmann's Encyclopaedia of Industrial Chemistry], vol. 13, pages
85-94 (Verlag Chemie, Weinheim, 1977).
The lubricants are in particular oils and fats, for example based on a mineral oil. Oils are
preferred.
A further group of lubricants which can be used are vegetable or animal oils, fats, tallows
and waxes or mixtures thereof with each other or mixtures with the mineral or synthetic
oils mendoned. Vegetable and animal oils, fats, tallows and waxes are, for example, palm
kernel oil, palm oil, olive oil, rapeseed oil or rape oil, linseed oil, groundnut oil, soya bean
oil, cotton oil, sunflower oil, pumpkin seed oil, coconut oil, maize oil, castor oil, walnut
oil and mixtures thereof, fish oils, tallow from slaughtered animals such as bovine tallow,
neatsfoot oil and bone oil and their modified, epoxidised and sulfoxidised forms, for
example epoxidised soya bean oil.
The mineral oils are based in particular on hydrocarbon compounds.
Examples of synthetic lubricants include lubricants based on aliphatic or aromatic
carboxylic esters, polymeric esters, polyalkylene oxides, phosphoric acid esters,
poly-~-olefins or silicones, on a diester of a dibasic acid with a monohydric alcohol, for
example dioctyl sebacate or dinonyl adipate, on a triester of trimethylolpropane with a
monobasic acid or with a mixture of such acids, for example trimethylolpropane
tripelargonate, trimethylolpropane tricaprylate or mixtures thereof, on a tetraester of
2048211
- 14-
pentaerythritol with a monobasic acid or with a mixture of such acids, for example
pentaerythritol tetracaprylate, or on a complex ester of monobasic and dibasic acids with
polyhydric alcohols, for example a complex ester of trimethylolpropane with caprylic and
sebacic acid or on a mixture thereof. Particularly suitable in addition to mineral oils are,
for example, poly-c~-olefins, lubricants based on esters, and phosphates, glycols,
polyglycols and polyalkylene glycols, and mixtures thereof with water.
Metal-working fluids and hydraulic fluids can be prepared based on the same substances
as described above for the lubricants. Frequently, these are also emulsions of such
substances in water or other fluids.
Incorporation into the organic materials can be carried out, for example, by mixing in the
compounds of the formula I and, if desired, other additives by the methods custornary in
industry. If they are polymers, in particular synthetic polymers, incorporation can be
carried out before or during moulding, or by applying the dissolved or dispersedcompounds to the polymers, if appropriate with subsequent evaporation of the solvent. In
the case of elastomers, these can also be stabilised as latices. A further possibility for
incorporation of the compounds of the formula I in polymers comprises their addition
before, during or immediately after polymerisation of the corresponding monomers or
before crosslinking. In this procedure, the compounds of the formula I can be added as
such but also in encapsulated from (for example in waxes, oils or polymers). In the case of
addition before or during polymerisation, the compounds of the formula I can also act as
regulators for the chain length of the polymers (chain terminators).
The compounds of the formula I or mixtures thereof can also be added to the plastics to be
stabilised in the form of a masterbatch which contains these compounds, for example in a
concentration of 2.5 to 25 % by weight.
The incorporation of the compounds of the formula I can expediently be carried out by the
following methods:
- as an emulsion or dispersion (for example addidon to latices or emulsion polymers)
- as a dry mixture during mixing of additive components or polymer mixtures
- by direct addition to the processing apparatus (for example extruders, internal mixers
etc.)
- as a solution or melt.
20~821~
- 15-
Polymer compositions according to the invention can be used in various forms or
processed to give various products, for example as (to give) sheets, fibres, tapes, moulded
maeerials, profiles or as binders for paints, adhesives or cement.
Lubricant compositions according to the invention are used, for exarnple, in internal
combustion engines, for exarnple in motor vehicles.
In addition to the compounds or mixtures according to the invention, the composi~ions
according to the invention can contain still other customary additives, in particular if they
contain organic, preferably synthetic polymers. Examples of such additives are:
1. Antioxidants
1.1. ALIcvlated monophenols, for example 2,6-di-tert-butyl-4-methylphenol,
2-tert-butyl-4,6-dimethylphenol, 2,6-di-tert-butyl-4-ethylphenol,
2,6-di-tert-butyl-4-n-butylphenol, 2,6-di-tert-butyl-4-isobutylphenol,
2,6-dicyclopentyl-4-methylphenol, 2-(-methylcyclohexyl)-4,6-dimethylphenol,
2,6-dioctadecyl-4-methylphenol, 2,4,6-tricyclohexylphenol,
2,6-di-tert-butyl-4-methoxymethylphenol, 2,6-dinonyl-4-methylphenol.
1.2. AL~cvlated hvdroquinones, for example 2,6-di-tert-butyl-4-methoxyphenol,
2,5-di-tert-butylhydroquinone, 2,5-di-tert-amylhydroquinone,
2,6-diphenyl-4-octadecyloxyphenol.
1.3. Hvdroxvlated thiodiphenvl ethers, for example 2,2'-thiobis(6-tert-butyl-
4-methylphenol), 2,2'-thiobis(4-octylphenol), 4,4'-thiobis(6-tert-butyl-3-methylphenol) ,
4,4 '-thiobis(6-tert-butyl-2-methylphenol).
1.4. AL~cvlidenebis~henols, for example 2,2'-methylenebis(6-tert-butyl-4-methylphenol),
2,2'-methylenebis(6-tert-butyl-4-ethylphenol),
2,2 '-methylenebis[4-methyl-6-(a-methylcyclohexyl)phenol],
2,2'-methylenebis(4-methyl-6-cyclohexylphenol),
2,2'-methylenebis(6-nonyl-4-methylphenol), 2,2'-methylenebis(4,6-di-tert-butylphenol),
2,2 '-ethylidenebis(4,6-di-tert-butylphenol),
2,2 '-ethylidenebis(6-tert-butyl-4-isobutylphenol),
2,2'-methylenebis[6-(c~-methylbenzyl)-4-nonylphenol],
20~8211
- 16 -
2~2~-methylenebis[6-(a~a-dimethylbenzyl)-4-nonylphenol]~
4,4 ' -methylenebis(2,6-di-tert-butylphenol),
4,4'-methylenebis(6-tert-butyl-2-methylphenol),
1, 1 -bis(S-tert-butyl-4-hydroxy-2-methylphenyl)butane,
2,6-bis(3-tert-butyl-5-methyl-2-hydroxybenzyl)-4-methylphenol,
1, 1 ,3-tris(5-tert-butyl-4-hydroxy-2-methylphenyl)butane,
1, 1 -bis(5-tert-butyl-4-hydroxy-2-methylphenyl)-3-n-dodecylmercaptobutane,
ethylene glycol bis[3,3-bis(3'-tert-butyl-4'-hydroxyphenyl)butyrate],
bis(3-tert-butyl-4-hydroxy-5-methylphenyl)dicyclopentadiene,
bis[2-(3 '-tert-butyl-2'-hydroxy-5 '-methylbenzyl)-6-tert-butyl-4-methylphenyl]
terephthalate.
1.5. Benzyl comPounds, for example
1 ,3 ,5-tris(3,5-di-tert-butyl-4-hydroxybenzyl)-2,4,6-trimethylbenzene,
bis(3,5-di-tert-butyl-4-hydroxybenzyl) sulfide, isooctyl 3,5-di-tert-butyl-
4-hydroxybenzylmercaptoacetate, bis(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)
dithioterephthalate, 1,3,5-tris(3,5-di-tert-butyl-4-hydroxybenzyl) isocyanurate,1,3,5-tris(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl) isocyanurate,
dioctadecyl 3,5-di-tert-butyl-4-hydroxybenzylphosphonate, calcium salt of monoethyl
3,5-di-tert-butyl-4-hydroxybenzylphosphonate, 1,3,5-tris(3,5-dicyclohexyl-
4-hydroxybenzyl) isocyanurate.
1.6. Acvlaminophenols, for example 4-hydroxylauranilide, 4-hydroxystearanilide,
2,4-bis(octylmercapto)-6-(3,5-di-tert-butyl-4-hydroxyanilino)-s-triazine, octyl
N-(3,5-di-tert-butyl-4-hydroxyphenyl)carbamate.
1.7. Esters of ~-(3~5-di-tert-butvl-4-hydroxvphenyl)propionic acid with mono- orpolyhydric alcohols, e.g. with methanol, octadecanol, 1,6-hexanediol, neopentyl glycol,
thiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol,
tris(hydroxyethyl) isocyanurate, N,N'-bis(hydroxyethyl)oxalamide.
1.8. Esters of ~-(5-tert-butvl-4-hvdroxv-3-meth~/lphenvl)propionic acid with mono- or
polyhydric alcohols, e.g. with methanol, octadecanol, 1,6-hexanediol, neopentyl glycol,
thiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol,
tris(hydroxyethyl) isocyanurate, N,N'-bis(hydroxyethyl)oxalamide.
~0~8211
- 17-
1.9. Esters of ~-(3.5-dicvclohexYl-4-hYdroxYphenYl)propionic acid with mono- or
polyhydric alcohols, e.g. with methanol, octadecanol, 1,6-hexanediol, neopentyl glycol,
Ihiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol,
tris(hydroxyethyl) isocyanurate, N,N'-bis(hydroxyethyl)oxalamide.
1.10. Amides of R-(3~5-di-tert-butvl-4-hvdroxYphenvl)proPionic acid for example
N,N '-bis(3 ,S-di-tert-butyl-4-hydroxyphenylpropionyl)hexamethylenediamine,
N,N ' -bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)trimethylenediamine,
N,N'-bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)hydrazine.
2. UV absorbers and li~ht stabilisers
2.1. 2-(2'-Hydroxvphenyl)benzotriazoles, for example the S'-methyl, 3',5'-di-tert-butyl,
S'-tert-butyl, 5'-(1,1,3,3-tetramethylbutyl), S-chloro-3',5'-di-tert-butyl,
S-chloro-3'-tert-butyl-5'-methyl, 3'-sec-butyl-S'-tert-butyl, 4'-octoxy, 3',5'-di-tert-amyl
and 3',5'-bis(a,a-dimethylbenzyl) derivative.
2.2. 2-Hydroxybenzo~henones, for example the 4-hydroxy, 4-methoxy, 4-octoxy,
4-decyloxy, 4-dodecyloxy, 4-benzyloxy, 4,2',4'-trihydroxy and
2'-hydroxy-4,4'-dimethoxy derivative.
2.3. Esters of variouslv substituted benzoic acids, for example 4-tert-butylphenyl
salicylate, phenyl salieylate, octylphenyl salieylate, dibenzoylresoreinol,
bis(4-tert-butylbenzoyl)resorcinol, benzoylresoreinol, 2,4-di-tert-butylphenyl
3,5-di-tert-butyl-4-hydroxybenzoate and hexadeeyl 3,5-di-tert-butyl-4-hydroxybenzoate.
2.4. Acrvlates, for example ethyl a-eyano-~"B-diphenylacrylate, isooctyl
a-eyano-~,~-diphenylaerylate, methyl a-earbomethoxyeinnamate, methyl
a-cyano-,B-methyl-p-methoxyeinnamate, butyl -eyano-,B-methyl-p-methoxycinnamate,
methyl a-earbomethoxy-p-methoxycinnamate and
N-(,B-earbomethoxy-,B-cyanovinyl)-2-methylindoline.
2.5. Nickel compounds, for example nickel complexes of
2,2'-thiobis[4-(1,1,3,3-tetramethylbutyl)phenol], such as the 1:1 or 1:2 complex, with or
without additional ligands such as n-butylamine, triethanolamine or
N-cyclohexyldiethanolamine, nickel dibutyldithioearbamate, nickel salts of monoalkyl
2048211
- 18-
4-hydroxy-3,5-di-tert-butylbenzylphosphonates, such as of the methyl or ethyl ester,
nickel complexes of ketoximes, such as of 2-hydroxy-4-methylphenyl undecyl ketoxime,
nickel complexes of l-phenyl-4-lauroyl-5-hydroxypyrazole, with or without additional
ligands.
2.6. Sterically hindered amines, for example bis(2,2,6,6-tetramethylpiperidyl) sebacate,
bis(1,2,2,6,6-pentamethylpiperidyl) sebacate, bis(1,2,2,6,6-pentamethylpiperidyl)
n-butyl-3,5-di-tert-butyl-4-hydroxybenzylmalonate, the condensadon product of
1-hydroxyethyl-2,2,6,6-tetramethyl-4-hydroxypiperidine and succinic acid, the
condensation product of N,N'-bis(2,2,6,6-tetramethyl-4-piperidyl)hexamethylenediamine
and 4-tert-octylamino-2,6-dichloro- 1,3,5-s-triazine, tris(2,2,6,6-tetramethyl-4-piperidyl)
nitrilotriacetate, tetrakis(2,2,6,6-tetramethyl-4-piperidyl) 1,2,3,4-butanetetracarboxylate,
1,1'-( 1 ,2-ethanediyl)bis(3,3,5,5-tetramethylpiperazinone).
2.7. Oxalamides, for example 4,4'-dioctyloxyoxanilide,
2,2'-dioctyloxy-5,5'-di-tert-butyloxanilide, 2,2'-didodecyloxy-5,5'-di-tert-butyloxanilide,
2-ethoxy-2'-ethyloxanilide, N,N'-bis(3-dimethylaminopropyl)oxalamide,
2-ethoxy-5-tert-butyl-2'-ethyloxanilide and its mixture with
2-ethoxy-2'-ethyl-5,4'-di-tert-butyloxanilide and mixtures of o- and p-methoxy-
disubstituted oxanilides, and o- and p-ethoxy-disubstituted oxanilides.
2.8. 2-(2-Hvdroxvphenyl)-1~3~5-triazines, for example
2,4,6-tris(2-hydroxy-4-octyloxyphenyl)- 1 ,3,5-triazine,
2-(2-hydroxy-4-octyloxyphenyl)-4,6-bis(2,4-dimethylphenyl)- 1 ,3,5-triazine,
2-(2,4-dihydroxyphenyl)-4,6-bis(2,4-dimethylphenyl)- 1 ,3,5-triazine,
2,4-bis(2-hydroxy-4-propyloxyphenyl)-6-(2,4-dimethylphenyl)- 1 ,3,5-triazine,
2-(2-hydroxy-4-octyloxyphenyl)-4,6-bis(4-methylphenyl)- 1 ,3,5-triazine,
2-(2-hydroxy-4-dodecyloxyphenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine.
3. Metal deacdvators, for example N,N'-diphenyloxalamide,
N-salicylal-N'-salicyloylhydrazine, N,N'-bis(salicyloyl)hydrazine,
N,N'-bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)hydrazine,
3-salicyloylamino-1,2,4-triazole, bis(benzylidene)oxalic dihydrazide.
4. Phosphites and phosphonites, for example triphenyl phosphite, diphenyl alkyl
phosphites, phenyl dialkyl phosphites, tris(nonylphenyl) phosphite, trilauryl phosphite,
20~8211
- 19-
trioctadecyl phosphite, distearyl pentaerythritol diphosphite, tris(2,4-di-tert-butylphenyl)
phosphite, diisodecyl pentaerythritol diphosphite, bis(2,4-di-tert-butylphenyl)
pentaerythritol diphosphite, tristearyl sorbitol triphosphite,
tetrakis(2,4-di-tert-butylphenyl) 4,4'-biphenylenediphosphonite,
3,9-bis(2,4-di-tert-butylphenoxy)-2,4,8,10-tetraoxa-3,9-diphosphaspiro[5.5]undecane.
5. Compounds which destrov peroxides, for example esters of ~-thiodipropionic acid, for
example the lauryl, stearyl, myristyl or tridecyl esters, mercaptobenzimidazole or the zinc
salt of 2-mercaptobenzimidazole, zinc dibutyldithiocarbamate, dioctadecyl disulfide,
pentaerythritol tetrakis(,~-dodecylmercapto)propionate.
6. Polvamide stabilisers, for example copper salts in combination with iodides and/or
phosphorus compounds and salts of divalent manganese.
7. Basic co-stabilisers, for example melamine, polyvinylpyrrolidone, dicyandiamide,
triallyl cyanurate, urea derivatives, hydrazine derivatives, amines, polyamides,polyurethanes, alkali metal salts and alkaline earth metal salts of higher fatty acids, for
example calcium stearate, zinc stearate, magnesium stearate, sodium ricinoleate and
potassium palmitate, antimony pyrocatecholate or tin pyrocatecholate.
8. Nucleatin~ a ents, for example 4-tert-butylbenzoic acid, adipic acid, diphenylacetic
acid.
9. Fillers and reinforcing agents, for example calcium carbonate, silicates, glass fibres,
asbestos, talc, kaolin, mica, barium sulfate, metal oxides and hydroxides, carbon black,
graphite.
10. Other addidves, for example plasticisers, lubricants, emulsifiers, pigments, fluorescent
whitening agents, flameproofimg agents, andstatic agents and blowing agents.
If the compositions according to the invention are those based on lubricants and hydraulic
fluids or metal-working fluids, they can also contain other additives which are added to
improve certain use properties, for example other antioxidants, metal deactivators, rust
inhibitors, viscosity index improvers, pour point reducers, dispersants/surfactants and wear
resistant additives.
20~8211
- 20 -
Examples of antioxidants are to be taken from the listing reproduced further above under
the title "1. Antioxidants", in particular items 1.1 to 1.10. Examples of other additional
additives are the following:
Examples of arnine antioxidants:
N,N'-di-isopropyl-p-phenylenediamine, N,N'-di-sec-butyl-p-phenylenediamine,
N,N '-bis(l ,4-dimethylpentyl)-p-phenylenediamine,
N,N '-bis(l-ethyl-3-methylpentyl)-p-phenylenediamine,
N,N'-bis(l-methylheptyl)-p-phenylenediamine, N,N'-dicyclohexyl-p-phenylenediamine,
N,N'-diphenyl-p-phenylenediamine, N,N'-di-(naphth-2-yl)-p-phenylenediamine,
N-isopropyl-N '-phenyl-p-phenylenediamine,
N-( 1 ,3-dimethylbutyl)-N'-phenyl-p-phenylenediamine,
N- ( 1 -methylheptyl)-N '-phenyl-p-phenylenediamine,
N-cyclohexyl-N'-phenyl-p-phenylenediamine, 4-(p-toluenesulfonamido)diphenylamine,
N,N'-dimethyl-N,N'-di-sec-butyl-p-phenylenediamine, diphenylamine,
N-allyldiphenylamine, 4-isopropoxydiphenylamine, N-phenyl-l-naphthylamine,
N-phenyl-2-naphthylamine, octylated diphenylamine, such as
p,p'-di-tert-octyldiphenylamine, 4-n-butylaminophenol, 4-butyrylaminophenol,
4-nonanoylaminophenol, 4-dodecanoylaminophenol, 4-octadecanoylaminophenol,
di-(4-methoxyphenyl)amine, 2,6-di-tert-butyl-4-dimethylaminomethylphenol,
2,4'-diaminodiphenylmethane, 4,4'-diaminodiphenylmethane,
N,N,N',N'-tetramethyl-4,4'-diaminodiphenylmethane,
1,2-di-[(2-methylphenyl)amino]ethane, 1,2-di-(phenylamino)propane, (o-tolyl)biguanide,
di[4-( 1 ' ,3 ' dimethylbutyl)phenyl]amine, tert-octylated N-phenyl- 1 -naphthylamine,
mixture of mono- and dialkylated tert-butyVtert-octyldiphenylamines,
2,3-dihydro-3,3-dimethyl-4H-1,4-benzothiazine, phenothiazine, N-allylphenothiazine.
Examples of other antioxidants: aliphatic or aromatic phosphites, esters of thiodipropionic
acid or thiodiacetic acid, or salts of dithiocarbamic or dithiophosphoric acid.
Examples of metal deactivators. for example for copper. are: triazoles, benzotriazoles and
derivatives thereof, tolutriazoles and derivatives thereof, 2-mercaptobenzothiazole,
2-mercaptobenzotriazole, 2,5-dimercaptobenzotriazole, 2,5-dimercaptobenzothiadiazole,
S,S'-methylenebisbenzotriazole, 4,5,6,7-tetrahydrobenzotriazole,
salicylidenepropylenediamine, salicylaminoguanidine and salts thereof.
2048211
- 21 -
Examples of rust inhibitors are:
a) Organic acids, their esters, metal salts and anhydrides, for example: N-oleoylsarcosine,
sorbitan monooleate, lead naphthenate, alkenylsuccinic anhydride, for example
dodecenylsuccinic anhydride, alkenylsuccinic monoester and monoamides,
4-nonylphenoxyacetic acid.
b) Nitrogen-containing compounds, for example:
i. Primary, secondary or tertiary aliphatic or cycloaliphatic amines and amine salts of
organic and inorganic acids, for example oil-soluble alkylammonium carboxylates.ii. Heterocyclic compounds, for example: substituted imidazolines and oxazolines.
c) Phosphorus-containing compounds, for example: amine salts of phosphoric mono- and
diester or phosphonic mono- and diester, zinc dialkyldithiophosphates.
d) Sulfur-containing compounds, for example: barium dinonylnaphthalenesulfonates,
calcium petroleumsulfonates.
Examples of viscosity index improvers are: polyacrylates, polymethacrylates,
vinylpyrrolidone/methacrylate copolymers, polyvinylpy rolidones, polybutenes, olefin
copolymers, styrene/acrylate copolymers, polyethers.
Examples of pour point reducers are: polymethacrylate, alkylated naphthalene derivatives.
Examples of dispersants/surfactants are: polybutenylsuccinamides or -imides,
polybutenylphosphonic acid derivadves, basic magnesium sulfonates and phenolates,
calcium sulfonates and phenolates, and barium sulfonates and phenolates.
Examples of wear-resistant additives are: sulfur- andJor phosphorus- and/or
halogen-containing compounds, such as sulfurised vegetable oils, zinc
dialkyldithiophosphates, tritolyl phosphate, chlorinated paraffins, alkyl and aryl di- and
trisulfides, triphenyl phosphorothionates, diethanolaminomethyltolyltriazole,
di(2-ethylhexyl)aminomethyltolyltriazole.
The invention also relates to novel compounds of the formula I, as defined above, with the
additional proviso that R2 is not hydrogen, C3 l2alkyl, phenyl, cyclopentyl or acetyl, if R3
is hydrogen and R is simultaneously hydrogen or methyl.
20~8211
- 22 -
Preferred novel groups of compounds are those p~eferred groups of compounds of the
formula I mentioned above in conjunction with the compositions according to the
invention. The same applies to the general and specific meanings of substituents.
In so far as the compounds of the formula I are not known (see the literature references
mentioned at the beginning), they can be prepared in analogy to processes known per se.
This is illustrated in more detail below by way of example.
Preparation of the compounds of the formula I
1. Starting compounds
The required starting compounds are prepared, for example, by the processes described in
WO-A-80/01566, p. 25, US-A-3,862,133 and in R. LAMER, J. HET. CHEM. 12, 1067
(1975).
The preparation can be illustrated by the following reaction scheme:
OH OH R
R ~ ~ [Nz
R4 Mandelic acid Ph
or
OH R
R~h f HO CH3CO2H ~o
CHO ~H2o R4
R4 Glyoxal
Mandelic acid and glyoxal are commercially available, and the substituted phenol is
prepared by processes known per se of organic chemistry, in so far as it is itself not
commercially available. When mandelic acid appropriately substituted on the phenyl ring
-23- 20~8211
or alkyl-substituted glyoxal is used, starting materials are obtained which take the
additional possible meanings of R1 into account.
2. Amidation with ring opening leads to the compounds according to the invention:
R~ + HNR2R3 ~CH~C,N\
R4 R4
or
O ~ OH R~ R1 OH
R~_ ~ C' `C~
The reaction can be carried out at 70 to 200C, preferably at 110 to 150C, with or without
solvent. When solvents are used (in particular in the presence of long-chain alkyl radicals),
high-boiling organic solvents are suitable, for example ligroin fractions, benzene, toluene,
xylene, further alkylated and also chlorinated benzene derivadves, DMF, DMA, DMSO,
sufficiently high-boiling alcohols, such as ethanol, propanol, butanol and hexanol. In the
case where R3 is hydrogen and R2 is -NHRI4 arnidadon is carried out directly with
hydrazine or derivatives thereof. In this case, the reaction temperature can be between 5
and 150C, preferably between 20 and 40C. Apart from the abovementioned solvents,
THF and dioxane can also be used. More details can also be gathered from the preparation
examples which follow,
The following examples further illustrate the invention without however limiting it.
Unless stated otherwise, parts and percentages given therein and in the remaining
description are by weight. t-Bu is the radical -C(CH3)3.
2048211
- 24 -
Example 1
~ ~OH e11
t-Bu~ + H2N--C8Hl 7 t-~u~ CH ~ NH-C8H,7
t-Bu
(IV) (V) t-Bu (VI)
161.2 g (0.5 mol) of compound IV are initially introduced into a 750 ml sulfonation flask
equipped with thermometer, condenser, and with N2 blankedng, and 64.6 g of
1-octylatnine (V) are added. The mixture is heated to 125C and left at this temperature
for 3 hours. The product is dissolved in 800 ml of hot isopropanoVwater (85/15), the
soludon is cooled in an ice bath, and the white residue forrned is filtered off with suction.
Washing with 200 ml of isopropanoVwater (85/15) and drying at 60C gives 213.4 g (94.5
% of theory) of a white powder (compound VI). Meldng point 118C.
Analysis: calculated: [%] found: [%]
C H N C H N
79.77 10.04 3.10 80.04 9.89 3.15
The compound of the formula IV is obtained according to WO-A-80/01566, p. 25.
Example 2
~ ~ OH ~
t-Bu~ + H2N--C~H37 t-Bu~¢ ~CH ~NH-C~8H37
t-Bu (VII)
(IV) (VIII)
- 25 -
32.2 g (0.1 mol) of compound IV are reacted analogously to Example 1 with 26.9 g (0.1
mol) of 1-octadecylamine (VII), the product obtained is recrystallised from 150 ml of
methanol and dried at 60C, giving 49 g (82.8 % of theory) of a white powder (compound
VIII). Melting point 98C.
Analysis: calculated: [%] found: [%]
C H N C H N
81.16 11.07 2.37 81.14 11.06 2.27
Example 3
t ~
t-Bu (IV) (IX) t-Bu (X)
16.1 g (0.05 mol) of compound IV are reacted analogously to Example 1 with 7.8 g (0.05
mol) of 4-amino-2,2,6,6-tetramethylpiperidine (IX) at 140C for 8 hours. The product is
suspended in n-hexane, filtered off with suction and dried at 60C, giving 22.8 g (95.3 %
of theory) of a white powder (compound X). Melting point: 199C.
Analysis: calculated: [%] found: [%]
C H N C H N
77.78 9.69 5.85 77.95 9.75 5.74
Example 4
64.5 g (0.2 mol) of compound IV are reacted analogously to Example 3 with 11.6 g (0.1
mol) of 1,6-diaminohexane (XI) and worked up, giving 58.3 g (76.6 % of theory) of a
white powder (compound XlI) having a melting point of 195C.
2048211
- 26 -
~0 + HZN(CH2); NH2-- ~1 13u~3 ~NH CH2-CH2-CH2' ¦
(IV) t-Bu 2
(XII)
Analysis: calculated: [%] found: [%]
C H N C H N
78.91 9.01 3.68 78.83 8.89 3.65
Exam~le 5
~ ~ OH ~3
tl3u~,~+ H2N--NH2 H20---- t-Bu~CH~c~ 2
t-Bu (XIII) t-Bu
(XIV)
64.5 g (Q.2 mol) of compound IV in 300 ml of ethanol are inidally introduced into a
750 ml sulfonation flask equipped with thermometer, reflux condenser, nitrogen
blankedng and dropping funnel. 10 g (0.2 mol) of hydrazine hydrate (XI~) are added
dropwise to this clear, colourless soludon over a period of 30 minutes. This gives a
crystalline paste, which is diluted with 250 ml of water and filtered off with suction. After
washing with 200 ml of ethanoVwater (l: 1), the white powder obtained (compound XIV)
is dried at 60C. Yield: 68 g (95.9 % of theory), meldng point 146C.
Analysis: calculated: [%] found: [%]
C H N C H N
74.54 8.53 7.90 74.59 8.55 7.77
,~
-27- 20~8211
Example 6: Stabilisation test for multiplv extruded polvpropvlene
1.3 kg of polypropylene powder (melt flow index 3.2 gllO min, measured at 230C using
2.16 kg) are mixed with 0.05 % of calcium stearate, 0.05 % of Irganox(~) 1010
(Ciba-Geigy) and 0.05 % of the compound from Example 1, 3 or 5. This mixture is
extruded in an extruder with a cylinder diameter of 20 mm and a length of 400 mm at 100
rpm, the three heating zones being set to the following temperatures: 260C, 270C and
280C. The extrudate is led through a waterbath for cooling and then granulated. After two
further extrusions carried out in the same manner, the melt flow index is measured (at
230C using 2.16 kg). A large increase in the melt flow index denotes extensive
degradadon of the chains, i.e. poor stabilisation.
The results are summarised in the table below:
Compound Melt flow index
from Example
1 4.6
without 16.5
addidve