Note: Descriptions are shown in the official language in which they were submitted.
2049889
- METHOD AND APPARATUS FOR STORING HEAT
IN ICE BY USING REFRIGERANT JET
BACKGROUND OF THE INVENTION
FIELD OF THE INVENTION
This invention relates to a method of storing heat in
ice by using refrigerant jet and an apparatus therefor. In
particular, the invention relates to a method and device for
storing heat in ice by using refrigerant jet, in which liquid
phase refrigerant is jetted together with water, and after being
jetted the refrigerant evaporates and water comes in contact with
the evaporating refrigerant so as to freeze.
BRIEF DESCRIPTION OF THE DRAWINGS
The present invention is described herein with
reference to the following drawings, of which:
204~889
Fig. I is a schematic block diagram showing an embodi-
ment of the device according to the invention;
Fig. 2 is a schematic illustration of a T-shape mixer to
be used in the device of the invention;
Fig. 3 is a simplified graph showing the pressure drop
in a nozzle to be used in the apparatus of the invention;
Fig. 4 is a graph showing a refrigerant heat cycle in an
embodiment of the invention, in which the refrigerant is
normal pentane;
Fig. 5 is a schematic illustration of a circula-
tion-type mixer to be used in the apparatus of the invention;
Fig. 6 is a schematic illustration of a motor-driven
impeller disposed in a T-shape mixer to be used in the appa-
ratus of the invention;
Fig. 7 is a schematic illustration of a sonar vibrator
mounted on a T-shape mixer to be used in the apparatus of the
invention;
Fig. 8 is a schematic block diagram of a conventional
device of refrigerant blowing type for storing heat in ice;
Fig. 9 is a schematic block diagram of a conventional
device of individual nozzle type for storing heat in ice;
Fig. 10 is a schematic block diagram of a conventional
device of chamber type for storing heat in ice; and
20~9839
Fig. 11 is a schematic illustration of a static mixer for
mixing refrigerant and water, which mixer uses a cylinder having
two kinds of twisted elements fixed therein in an alternate
fashion.
Like parts are designed by like numerals and symbols
throughout the different drawing figures.
DESCRIPTION OF THE PRIOR ART
From the standpoint of reducing the size of heat storing
apparatus, attention has been paid to direct-contact-type
heat exchange in which water is brought to direct contact
with liquid-phase refrigerant having a low water solubility
(including water-insoluble refrigerant, to be referred to as
"hardly-water-soluble refrigerant), so as to cool the water
with the latent heat of evaporation of evaporating hardly-
water-soluble refrigerant until the u~ater freezes. The
following three kinds of structures have been proposed to
practice such direct-contact-type heat exchange.
A blowing type as shown in Fig. 8: Liquid-phase refrig-
erant is blown into cooling ~ater 2b in a water tank 1, so as
to produce sherbet-like ice 2a.
An individual nozzle type as shown in Fig. 9: Liquid-
phase refrigerant from a liquid refrigerant pipe
A
2 ~ 1 ~ 8 8 ~
and cooling water from a cooling water return pipe
18 are simultaneously blown into a water tank 1
through refrigerant nozzles 4 and water nozzles 5,
respectively, so as to produce water-ice mixture 2.
A chamber type as shown in Fig. 10: Water and refriger-
ant are mixed in a chamber 25 which is provided in
the space above water surface of a water tank 1,
and ice slurry produced by the mixing slides down
10onto the water in the tank 1 through lower opening
of the chamber, while evaporated refrigerant gas
moves upward to a refrigerant gas outlet pipe 6
through an upper opening of the chamber.
15Operation of the blowing type in Fig. 8 will be briefly
described in the case of cooling operation. Refrigerant gas,
which has evaporated by chilling the cooling water in the
water tank 1 after being jetted thereto from a liquid refrig-
erant pipe 12, moves upward to a refrigerant gas outlet pipe
6 leading to a compressor 7, and after being compressed it is
fed to a compressed refrigerant gas pipe 8 leading to a
refrigerant condenser 9. After liquefied, the refrigerant
returns to the refrigerant liquid pipe 12 through an expan-
sion unit 11, and completes one heat cycle of the refriger-
ant. The refrigerant condenser 9 is cooled by the outsideair. Water-cooled refrigerant condenser 9 can be also used.
The cooling water 2b in the water tank 1, which holds stored
heat from the jetted refrigerant, is sucked to a cooling
water outlet pipe 14 through the lower portion of the tank 1
by a cooling water circulating pump 15.
The cooling water from the circulating pump 15 enters
into a cooling water heat-exchanger 16, and gives its heat to
load-side piping 17, and then it returns to the water tank 1
through a cooling water return pipe 18, and completes one
2~9889
cycle of cooling water. To separate water and water drop from
refrigerant, an eliminator 13 may be provided at the junction
between the water tank 1 and the refrigerant gas outlet pipe
6, as shown in Fig. 9.
In the example of Fig. 8, the load-side piping 17 is
connected to an air blower 21 which sends cooled air to an
air conditioning apparatus 22, so as to accomplish the de-
sired cooling function. A cooling unit 20, which is provided
on the cooling water return pipe 18, has refrigerant passages
connected to a branch refrigerant pipe extending from a cross
valve 19 on the liquid refrigerant pipe 12 to another cross
valve 19 on the refrigerant gas outlet pipe 6. Numeral 9a in
the drawing shows a liquid receptacle unit for receiving
liquid refrigerant dripped from the condenser 9.
In the case of heating operation, the condenser 9 is
switched by a suitable switching means (not shown) so as to
cause the refrigerant to absorb heat, and the refrigerant
gives its absorbed heat to water in the water tank 1 so as to
make it warm water.
The operations of the systems of Figs. 9 and 10, are
apparent to those skilled in the art from the foregoing
description with respect to the example of Fig. 8.
SUMMARY OF THE INVENTION
The blowing type of Fig. 8 has a shortcoming in that,
when the amount of ice in the cooling water of the water tank
1 increases in excess of a certain limit, the ice piles up on
the water surface and tends to intervene with the mixing of
the refrigerant with water, causing disturbance in ice forma-
tion thereafter. Such disturbance leads to reduction of
A
6 2a~988~
overall efficiency of heat exchange and ice production.
To solve the above shortcoming, it has been proposed to
add a fluidization agent in the cooling water to facilitate
production of soft sherbet-like ice 2a. Examples of such
fluidization agent include ethylene glycol, propylene glycol
and the like. These fluidization agents exhibit properties
as antifreezing fluids and they reduce the freezing point of
cooling water to below 0C. Thus, the use of fluidization
agents tends to cause a problem in that the refrigerant
evaporating temperature is lowered and the coefficient of
performance (COP) of freezing cycle is reduced. Further, the
use such agents also results in a cost increase and, in
addition, possible environmental problem at the time of
removing the cooling water from the water tank 1, e.g., for
maintenance and repair of various apparatuses in the system.
The individual nozzle type of Fig. 9 is free from the
above problem due to ice floating on water surface, because
the refrigerant and water come in contact with each other
substantially above the water surface and heat exchange takes
place in air. It has, however, a different problem. Namely,
gas-phase refrigerant, which is called flash gas, is generat-
ed at the gas trap (numeral 9a in Fig. 1) or the like, and
the refrigerant flow through each refrigerant nozzle 4 tends
to have two, gas and liquid, phases. With an ordinary noz-
zle, the presence of gas in the refrigerant flow therethrough
reduces the centrifugal force at the outlet thereof, so that
the spreading area of the refrigerant from the nozzle outlet
tends to shrink. As the spreading area shrinks, the contact
surface area between water and refrigerant becomes smaller,
resulting in a reduction of heat exchange therebetween, which
reduction leads to drop in both evaporating pressure and
evaporating temperature of the refrigerant. Hence, the heat
exchange efficiency is reduced and efficient ice formation is
A
- 2~988~
hampered. Besides, when the spreading radius of Ice is small
if the amount of ice increases, an ice pile is inevitably
formed immediately below the refrigerant nozzles 4, and such
ice pile tends to disturb contact between ice and water. Once
the ice pile is formed, deterioration of the contact heat
exchange between refrigerant and water is accelerated, and
the performance of ice formation rapidly erodes. The inven-
tors confirmed such phenomena through experiments.
The chamber type of Fig. 10 appears to aim at prevention
of the above-mentioned deterioration of the heat exchange
performance by using the chamber 25, instead of the nozzles 4
and 5, for mixing the refrigerant and water. However, since
ice produced in the chamber 25 falls down substantially
vertically together with water through a lower opening there-
of, ice pile is inevitably formed on water surface in the
water tank 1 immediately below the lower opening of the
chamber 25 when the amount of ice from the chamber 25 in-
creases. Thus, suitable fluidization agent must be added to
prevent formation of ice pile and to facilitate breakdown of
ice pile when formed.
The chamber 25 is complicated in construction, and it is
costly to make. Besides, from practical standpoint, it is
difficult to design such chamber 25 so as to ensure continu-
ous presence of water within it for mixing with liquid re-
frigerant while preventing both overflow and fall down
through its upper opening and lower opening, respectively.
Further, it is also difficult to operate such chamber 25 in
line with the intention of its designer. The reason for such
difficulty is in that flow rates of the liquid refrigerant
and water vary depending on the running conditions or the
overall thermal system of which the heat storing device is a
part.
A ~
2a4~8~
Therefore, an object of the present invention is to
dissolve the above-mentioned shortcomings of the prior art by
providing a method and an apparatus for storing heat in ice
by using refrigerant jet, said refrigerant jet consisting of
a mixture of liquid refrigerant and water and being jetted
after the mixture is formed.
The inventors noted the fact that if a hardly-water-
soluble refrigerant having boiling point lower than freezing
point of water is merely mixed with water under normal pres-
sure at a temperature below the water freezing point, the
water thus mixed will freeze immediately after the mixing,
but if pressure of the hardly-water-soluble refrigerant at
the time of mixing is suitably selected, the freezing of
water at the time of mixing can be avoided and the water thus
mixed is allowed to freeze after jetting of the mixture
through a nozzle to a pressure suitable for the freezing.
More specifically, at a location upstream of the nozzle,
if the hardly-water-soluble refrigerant and water are mixed
at a pressure higher than saturation pressure of the refrig-
erant for a temperature equivalent to water freezing point,
and if the thus mixed mixture passes through a nozzle and is
jetted toward downstream of the nozzle at a pressure lower
than the saturation pressure of the refrigerant for the water
freezing point, then the refrigerant stays in liquid phase
without evaporation at the time of mixing and its evaporation
immediately after the mixing is prevented, and yet the re-
frigerant in the mixture evaporates toward a wide area after
being jetted through the nozzle so as to cause the water in
the mixture to freeze after being jetted and the frozen ice
to be dispersed over a wide area.
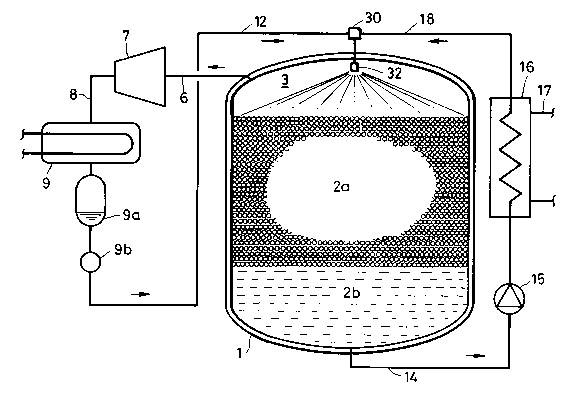
Referring to Fig.1 through Fig. 3, in an embodiment of
the method of storing heat in ice by using refrigerant jet
A
- 9 2~4~8~9
according to the invention, the pressure of space 3 above
water surface in a heat-insulating water tank 1 is set at P2
that is lower than saturation pressure P0 of a hardly-water-
soluble refrigerant for a temperature equivalent to water
freezing point (P2<P0). The refrigerant of liquid phase is
mixed with water at a pressure P1 higher than the saturation
pressure P0 (PO<P1). The thus mixed liquid mixture is jetted
into the space 3 above water surface of the water tank 1
through a nozzle 32 that is disposed in the space 3. U'here-
by, the refrigerant of the thus jetted liquid mixture iscaused to evaporate at its saturation temperature for the
pressure P2 in the space 3 while deriving latent heat of
evaporation from the water of the jetted liquid mixture, so
that the water of the jetted liquid mixture freezes into
sherbet-like ice 2a for storing heat in the thus frozen ice
2a.
.
An embodiment of the apparatus for storing heat in ice
according to the invention is to freeze water with latent
heat of evaporation of a hardly-water-soluble refrigerant,
and the apparatus uses a heat-insulating water tank 1 whose
inside pressure P2, such as the pressure in top space 3
thereof, is kept lower than saturation pressure P0 of a
hardly-water-soluble refrigerant for a temperature equivalent
to water freezing point (P2<P0). A mixer 30 mixes the re-
frigerant of liquid phase with water at a pressure P1 which
is higher than the above-referred saturation pressure P0
(PO<P1). Output from the mixer 30 is connected to the inlet
end of a nozzle 32, and outlet orifice of the nozzle 32 opens
in the top space 3 of the water tank 1.
A
.
9a 2~ 889
DETAILED DESCRIPTIO~ OF PREFERRED EMBODIME~TS
Before entering details of preferred embodiments, the
operating principles of the invention will be described.
Referring to Fig. 4 showing a heat cycle of hardly-
water-soluble refrigerant to be used in the invention, the
abscissa shows enthalpy i and the ordinate shows pressure P.
The refrigerant is in liquid phase on and to the left of a
saturation liquid line SL, and the refrigerant is in over-
heated gas phase to the right of a saturation gas line SG,
and the refrigerant is in moist gas phase bet~een the satura-
tion liquid line SL and the saturation gas line SG. In the
moist gas phase, when heated the refrigerant evaporates while
absorbing latent heat of evaporation.
To show the heat cycle in numerical terms, normal pen-
tane will be used in the following description as an exampleof the hardly-water-soluble refrigerant. The refrigerant to
be used in the invention, however, is not restricted to
normal pentane, and in fact, it is possible to use isobutane,
neopentane, and other suitable refrigerants. As shown in
Fig. 4, saturation pressure of normal pentane for a tempera-
ture equivalent to water freezing point O C is approximately
188 Torr. With increase of pressure, the saturation tempera-
ture of normal pentane increases; for example, at a pressure
A
of 400 Torr, the normal pentane has a saturation temperature
of 20 C.
More specifically, if the pressure is kept at 400 Torr,
liquid normal pentane will not boil at 0 oC, and it boils
only when the temperature is at 20 C or higher. It means
that, when liquid normal pentane is mixed with water under
the pressure of 400 Torr, boiling temperature of the liquid
normal pentane is not below 20C because its saturation
temperature for this pressure 400 Torr is 20 oC. The gas-
liquid ratio of normal pentane may vary depending on evapora-
tion and condensation, but liquid normal pentane will never
boil at temperatures below 20C as long as the pressure is at
400 Torr. Thus, mere mixing of liquid normal pentane with
water under the pressure of 400 Torr will not cause the thus
mixed mixture to be cooled to 0 C or below, and the water
will not freeze by the mixing alone.
One may conclude that at a pressure higher than 188
Torr, which is the saturation pressure of the normal pentane
for water freezing point 0 C, for example, at 400 Torr, even
if water and liquid normal pentane are mixed by the mixer 30,
the water in the thus mixed mixture will not freeze and a
liquid mixture of water and normal pentane is produced. When
such liquid mixture is fed to nozzle 32 having an orifice to
a lower pressure space, it is possible to disperse the fluid
mixture over a wide range by blowing it to the lower pressure
space from the orifice of the nozzle 32.
Fig. 4 also shows that, at a pressure lower than 188
Torr that is the saturation pressure of the normal pentane
for water freezing point of 0 C, for example, at 180 Torr,
the saturation temperature of the normal pentane is -1 C.
If the pressure in the water tank 1 is kept at, for example,
this pressure 188 Torr, liquid normal pentane in the above
2~ g~
ll
fluid mixture dispersed form the nozzle 32 in the top space 3
of the water tank 1 starts to boil at -1 C. This boiling can
be compared with the well-known fact that, if water with a
pressure higher than 1 atm and having a temperature of 100 C
or higher is decompressed to 1 atm, the water starts to boil
at 100C, and if the water is continuously heated so as to be
kept at 100 C or higher, then the water continues to boil
until the entire water is converted into vapor.
In the embodiment of Fig. 1, when the refrigerant normal
pentane jetted from the nozzle 32 boils at -1 C, the water
jetted together with the refrigerant gives the latent heat of
evaporation to the refrigerant and freezes into ice. In
actual operation, the boiling temperature of the refrigerant
often varies in a range from about 0 oC to -5 oC depending on
the manner in which the refrigerant comes in contact with
water. For simplicity, however, it is assumed to be -1 C in
the foregoing description.
Fig. 3 shows pressure in the nozzle 32. A pressure drop
is produced across the nozzle 3Z, i.e., from the pressure P1
at inlet side piping thereof to the pressure P2 at outlet
orifice which opens to the top space 3 of the water tank 1.
Once the refrigerant starts to boil, water jetted from
the nozzle 32 is derived of the latent heat of evaporation by
the refrigerant and the water itself freezes into ice. With
the invention, the refrigerant is dispersed by the nozzle 32
over a wide range, and the ice thus produced is also scat-
tered to a wide area, so that no ice piles are formed immedi-
ately below the nozzle 32.
In the embodiment in Fig. 1, the refrigerant extracted
from a water tank 1 through a refrigerant gas outlet pipe 6
is compressed by the compressor 7 up to, for example, 700
12 2049~89
Torr as shown in Fig. 4. The compressed refrigerant, which is
at a high temperature such as 34 C, is fed to a condenser 9
through a compressed refrigerant gas pipe 8 so as to be
cooled and liquefied. The liquid refrigerant from the con-
denser 9 is delivered to a liquid refrigerant pipe 12 througha liquid receptacle unit 9a and a gas trap 9b, and the liquid
refrigerant thus delivered has a temperature of about 20 C
and a pressure of about 400 Torr. On the other hand, cooling
water 2b in the water tank 1 is fed to a cooling water heat
exchanger 16 by a cooling water outlet pipe 14 and a cooling
water circulating pump 15. At the heat exchanger 16, heat is
transferred from the cooling water 2b to, for example, load-
side piping 17. The cooling water then flows into a return
pipe 18, where the pressure of the cooling water is at about
400 Torr.
The mixer 30 mixes the liquid refrigerant from the
liquid refrigerant pipe 12 with the cooling water 2b from the
cooling water return pipe 18 at a pressure of about 400 Torr,
and it feeds the thus mixed liquid mixture to the nozzle 32
which is disposed in the top space 3 of the water tank 1. When
normal pentane is used as the refrigerant, its saturation
temperature for the pressure 400 Torr is high, and the water
in the liquid mixture does not freeze before entering and
being dispersed by the nozzle 32. If the pressure at the top
space 3 is at 180 Torr, the refrigerant jetted from the
nozzle 32 boils at the saturation temperature of -1 C for
the pressure 188 Torr, and waterdrops in contact with such
boiling refrigerant is deprived of the latent heat of evapo-
ration of the refrigerant and freezes into sherbet-like ice
2a. Thus, heat is stored in the sherbet-like ice 2a, which
falls onto the cooling water 2b and cools it.
Fig. 2 shows a T-shape mixer 33 as a modification of the
mixer 30 of Fig. 1. The T-shape mixer 33 has a horizontal
13 2~8~
straight tubular portion and a central leg portion depending
from an intermediate section of the horizontal portion. The
horizontal portion receives the refrigerant of liquid phase
at one end and also receives water at the opposite end there-
of, so as to mix the refrigerant and water therein. Thecentral leg portion communicates with the horizontal portion
at its intermediate section, so as to extract the thus mixed
liquid mixture therefrom while further mixing the refrigerant
and water therein. The illustrated T-shape nozzle 33 is
connected to a single-orifice nozzle 32, but it is also
possible to connect such T-shape mixer 33 to a multi-orifice
nozzle of Fig. 5 for expanding the area of dispersing the
sherbet-like ice 2a.
Fig. 5 shows a circulation-type mixer 34 as another
modification of the mixer 30 of Fig. 1. The circulation-type
mixer has a circular portion and a central leg portion
depending from a central section of the circular portion.
The circular portion receives the liquid refrigerant at one
peripheral part thereof in a tangential direction thereat,
and the circular portion also receives water at a diametri-
cally opposite peripheral part thereof to the above one
peripheral part in a tangential direction thereat. The thus
received refrigerant and water circulate in the circular
portion and get mixed with each other while circulating. The
central leg portion communicates with the circular portion at
its central section, so as to extract the thus mixed liquid
mixture therefrom. The circulation-type mixer 34 ensures
thorough mixing of the liquid refrigerant and water without
using any power form the outside. The example of Fig. 5
expands the area of dispersion of sherbet-like ice 2b by
connecting the mixer 34 to a multi-orifice combination of
nozzles 32. It is also possible to connect the circulation-
type mixer 34 to a single-orifice nozzle.
2~49889
14
Fig. 6 shows a modification of the T-shape mixer 33, in
which a motor-driven impeller 35 and its driving motor 36 are
disposed in the intermediate section of the horizontal por-
tion of the mixer 33. In the example, the impeller 35 is in
the intermediate section and the motor 36 is attached to the
outside of the intermediate section. The use of the impeller
improves the degree of mixing of the liquid refrigerant with
water. Although the illustrated mixer 33 with the impeller
35 is connected to a multi-orifice nozzle, it can be also
connected to a single-orifice nozzle.
Fig. 7 shows another modification of the T-shape mixer
33, in which an ultrasonic mixer 37 is attached to the in-
termediate section of the horizontal portion of the mixer 33.
The ultrasonic vibrator 37 thus attached pulverizes the
liquid refrigerant and water into very fine particles so as
to enlarge the contact area therebetween and improve the heat
exchange efficiency therebetween. The mixer 33 with the
ultrasonic vibrator 37 may be connected to either a single-
orifice or a multi-orifice nozzle.
To mix refrigerant and water, one can use a static mixer
40 as shown in Fig. 11. The static mixer 40 has a cylinder,
which cylinder has an inlet opening receiving both refriger-
ant and water and an outlet opening to be connected to thenozzle 32. Two kinds of twisted elements 41 and 42 are
connected alternately in the cylinder. The first kind ele-
ment 41 is made by twisting rightward a rectangular plate by
180, and it may be called a rightward element. The second
kind element 42 may be called a leftward element as it is
twisted similarly as the first element but in opposite direc-
tion. In the static mixer 40, an angular displacement of 90
is provided between the adjacent two kinds elements; namely,
between the first kind element 41 and the second kind element
42. Thus, the two kinds elements in the cylinder are con-
2~ ~9~8g
nected alternately in series with a 90 displacement at thejunction between the adjacent two kind elements. With such
disposition of the rightward and leftward elements, it is
known to those skilled in the art that fluid in the cylinder
is bisected each time it passes through one element.
In the mixer 40 of Fig. 11, six elements, three right
ward and three leftward, are used, and the fluid in the inlet
end of the mixer 40 is divided into 64(=26) sections at its
outlet. In addition to such division, the fluid entering the
inlet of the mixer 40 is turned as it proceeds through the
cylinder and the turning direction is reversed when it moves
from one element to the next, and the fluid flow shifts
between the twisted surface of the elements 41, 42 and the
inside surface of the cylinder. Such combination of divi-
sion, reversion of turning direction, and shifting of the
flow results in thorough mixing of the fluid. Thus, when
liquid mixture of refrigerant and water passes through such
static mixer 40, the refrigerant and water are thoroughly
mixed to ensure highly efficient heat exchange therebetween.
As described in detail in the foregoing, the method and
device for heat storage in ice according to the invention
mixes liquid refrigerant and water and then jets the mixture
through a nozzle unit, and the following outstanding effects
are achieved.
(1) The jetting of liquid refrigerant together with
water enables dispersion of the resultant sherbet-like
ice over a wide area, so as to assure high efficiency in
heat exchange.
(2) Sherbet-like ice is produced without being affected
by water in a water tank.
16 2D ~9 889
(3) No fluidization agent is required, and the cost
therefor is saved.
(4) It is possible to avoid any drop of freezing point
of the cooling water because fluidization agent is not
used, and high efficiency of heat exchange is achieved.
(5) Being simple in construction, the apparatus of the
invention can be made at a low cost.
(6) A number of schemes are available for mixing liquid
refrigerant with water; namely, simple confluent scheme,
natural circulation scheme, forced circulation scheme
with a rotary impeller, fine pulverization scheme with
an ultrasonic vibrator, and a combination of any of the
above schemes.