Note: Descriptions are shown in the official language in which they were submitted.
~ W 0 90/123l8 2 ~ 5 0 8-8 ~ pcT/GB9o/no5s~
ASSAYS USING ALBUMIN - TETRAZOLIUM IN1ERACTION
This invention relates to an improved method for reduction of
tetrazolium compounds to coloured formazans and to the use of this
method in assays (ie detection and/or quantitative analysis) of
analytes, in particular albumin.
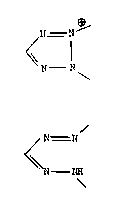
Tetrazolium salts contain -the cationic tetrazolium nucleus:
< r~ ~_ N
li I~
which is opened by reduction to produce a formazan containing the
ring-opened group: /
r~
r~
The formation of a formaxan will produce a co~our chanp~, as the
forma~an ~roup li~ a stron~ coloured chromophor~, and t~tra7~011urn
salts are often colourless. Ther~fore the presence and/or amount of
formazan produced may conveniently be detected visually or
colorimetrically, for example by observing the absorbance change in
the region between 505 and 660 nm, especially around 590 ntn.
Generally weak reducing agents can only reduce tetrazolium salts to
formazans in the absence of proteinaceous materials. This colour
change on reduction may be used in assay methods for example as
described in W088/08882.
Albumin is a protein, in one form having a relative molecular mass
of 67000 and consisting of a single chain of 584 amino acids. It is
present in human serum at a higher concentration than any other
single protein. The primary functions of albumin in serum include
~0 the maintenance of osmotic pressure, and acting as a carrier protein
for metabolites such as bilirubin, fatty acids, inorganic compounds
such as calcium, hormones and many drugs; it also provid~s a source
of amino acids.
~5 ~or several reasons albumin is often assayed in the clinical
W 0 90/1~318 5~4 PCT/GB90/0055
biochemistry laboratory. For example it is used as an indicator of
liver disease, is an essential requirement in albumin replacement
therapy, it can indicate levels of unbound (unconjugated) bilirubin,
it can indicate cases of myeloma and may be used in nutritional
treatment regimes (Whicher and Spencer, Ann. Clin. Biochem.
24 572 (19~7)).
There are several known methods of quantltatively measuring serum
albumin. It can be measured by electrophoresis, or by immunological
methods. However, one of the most commonly used methods i5 based on
dye-binding, using either bromocresol green (BCG)(Rodkey, F.L.,
Clin Chem. ll 478 (1965)) or bromocresol purple (BCP) (Louderback, A.,
Mealey, E.~1., & Taylor, N.A. Clin. Chem. 1~ 793 tl968)). ~romocresol
green methods give low recoveries at high albumin concentrations and
high recoveries at low albumin concentratlons ~Webster, D., ~lgn~ll,
A.~1.C. & Akwood, E.C., Clin. Chim. Acta. 53 ~Ol (197~) ), and ar(!
generally non~sp~clfic ~Slater, L., Carr,er, P.M. & ~10bbs, J.11., ~nn.
Chim. Biochim. 12 33 (1975). Methods bnsed on bromocresol purple
are generally more specific (Pinnell, A.E. & Northam, B.E., Clin.
Chem. 2 80 (1978)). However, bromocresol purple does not ilave
interspecies albumin specificity; many calibrators contain either
bovine or equine albumin standards, which give an absorbance too far
below that of human albumin to be of value. Further, heparin is
reported to interfere (Perry, B.W. & Douman, B.T., Clin.Chem.
25 1520 (1979)~, which precludes the use of heparin~sed blood.
Bilirubin is also believed to interfere.
Therefore a method based on the formation of a~eoioured product in
response to the presence and concentration of albumin which did not
~0 suffer interference from other components which may be present in the
sample would be of great practical advantage. It is an object of the
present invention to provide a new method which does not suffer the
disadvantages described and can be used for the quantitation of
albumin by a procedure generating a coloured product. Oth~r objects
and advantages of this invention will be apparent from khe following
description.
W O 90/1Z318 2 0 5 0 ~ 8 4 PCT/GB90/00552
According to a first aspect of this invention a method for the
reduction of a tetrazolium compound containing the cationic nucleus:
< ;~
to produce a ring-opened formazan compound containing the ~roup:
10 is characterised by the step of causing the tetrazolium compound to
interact with albumin, and reducing the tetrazolium compound - albumin
interaction product with a reducing agent in an aqueous solution at a
pH between 6 and 10.
15 The invention deriveS from the unexpected discovery that although
weak reducing agents can only reduce many t~tra~oliurn salts at p~l
above 10 and in the abs~nce of albumin, at the lower pll rnng~ Or the
invention the reduction of tetrazoliLIm s~lts is ~ub~t~ntlAlly
enhanced by interaction wlth albumln. The method of the inv~ntiorl ls ?
20 particularly applicable to reductlon of the leuco forrn of 2-(2' -
benzothiazolyl~ - 5-styryl-3-(4' phthalhydrazidyl)-tetrazolium,
tabbreviated herein to "BSPT"), especially the hydrochloride.
BSPT contains the tetrazolium cation:
. ~ .
/N = N-~
3 M S
.'. - .
, ~ :`
The interaction between the tetrazolium salt and the albumin is of a
type that results in enhancement, acceleration or catalysis of the
,5 reduction of the tetrazolium salt to the coloured formazan dye.
Although the precise nature of interaction between the tetrazolium
salt and albumin in the rnethod of the invention is not fully under-
stood, it is believed that the interaction may involve formation of
- ~:
.
W O 90/12318 PCT/GB90/00552~
~5~8~4
a complex between the salt and the albumin. The interaction and/or
complex formation may result from the presence of the substituent
groups on the tetraæolium nucleus. Hence the method of the invention
may be particularly applicable to tetrazolium salts in which the
nucleus is substituted with one or more of the substituents present in
BSPT ie benzothiazolyl, styryl or phthalhydrazidyl groups which may
themselves carry substituent(s) on their ring systems, or a combina-
tion of such groups.
Many reducing agents are suitable for use in the rnethod of the
invention, but preferably a weak reducing agent is used. Examples of
those found to be suitable include dithiothreitol ("DTT"),
mercaptoethanol eg betamercoptoethanol, cysteine, nicotinamide adenine
dinucleotide and aminophenol. A particularly preferred reducing agent
~5 is NADH (reduced form of nicotinaminde aderlin~ dinucleotide).
An electron carrier may be used in the r~action medi-lm of the m~khod
to enhance the speed of el~ctron trnnP.f~r betwe~n th~ r~d~lGLn~ a~,ent
and the tetrazolium salt. A pr@f~rred alactron c~rricr is a
phenazinium salt, ie compounds containing the cationic nucleus:
~X`
R ~
where R is hydrogen or alkyl. A preferred phenaæinium salt is
5 methox~ N-methylphenazinium methyl sulphate ~"MPMS"), ie
OCH
~ H_SO4~
although other suitable but less preferred phenazinium salts includeO phenazinium methosulphate ("PMS") and phenazinium ethosulphate ("PES").
Any buffer capable of maintaining th0 pH between 6 and lO may be used
a preferred buffer being tris hydroxymethylaminomethane HCl ("Tris-
HCl"). A preferred solution pH is 8.5.
It is also preferred to include a s~rfactant in the reaction solution
of the method, as this aids the solubilisation of the formaæan
W O 90/12318 PCT/GB90/00552
2~;5~84
produced. A preferred surfactant is a non-ionic surfactant
especially a polyoxyethylene sorbitol ester, in particular Tween 80
~Trade Mark).
The degree of reduction of the tetrazolium compound and consequent
formation of formazan quantitatively relate8 to the quantity of
albumin and/or reducing agent present in the reaction solution, and
therefore when one of these two is the limlting factor in the reaction,
ie all the other reactants are present in excess, the method of the
invention may be used i3S the basis of assays for albumin or reducing
agents.
Therefore in a second aspect this invention provides a method for the
assay of human or rnammalian albumin in a sample comprises reacting a
tetrazolium salt which is capable of interacting with albumin, with a
reducing agent to produce a formazan ln an aque ou~ solutlon ~t a pH
between 6 and 10 i~ the pre~ence of the saMple, and relatln~ trihe
presence and/or arnount of formazan produced t,o th~ pr~sence and~or
amount of albumin in the sample.
Although other tetrazolium salts which can only be significantly
reduced ~within a time period acceptable to clinical analysis) by
weak reducing agents in the pH range 6 - 10 after interaction with
albumin may be used, preferred tetrazolium salts are those of BSPT,
25 especially the hydrochloride, or other tetrazolium salts in which the ;~ .
nucleus is substituted with one or more of the substituents present in
BSPT as mentioned abo~e, which may themselves carry subs~iiiuents on
~heir ~ing systems or-a combination o~ such groups.
The method of assay of albumin is suitable for use with any sample
believed to contain albumin provided that at least a proportion of
any albumin contained therein can be dissolved to form the necessary
aqueous solution if the solution does not already comprise an aqueous
solution. The me~hod is therefore most suited to the assay of aqueous
samples believed to contain albumin, especially biologlcal samples
such as bodily fluids, and in particular blood serum, which may be
used directly without any pretreatment~ or urine etcO ~;
':
. ' ' !,
W O 90/123i8 PCr/~B90/005~
.~ '' 2~'5~8~
In performing the assay method it is desirable that the quantity of
tetrazolium salt and reducing agent present in the reaction mixture
should be in excess of that expected to interact with the albumin in
the sample so that the quantity of albumin present is the limiting
factor.
Suitable and preferred reaction conditions and reagents for the
albumin assay method of this invention are as discussed above with
reference to the first aspect of the invention, ie the choice of
reducing agent, the use of an electron carrier, buffers and pH, and
use of a surfactant etc. I'hese parameters are summarised in Table l -
below:
Table l
Parameter Typical Ran~e
Concn. of tetrazolium salt lO~m - lOO~M 75 - 95~M
Concn. of reducing ag0nt l~M - lOOmM 0.05 ~ 0.2mM
Concn. of electron carrier l~M - lmM 5~M - 0.5mM
Concn. of pH buffer LmM - lM 50 - l50 mM
Concn. of surfactant ~W/V) O.Ol - 5% 0.2 - 0.5%
Temperature 5 - 60C20 - 37 C
Under these conditions reduction of the tetrazolium salt takes place
rapidly enough for the assay to be carried out in a few minutes, a
time scale convenient for clinical use.
The method is sensitive to albumin levels below those of clinical
significance and the colorimetic analysis has a linear rel~tionship
with initial albumin levels up to a~out-lOOmg~ml of original serum
sample. Serum itself does not significantly absorb over the range
of~wavelengths used for measurement and so a sample blank is not
normally required. The method appears to be specific to albumin,
requires no pretreatment of a serum sample and is not significantly
subject to interference. The method of estimating albumin of the
present invention will have many applications, but will be
particularly useful if a quick and routine method of analysis is
required, most especially in the field of clinical chemistry. Other
uses may include quality control measurement in albumin production,
~ ~.~ .. .. .. . .. .. .
W O 90/12318 PCTI~B90/00552
assessment of albumin content in other blood products and structural
investigations of albumin.
Accordingly in a third aspect the invention provides a method for the
assay of reducing agents in a sample which comprises reacting a
tetrazolium salt which is capable of interacting with albumin, with
the sample in the presence of albumin in an aqueous solution at a pH
between 6 - lO and relating the presence and/or amount of formazan
produced to the presence and/or amount of reducing agent in ~he
sample, the quantity of tetrazoliunl salt used being in excess of the
amount expected to be reduced by the reducing agent in the sample.
Suitable and preferred reaction conditions and reagents for this
method of assay of reducing agents are as discussed above with
1; reference to the first and second aspects, ie the choice of
tetrazolium salt, us~ of an electron carri~r, buff~r~ and p~i, and us~
of a surfactant etc. It is desirable to arran~ that th~ p~ram~ters
of` th~ reaction solution of th~ mcthod ar~ wlthin thH rangc~ indlcnk~d
in Table l, if the approxima~e concentration of reducing agent in the
sample can be approximated in advance. An albumin concentration of up
to about lOOrng/ml appears to be suitable.
This method of assay of reducing agents may be used to assay many
different chemical reducing agents. It is particularly suitable for
~5 the assay of reducing agents which are of biochemical clinical or
diagnostic importance, such as aminophenols, NADH or co-en~yme A
(CO-A~ and-using this method they may be assayed in samples OI- bodily
fluids such as serum-j l1ri~e elc.
As a further mcdification, the method of assay of reducing agents may
be used to assay compounds which can participate in a chemical
reaction in which a reducing agent is formed which can be assayed by
the method of the invention and related to the presence and/or
quantity of the compound.
In such a reaction the compound to be assayed may itself be converted
into the reducing agent. For example the drug paracetamo}
.
W O 90~123t~ PCT/GB90/005
(p-hydroxy acetanilide) may be quantitatively converted into the
reducing agent para-aminophenol using for example an aryl acylamidase
enzyme in a well known reaction. The para-aminophenol formed may then
be assayed and related to the amount of paracetamol.
Alternatively in such a reaction one or more of the participating
reagents other than the compound may be converted into a reducing
agent in an amount related to the quantity of the compound present,
10 For example the antibiotics chloranlphenicol and thiamphenicol, or
genta~icins may be acetylated in a reaction in which acetyl Co-A is
used as an acetylating reagent and which is consequently converted
into Co-A. Such reactions proceed readily under the mediation of the
enzymes chloramphenicol acetyl tnansf~rase ("CA~ or gentanicin
1~ acetyl transferace ("GAT") respectively. In each case th~ presence
and/or arnount of the Co A produced mny be quantitatively r~lated to
the presence and/or amount of antibiotic,
These assay rnethod6 Or the invention may be perforrned either manually
20 or automatically in a number of ways. For example an aqueous solution
may be made up containing at least the sarnple, the tetrazolium salt
and the reducing agent or albumin as appropriate at the indicated pH,
optionally also containing the other reagents referred to above, and
incubated at a suitable temperature. The colour produced is then
~5 detected and for example compared with a standard or measured
colo rimetrically. Although the reaction solution is aqueous it may
be necessary to include additional water-miscible solvents tO assist
in dissolution OI all OI the reagents, in pariicular the te~razolium
salt. One preferred example of such a solvent is dimethylformamide
30 ("DMF"). When performing the method of the invention in this way the
order in which the reagents and sample are mixed does not appear to
be critical.
Alternatively1 suitable reagents such as the tetrazolium salt,
35 reducing agent or albumin as appropriate, buffer etc may be
immobilised on a solid support by for example impregnation, adsorption
or absorption. Exposure to a soluti~n sample containing albumin or
~W O 90/123l8 PCT/~B90/00552
9 2050~8~i :
reducing agent as appropriate then causes a colour change in the
support which can be detected.
~o make the assay methods of the invention convenient for laboratory
and clinical use, the invention also provides an assay kit including
in combination one or more ready made reagents as described above for
the performance of the method. For exarnple a kit may comprise
separate solutions containing respectively the tetrazolium salt
capable of inter2cting with albumin, buffer, and reducing agent or
albumin as appropriate, at concentrations suitable to enable them to
be easily used in the method of the invention, optionally together
with the other reagents, eg surfactant, electron carrier referred to
above. Such a kit may alternatively comprise a solid substrate
having immobilised thereon suitable reagents. The kit may also ~,
include standards and instructions which may lnclude a colour
comparison chart. In such a test kit the reagentq have been found to
be stable on storage for at least 30 day9, but it i5 de~irabl~ to make
up the solution Or the tetrazolium 8alt in ~n aold ~olution, ~ O.Ol -
0.2M hydrochloric, mi~lie or ~speclally cltric acid, at a p~l of 3 - 6
for stability on long term storag~. Methods of making such Kits
embodying the methods of the invention will be apparent to those
skilled in the art. ~-
Examples illustrating the invention will now be described with
reference to:
Fig l which shows the hyperchromic shift on reduction of a
3SPT-albumin interaction-product.
Fig 2 which shows the absorbance at 590nm versus albumin ~
concentration for the method of Example l. ~ -
3 Fig 3 which shows the stability of the reagents on storage.
Fig 4 which shows comparison between the method of the
invention and a conventional method used manually. `
Fig 5 which shows comparison between the method of the
invention and a conventional method used on an
automated apparatus.
; Fig 6 which shows comparison between the method of the
invention and a conventional immunological method.
: :
. ~ . ~ ,.. .... , , . . ~ . .. ..
W O 90/12-l8 ~ ~ 4 PCT/GB90/005
'^ ' 10
Fig 7 which shows absorbance versus Co-A concentration.
Fig 8 which shows absorbance versus paracetamol concentration.
Fig 9 which shows absorbance versus chloramphenicol
concentration.
EXAMPLE 1 DIAGNOSTIC KIT FOR ALBUMIN (I)
REAGENTS
Stock Bolution of tetrazoliutn salt:
(i) BSPT hydrochloride: lOmg dissolved in 10.5ml
dimethyl formamide.
(ii) 9.4ml 12mmol/L citric acid.
working solution of tetrazolium salt:
an 18ml volume of the stock solution is diluted by
adding76ml 12mmol/L citric acid, 4ml 15% (w/v) Tween
80 and 2rnl lmmol/L MPMS.
~:
20rrlmo.l/L Tri~ ~ICl, p~l 8 . 5
Rea8ent C:
5mmol~L nicotinamide adenine dinuclectide (reduced form).
METHOD
l. To 0.5ml reagent A, add 0.5ml reagent B and 40 ~l of
reagent C.
2. Equilibrate for 3 minutes at room temperature.
3. Add 50 yl sample~and mix well.
A Read.absorrbance at 590nm aIter i minute.
The results for the A590nm given by various initial concentrations of
30 albumin are given in the calibration curve in Figure 2. The colour
produced is stable for at least one hour.
' :
~ W O 9U/12318 PCT/GB90/00552
~ 2~88~ ~
11
EXAMPLE 2 DIAGNOSTIC KIT FOR ALBUMIN (II)
REAGENTS
Reagent A:
12.0 mmol/L citric acid
0.18 mmol/L BSPT Hydrochloride
0.5 mmol/L MPMS
Rea~ent B:
200 mrnoL/L Tris ~ICl pH 8.5
35% v/v Tween - 80
Reagent C:
-
2.5 mmol/L NADH
THOD
l. Prepare a working reagent by mixing 50rnl of Rea~ent A,
50ml of Reagent B and 4.0ml of Reagent C. This mixture
is stable for at least 12 hours.
2. To l.Oml of workin~ ren~l~nt add 5~L s~rurn (or a sflmplo
containin~ albumin). Read absorbance at 590nm a~aln~:t a
reagent blank after l minute.
The reagents A, B and C as prepared and stored separately are stable
for a minimum of 30 days as demonstrated in Fig 3 by the stability ,
of the absorption at 59Qnm resulting from addition of various albumin
concentrations to a working reagent prepared from reagents stored for
the indicated periods.
'~ `' " .:
EXAMPLE 3 AL~UMIN ASSAY WITH OTHER REDUCING AGENTS
Table 2 below demonstrates the use of other reducing agents than `
~0 NADH at indicated concentrations. The range of albumin concentrations
over which the relationship between formazan production and albumin
concentrations is linear is also shown, with NADH being used as a
comparative standard. In each case the tetrazolium salt was BSPT
hydrochloride.
~5
.: . . .; ,, " .. . : .. . .. . .
wo go/12318 PCr/GB9n/005~
~5~8~ 12 ~`~d
Table 2
Reducin~ Agent Concentration Linearit~ A590 Colour
.... .
(mM) (mg/ml ? o~OYield
NADH 5.0 0-80 1.70lO0.00
L-Cysteine 2.5 0-55 1.0561.76
L-Cysteine 5.0 0-70 1.5088.24
L-Cysteine lO.0 0-70 1.5892.94
Mercaptoethano} 2.5 . 0-60 0.99 58.24
Mercaptoethano]. 5.0 0-70 1.42 83.53
Mercaptoethanol 10.0 0-80 1.48 87.06
DTT 1.0 0-50 1.0 58.82
DTT 2.5 0-80 1.6798.24
DTT 5.0 0~80 1.6999.4
EXAMPLE 4 INTERFERENCE IN AL~UMIN A SAYS
InterIerence with albumin assays using the kit of Exampl~ 1 or 2 wn~
investlgated by ~omparing te~t ~mpl~s containing v~rlou~ pot~nt.Lall.y
i.nt~rfering materlals. Th~ r~ult~ are indic~ted in table 3 below:
Table 3
Blood Constituent Concentration Ran~e Interference
Tran~ferrin 1.67 - 7.14 mg/ml None
Creatinine 0.05 - 0.3 mM None
Creatine 0.05 - 0.3 mM None
25 Urea 0.02 - 0.3 mg/ml Slight
Glucose 0.3 - 1.5 mg/ml None
MgCI. 0.3 --1.5 mM None~
NaC1 100 - 200 mM None
KCl 2 - 8 mM None
3o Ascorbic Acid 10 mM None
Ascorbate 10 mM None
Bilirubin 0.001- 0.3 mg/ml None
Heparin 3 - 25 Units/ml None
"- ~ ". ~ -. . ,
.'; ` `` . . ` . . . . ., .. ~. . .. . .~
.,~VO 90/12318 PCT/GB90/00552
-l 2~i08
~ ~ r
13
Therapeutic Agents Concentration Range Interference
Salicylic AcidO.l - 0.4 mg/ml None
TheophyllineO.l - 0.5 mg/ml None
Acetaminophen0.02 - l.O mg/ml None s
Lidocaine 0.002-0.01 mg/ml None
Barbital O.Ol -~.l mg/ml None
Caffaine O.l mg/ml None
Blood ConstituentConcentratlon RangeInterference
. .
Transferrin1.67 - 2.86 mg/ml None
Creatinine 50 - 300 mg/ml None
"~' '.
Blood ProteinsConcentration (m~/ml)Interference
Globulins Bovine 20 Nona
Bovine Globulins 20 None
Insulin Bovine 20 None
Blood ProteinConcentration (m~/ml)Ab~orbance 590nrn ;. .
Albumin ~Control) 50 0,54l
Insulin Bovine 20 0.556
Globulins Bovine 20 0.542
Bovine Globulins 20 0.5~2
.. .
As shown by above, all the blood constituents gave no interference.
The concentrations studied were above the normal range found in
blood. Heparin is added to blood to step coagulation at a
concentration~of 6.7 units/ml~ -
:~ . .
The only noticeable interference~was obtained with urea.
EXAMPLE 5 CORRELATION WITH "CONVENTIONAL" ALBUMIN ASSAY METHODS
Th~ BSPT method of albumin determination was compared to other
methods which are~commonly in use in hospitals. These were a dye-
binding method based on bromocresol green ~8CG) and an immunological
method. A total of 71 pathological ser~m samples were tested
manually using the BSPT method and compared to an automated BCG
method. In these samples the amount of albumin varied between
W O 90/123l8 PCT/CB90/005~
20S~
14
about 15 and 60 mg/ml. This data was then statistically analysed to
give a regression equation of Y = 9.268 ~ 0.7X9 indicating good
correlation between the BSPT and BCG methods. This data relates to
use of the BSPT and BCG methods manually; results are shown in Fig 4.
The methods used above (BSPT and BCG) can also be used in automated
instruments. Similar analyses were carried out using the BSPT and
BCG methods as automated procedures on the "Multistat III plus" and
resultc are illustrated in Figure 5.
The BSPT method also correlates well with the immunological technique
as shown in Fig 6. (Results data not shown)
EXAMPLE_6 ASSAY OF COENZYME-A
~5 A solution was prepared containing BSP'I' hydrochloride, albumin, MPMS,
Tris-HCl buffer and Tween-80 ~t concentration~ within the range~J
indicated in Table 1. To allquots of this was added Co-~ ovor th@
concPntration ran~e 0.25 mM o~ Co-A final GCnCentrntiOn, F'l~ 7 show~
that over this range the absorbance at 590 nm was linearly related to
ZO Co-A concentration. '~
EXAMPLE 7 ASSAY OF PARACETAMOL
Paracetamol was quantitatively cleared to form p-aminophenol using
the enzyme aryl acylamidase in a known reaction. A solution was
prepared containing BSPT hydrochloride, albumin, MPMS, Tris-HCl buffer
and Tween-80 at concentrations within -the ranges indicated~in Table l.
To a1iquots of this was added p-aminophenol. The grapn of 4-
aminophenol concentration against absorbance of 590 shows an '~ '
absorbance change up to a concentration of 3.0 mM final concentration '
of p-aminophenol. This could be related to paracetamol concentration
versus absorbance at 590 nm which showed a good linear relationship
up to a concentration of 2.0 mM as shown in Fig 8.
EXAMPLE 8 ASSAY OF CHLORAMPHENICOL
Chloramphenicol was acetylated using acetyl Co-A and the enzyme CAT
by a known reaction. This resulted in production of a quantity of
Co_A which was quantitatively related to the amount of chloramphenicol
;. ~ .: . , .. . : . . . , : , ~.
W O 90/~23l8 PCT/GPgO/00552
15 '`. .:2~884 ~
originally present. The Co-A produced was assayed using the ~ :
procedure of example 6. The absorbance at 590 nm against ~:
chloramphenicol concentration was linear over the chloramphenicol
range 10 - 200 ~M as shown in Fig 9.
`~ '
L6