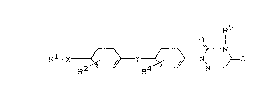Note: Descriptions are shown in the official language in which they were submitted.
The present invention relates to new substituted 1,2,4-
triazinediones, to processes for their preparation, to
intermediates for carrying out this process, and to their
use for controlling parasitic protozoa and in particular
coccidia and fish parasites.
The use of substituted 1,2,4-triazinediones for control-
ling coccidia is known. However, the reaction of these
compounds is not sa~isfactory in every case.
The present invention relates to
1. New compounds of the formula ( I ) R5
R1-X ~ y ~ ~ N (I)
in which
A-~ represents -N=CH- or -N-CH2-,
R5
X represents O or S, SO or SO2,
Y represents O, S, CO, OH or CN,
-CH- -CR -
Rl represents Cl4-halogenoalkyl,
R2 represents hydrogen, halogen or C14-halogeno-
alkyl,
Le A 27 916 - 1 -
R3 represents hydrogen or C14-alkyl,
R4 represents one or more identical or different
radicals from the series consisting of
hydrogen, halogen, halogenoalkyl and C14-alkyl,
and
R5 and R5, independently of one another, represent
hydrogen, C14-alkyl, halogenoalkyl, aralkyl or
alkinyl.
2. Process for the preparation of the new compounds of
the formula (I) according ~o (1), characterised in
that in the cases in which A-D representæ -N=CH-,
a) in the case in which R5 represents a radical other
than hydrogen, compounds of the formula (Ia)
O
R1-X ~ R N-- ~ (Ia)
in which
Y, X, R1, R2, R3 and R4 have the meaning indicated in
( 1 ) ,
are reacted with compounds of the formula (II)
Rs_B (II)
in which
R5 represents optionally substituted alkyl, alkenyl,
alkinyl or aralkyl and
Le A 27 916 - 2 -
B represents halogen, -O-SO2-alkyl, -O-SO2-aryl or
-O-SO2-halogenoalkyl,
or
b) in that compounds of the formula (III)
R5
R1-X ~ Y ~ ~ (III)
R4 COOH
in which
Y, X, Rl, R2, R3, R4 and R5 have the meanings indi-
cated in (1),
are decarbcxylated by heating, and additionally in
that in the cases in which A-D represents -N-CH2-
R6
c) compounds of the formula (Ib)
R5
R1-X ~ ~ ~ N~ - ~ (Ib)
are hydrogenated, or
Le A 27 916 - 3 -
d) in that compounds of the formula
r==~ r==~ ~ N-H
R1-X ~ Y- ~ N~ ~ o
R4
H
in which
Y, X, Rl, R2 and R4 have the abovemention~d meaning,
are reacted with compounds of the formula II
R5-B (II)
in which
R5 represents optionally substituted alkyl, alkenyl,
alkinyl or aralkyl and
B represents halogen, -O-SO2-alkyl, -O-SO2-aryl or
-O-SO2-halgoenalkyl,
or
e) in that compounds of the formula
~5
R1-X ~ y
R4
H
in which
Y, X, R1, R2, R4 and Rs have the abovementioned
meaning, but R5 does not represent hydrogen,
are reacted with compounds of the formula VI
R6_B (VI)
in which
R6 and B have the abovementioned meaning.
Le A 27 916 - 4 -
3. New compounds of the formula (III)
RS
R1-X ~ I (III)
R4 COOH
in which
Y, X, R1, R2, R3, R4 and R5 have the meaning indicated
in (1)-
4. Process for the preparation of the new compounds of
the formula (III) according to (3), characterised in
that compound6 of the formula (IV)
R5
R l - x~r~ ( IV)
in which
Y, X, R1, R2, R3, R4 and R5 have the meaning indicated
in (1),
R7 represents CN or the radical -Co-N(R5)-CooRB, and
R3 represents alkyl or aryl,
are heated in the presence of aqueous mineral acids.
5. New compounds of the formula (IV)
R5
R1-X ~ ~4 ~ (IV)
Le A 27 916 - 5 -
in which
Y, X, R1, R2, R3, R4, R5 and R7 have the meaning
indicated in (4).
6. Process for the preparation of the new compounds of
the formula (IV) according to (5), characterised in
that compounds of the formula (V)
X~ _ N - N = C - C O - N - C OOR 8 ( V )
in which
Y X R1 R2 R3 R4 R5 R7 and R9 have the meaning
indicated in (5),
are heated in the presence of bases.
7. New compounds of the formula (V)
R7
~ N-N=C-CO-N-CoOR8 ~V)
in which
X, R1, R2, R3, R4, R5, R7 and Ra have the meaning
indicated in (5) and R7 can additionally represent
hydrogen.
8. Proces~ for the preparation of the new compounds of
the formula (V), characterised in that compounds of
Le A 27 916 - 6 -
the formula (VI)
R1-x ~ Y NH2 (
in which
Y, X~ R1, R2, R3 and R4, have the meaning indicated
in (1),
are diazotised in a manner known per se and subse-
quently reacted with compounds of the formula (VII)
R7
-Co-N-COOR8 (VII
in which
R5, R7 and Ra have the meaning indicated in (5).
The compounds of the formula (I) and their salts with
bases or acids are outstandingly suitable for the control
of parasitic protozoa and in particular of coccidia and
fish parasites.
Preferred compounds of the formula (I) are compounds in
which
X represents O or S,
Y represents 0, S, CO, fH or CN,
-CH- -CR3-
R1 represents Cl4-halogenoalkyl,
Le A 27 916 - 7 -
R~ represents hydrogen, halogen or Cl4-halogeno-
alkyl,
R3 reprèsents hydrog~n or C14-alkyl,
R4 represents one or more identical or different
radicals from the series consisting of hydrogen,
halogen, halogenoalky:L and C14-alkyl, and
R5 and R6 represent hydrogen.
Particularly preferred compounds of the formula (I) are
those
~0 in which
X represents O or S,
Y represents 0, S or CN,
-CH- -
R1 represents C14-halogenoalkyl,
R2 represents hydrogen or halogen,
R4 represents one or more identical or different
radicals from the series consisting of hydrogen,
halogen, C14-alkyl and trifluoromethyl, and
R5 and R6 represent hydrogen.
Very particularly preferred compounds of the formula (I)
are those
in which
X represents O or S,
Y represents 0, S or CN,
-CH-
R1 represents C14-halogenoalkyl, in particular
trifluoromethyl,
Le A 27 916 - 8 -
R2 represents hydrogen,
R4 represents one or mor~e identical or different
radicals from the series consisting of hydrogen,
fluorine, chlorine and bromine, in particular
chlorine, and
R5 and R6 represent hydrogen.
The following compounds may be mentioned in detail
R4 o
f==\ >=\ '~N
F~C-X~r--~ ,~N ~0
~ N~
X y R R4
O O Cl Cl
S O Cl Cl
O S Cl Cl
S S Cl Cl
O CHCN Cl Cl
S CHCN Cl Cl
The following compounds may furthermoro be mentioned
R2 R4 o
R1-X ~ Y ~N~
Le A 27 916 - 9 -
231~9-7270
x r Rl R2 ~ R4
O O CHF2 H Cl Cl
o O CHF2 Cl Cl Cl
O O CF2CHFz H Cl Cl
CF2CHF2 C 1 C 1 C 1
O O CF3 H CH3 CH3
O S CHF2 H Cl Cl
O S CHF2 Cl Cl Cl
O S CF2CHF2 H Cl Cl
S CF2CHF2 C 1 C 1 C 1
: o S CF3 Cl CH3 CH3
O CHCN CHF2 H Cl Cl
O CHCN CHF2 Cl Cl Cl
o CHCN CF2CHF2 H Cl Cl
O CHCN CF2CHF2 Cl Cl Cl
O CHCN CF3 H CH3 CH3
S CHCN CF3 H CH3 CH3
S CHF2 H Cl Cl
S O CF3 H CH3 CH3
S O CHF2 Cl Cl Cl
CF2CHF2 H Cl Cl
If 2-~4-(4'-trifluoromethylthiophenyl)-3,5-dichloro-
phenoxy]-1,2,4-triazine-3,5-(2H,4H)dione is employed as
the compound of the formula (Ia) and methyl iodide is
employed as the compound of the formula (II) in process
2a) for the preparation of the compounds of the formula
(I), in which R5 does not represent hydrogen, the process
can be described by the following equation:
Le A 27 916 - 10 -
23189-7270
O
F 3 C 5~3~C~N~,>=o + CH 3
Cl
F3CS~ Cl ~N
O--~N _~=O
Cl
The compounds of the formula (Ia) are prepared as des-
cribed in process 2b).
The compounds of the formula (II) are known or can be
prepared by known methods. Methyl iodide and ethyl
bromide may be particularly mentioned.
The process i8 carried out by reacting a compound of the
formula (Ia) with compounds of the formula (II) in the
presence of a base and of a diluent.
Suitable diluents are virtually all the inert organic
solvents. These preferably include aliphatic and aroma-
tic, optionally halogenated hydrocarbons, such as benzine,
ligroin, benzene, toluene, xylene, methylene chloride,
ethylene chloride, chlorobenzene and o-dichlorobenzene,
ethers such as diethyl ether and dibutyl ether, glycol
dimethyl ether and diglycol dimethyl ether, tetra-
hydrofuran and dioxane, ketones such as acetone, methyl
Le A 27 916 - 11 -
231~9-7270
ethyl ketone, methyl isopropyl ketone and methyl isobutyl
ketone, esters such as methyl acetate and ethyl acetate,
nitriles such as, for example, acetonitrile and propio-
nitrile, amides such as, for example, dimethylformamide,
dimethylacetamide ~nd N-methylpyrrolidone and also
dimethyl sulphoxide, tetramethylene sulphone and hexa-
methylphosphoric triamide.
The process is carried out in the presence of bases.
Preferred bases which may he mentioned are the alkali
metal hydroxide4 such as sodium hydroxide, alkali metal
alkoxides such as sodium methoxide or potas~ium butoxide,
metal hydrides -~uch as sodium hydride or organic bases
such as 1,8-diazabicyclo[5.40]undec-7-ene (DBU).
The process i~ carried out at normal precsure and at
temperatures between 20~ and 140C.
The reaction is carried out by combining equimolar
amounts of the compound of the formula (Ia) and base,
adding an equimolar amount of the compound of the formula
(II) to this mixture and heating to the reaction
temperature.
Both the compounds of the formula (I) and the compounds
of the formula (Ia) can be prepared by process 2b)
described below.
If 2-[4-(4'-trifluoromethylthiophenyl)-3,5-dichlorophenoxy]-
1,2,4-triazine-3,5(2H,4H)dione-6-carboxylic acid is employed
as the
Le A 27 916 - 12 -
23189-7270
compound of the formula III in process 2b) for the
preparation of the compounds of the formula I, the
process can be described by the following equation:
H
F3CS~ Cl O
C 1 C02H
H
F3CS~_ ~N
The compounds of the formula (III) are prepared by ~he
process described further below (4). Compounds of the
formula (III) are preferably employed in which Y, X, Rl,
R2, R3, R4 and R5 have the preferred meanings indicated
for the compound~ of the formula tI).
Individual compounds of the formula (III) which may be
mentioned are
H
F3CX~Y~
C02H
Le A 27 916 - 13 -
23189-7270
x r R4
O 3,5-C12
0 3,5-C12
S 3~5-C~2
S S :~,S-C12
O CHCN 3,5-C12
CHCN 3~5-C~2
The decarboxylation i5 optionally carried out in the
presence of inert organic diluents. These include alipha-
tic and aromatic, optionally halogenated hydrocarbons
such as nonane, decane, dodecane or xylenes, alcohols
such as diethylene glycol, ethers such as ethylene glycol
monobutyl ether or diethylene glycol dibutyl ether,
sulphoxides such as dimethyl sulphoxide and sulphones
such as tetramethylene sulphone.
The reaction can moreover be carried out in the presence
of mercap~o group-containing carboxylic acids such as,
for example, mercaptoacetic acid or thiosalicylic acid.
The reaction i8 carried out at temperatures between 150
and 300C, if appropriate in the presence of mercapto
group-containing caxboxylic acids such as, for example,
mercaptoacetic acid, preferably between 160 and 250C,
in particular between 180 and 210C.
The reaction i8 carried out at normal pressure. The
compounds of the formulae (III) are heated in substance
or in the form of a solution or suspension in the
respective diluent.
Le A 27 916 - 14 -
23189-7270
If 2-[4-~4'-trifluoromethylthiophenyl)-3,5-dichloro-
phenoxy]-1,2,4-triazine-3,5-[2H,4H)dione is employed as
the compound of the formula Ib in process 2c, the process
can be described by the following equation:
Cl O
F3CS{~}`N~
cl
Cl O
F3CS~7N~
Cl
The compounds of the formula Ib are new and are obtained,
for example, by the processes 2a-c).
Compounds of the formula Ib may preferably be mentioned
in which X, Y, R1, RZ, R3, R~ and R5 haYe the preferred
meanings mentioned for the compoundq of the formula I.
In particular, the following compounds of the formula Ib
may be mentioned:
o
F3CX~R~}N~J
Le A 27 916 - 15 -
X Y R1 ~R4
O 0 Cl Cl
S O Cl Cl
O S Cl Cl
S S Cl C1
O CHCN Cl ~1
S CHCN Cl Cl
SO2 CHCN Cl Cl
Process 2c) is carried out by heating a compound of the
formula Ib in the presence of a reducing agent and of an
acid. Reducing agents which can be used are metals such
as, for example, zinc and metal salts such as, for
example, tin(II) chloride, metal hydrides such as lithium
aluminium hydride and catalytically activated hydrogen.
Acids employed are dilute mineral acid~ such as, for
example, hydrochloric acid and organic acids such as, for
example, glacial acetic acid. The reaction can optionally
be carried out in the presence of a diluent. Diluents
which can be used as inert organic solvents. These
include hydrocarbons such as, for example, toluene,
ethers such as, for example, dioxane, ketones such as,
for example, acetone and alcohols such as, for example,
ethanol. The reduction is carried out at temperatures
between 80 and 120C at normal pressure or at elevated
pres~ure.
Proce~ses 2d and 2e are carried out according to the
Le A 27 916 - 16 -
23189-7270
conditions indicated for proce!ss 2a.
If 2-[4-(4~-trifluoromethylthiophenyl)-3,5-dichloro-
phenoxy]-6-cyano-1,2,4-triazine-3,5(2H,4H)dione is
employed as the compound of the formula (IV) in process
4 for the preparation of the compounds of the formula
(III), the process can be described by the following
equation:
F3CS~3 = N ~=0
Cl CN
C 1 0
F3Cs~3n ,~o
C 1 C02H
The compoundq of the formula (IV) are new. They are
prepared by the process described under (6).
0 Compounds of the formula (IV) are preferably employed in
h X R1 R2 R3 R4 and R5 has the preferred mean
ings indicated for the compounds of the formula (I) and
R7 represents CN.
Hydrolysis of the compounds of the formula ~IV) is
carried out under acidic conditions. Acids used are
mineral acids such as, for example, hydrochloric acid,
hydrobromic acid, sulphuric acid and mixtures of mineral
Le A 27 916 - 17 -
23189-7270
acids and organic acids such as, for e~ample acetic acid
or propionic acid.
The reaction is carried out at temperatures between 80
and 120C. It is carried out under normal pressure.
The compounds of the formula (IV) are dissolved in 10-30
times the volume of the acid or of the acid mixture and
the mixture is heated until hydrolysis is complete.
If ethyl N-[[[cyano(3,5-dichloro-(4'-trifluoromethyl-
phenyl)phenoxy)-hydrazinylidene]methyl]carbonyl]-carbamate
is employed as the compound of the formula V in process
6 for the prepara~ion of the compounds of the formula
(IV), the process can be described by the following
equation:
Cl CN
,~G~}N-N=c-c-N-co2c2H5
F3CS Cl o H
Cl O
~ ~ N ~
F3C Cl CN
The compounds of the formula (V) are new. They are
prepared by the process described under (8). Compounds
Le A 27 916 - 18 -
of the formula (V) are preferably employed in which Y,
X, Rl, R2, R3, R4 and R5 have the preferred meanings
indicated for the compounds of the formula (I), R8
represents C14-alkyl, in particular methyl or ethyl and
phenyl, and R7 represents CN.
Individual compounds of the formula (V) which may be
menti.oned are
F3CX~ R4 N-N=C-CONHCOO-ethyl
X Y R4
Q 3,5-C12
0 3,S-C12
O S 3,5-C12
3,S-C12
O CHCN 3,S-C12
S CHCN 3,S-C12
Process 6) is carried out by heating a compound of the
formula (V), if appropriate in the presence of a solvent
and of a base.
Solvents and bases used are the solvents and bases
mentioned for the preparation of the compounds I. Further
particularly preferred organic solvents employed are
Le A 27 916 - 19 -
23183-7270
alcohols such as, for example, ethanol or organic acids
such as, for example, glacial acetic acid.
Particularly preferred bases are the hydroxides and
acetates of the alkali metals or alkaline earth metals
S such as, for example, NaOH or sodium acetate and potas-
sium aceta e.
The reaction is carried out under normal pressure at
temperatures between 70 and 150C, preferably between 70
and 100C.
~he base used is employed in a 10-80 % molar excess. The
reaction mixture is preferably acidified using a dilute
mineral acid such as, for example, hydrochloric acid
after cyclisation is complete and the product obtained aæ
a solid i8 filtered off.
The compounds of the formula V employed in process 6)
are new. They are prepared by the process described under
(8).
If 4-(4'-trifluoromethylthiophenyl)oxy-3,5-dichloroaniline
is employed as the compound of the formula (VI) and ethyl
cyanoacetylurethane is employed as the compound of the
formula (VII) in process 8) for the preparation of the
compound3 of the formula (V), the process can be des-
cribed by the following equation:
Le A 27 916 - 20 -
F3CS~ NH2 IF3CS~ >~ r~r ~N--N+~
C1 CH3
NCCH2-C-N-COOC2H5
CN
~3CS~
>-N-N=C-ICI-N-CO2C2H5
CH3 H O
The compounds of the formulae (VI) and ~VII) are known
or can be prepared analogously to known processes (cf.
DE-OS (German Publi~hed Specification) 2,413,722;
2,718,799; US Patent 4,005,218; Le A 26 382).
The process is carried out by reacting an aniline of the
formula (VI) with NaNO2 and conc. mineral acid such as,
for example, HCl, if appropriate in the presence of a
diluent.
Diluents used are the wa~er-miscible diluents such as
alcohols, for example methanol, ethanol, glycol ether.
such as monomethylglycol ether, nitrile~ such as aceto-
nitrile, dimethyl sulphoxide, organic acids such as, for
example, glacial acetic acid, formic acid or propionic
acid, or mixtures of organic acids, preferably a mixture
Le A 27 916 - 21 -
of glacial acetic acid and propionic acid.
The diazonium salt generated in this way is reacted in
situ with a compound of the formula (VII) such as, for
example, malonyldiurethane or cyanoacetylurethane in the
presence of a base. Bases used are hydroxides and car-
bonates of the alkali metals and alkaline earth metals
and acetates of sodium, potassium and ammonium.
Organic bases such as pyridine or triethylamine can
additionally be used.
Diazotisation is carried out at normal pressure and at
temperatures between 0C and 10C. The addition of the
compounds of the formula (VII) is carried out at 0 to
20C. Aniline and nitrite are reacted in equimolar
amounts in an excess of acid which is preferably
2-3 time~ the molar amount. The CH-acid compound is added
in a 0 to 30 % molar excess, preferably a 10% excess. The
base is added in a 1.5-2.5 times molar excess. Advant-
ageously, a reverse addition can also be carried out,
i.e. the diazonium salt of the compound of the formula
(VI) generated by diazotisation is added dropwise at 0
to 10C to a mixture of a compound of the formula (VII)
and the respective solvent or solvent mixture.
The coupling product of the diazonium salt and CH-acid
compound is insoluble in the reaction medium and can be
isolated as a solid.
Le A 27 916 - 22 -
The process can also be carried out such that compounds
of the formula (VI) are formed directly without isolation
of the compound of the formula (V). To do this, the
diazotisation of the anilines of the formula (VI) and the
reaction with the urethanes of the formula (VII) is
carried out in a diluent suitable for the cyclisation.
The reaction mixture is heated after diazotisation and
coupling have been carried out and the triazinedione of
the formula (IV) is then isolated.
Diluents which may be mentioned are: alcohols such as
methanol and ethanol.
j For cyclisation, the reaction mixture is heated to about
80 to 120C, preferably about 80 to 100C.
Working-up i~ carried out as indicated further above for
process (6) for the preparation of the compounds of the
formula (IV).
The active compounds are suitable for combating parasitic
protozoa which are encountered in the keeping and raising
of animals with productive, breeding, zoo, laboratory,
experimental and pet animals, and have favourable toxi-
city to warm-blooded animals. They are active against all
or individual stages of development of the pests and
against resistant and normally sen~itive strains. By
` combating the parasitic protozoa, disease, cases of death
and yield reductions (for example in the production of
meat, milk, wool, hides, eggs, honey etc.) should be
:
Le A 27 916 - 23 -
.
reduced so that more economical and simpler keeping of
animals is possible through the use of the active com-
pounds.
The parasitic protozoa includes
Mastigophora (Flagellata) such as, for example, Trypano-
somatidae, for example Trypanosoma b. brucei, T.b.
gambiense, T.b. rhodesiense, T. congolense, T. cru~i, T.
evansi, T. equinum, T. lewisi, T. percae, T. simiae, T.
vivax, Leishmania brasiliensis, L. donovani, L. tropica,
such as, for example, Trichomonadidae, for example
Giardia lamblia and G. canis.
Sarcomastigophora (Rhizopoda) such as Entamoebidae, for
example Entamoeba histolytica, Hartmanellidae, for
example Acanthamoeba sp. and Hartmanella sp.
Apicomplexa (Sporozoa) such as Eimeridae, for example
Eimeria acervulina, E. adenoides, E. alabahmensis, E.
anatis, E. anseris, E. arloingi, E. ashata, E.
auburnensis, E. bovis, E. brunetti, E. canis, E. chin-
chillae, E. clupearum, E. columbae, E. contorta, E.
crandalis~ E. dabliecki, E. dispersa, E. ellipsoidales,
E. falciformis, E. faurei, E. flavescens, E. gallopavo-
nis, E. hagani, E. intestinalis, E. iroquoina, E. irresi-
dua, E. labbeana, E. leucarti, E. magna, E. maxima, E.
media, E. meleagridis, E. meleagrimitis, E. mitis, E.
necatrix, E. ninakohlyakimovae, E. ovis, E. parva, E.
pavonis, E. perforans, E. phasani, E~ piriformis, E.
Le A 27 916 - 24 -
praecox, E. residua, E. scabra" E. spec., E. stiedai, E.
suis, E. tenella, E. truncatal~ E. truttae, E. zuernii,
Globidium spec., Isospora belli, I. canis, I. felis, I.
ohioensis, I.rivolta, I. spec., I. suis, Cystisospora
spec., Cryptosporidium spec. such as Toxoplasmadidae, for
example Toxoplasma gondii, such as Sarcocystidae, for
example Sarcocystis bovicanis, S. bovihominis, ~. ovi-
canis, S. ovifelis, S. spec., S. suihominis such as
Leucozoidae, for example Leucozytozoon simondi, such as
Plasmodiidae, for example Plasmodium berghei, P. falci-
parum, P. malariae, P. ovale, P. vivax, P. spec., such as
Piroplasmea, for example Babesia argentina, B. bovis, B.
canis, B. spec., Theileria parva, Theileria spec., such
as Adeleina, for example Hepatozoon canis, H. spec.
In addition Myxospora and Microspora, for example Glugea
spec. and Nosema spec.
In addition Pneumocystis carinii and Ciliophora (Ciliata)
such as, for example, Balantidium coli, Ichthiophthirius
spec., Trichodina spec. and Epistylis spec.
The compounds according to the invention are also active
against protozoa which occur as parasites in insects.
Those which may be mentioned are parasites of the Micro-
sporida strain, in particular the genus Nosema. Nosema
apis in the honey bee may be particularly mentioned.
The productive and breeding animals include mammals such
as, for example, cattle, horses, sheep, pigs, goats,
Le A 2~ 916 - 25 -
camels, water buffalo, donkeys, rabbits, fallow deer,
reindeer, animals having a valuable coat such as, for
example, mink, chinchilla, racoons, birds such as, for
example, hens, geese, turkeys, ducks, doves, and species
of bird for keeping at home and in the zoo. In addition,
productive and ornamental fish are included.
The laboratory and experimental animals include mice,
rats, guinea pigs, golden hamsters, dogs and cats.
The pet animals include dogs and cats.
The fish include productive, breeding, aquarium and
ornamental fish of all ages which live in fresh and salt
water. The productive and breeding fish include, for
example, carp, eel, trout, whitefish, salmon, bream,
roach, rudd, chub, sole, plaice, halibut, Japanese
yellowtail (Seriola quinqueradiata), Japanese eel
(Anguilla japonica), red seabream (Pagurus major), sea
bass (Dicentrarchus labrax), grey mullet (Mugilus
cephalus), pompano, gilthread seabream (Sparus auratus),
Tilapia spp., chichlidae specie~ such as, for example,
Plagioscion or Channel catfish. The agents according to
the invention are particularly suitable for the treatment
of fry, for example carp of 2-4 cm body length. The
agents are also very highly suitab~e in the feeding of
eels.
Administration can be carried out both prophylactically
and therapeutically.
Le A 27 ~16 - 26 -
The administration of the active compounds is carried out
directly or enterally, parenterally, dermally or nasally
in the form of suitable preparations.
Enteral administra~ion of the active compounds is carried
out, for example, orally in the form of powders, sup-
positories, tablets, capsules, pastes, drinks, granules,
drenches, boli, medicated feed or drinking water. Dexmal
administration is carried out, for example, in the form
of dipping, spraying, bathing, washing, pouring-on and
spotting-on and powdering. Parenteral administration is
carried out, for example, in the form of injection
(intramuscular, subcutaneous, intravenous,
intraperitoneal) or by implants.
Suitable preparations are:
solutions such as injection solutions, oral solutions,
concentrates for oral administration after dilution,
solutions for use on the skin or in body cavities~
pouring-on formulations and gels;
emulsions and suspensions for oral or dermal administra-
tion and also for in~ection; semi-solid preparations;
formulations in which the active compound is processed in
an ointment base or in an oil-in-water or water-in-oil
emulsion base;
solid preparations such as powders, premixes or concen-
trates, granule~, pellets, tablets, boli, capsules;
aerosols and inhalants, and moulded articles containing
active compound.
Le A 27 ~16 - 27 -
Injection solutions are administered intravenously,
intramuscularly and subcutaneously.
Injection solutions are produced by dissolving the active
compound in a suitable solvent and, if necessary, adding
additives such as solubilisers, acids, bases, buffer
salt3, antioxidants and preservatives. The solutions are
sterile filtered and bottled.
Solvents which may b~ mentioned are: physiologically
tolerable solvents such as water, alcohols such as
ethanolj butanol, benzyl acohol, glycerol, hydrocarbons,
propylene glycol, polyethylene glycols,
N-methylpyrrolidone, and mixtures of these.
The active compounds may optionally also be dissolved in
physiologically tolerable vegetable or synthetic oils
which are suitable for injection.
Solubilisers which may be mentioned are: solvents which
promote the solution of the active compound in the main
solvent or prevent its precipitation. Examples are
polyvinylpyrrolidone, polyoxyethylated castor oil and
polyoxyethylated sorbitan esters.
Preservatives are: benzyl alcohol, trichlorobutanol,
p-hydroxybenzoic acid esters and n-butanol.
Oral solutions are administered directly. Concentrates
are administered orally after previously diluting to the
Le A 27 916 - 28 -
administration concentration. Oral solutions and con-
centrates are prepared as described above for the injec-
tion solutions, it being possible to dispense with
sterile working.
Solutions for use on the skin are poured on dropwise,
spread on, rubbed in, sprinkled on, sprayed on or applied
by dipping, bathing or washing. These solutions are
prepared as described above for the injection solutions.
It may be advantageous to add thickeners during the
preparation. Thickeners are: inorganic thickeners such
as bentonites, colloidal silica, aluminium monostearate,
organic thickeners such as cellulose derivatives,
polyvinyl alcohols and their copolymers, acrylates and
metacrylates.
Gels are applied to or spread on the skin or introduced
into body cavities. Gels are prepared by adding such a
quantity of thickener to solutions which have been
prepared as described for the injection solutions that a
clear composition having an ointment-like consistency
results. The thickeners indicated above are employed as
thickeners.
Pouring-on formulations are poured onto or ~prinkled onto
limited areas of the skin, whereupon the active compound
either penetrates the skin and acts systemically or is
di~tributed on the body surface.
Le A 27 916 - 29 -
Pouring-on formulations are prepared by dissolving,
suspending or emulsifying the active compound in suitable
skin-compatible solvents or solvent mixtures. If appro-
priate, further auxiliaries such as colorantR, absorp-
tion-promoting substances, antioxidants, light screens
and adhesives are added.
Solvents which may be mentioned are: water, alkanols,
glycols, polyethylene glycols, polypropylene glycols,
glycerol, aromatic alcohols such as benzyl alcohol,
phenylethanol, phenoxyethanol, esters such as ethyl
ace~ate, butyl acetate, benzyl benzoate, ether6 such as
alkylene glycol alkyl ethers such as dipropylene glycol
monomethyl ether, diethylene glycol mono-butyl ether,
ketones such as acetone, methyl ethyl ketone, aromatic
and/or aliphatic hydrocarbons, vegetable or synthetic
oils, DMF, dimethylacetamide, N-methylpyrrolidone and
2-dimethyl-4-oxy-methylene-1,3-dioxolane.
Colorants are all colorants which may be dissolved or
suspended and which are permitted for administration to
animals.
Absorption-promoting substances are, for example, DMSO,
spreading oils such as isopropyl myristate, dipropylene
glycol pelargonate, silicone oils, fatty acid esters,
triglycerides and fatty alcohols.
Antioxidants are sulphites or metabisulphites such
as potassium metabisulphite, ascorbic acid,
Le A 27 916 - 30 -
butylhydroxytoluene, butylhyd:roxyanisole and tocopherol.
Light screens are, for example, substances of the benzo-
phenone class or novantisolic acid.
Adhesives are, for example, cellulose derivatives, starch
derivatives, polyacrylates, and natural polymers such as
alginates and gelatin.
Emulsions may be administered orally, dermally or as
injections.
Emulsions are either of the water-in-oil type or the
oil-in-water type.
They are prepared by dissolving the active compound
either in the hydrophobic or the hydrophilic phase and
homogenising this with the solvent of the other phase
with the aid of suitable emulsifiers and, if appropriate,
further auxiliaries such as colorants, absorption-
promoting substances, preservatives, antioxidants, light
screens and viscosity-increasing substance
Hydrophobic phases (oils) which may be mentioned are:
paraffin oils, silicone oils, natural vegetable oils such
as sesame oil, almond oil, castor oil, synthetic tri-
glycerides such as caprylic/capric acid biglyceride,
triglyceride mixture with vegetable fatty acids of chain
l~ngth C~l2 or other specially selected natural fatty
acids, partial glyceride mixtures of saturated or
:
Le A 27 916 - 31 -
~'
unsaturated fatty acids possibly also containing hydroxyl
groups, and mono- and diglyce:rides of C8/C10-fatty acids.
Fatty acid esters such as ethyl stearate, di-n-butyryl
adipate, hexyl lauroate, dipropylene glycol pelargonate,
esters of a branched fatty acid of medium chain length
containing saturated fatty alcohols of chain lenqth
C~6-Cl3, isopropyl myristate, isopropyl palmitate,
caprylicJcapric acid esters of ~aturated fatty alcohols
of chain length C~2-C1a, isopropyl stearate, oleyl oleate,
decyl oleate, ethyl oleate, ethyl lactate, waxy fatty
acid esters ~uch as dibutyl phthalate, diisopropyl
adipate, ester mixtures related to the latter, inter alia
fatty alcohols such as isotridecyl alcohol, 2-octyl-
dodecanol, cetyl stearyl alcohol and oleyl alcohol.
Fatty acids such as, for example, oleic acid and its
mixtures.
Hydrophilic phases which may be mentioned are:
water, alcohols such as, for example, propylene glycol,
glycerol, sorbitol and their mixtures.
Emulsifiers which may be mentioned are: non-ionic surfac-
tants, for example polyoxyethylated castor oil, polyoxy-
ethylated sorbitan monooleate, sorbitan monostearate,
glycerol monostearate, polyoxyethyl stearate and alkyl-
phenol polyglycol ethers;
: 25 ampholytic surfactants such as di-Na N-lauryl-~-imino-
~ dipropionate or lecithin;
;'
: Le A 27 916 - 32 -
anionic surfactants, such as Na lauryl sulphate, fatty
alcohol ether sulphates, mono/dialkyl polyglycol ether
orthophosphoric acid ester monoethanolamine salt;
cationic sur~actants such as cetyltrimethylammonium
S chloride.
Other auxiliaries which may be mentioned are: substances
increasing viscosity and ~tabilising the emulsion such as
carboxymethylcellulose, methylcellulose and other cel-
lulose and starch derivatives, polyacrylates, alginates,
gelatin, gum arabic, polyvinylpyrrolidone, polyvinyl
alcohol, copolymers of methyl vinyl ether and maleic
anhydride, polyethylene glycols, waxes, colloidal silica
or mixtures of the substances mentioned.
Suspensions may be administered orally, dermally or as
an in~ection. They are prepared by suspending the active
compound in an excipient liquid, if appropriate with the
addition of other auxiliaries such as wetting agents,
colorants, absorption-promoting substances, preserva-
tives, antioxidants light screens.
Excipient liquids which may be mentioned are all homo-
geneous solvents and solvent mixtures.
Wetting agents (dispersants) which may be mentioned are
the surfactants indicated above.
Le A 27 916 - 33 -
Other auxiliaries which may be mentioned are ~hose
indicated above.
Semi~solid preparations can be administered orally or
dermally. They differ from the suspensions and emulsions
described above only by their higher viscosity~
In order to prepare solid preparations, the active
compound is mixed with suitable excipients, if appropri-
ate with the addition of auxiliaries, and brought into
the desired form.
Excipients which may be mentioned are all physiologically
tolerable solid inert substances. Those used are inor-
ganic and organic substances. Inorganic substances are,
for example, sodium chloride, carbonates such as calcium
carbonate, hydrogen carbonates, aluminium oxides, silicic
acids, aluminas, precipitated or colloidal silica and
phosphates.
Organic substances are, for example, sugars, cellulose,
foodstuffs and feeds such as milk powder, animal meal,
cereal meal and shreds, and starches.
Auxiliaries are preservatives, antioxidants and colorants
which have already been mentioned above.
Other suitable auxiliaries are lubricants and glidants
~uch as, for example, magnesiu~ stearate, stearic acid,
talc, bentonites, disintegration-promoting substances
Le ~ 27 916 - 34 -
such as starch or crosslinked polyvinylpyrrolidone,
binders such as, for example, starch, gelatin or linear
polyvinylpyrrolidone and dry binders such as microcrys-
talline cellulose.
The active compounds may also be present in the prepara-
tions as a mixture with synergists or with other active
compounds.
Ready-to-use preparations contain the active compound in
concentrations of 10 ppm - 20 per cent by weight, prefer-
lQ ably from 0.1 - 10 per cent by weight.
Preparations which are diluted before use contain the
active compound in concentrations of 0.5 - 90 per cent
by weight, preferably from 1 to 5~ per cent by weight.
In general, it has proved advantageous to administer
amounts of about 0.5 to about 50 mg, preferably 1 to
20 mg, of active compound per kg of body weight per day
to attain effective results.
The active compounds can also be administered together
with the feed or drinking water of the animals.
Feeds and foodstuffs contain 0.01 to 100 ppm, prefexably
0.5 to 50 ppm of the active compound in combination with
a suitable edible material.
Such a feed or foodstuff can be used both for healing
Le A 27 916 - 35 -
purposes and for prophylactic purposes.
The preparation of such a feed or foodstuff is carried
out by mixing a concentrate or a premix which contains
0.5 to 30 %, preferably 1 to 20 % by weight, of an active
S compound in a mixture with an edible organic or inorganic
excipient with customary feeds. Edible excipients are,
for example, maize flour or maize and soya bean flour or
mineral salts which preferably contain a ~mall amount of
an edible dust-preventing oil, for example maize oil or
soya oil. The premix obtained in this way can then be
added to the complete feed before feeding it to the
animals.
Use in coccidiosis may be mentioned as an example:
For the curing and prophylaxis, for example, of cocci-
diosis in poultry, in particular in hen~, ducks, geeseand turkeys, 0.1 to 100 ppm, preferably 0.5 to 100 ppm,
of an active compound are mixed with a suitable edible
material, for example a nutritious feed. If desired,
these amounts can be increased, particularly if the
active compound is well tolerated by the recipient.
Administration can correspondingly be carried out via the
drinking water.
For the treatment of individual animals, for example in
the case of the treatment of coccidiosis in mammals or
toxoplasmo6is, preferably amounts of active compound of
0.5 to 100 mg/kg of body weight are administered daily
in order to obtain the desired results. In spite of this
Le A 27 916 - 36 -
it may be periodically necessary to deviate from the
amounts mentioned, in particular depending on the body
weight of the test animal or the type of administration
method, but also on account of the type of animal and its
individual reaction to the active compound or the manner
of formulation and the time or the interval at which it
is administered. Thus, in certain cases it may be
- sufficient to manage with less than the minimum amount
previously menti.oned, whereas in other cases the upper
lLmit mentioned must be exceeded. With the administration
of larger amounts, i~ may be expedient to divide these
into a number of individual administrations over the
course of the day.
The compounds according to the invention are moreover
active against various fish parasites which belong to the
helminths (worms).
The fish parasites include from the sub-kingdom of the
protozoa, species of the Ciliata strain, for example
Ichthyophthirius multifiliis, Chilodonella cyprini,
Trichodina spp., Glossatella spp., Epistylis 5pp. of the
Myxosporidia strain, for example Myxosoma cerebralis,
Myxidium spp., Myxobolus spp., Heneguya spp., Hoferellus
spp., the Microsporidia class, for example Glugea spp.,
` Thelohania spp., Pleistophora spp., from the plat hel-
:~ 25 minths strain: trematodes; Monogenea, for example Dactyl-
` ogyru~ 8pp., Gyrodactylus spp., Pseudodactylogyrus spp.,
- Diplozoon ~pp., cestodes, for example from the groups of
the Caryphyllidea (for example Caryophyllaeus laticeps),
~.
~,
Le A 27 916 - 37 -
:,
Pseudophyllidea (for example Diphyllobothrium spp.),
Tetraphyllidea (for example Phyllobothrium spp. ) and
Protocephalida (for example species of the genus Proteo-
cephalus) and from the strain of the Arthropoda, various
parasitic crustaceae, in particular from the sub-classes
of the Branchiura (fish-lice) and Copepoda (copepods) and
the orders of the Isopoda (isopods) and Amphipoda
(amphipods).
The treatment of the fish is carried out either orally,
for example via the feed or by short-term treatment,
"medicinal bath", into which the fish are put and in
which they are kept for some time (minutes up to a number
of hours), for example when transferring from one breed-
ing pond to the other.
However, temporary or permanent treatment of the living
space of the fish (for example entire pool units,
aquaria, tanks or ponds), in which the fish are kept, can
also be carried out.
The active compound is administered in preparations which
are suited to the applications.
The concentration of the active compound in the prepara-
tions i8 1 ppm to 10 ~ by weight.
Preferred preparations for short-term treatment in the
course of uRe as a "medicinal bath", for example in the
treatment when transferring the fish or for the treatment
Le A 27 916 - 38 -
of the living space (pool treatment) of the fish, are
solutions of the active compound in one or more polar
solvents which give an alkaline reaction on diluting with
water.
For the preparation of these solutions, the active
compound is dissolved in a polar, water-soluble solvent
which either gives an alkaline reaction or to which is
added an alkaline water-soluble substance. The latter is
advantageously also dissolved in the solvent, bu~. can
also be suspended in the solven~ and only dissolve in the
water. After addition of the active compound solution,
the water should have a pH of 7-10, but preferably a pH
of 8-10.
The concentration of the active compound can be in the
range from 0.5 - 50 %, but preferably in a range from
1 25 %.
Suitable solvents are all water-soluble solvents in which
the active compound is soluble at a sufficient concentra-
tion and which are physiologically acceptable.
These are ethyl alcohol, isopropyl alcohol, benzyl
alcohol, glycerol, propylene glycol, polyethylene
glycols, poly(oxoethylene)-poly(oxypropylene) polymexs,
basic alcohols such as mono-, di- and triethanolamine,
ketones such as acetone or methyl ethyl ketone, esters
such as ethyl lactate, in addition N-methylpyrrolidone,
dimethylacetamide, dimethylformamide, and in addition
.~
Le A 27 916 - 39 -
'
dispersants and emulsifiers such as polyoxyethylated
castor oil, polyethylene glycol sorbitan monooleate,
polyethylene glycol stearate or polyethylene glycol
ethers and polyethylene glycol alkylamines.
Bases which may be mentioned for adjusting the alkaline
pH are organic bases such as basic amino acids such as
L-or D,L-arginine, L- or D,L-lysine, methylglucosamine,
glucosamine, 2-amino-2-hydroxymethylpropane-1,3-diol and
in addition such as N,N,N',N'-tetrakis-(2-hydroxypropyl)-
ethylenediamine or polyether tetrol based on ethylene-
diamine (~.W. ~80-420), inorganic bases, such as ammonia
or sodium carbonate - if appropriate with the addition of
water.
The prepara~ions may also contain 0.1 to 20 % by weight,
preferably 0.1 - 10 % by weight, of other formulation
auxiliaries, such as antioxidants, surfactants, suspen-
sion stabilisers and thickeners such as, for example,
methylcellulose, alginates, polysaccharides, galacto-
mannans and colloidal silicic acid. The addition of
colorants, flavouring and builders for animal nutrition
is also possible. Even acids which, together with the
base initially introduced, form a buffer ~ystem or reduce
the pH of the solution, can be mentioned here.
The concentration of the active compound during use
depends on the type and duration of the treatment, and
the age and condition of the treated fish. It is, for
example, for a short-term treatment, 2-50 mg of active
Le A 27 916 - 40 -
compound per litre of water, preferably 5-10 mg per
litre, for a treatment period of 3-4 hours. For the
treatment of young carp, for example, a concentration of
5-10 mg/l and a treatment per:iod of about 1-4 hours are
used.
Eels are treated using concentrations of about 5 mg/l for
about 4 hours.
For a relatively long treatment period or for continuous
treatment, the concentration can be chosen to be cor-
respondingly lower.
For pool treatments, 0.1 - 5 mg of active compound per
litre of water can be used.
Preparations for use as a food additive are, for example,
composed as follows:5 a) Active compound 1 - 10 parts by weightof the formula I
Soya bean protein 49 - 90 parts by weight
b) Active compound 0.5 - 10 parts by weight
of the formula I
Benzyl alcohol 0.08 - 1.4 parts by weight
Hydroxypropyl- 0 - 3.5 parts by weight
methyl cellulose
Water remainder to 100
Preparations for use in "medicinal baths" and for pool
treatment are, for example, composed and prepared as
Le A 27 916
~ollows.
c) 2.5 g of active compound of the formula (I) are
dissolved in 100 ml of triethanolamine
with warming.
d) 2.5 g of active compound of the formula (I)
12.5 g of lactic acid are dissolved in 100 ml of
triethanol ~mine with warming and
stirring.
e) lO.0 g of active compound of the formula (I) is
dissolved in lO0 ml of monoethanolamine.
f) Active compound of the formula I 5.0 g
Propylene glycol 50.0 g
Sodium carbonate 5.0 g
Water to 100 ml
g) Active compound of the formula I 5.0 g
Monoethanolamine lO g
N-Methylpyrrolidone to 100 ml
h) Active compound of the formula I 2.5 g
Sodium carbonate 5.0 g
Polyethylene glycol200 to 100 ml
The active compound i8 dissolved in polyethylene glycol
with werring and sodiuI carbonete is suspended therein.
''
,
Le A 27 916 - 42 -
;~ .
Example A
Coccidiosis in hens
9 to 11 day-old chicks were infected with
40,000 sporulated oocysts of strongly virulent strains
of ~iveria acervulina, E. maxima and E. tenella, the
disease pathogens of intestinal coccidiosis.
From 3 days before infection until 8 days after infection
(end of the experiment), active compound was administered
mixed in the food of the animals in the concentration
indicated.
The number of oocysts in the faeces was determined with
the aid of the McMaster chamber (see Engelbrecht and co-
workers 'IParasitologische Arbeitsmehoden in Medizin und
Veterinarmedizin" (Parasitological Working Methods in
Medicine and Veterinary Medicine), p. 172, Akademie-
Verlag, Berlin (1965)).
Those doses are regarded as effective which prevent the
excretion of oocysts and/or clinical symptoms of cocci-
diosis including mortality completely or to a great
extent. The effective doses are indicated in the follow-
ing table:
Le A 27 916 - 43 -
o ~
~ 0
x a
~ s 0 ~ -
~ ~ o
o ~
o ~
~a ~ O
0
u~ h ~
al a O h
o a) ~ In o o
S ~ o o
~t
~ .,~ O ~ O
,C ~ t.) h
dP ~ ~
O ~ O
~1 IQ r~ U
I
O r~
.,~ ~ I ~
0 ~ O
h ~;
o a) ~
X o S ~1 o o o
al ~ O
.rl O ~ O
h
~ dP ~ ~
g ~ O a~ O
O -~ LQ ~ O
G)
0
h
51 0 tl~ N O O
a ~ ~
tQ
o ~ O
~: Q
.,
O
_, ,1 a) ~ a~ --
~ ~i 0 ~ O
a~ ~ h
0
0 O X O ~ ~: O ~D
E~ ~ ~ æ
Le A 27 916 - 44 -
i
23189-7270
Preparation Examples
I Example of process 2a)
Example 1
2-[4-(4-Trifluoromethylthiophenyl)-3,5-dichlorophenoxy]-
N-methyl-3,5-(2H,4H)-dioxo-1,2,4-triazine
H
F3CS~\ c ' 0~ 2 ) CH3 I
,,~
:
F3CS
Cl
3.1 g (7 mmol) of a~auracil are diRsolved in 20 ml of
absolute DNSO and 0.16 g (6 mmol) of sodium hydride is
added. The mixture i8 stirred at ~T for 20 min and l.S g
(9 mmol) of methyl iodide in 5 ml of DMSO are then added
under argon. The reduction mixture i~ warmed to 50C and
kept at thi temperature for 3 h. It is subsequently
concentrated in vacuo and water i8 then added. After
filtering off the precipitated ~olid with suction, 2.3 g
(72% of theory) of the N-methyl compound are obtained.
:,
~,~
Le A 27 916 - 45 -
'"
:
23189-7270
II Example of process 2b)
Example 2
2-~4~4'-Trifluoromethylthiophenyl~-3,5-dichlorophenoxy]-
1,2,4-triazine-3,5(2H,4H)dione
Cl O H
F3CS~=D HSCHzCOzH
C 1 COzH
Cl O H
F3CS } N~=O
14.8 g (0.03 mol) of carboxylic acid are heated ~o 170C
in 20 ml of mercaptoacetic acid. After 1.5 h, the mixture
is allowed to cool, water is added and after filtering
off 11.5 g ~85~ of theory) of decarboxylated product are
obtained.
The following compounds are prepared analogously
CN Cl O H
F~CX~--~NN~
H Cl
Ex. 3 X = O m.p. 110C
Ex. 4 ~ = S m.p. 191C
Le A 27 916 - 46 -
23189-7270
II Example of process 2c)
Example 4a
10 q (23 mmol) of 2-[4-(4'-trifluoromethylthiophenyl)-3~5
dichloro-phenoxy]-1,~,4-triazine-3,5(2H, 4H)dione are
stirred under reflux for 1.5 h with 10 g of zinc in
100 ml of glacial acetic acid.
The solid is then filtered off hot with suction and the
residue is boiled twice with DMF. The filtrates are
concentrated stirred with water and the precipitated
solid is filtered off with suction. 7.8 g (78~ of theory)
of the dihydro compound are thus obtained as a colourless
i~ solid.
.
The following are prepared analogously
H
~N
F3C~ N~
Cl
Ex. No. X Y m.p.
4b O CHCN
4c S CHCN
4d S O
.,
Le A 27 916 - 47 -
;~`
23189-7270
III Example of process 4
Example S
2-[4-(4'-Trifluoromethylthiophenyl)-3-chlorophenoxy]-3,5-
(2H,4H)-dioxo-1,2,4-triazine-6-carboxylic acid
o H
F3CS~ ~=0 HC 1 / HOAc
Cl CN
o H
F3CS~3--1 S3N ~
C 1 C02H
8.5 g (0.02 mol) of cyanoazauracil are boiled in 50 ml
of glacial acetic acid and 50 ml of conc. HCl for
4 hours. The mixture is subsequently cooled and diluted
with water. The precipitated solid is filtered off with
suction and dried. 6.6 g (74~) of the carboxylic acid are
thus obtained.
The following compounds are prepared analogously
Cl o H
F3C-X{~Y ~N--~
C 1 C02H
Le A 27 916 - 48 -
23189-7270
Ex. 6 X = O Y - CHCN
Ex. 7 X = S Y = CHCN
IV Example of process 6
Example 8
2-[4-(4'-Trifluoromethylthiophenyl)-3-chlorophenoxy]-3,5-
(2H,4H)-dioxo-6-cyano-1,2,4-triazine
Cl CN H
/~\ ~ I ¦ NaOCOCH3
F3CS ~;=~H C ll_N_C02Et. HOCOCH3
O H
F3CS{~)~N~
Cl CN
lS g (0.029 mol) of the hydrazonocyanourethane and 3.3 g
(0.44 mol) of sodium acetate are heated under reflux in
50 ml of glacial acetic acid for 2 h. The mixture i9
subsequently cooled and concentrated in vacuo. The
residue is ctirred with water and the precipitate deposi-
ted i~ filtered off with suction. lO.S g (83~ of theory)
of cyanoazauracil are thus obtained after drying.
,~
lS The following compounds are prepared analogou~ly
Le A 27 916 - 49 -
23189-7270
Cl O H
F 3 CX~ C 1 NN--
Ex. 9 X = 0 Y = CHCN
Ex.10 x = S Y = CHCN
.~
V Example of process 8
`~ Example 11
Ethyl N-[[[cyano(4-(4'-trifluoromethylthiophenyl)-3,5-
dichlorophenoxy)-hydrazinylidene]-methyl]-carbonyl]-carbamate
3CS Cl - o
1 . HC l / NaN02
NH2 ~ NCH2C-C-N-CO2E~
>'=/ H 2. NaOCOCH~
Cl
`:
F3CS~}N-N=C-C-N-C02Et.
Cl H O H
15.8 g (0.045 mol) of aniline are dissolved in 10 ml of
conc. HCl and 100 ml of ethanol and a solution of 3.2 g
(0.045 mol) of sodium nitrite in 30 ml of water is added
dropwise at 0-5C. The mixture is ~ubsequently stirred
until the solution i8 clear, then a mixture of 7.1 g
(0.045 mol) of cyanoacetylurethane and 11 g (0.13 mol) of
.
.
. .
Le A 27 916 - 50 -
'`"
"
.::
,
~.
.... .
:,:
:
'~
sDdium acetate is added and the mixture is subsequently
stirred at 10C for 3 h. The reaction mixture is
concentrated in vacuo, stirred with water and the solid
is filtered off with suction, 19 g (82%) of product are
thus obtained as a finely crystalline yellow powder.
Example of process 8
Example 12
CN 1) HCl/NaNO2
F3Cs~3C{~NHz 2) NaOCOCH3/NCCH2CONNC02C2H5
H C1
CN CN
F3Cs ~ ~ 3 N-N=C-C-N-Co2Et.
H Cl H O H
8.9 g (0.026 mol) of aniline are di~solved in 5.6 ml of
conc. HCl and a mixture of 50 ml of glacial acetic acid
and 50 ml of propionic acid and 1.8 g (0.026 mol) of
sodium nitrite are added dropwise at 0C in 5 ml of
water. The mixture is subsequently stirred for 30 min and
the diazonium salt solution thus prepared is added
dropwise to a mixture, cooled to 0C, of 4 g (0.026 mol)
of cyanoacetylurethane and 5.3 g (00065 mol) of sodium
acetate in 40 ml of glacial acetic acid and 40 ml of
propionic acid ans the mixture is subsequently stirred at
10C for 3 hours. The reaction mixture is concentrated in
Le A 27 916 - Sl -
vacuo, water is added and the solid is filtered off with
suction. 3 g (76~) of the hydrazinyl compound are thus
obtained as a yellow solid.
The following compounds are prepared analogously
CN Cl CN
S F3CX~7-N=C-C-N-C02Et-
H Cl H o H
Ex. 13 X = O
Ex. 14 X = S
Le A 27 916 - 52 -