Note: Descriptions are shown in the official language in which they were submitted.
2~7~
The present invention relates to a one time use Teflon~M
injection needle set, and is aimed to smooth the transfusion
of blood, and injections and to cut off intrusion of bacteria
into the cylindrical rods of such a set, in the course of the
S transfusion, by sealing the rod and the cylinder with a thin
polyethylene shield.
TeflonTM vein needle sets made of metal currently in use
are pricked into a blood vessel after which the metal needle
of same is removed and the injector connected again. The
TeflonTM vein injection needle in use is used in the following
manner: 1) The TeflonTM vein injection tube is pricked into
the blood vessel by using a metal needle and then the metal
needle is removed; 2) The transfusion tube of a blood
transfu~ion set, an injection set or an injector is connected
to the upper portion of the stopper; and 3) Transfusion of
blood or an injection is made by the injector.
Since thi~ injection is made by the use of a metal
needle, and the needle is to be removed immediately after the
injection and connected to the blood or injection transfusion
tube or to the injector, the injection has deficiencies of:
1) Leaking of blood or injection during the connection and
injection; 2) Connection not only takes much time and requires
that many details be attended to, it require~ an additional
replacement Teflon~M tube when the injection tube under
2~ i 71~
injection in one blood vessel is moved to another since the
metal needle is removed.
In particular, to inject into another blood vessel, the
TeflonTM tube has to be replaced by a new one after the blood
or injection transfusion tube is disconnected, and the metal
needle must be immediately removed after the replacement
needle is pricked into the blood vessel and the tube must be
connected to the injector. Therefore, these processes cause
a grave deficiency of allowing bacterial infection.
The present invention is designed to provide a one time
use TeflonTM vein injection tube set that allows the speedy
and smooth transfusion of blood or injection, and is also
aimed to keep the needle set free from bacterial infection,
by sealing its rod from the atmosphere when injection is
taking place with a thin polyethylene shield.
In a broad aspect, the present invention relates to a
device for use in effecting intra-venous injection or blood
transfusion including: a metal needle having a bore through
which blood or fluid to be injected may flow, and a sharp tip
capable of penetrating into a vein; a rod co-axial with said
needle and having a bore communicating with the bore of said
needle which extends from said rod; a cylinder encasing said
rod, having a first end from which a Teflon~M tube extends
said TeflonTM tube having a bore which snugly fits said
needle, and a second end from which the end of said rod remote
2~ 7~
from said needle extends; and a connecting socket extending
from and co-axia] with said rod and having a bore
communicating with the bore of said rod, said socket being
adapted to engage the end of a transfusion or injection tube,
wherein said rod is moveable in said cylinder from a first
position in which said metal needle protrudes slightly from
said teflon tube to a second position in which said metal
needle is withdrawn from said TeflonTM tube.
In drawings that illustrate the present invention by way
of example:
Fig. 1 is a perspective view of the device of the present
invention, showing its overall connections;
Fig. 2 is an exploded view of the device illustrated in
Fig. 1;
Fig. 3 is a longitudinal sectional view of the device of
the present invention in a compressed state;
Fig. 4 is a longitudinal sectional view of the device of
the present invention in an extended state;
Fig. 5 is a sectional view along line A-A in Fig. 3; and
Fig. 6 is a sectional view along line B-B in Fig. 4.
2~
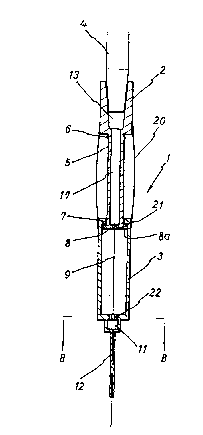
Referring now to the drawings, the transfusion tube (4)
is connected from the bottom of the injection container (15)
to the top of the TeflonTM needle assembly, and the flow
regulator (16) is installed between the two points.
The transfusion tube (4) is inserted into the connecting
socket (13) of the connector (2). Protrusions (6) and (7) at
the top and bottom of rod (5) co-axial with connector (2)
limit the up-and-down movement of the rod (5) within a
cylinder (3). Hole (8a) on round plate (8) at the bottom of
the rod (5) is aligned to a Teflon~ tube (12) and to a metal
needle tightly fitting within the tube (12). The end of the
metal needle (9) protrudes approximately 1 mm at the tip of
the tube (12) at the time of injection, as can be seen in
Fig. 3.
A polyethylene shield is sealed between the upper portion
of the cylinder (3) and the lower portion of the connector (2j
to keep the rod (5) from the atmosphere and intrusion of
bacteria. The shield (20) is made of transparent elastic
polyethylene, so the polyethylene shield (20) is transparent
and flexible 80 that rod (5) is easily moveable, and
constantly visible.
As ~hown on the Figs. 5 and 6, the passage (21) in the
round plate (8) and the passage hole (22) in cylinder (3) are
furnished and aligned to make the smooth flow of blood or
2 ~ PI ~ ~
injection from the rod bore (17) to the cavity compartment
(11~ .
This device is so designed and is used in the following
manner, and has the following advantages. Connector (2) is
depressed toward cylinder (3), thereby making the metal needle
tip protrude about 1 mm at the end of the TeflonTM tube after
the transfusion tube (4) is connected to the connecting socket
(13) of the connector (2). The metal needle (9) is pricked
into the blood vessel and the cylinder (3) held with one hand
-and the connector (2) i5 pulled upward with the other hand.
This extends the device to the shape illustrated in Fig. 4.
Then the injection in the container (15) flows into the blood
vessel through the transfu~ion tube (4), connecting socket
(13), rod bore (17), the metal needle (9), cavity compartment
(11), TeflonTM tube (12) and finally into the blood vessel.
The thinner the TeflonTM tube (12) is, the better, as it must
have some flexible tension not to hurt the blood vessel of the
patient receiving the injection. The metal needle (9) has a
vertical bore (14), and moves up and down tightly inside the
TeflonTM tube. As shown in Figs. 3 and 4, the flexible
polyethylene shield (20) is sealed to the upper part of the
cylinder (3) and to the lower part of the connector (2) to
protect the rod (5) from being infected by bacteria.
The upper protrusion (6) at the top of the rod (5)
maintains proper connections to the connector (2), and the
cylinder (3) and the lower protrusion (7) also maintains
2~ 7~ ~
proper connection to the concave groove (10) at the point that
the connector (2) is pulled upward to the maximum, and adjusts
the moving distance of the connector (2) accordingly. At this
point, the tip of the metal needle ~9~ locates at the top of
the cavity compartment (11), and makes the transfusion of
blood or injection easier, and maintains close contact with
the upper part of the cavity compartment (11) whereby reverse
flow of blood or injection in the cavity compartment (11~ into
the cylinder (3) while the transfusion process is under way
is prevented.
Since the transfusion of blood or injection made through
the metal needle (9) is small in volume and slow in its flow
speed, transfusion is also made through the passage (21) at
the bottom of the rod bore (17). A part of the blood or the
injection is collected in the cylinder (3), and is again
collected in the cavity compartment (11) after passing through
the passage hole (22). After the blood or the injection is
being collected in the compartment (11), then the transfusion
is easily made into the blood vessel through the TeflonTM tube
(12).
In the event of relocating the TeflonT~ needle assembly
set (1) from one blood vessel to another, the TeflonTM tube
( 12 ) i5 removed from one blood vessel, the connector (2) is
returned to the original position (Fig. 3). Injection with
the metal needle tip (9) is then carried out, with the tip of
the needle (9) protruded about 1 mm out from the tip of the
TeflonTM tube (12). At this point, protrusion (6) fits in the
concave groove (10).
Hole (8a) on round plate (8) keeps the air pressure
inside the cylinder (3) stable to that of the atmosphere.
The tip of the metal needle (9) must be very sharp and
the connector should have proper tension.
Since there is a frequent need to move the rod (5) to
relocate the TeflonTM tube under the transfusion from one
blood vessel to another, polyethylene shield is provided to
protect the rod (5) from being infected by bacteria.
This device, therefore, can be used for transfusion, in
a simple and speedy manner with the transfusion tube and the
injector connected and without any leaking or wasting of the
needle by following the simple procedures previously
mentioned. And also, this device can make for bacteria free
transfusion of blood or injection from one blood vessel to
another by sealing the rod that has to be moved during the
process, and provides the smooth and stable transfusion effect
to the maximum extent by means of the rod bore, passage holes
and the cavity compartment.
Furthermore, this device requires no replacing of the
transfusion tube or the injector while the transfusion is in
2~17~ ~
process, can make the transfusion by following the simple
processes and provides bacterial infection free transfusion.