Note: Descriptions are shown in the official language in which they were submitted.
AHP-9675/9675-1-N1
5~"
AMINOE~ l'cS OF RAP~Y~IN
BACKGROUND OF THE INVENTION
This invention relates to novel esters of rapamycin and a method for using them
in the treatment of transplantation rejection, host vs. graft disease, autoimmune
diseases, diseases of inflammation, and fungal infections.
Rapamycin is a macrocyclic triene antibiotic produced by Streptomvces
hvgroscopicus, which was found to have antifungal activity, particularly againstCandida albicans, both ~ vitro and in yivo [C. Vezina et al., J. Antibiot. 28, 721
(1975); S.N. Seghal et al., J. Antibiot. 28, 727 (1975); H. A. Baker et al., J. Antibiot.
31, 539 (1978); U.S. Patent 3,929,992; and U.S. Patent 3,993,749].
Rapamycin alone (U.S. Patent 4,885,171) or in combination with picibanil
(U.S. Patent 4,401,653) has been shown to have antitumor activity. R. Martel et al.
[Can. J. Physiol. Pharmacol. 55, 48 (1977)] disclosed that rapamycin is effective in
the experimental allergic encephalomyelitis model, a model for multiple sclerosis; in the
adjuvant arthritis model, a model for rheumatoid arthritis; and effectively inhibited the
formation of IgE-like antibodies.
The i.nmunosuppressive effects of rapamycin have been disclosed in FASEB 3,
3411 (1989), rapamycin has been shown to be effective in inhibiting transplant
rejection (U.S. Patent Application Ser. No. 362,544 filed June 6, 1989). Cyclosporin
A and FK-506, other macrocyclic molecules, also have been shown to be effective as
irnmunosuppressive agents, therefore useful in preventing transplant rejection [FASEB
3, 3411 (1989); FASEB 3, 5256 (1989); and R. Y. Calne et al., Lancet 1183 (1978)].
Mono- and diacylated derivatives of rapamycin (esterified at the 28 an~ 43
positions) have been shown to be useful as antifungal agents (U.S. Patent 4,316,885)
and used to make water soluble prodrugs of rapamycin (U.S. Patent 4,650,803).
Recently, the numbering convention for rapamycin has been changed; therefore
according to Chemical Abstracts nomenclature, the esters described a'oove would be at
the 31- and 42- positions.
AHP-9675/9Ç75-1-N1
- 2 - ~5~
DESCRIPI~ON OF THE INVENTION
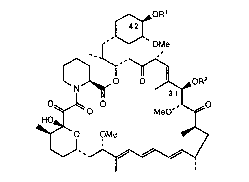
This invention providcs derivatives of rapamycin which are useful ai
immunosuppressive, anti-inflammatory, and anti-fungal agents having the structure
~OMe
~oR2
~o MeO'
~0 OMe ,
" ~~
S wherein Rl and R2 are each, independently, hydrogen or
o
--[C(CH2)mCH(CH2)nN]pCo2R5
R3 R4
with the proviso that Rl and R2 are not both hydrogen;
R3 is hydrogen, alkyl of 1-6 carbon atoms, aralkyl of 7-10 carbon atoms,
-(CH2)qCO2R6~ -(CH2);NR7Co2R8~ carbamylalkyl of 2-3 carbon atoms,
aminoalkyl of 1-4 carbon atoms, hydroxyalkyl of 1-4 carbon atoms,
guanylalkyl of 2-4 carbon atoms, mercaptoalkyl of 1-4 carbon atoms,
alkylthioalkyl of 2-6 carbon atoms, indolylmethyl, hydroxyphenylmethyl,
imidazoylmethyl or phenyl which is optionally mono-, di-, or tri-substituted
with a substituent selected from alkyl of 1-6 carbon atoms, alkoxy of 1-6
carbon atoms, hydroxy, cyano, halo, nitro, carbalkoxy of 2-7 carbon atoms,
trifluoromethyl, amino, or a carboxylic acid;
R4 and R7 are each, independently, hydrogen, alkyl of 1-6 carbon atoms, or aralkyl
of 7-10 car'oon atoms;
AHP-9675/9675-1-N1
5~L 7~ ~ rJ
~ 3 ~
R5, R6, and R8 are each, independently, alkyl of 1-6 carbon atoms, aralkyl of 7-10
carbon atoms, fluorenylmethyl, or phenyl which is optionally mono-, di-, or ~i-
substituted with a substituent selected from alkyl of 1-6 carbon atoms, alkoxy
of 1-6 carbon atoms, hydroxy, cyano, halo, nitro, carbaL~coxy of 2-7 carbon
S atoms, trifluoromethyl, arnino, or a carboxylic acid;
misO-4;
n is O - 4;
pis 1 - 2;
qisO-4;
risO-4;
wherein R3, R4, m, and n are independent in each of the [C(CH2)mCH(CH2)nN]
subunits when p = 2;
or a pharmaceutically acceptable salt thereof. R3 R4
Of the compounds, preferred members are those in which m = O, n = O, and
p = 1; m = O, n = O, and p = 2; n = O, and R3 is -(CH2)qC02R6; m = O, n = O, and R3
is ~(CH2)rNR7Co2R8; and m = O, n = O, and R3 is hydrogen.
The pharmaceutically acceptable salts may be formed from inorganic cations
such as sodium, potassium, and the like, and organic acids such as acetic, lactic, citrict
tartaric, succinic, maleic, malonic, gluconic, and the like, when R3 contains a basic
20 amino group.
The compounds of this invention can be prepared by acylating rapamycin with
an acylating agent having the general structure
1l
X- [c(cH2)mcH(cH2)nNlpco2Rs
1 3 l 4
where X is OH in the presence of a coupling reagent, such as dicyclohexyl-
25 carbodiimide. The compounds of this invention also can be prepared using a mixed
anhydride of the above described carboxylic acid as the acyla~ing species.
Alternatively, the acylating species can be an acid halide, where X can be Cl, Br, or I.
The acylating groups used to prepare the compounds of this invention are commercially
available or can be prepared by methods that are disclosed in the literature.
AHP-9675/9675-1-Nl
- 4-
Immunosuppressive activity was evaluated in an in vitro standard
pharmacological test procedure to measure lymphocyte proliferation (LAF) and in two
in vivo standard pharmacological test procedures. The first in ~ivo procedure was a
popliteal Iymph node (PLN) test procedure which measured the effect of compounds of
S this invention on a mixed Iymphocyte reaction and the second in ~Q procedure
evaluated the survival time of a pinch skin graft.
The comitogen-induced thymocyte proliferation procedure (LAF) was used as
an in vitro measure of the immunosuppressive effects of representative compounds.
Briefly, cells from the thymus of normal BALB/c mice are cultured for 72 hours with
10 PHA and lL-l and pulsed with tritiated thymidine during the last six hours. Cells are
cultured with and without various concentrations of rapamycin, cyclosporin A, or test
compound. Cells are harvested and incorporated; radioactivity is determined.
Inhibition of Iymphoproliferation is assessed in percent change in counts per minute
from non-drug treated controls. The results are expressed by the following ratio, or as
15 the percent inhibition of Iymphoproliferation of 1 ',lM.
3H-control thvmus cells - H3-rapamvcin-treated thvmus cells
3H-control thymus cells - H3-test compound-treated cells
A mixed Iymphocyte reaction (MLR) occurs when Iymphoid cells from
genetically distinct animals are combined in tissue culture. Each stimulates the other to
undergo blast transformation which results in increased DNA synthesis that can be
quanti~led by the incorporation of tritiated thymidine. Since stimulating a MLR is a
function of disparity at Major Histocompatibility antigens, an in vivo popliteal Iymph
node (PLN) test procedure closely correlates to host vs. graft disease. Briefly,irradiated spleen cells from BALB/c donors are injected into the right hind foot pad of
recipient C3H mice. The drug is given daily, p.o. from Day 0 to Day 4. On Day 3 and
Day 4, tritiated thymidine is given i.p., b.i.d. On Day 5, the hind popliteal lymph
nodes are removed and dissolved, and radioactivity counted. The corresponding left
PLN serves as the control for the PLN from the injected hind foot. Percent
suppression is calculated using the non-drug treated animals as allogenic control.
Rapamycin at a dose of 6 mg/kg, p.o. gave 86% suppression, whereas cyclosporin Aat the same dose gave 43% suppression. Results are expressed by the following ratio:
3H-PLN cells control C3H mouse - 3H-PLN cells rapamvcin-treated C3H mouse
3H-PLN cells control C3H mouse - 3H-PLN cells test compound-treated C3H mouse
., .
AHP-9675/9675- 1 -Nl
~5:~l'7~
The second in vivo test procedure is designed tO determine the survival time of
pinch skin graft from male DBA/2 donors transplanted to male BALB/c recipients. The
method is adapted from Billingham R.E. and Medawar P.B., J. Exp. Biol. 28:385-
402, (1951). Briefly, a pinch skin graft from the donor is grafted on the dorsum of the
5 recipient as a homograft, and an autograft is used as control in the same region. The
recipients are treated with either varying concentrations of cyclosporin A as test control
or the test compound, intraperitoneally. Untreated recipients serve as rejection control.
The graft is monitored daily and observations are recorded until the graft becomes dry
and forms a blackened scab. This is considered as the rejection day. The mean graft
10 survival time (number of days + S.D.) of the drug treatment group is compared with
the control group.
The following table summarizes the results of representative compounds of this
invention in these three standard test procedures.
TABLE 1
LAF* PLN* Skin Graft
Compound (ratiol fratio)__ (davs + Sl)!
Examplel 1.8 0.61 12.0+ 1.6
Example 2 0.33 0.62 11.5 + 0.6
Example 3 0.20 + 9.0 + 0.9
Example 4 4.9 0.18 12.3 + 0.5
Example 5 0.006 + 8.8 + 0.9
Example 6 5.4 0.33 11.5 + 3.5
Example 7 3% at l ~,lM** + 7.7 + 1.5
Example 8 0.03 0.41 +
Example9 0.96 1.34 10.3 + 0.8
Example 10 2.0 0.96++ 12.7 + 1.2
Example 11 0.004 + 10.5 + 1.3
Example 12 19.8 -2.87 12.0 + 2.0
Example 13 22% at l~M** + 7.0 + 0.6
Rapamycin 1.0 1.0 12.0 + 1.7
* Calculation of ratios was described supra.
Result expressed as percent inhibition of Iymphoproliferation at 1 ~M.
+ Not evaluated
++ Results obtained using cremophore/ethanol as a vechicle for administration.
Ratios of 0.33 and 1.07 were also obtained using carboxymethyl cellulose
as a vehicle for administration.
AHP-9675/9675-1-Nl
2~5~
- 6 -
The results of these standard pharmacological test procedures demonstrate
immunosuppressive activity both in vitro and in viVO for the compounds of this
invention. Positive ratios in the LAF and PLN test procedures indicate suppression of
T cell proliferation. As a transplanted pinch skin grafts are typically rejected within ~7
5 days without the use of an immunosuppressive agent, the increased survival time of the
skin graft when treated with the compounds of this invention further demonstrates their
utility as immunosuppressive agents. While it appears that the compound disclosed by
Example 12 may cause T cell proliferation in the PLN test procedure, it is believed a
negative ratio in this test procedure coupled with an increased survival time observed in
10 the skin graft test procedure indicates a proliferation of TSuppressor cells, which are
implicated in suppressing the immune response. (see, I. Roitt et al. Immunology,C.V.Moseby Co. 1989, p 12.8-12.11).
Andfungal activity of the compounds of this invention was measured against 5
15 strains of Candida albicans using a plate test procedure for measurement of inhibition.
The following represents the typical procedure used. Compound to be tested was
placed on st~rile dried 1/4" plate disks, and allowed to dry. Agar plates were seeded
with fungi and allowed to solidify. The impregnated disks were placed on the seeded
Agar surface and incubated for the time required for the particular culture. Results are
20 expressed in MIC ( ,ug/ml) to inhibit growth. The results of this test procedure showed
that the compounds of this invention have antifungal activity; however, it was
surprising that the compounds of this invention were less active than the parentcompound, rapamycin
AHP-9675/9675-1-N1
~5~ 7~ ~?
- 7 -
Table 2*
Strain of Candida albicans
CompoundATCC 10231ATCC 38246ATCC 38247ATCC 38248 3669
Examplel>0.4 >0.4 >0.4 >0.4 >0.4
S Example20.1 0.2 0.2 0.2 0.1
Example 30.4 > 0.4 > 0-4 >0.4 0-4
Exarnple40.1 0.4 0.1 0.1 0.2
Example 5> 0.4 > 0.4 > 0.4 >0.4 >0.4
Example 60.1 > 0.4 0.2 0.4 >0.4
Example 7 + + + + +
Example 8> 0.4 > 0.4 > 0-4 ~0-4 >0-4
Example 90.4 > 0.4 0.4 >0.4 >0.4
Example 100.2 > 0.4 0.2 0.4 0.4
Example 11> 0.4 > 0.4 > 0.4 >0.4 >0.4
lS Example 120.2 > 0.4 0.1 0.2 0.4
Example 13> 0.4 > 0.4 > 0.4 >0.4 >0.4
Rapamycin0.003 0.025 0.003 0.006 0.025
* expressed as MIC (~g/ml)
20 + not evaluated
Based on the results of these standard pharmacological test procedures, the
compounds are useful in the treatrnent of transplantation rejection such as, heart,
kidney, liver, bone marrow, and skin transplants; autoimmune diseases such as, lupus,
rheumatoid arthritis, diabetes mellitus, myasthenia gravis, and multiple sclerosis; and
25 diseases of inflammation such as, psoriasis, dermatitis, eczema, seborrhea,
inflammatory bowel disease; and fungal infections.
The compounds may be administered neat or with a pharmaceutical carrier to a
rnarnmal in need thereof. The pharmaceutical carrier may be solid or liquid.
A solid carrier can include one or more substances which may also act as
30 flavoring agents, lubricants, solubilizers, suspending agents, fillers, glidants,
compression aids, binders or tablet-disintegrating agents; it can also be an encapsulating
material. In powders, the carrier is a finely divided solid which is in admixture with the
finely divided active ingredient. In tablets, the active ingredient is mixed with a carrier
having the necessary compression properties in suitable proportions and compacted in
AHP-9675/9675-1-Nl
2~S~7
- 8 -
the shape and size ~esired. The powders and tablets preferably contain up to 99% of
the active ingredient. Suitable solid carriers include, for example, calcium phosphate,
magnesium stearate, talc, sugars, lactose, dextrin, starch, gelatin, cellulose, methyl
cellulose, sodium carboxymethyl cellulose, polyvinylpyrrolidine, low melting waxes
5 and ion exch~nge resins.
Liquid carriers are used in preparing solutions, suspensions, emulsions,
syrups, elixirs and pressuriæd compositions. The active ingredient can be dissolved or
suspended in a pharmaceutically acceptable liquid carrier such as water, an organic
solvent, a mixture of both or pharmaceutically acceptable oils or fats. The liquid carrier
10 can contain other suitable pharmaceutical additives such as solubilizers, emulsifiers,
buffers, preservatives, sweeteners, flavoring agents, suspending agents, thickening
agents, colors, viscosity regulators, stabilizers or osmo-regulators. Suitable examples
of liquid carriers for oral and parenteral administration include water (partially
containing additives as above, e.g. cellulose derivatives, preferably sodium
15 carboxymethyl cellulose solution), alcohols (including monohydric alcohols and
polyhydric alcohols, e.g. glycols) and their derivatives, and oils (e.g. fractionated
coconut oil and arachis oil). For parenteral administration, the carrier can also be an
oily ester such as ethyl oleate and isopropyl myristate. Sterile liquid carriers are useful
in sterile liquid form compositions for parenteral administration. The liquid carrier for
20 pressurized compositions can be halogenated hydrocarbon or other pharmaceutically
acceptable propellent.
Liquid pharmaceutical compositions which are sterile solutions or suspensions
can be utilized by, for example, intramuscular, intraperitoneal or subcutaneous
injection. Sterile solutions can also be administered intravenously. The compound can
25 also be administered orally either in liquid or solid composition form.
Preferably, the pharmaceutical composition is in unit dosage form, e.g. as
tablets or capsules. In such form, the composition is sub-divided in unit dose
containing appropriate quantities of the active ingredient; the unit dosage forms can be
packaged compositions, for example, packeted powders, vials, ampoules, prefilled30 syringes or sachets containing liquids. The unit dosage form can be, for example, a
capsule or tablet itself, or it can be the appropriate number of any such compositions in
package form. The dosage to be used in the treatment must be subjectively determined
by the attending physician.
AHP-9675/9675- 1 -N 1
2~5~7~
In addition, the sompounds of this invention may be employed as a solution,
cream, or lotion by formulation with pharmaceutically acceptable vehicles containing
0.1-0.5 percent, preferably 2%, of active compound which may 'De administered to a
fungally affected area.
s
The ~ollowing examples illus~ate the preparation of representalive compounds
of this invention.
10 E~ample 1
Rap~mycin-42-ester with N-r(l~l-dimethvlethoxv!carbony~ lvcvl~lvcine
Under anhydrous conditions, a solution of rapamycin (3 g, 3.28 mmole) and
N-[(1,1-dimethylethoxy)carbonyl]-glycylglycine (3.04 g, 13.1 mmole) in 40 mL of
anhydrous dichloromethane was treated with dicyclohexylcar'oodiimide (1.35 g, 6.56
mmole) followed by 4-dimethylaminopyridine (0.8 g, 6.56 mmole). After stirring at
ambient temperature for 48 hours, the precipitated solid was collected and washed with
dichloromethane. The combined filtrates were absorbed directly onto silica gel Merck
60 by adding the gel and evaporation to dryness. Flash chromatography of the
preabsorbed material (using a gradient elution with ethylacetate-toluene from 2:1 to 1:0
v/v) afforded 1.05 g (28.3 %) of the title compound isolated as a three quarter toluene
solvate, along with the 31,42-diester of Example 2. HPLC analysis showed that the
monoester is a 8.3:1 mixture of two conformers.
lH NMR (CDC13, 400 MHz): o 1.46 (m, 9H, COOBut), 1.654 (s, 3H,
CH3C=C), 1.751 (s, 3H, CH3C=C), 3.14 (s, 3H, CH30), 3.33 (s, 3H, CH30~,
3.36 (s, 3H, CH30), 4.18 (d, lH, CHOH), 4.75 (m, lH, 42-CHO ), 4.79 (s, lH,
OH); High Res. MS (neg. ion FAB) Calcd for C60Hg3N3017: 1127.6504, measured
mass 1127.6474.
Anal. Calcd for C6nHg3N3017 0.75 PhCH3: C, 65.45; H, 8.33; N, 3.51
Found: C, 65,23; H, 8.32; N, 3.86
AHP-9675/9675- 1 -N 1
2 ~S~ d
- 10-
The following representative compounds can be prepared from rapamycin and the
appropriate terminally-N-substituted amino acid by employing the method used to
prepare the title compound in Example 1.
Rapamycin-42-ester with N-[(fluorenylmethoxy)carbonyl]-alanylserine
S Rapamycin-42-ester with N-[(fluorenylmethoxy)carbonyl]-glycylglycine
Rapamycin-42-ester with N-[(ethoxy)carbonyl]-arginylmethionine
Rapamycin-42-ester with N-~(4'-chlorophenoxy)carbonyl]-histidylarginine
Rapamycin-42-ester with N-[(phenoxy)carbonyl]-tryptophanylleucine
Rapamycin-42-ester with N-[(phenylmethoxy)carbonyl)]-N-methylglycyl-N-ethyl-
10 alanine
Rapamycin-42-ester with N-[(phenylmethoxy)carbonyl]-N-methyl-,l~-alanylphenyl-
alanine
Rapamycin-42-ester with N-[(l,l-dimethylethoxy)carbonyl]-cysteinylglycine
Example 2
Rapamvcin-31.42-diester with N-f(l .l-dimethylethoxy!carbonvll-glvcylglYcine
The title compound (1.85 g, 42%) was separated from the 42-monoester as
described in Example 1 and isolated as a three quarter toluene solvate. HPLC analysis
showed that the diester is a 8.1: 1 mixture of conformers.
lH NMR (CDC13, 400 MHz): ~ 1.452 (m, 18H, COOBut), 1.6612 (s, 3H,
CH3C=C), 1.7815 (s, 3H, CH3C=C), 3.14 (s, 3H, OCH3), 3.34 (s, 3H, OCH3),
3.35 (s, 3H, OCH3), 4.52 (s, lH, OH), 4.79 (m, lH, 42-CHO ); High Res. MS (neg.
ion FAB): Calcd for C6gH107Nso2l 1341.7458, measured mass: 1341.7463.
Anal. Calcd for C6gH107Nso2l 0.75 PhCH3: C, 63.17; H, 8.06; N, 4.96
Found: C, 62.83; H, 8.09; N, 5.00
.:
AHP-9675/9675-1-N1
S~
The following representative compounds can be prepared from rapamycin and the
appropriate terminally-N-substituted amino acid by employing the method used to
prepare the title compound in Exarnple 2.
S Rapamycin-31,42-diest~r with N-[(fluorenylmethoxy)carbonyl]-alanylserine
Rapamycin-31,42-diester with N-l(fluorenylmethoxy)carbonyl]-glycylglycine
Rapamycin-31,42-diester with N-[(ethoxy)carbonyl]-arginylrnethionine
Rapamycin-31,42-diester with N-[(4'-chlorophenoxy)carbonyl]-histidylarginine
Rapamycin-31,42-diester with N-[(phenoxy)carbonyl]-tryptophanylleucine
10 Rapamycin-31,42-diesterwith N-[(phenylmethoxy)carbonyl)]-N-methylglycyl-N-
ethyl-alanine
Rapamycin-31,42-diester with N-[(phenylmethoxy)carbonyl]-N-methyl-~-
alanylphenyl- alanine
Rapamycin-31,42-diester with N-[(1,1-dimethylethoxy)carbonyU-cysteinylglycine
Example 3
l~amvcin-31~42-dieste~with N-r(l.l-dimethvlethoxv~carbonvll-N-methvl~lvcine
Under anhydrous conditions, an ice cold solution of rapamycin (2 g, 2.18
mmole) and Na-Boc sarcosine (1.65 g, 8.75 mmole) in 20 ml of anhydrous
dichloromethane was treated with dicyclohexylcarbodiimide (1.8 g, 8.7 mmole)
followed by 4-dimethylaminopyridine (1 g, 8.7 mmole). After stirring overnight at
ambient temperature, the precipitated solid was collected and washed with
dichloromethane. The combined filtrates were evaporated to dryness to give an
amorphous amber solid (3 g). The crude product was purified by flash chromatography
( on silica Merck 60, elution with hexane-ethylacetate 1: 1, v/v) to provide the title
compound (0.75 g, 27.4%) along with the 42-monoester of Example 4. HPLC analysisshowed that the diester is a 19.8:1 mixture of two conformers. The multiplicity of the
NMR peaks suggests the presence of amide rotamers.
1H NMR (CDC13, 400 MHz): ~ 1.411, 1.438, 1.448 and 1.474 (m, 18 H,
COOBut), 2.91 (m, 6H, NCH3), 3.14 (s, 3H, CH30), 3 34 (s, 3H, CH30), 3 37 (s,
3H, CH30), 4 73 (broad, lH, 42-CHO), 4 82 (2s, lH, OH); High Res. MS (neg. ion
~AB): Calcd. for C67H10sN3ol9 1255.7342, measured mass 1255.7289.
Anal. Calcd for C67H105N3019: C, 64.04; H, 8.42; N, 3.34
Found: C, 64.14; H, 8.74; N, 3.63
AHP-9675/9675-1-N1
- 12 - 2~S~l'7~3~
The following representative compounds can be prepared from rapamycin and
the appropriate terminally-N-substituted amino acid by employing the method used to
prepare the title compound in Example 3.
s
Rapamycin-31,42-diester with N- [(ethoxy)carbonyl]-tyrosine
Rapamycin-31,42-diester with N-~(fluorenylmethoxy)carbonyl]-phenylalanine
Rapamycin-31,42-diester with N- [(3',4' ,S'-trihydroxyphenoxy)carbonyl] -isoleucine
Rapamycin-31,42-diester with N- [(1,1 -dimethylethoxy)carbonyl)-glutamine
10 Rapamycin-31,42-diester with N- [(phenoxy)carbonyl] -N-methylalanine
Rapamycin-31,42-diester with N-[(propyloxy)carbonyl]-4-aminobutryic acid
Rapamycin-31,42-diester with N-[(phenylmethoxy)carbonyl]-7-aminoheptanoic acid
Rapamycin-31,42-diester with N-[(fluorenylmethoxy)carbonyl]-serine
15 Exampl~ 4
RaDanly~ilL-4~-e~t~with N-~ l-dim~hy]ethoxv!carbonyl]-N-methyl~ly~ç
Under anhydrous conditions, an ice cold solution of rapamycin (0.95 g, 1.02
20 mmole) and Na-Boc sarcosine (0.21 g, 1.1 mmole) in 20 mL of anhydrous
dichloromethane was treated with dicyclohexylcarbodiimide 0.21 g, 1 mmole) followed
by 4-dimethylaminopyridine (0.12 g, 1 mmole). After stirring for 4 hours at ambient
temperature, the precipitated solid was collected and washed with dichloromethane. The
combined filtrates were concentrated in vacuo to give an amorphous amber solid. Flash
25 chromatography of the crude product (on silica Merck 60, elution with hexane-ethylacetate 1:1 v/v to remove the diester of Example 3, followed by chloroform-ethylacetate-methanol 75:25:1 v/v) provided partially purified title compound (0.38 g,
35%). Pure product was obtained by preparative HPLC (Waters Prep 500, silica gel,
chloroform-ethylacetate-methanol 75:25: 1 v/v, flow rate 250 mL/min). HPLC analysis
30 showed that the ester is a 6.6:1 mixture of two confolTners. The multiplicity of NMR
peaks suggests the presence of amide rotamers.
lH NMR (CDCl3, 400 MH~ 1.42-1.46 (ds, 9H, COOBut), 2.91 (ds, 3H,
NCH3), 1.644 (s, 3H, CH3C=C), 1.738 (s, 3H, CH3C=C), 3.12 (s, 3H, CH30),
3.32 (s, 3H, CH30), 3.35 (s. 3H, CH30), 4.18 (d, lH, CHOH), 4.71 (broad, lH,
35 42-CHO), 4.78 (broad s, lH, OH); High Res. MS (neg. ion FAB): Calcd for
Cs9H92N2O16 1084.6446, measured mass 1084.6503.
AHP-9675/9675-1-
2~S~
- 13-
Anal. Calcd for CsgHg2N2016: C, 65.29; H, 8.54; N, 2.58
Found: C, 65.25; H, 8.52; N, 2.42
The following representative compounds can be prepared from rapamycin and
5 the appropriate terminally-N-substituted amino acid by employing the method used to
prepare the title compound in Example 4.
Rapamycin-42-ester with N-[(ethoxy)carbonyl]-tyrosine
Rapamycin-42-ester with N-~(fluorenylmethoxy)carbonyl]-phenylalanine
Rapamycin-42-ester with N-[(3',4',5'-trihydroxyphenoxy)carbonyl]-isoleucine
Rapamycin-42-ester with N-[(l,l-dimethylethoxy)carbonyl)-glutamine
Rapamycin-42-ester with N-[(phenoxy)carbonyl]-N-methylalanine
Rapamycin-42-ester with N-[(propyloxy)carbonyl]-4-aminobutryic acid
Rapamycin-42-ester with N-[(phenylmethoxy)carbonyl]-7-aminoheptanoic acid
Rapamycin-31,42-diester with N-[(fluorenylmethoxy)carbonyl]serine
Example S
Rap~amycin-31 ~42-diester with S-( l . I -dimethvlethoxv!-2-rrf 1 ~ I -dimethvlethoxyl-
calrl2Qny~ oxopentanoic acid
Under anhydrous conditions, an ice cold solution of rapamycin (4 g, 4.37
mmole) and L-glutamic acid Na~Boc-~tert-butylester (4.9 g, 16.1 mmole) in 40 mL
of dry dichloromethane was treated with dicyclohexylcarbodiimide (1.8 g, 8.7 mmole)
followed by 4-dimethylaminopyridine (1 g, 8.7 mmole). After stirring overnight at
room temperature, the precipitated solid was collected and washed with
dichloromethane. The combined filtrates were concentrated in vacuo to provide 11 g of
an amorphous amber solid. The crude product was purified by flash chromatography(on silica Merck 60, gradient elution with hexane-ethylacetate from 2: 1 tO 1: 1, v/v) tO
yield 4.52 g (69.6%) of the title compound along with the 42-monoester of Example 6.
HPLC analysis showed that the diester con.sists of a 6.6: 1 mixture of two conformers.
lH NMR (CDC13, 400 MHz): ~ 1.42 (m, 36 H, COOBut), 1.646 (s, 3H,
CH3C=C), 1.701 (s, 3H, CH3C=C), 3.13 (s, 3H, CH30), 3.34 (s, 3H, CH30),
3.36 (s, 3H, CH30), 4.735 (m, 2H, OH~42-CH-O); High Res. MS (neg. ion FAB):
calc. for qgH12sN3023 1483.8715, measured mass 1483.8714.
AHP-9675/9675-1-N1
7~
- 1 -
Anal. Calcd for C7gH12sN3023: C, 63.90; H, 8.49; N, 2.83
Found: C, 63.63; H, 8.41; N, 2.44
The following representative compounds can be prepared from rapamycin and
S the appropriately terminally-N-substituted amino diacid monoester by employing the
method used to prepare the title compound in Example S.
Rapamycin-31,42-diester with 6-(phenylmethoxy)-2-[~fluorenylmethoxy)carbonyl]-
amino]-~oxohexanoic acid
Rapamycin-31,42-diester with 6-(4'-methylphenoxy)-3-[[(phenylmethoxy)carbonyl]-
amino-6-oxohexanoic acid
Rapamycin-31,42-diester with 6-(ethoxy)-4-[[(phenoxy)carbonyl]amino]-6-oxo-
hexanoic acid
Rapamycin-31,42-diester with 6-(methoxy)-S-[[(ethoxy)carbonyl]amino]-6-ox~
hexanoic acid
Rapamycin-31,42-diester with 4-(phenoxy)-2-[N-[(1,1-dimethylethoxy)carbonyl]-N-
methylamino]-40xobutanoic acid
Rapamycin-31,42-diester with 4-(phenylmethoxy)-3-[N-[(methoxy)carbonyl]-N-
methylamino]-4-oxobutanoic acid
Example 6
Ra~a~y~in-42-ester with 5-(1~1-dimethvlethoxvl-2-~r(l~l-dimethvlethoxv)-
carbony~ ~oxo~entanoic acid
The title compound (1.14 g, 20.6%) was separated from the 31,42-diester as
described in Example S and isolated as the quarter hydrate/mono-ethyl acetate solvate.
HPLC analysis showed that the monoester is a l l.S: 1 mixture of two conformers.1H NMR (CDC13, 400 MHz): o 1.425 (m, 18H, COOBut), 1.643 (s, 3H,
CH3C=C), 1.737 (s, 3H, CH3C=C), 3.13 (s, 3H, CH30), 3.32 (s, 3H, CH30),
3.36 (s, 3H, CH30), 4.17 (d, IH, CHOH), 4.71 (M, lH, 42-CHO), 4.785 (s, lH,
OH); High Resolution MS ( neg. ion FAB): C~lc. for C65H102N218 1198-7127
measured mass 1198.7077.
AE IP-9675/9675-1-Nl
- 15- ~ L7~?,
Anal. Calcd for C6sH102N2olg CH3COOEt 0-25 H2O:
C, 64.13, H, 8.60; N, 2.17
Found: C, 64.18; H, 8.52: N, 2.01
S The following representative compounds can be prepared from ~apamycin and
the appropriately terminally-N-substituted amino diacid monoester by employing the
rnethod used to prepare the tide compound in Example 6.
Rapamycin-42-ester with 6-(phenylmethoxy)-2-[[fluorenylmethoxy)carbonyl]-amino]-~oxohexanoic acid
Rapamycin-42-ester with ~(4'-methylphenoxy)-3-[[(phenylmethoxy)carbonyl]-amin~
~oxohexanoic acid
Rapamycin-42-iester with ~(ethoxy)-4-[[(phenoxy)carbonyl]amino]-~oxo- hexanoic
acid
l S Rapamycin-42-ester with 6-(methoxy)-S-[[(ethoxy)carbonyl]amino~-6-oxo- hexanoic
acid
Rapamycin-42-ester with 4-(phenoxy)-2-[N-[(1,1-dimethylethoxy)carbonyl]-N-
methylamino]-~oxobutanoic acid
Rapamycin-42-ester with 4-(phenylmethoxy)-3-[N-[(methoxy)carbonyl]-N-methyl-
amino]-4-oxobutanoic acid
Example 7
Rapamvcin-31.42-diester with 2-~r( l . l -dimethvlethoxv~carbonvllaminol-4-oxo-4-
(Dhenylme~hg~ butanoic acid
Under anhydrous conditions, 295mg (1.21mmol) of 2,4,6 trichlorobenzoyl
chloride was added to a solution of 391mg(1.21mmol) of Na-Boc-L-aspartic acid-~-benzyl ester and 17011L (1.21mmol) of Et3N in 1 mL of THF at room temperature.
After stirring for 30 minutes, 500 mg (0.SSmmol) of rapamycin and 295 mg ( 2.42
mmol) of dimethylaminopyridine was added and the reaction was left to stir overnight.
The reaction mixture was then filtered and the filtrate concentrated in vacuo. Pure
product (200 mg, 25%) was obtained by preparative HPLC (S cm column, 40 % ethyl
acetate-hexane). The product was isolated as the heptahydrate.
AHP-9675/9675- 1 -N1
2~5~7~3~
- 16-
IH NMR (CDC13, 400 MHz) ~ 7.347 (s, 10 H, Ar), 6.223, 5.126 (s, 4 H, CH2Ph),
4.698 (m, 1 H, CH-CO2), 4.587 (m, 2 H, NH), 3.353 (s, 3 H, CH30), 3.337 (s, 3
H, CH30), 3.301 (s, 3 H, CH30), 2.775 (m, 4 H, CH2CO2); IR ~KBr) 3420 (OH),
2935 (CH), 2920 (CH), 1730 (C=O), 1650, 1500, 1455, 1370, 1170 cm-l; MS (neg.
S ion FAB) 1523 (M-), 1433, 297, 248, 205, 148, 44, 25 (100).
Anal. Calcd for Cg3HI l7N3O23-7H2O C, 60.40; H, 7.09; N, 2.54
Found: C, 60.54; H, 7.28; N, 2.56
Example 8
Rapamycin-~42-diester with 3-r~(l.l-dimethvlethoxy!carbc,nvllaminol-4-ox~4-
(~henvlmethoxv! butanoic acid
Under anhydrous conditions, 532 mg (2.18 mmol) of 2,4,6 trichloro'~enzoyl
chloride in 1 mL THF was added to a solution of 704 mg (2.18 mrnol) of Na-Boc-L-aspartic acid-a-benzyl ester and 303 ',IL (2.18 mmol) of Et3N in S mL of THF at room
temperature. After stirring for 20 minutes, the reaction mixture was ~lltered over
sintered glass, and the precipitate was washed with THF. The filtrate was concentrated
in vacuo to give a thick oil. l'he oil was dissolved in S rnL of 'Denzene and 1.00 g (1.09
mmol) of rapamycin and 532 mg (4.36 mmol) of dimethylaminopyridine in 1 mL of
benzene was added dropwise. The reaction was stirred for 2 hr, poured into ethylacetate, and washed consecutively with 0.5 N HCI and brine. The solution was dried
over sodium sulfate, decanted, concentrated in vacuo to give a white foamy solid,
which was purified via flash chromatography on a 60 mm x 100 mm silica column (20-
40 % ethyl acetate/hexane as eluant) to give 532 mg (33 %) of the title compound which
was isolated as the hydrate.
lH NMR (CDC13, 400 MHz) ~ 7.362 (s, 10 H, Ar), 5.193 (s, 4 H, CH2Ph),
4.596 (m, 1 H, CH-CO2), 4.586 (m, 2 H, NH), 3.336 (s, 3 H, CH30), 3.306 (s, 3
H, CH30), 3.145 (s, 3 H, CH30); IR (KBr) 3410 (OH), 2950 (CH), 2920 (CH),
1735 (C=O), 1710 (C=O), 1640, 1490, 1445, 1350, 1150 cm -1; MS (neg. ion FAB)
1524 (M-), 1434, 297, 248, 232, 214, 205, 167, 148, 42 (100), 26.
Anal. Calcd for C83H1l7N3O23 - H2O: C, 65.38; H, 7.73; N, 2.76
Found: C, 64.85; H, 7.67; N, 2.56
AHP-9675/9675 - 1 -N 1
2~315
- 17-
Example 9
Rapamycin-42-ester with 3-rr(l.l-dimethvlethoxv!carbonvllaminol-4-oxo-4-
(phenylmethoxv~ butanoic acid
s
The title compound (374 mg, 23%~ was prepared by the method described in
the previous Example and separated from the compound described in the previous
Example by flash chromatography (20-40% ethyl acetate/hexane as the eluant) and
isolated as the sesquihydrate.
lH NMR (CDC13, 400 MHz~ o 7.356 (s, 5 H, Ar), 5.185 (s, 2 H, CH2Ph),
4.635 (m, 1 H, CH-CO2), 4.582 (m, 1 H, NH), 3.330 (s, 6 H, CH30), 3.135 (s, 3
H, CH30); IR (KBr) 3410 (OH), 2950 (CH), 2920 (GH), 1735 (C=O), 1710 (C=O),
1640, 1490, 1445, 1350, 1150 cm -1; MS (neg. ion FAB) 1218 (M-), 1127, 590, 168,42, 25, 17 (1~0).
Anal. Calcd for C67Hg8N2O18 - 1.5 H2O: C, 63.64; H, 8.21; N, 2.22
Found: C~ 63.64; H, 7.51; N, 2.13
Example 10
Ra~amvcin-42-ester with 5-(l.l-dimethyloxv~-4-lr(1.1-dimethvlethoxv!carbonvll
aminol-5-oxopentanoic acid
IJnder anhydrous conditions, an ice cold solution of rapamycin (4 g, 4.37
mmole) and L-glutamic acid Na-Boc-a-tert-butylester (4.9 g, 16.1 mmole) in 40 mLof anhydrous dichloromethane was treated with dicyclohexylcarbodiimide (1.8 g, 8.7
mmole) followed by 4-dimethylamino pyridine (1 g, 8.7 mmole). After stirring
overnight at ambient temperature, the precipitated solid was collected and washed with
dichloromethane. The combined filtrates were concentrated in vacuo to give 9 g of an
amorphous amber solid. The crude product was purified by flash chromatography (on
silica Merck 60, gradient elution with hexane-ethylacetate from 2:1 to 3:2, v/v) to
provide 1.35 g (25.7%) of the title compound along with the 31,42-diester of
Example 11. HPLC analysis showed that the monoester is a 7.5: I mixture of two
conformers.
,
AHP-9675/9675-1-N1
~5 iL~;3
- 18-
1H NMR (CDC13, 400 MHz): ~ 1.43 (s, 9H, COOBut) and 1.46 (s, 9H,
COOBut), 1.65 (s, 3H, CH3C=C), 1.75 (s, 3H, CH3C=C), 3.14 (s, 3H, CH30),
3.34 (s, 3H, CH30), 3.38 (s, 3H, CH30), 4.18 (d, lH, CH-OH), 4.65 (m, lH, 42-
CHO), 4.80 (s, lH, OH);
S High Res. MS (neg. ion FAB): Calc. for C6sH102N2olg: 1198.7126, measured
mass 1198.7135.
Anal. Calcd for C6sH102N2ol8: C~ 65-09; H~ 8-57; N~ 2-34
Found C, 65.04; H, 8.33; N, 2.64
Example 11
Rapamvcin-31.42-diester with 5-(1.1-dimethvlethoxv)-4-rL(1.1-dimethvlethoxv)-
carbonvll- amino3-5-oxopentanoic acid
The title compound was prepared (0.83 g, 12.8%) along with the 42-
monoester as described in Example 10. HPLC analysis showed that the diester is a7.7:1 mixture of two conformers.
lH NMR (CDC13, 400 MHz): ~ 1.43 (s, 18H, COOBut), 1.46 (s, 18H,
COOBut), 1.659 (s, 3H, CH3C=C), 1.759 (s, 3H, CH3C=C), 3.14 (s, 3H, CH30),
3.34 (s, 3H, CH30), 3.38 (s, 3H, CH30), 4.66 (m, IH, 42-CHO), 4.72 (s, IH,
OH); High Res. MS (neg. ion FAB): Calcd for C7gH12sN3O23: 1483.8704,
25 measured mass 1483.8636.
Anal. Calcd for C7gH125N3O23: C, 63.90; H, 8.49; N, 2.83
Found: C, 63.68; H, 8.60; N, 3.20
AHP-9675/9675- 1 -N 1
~S~,~B,~.
- 19-
Example 1~
Rapamycin-4~-çster with N~-bis~ l-dimethylethoxv!carbonyll-L-lvsine
Under anhydrous conditions, a solution of rapamycin (3 g, 3.28 mmole) and
Na, N-bis-Boc-L-lysine (4.5 g, 13 mmole) in 40 mL of anhydrous dichloromethane
was treated with dicyclohexylcarbodiimide (1.35 g, 6.56 mmole) followed by 4-
dimethylaminopyridine (0.8 g, 6.56 m mole). After stirring overnight at ambient
temperature, the pr~cipitated solid was collected and washed with dichloromethane. The
combined filtrates were concentrated in vacuo to give an amorphous amber solid. Flash
chromatography of the crude product (on silica Merck 60, elution with hexane-
ethylacetate 1:1 v/v) gave partially purified title compound. Pure product (0.8 g,
19.6%) was obtained by preparative HPLC (Waters Prep 500, silica gel, hexane-
ethylacetate 3:2 v/v, flow rate 250 mLlmin). HPLC analysis showed that the monoester
is a 9:1 mixture of two conformers.
lH NMR (CDC13, 400 MHz): o 1.438 (m, 9H, ~OOBut), 1.455 (s, 9H,
COOBut), 1.652 (s, 3H, CH3C=C), 1.752 (s, 3H, CH3C=C), 3.14 (s, 3H, CH30),
3.33(s, 3~I, CH30), 3.37 (s, 3H, CH30), 4.18 (d, lH, CHOH), 4.72 (m, lH, 42-
CHO), 4.79 (s, lH, OH); High Res. MS (neg. ion FAB): Calcd for C67H107N3Olg:
201241.7549, measured mass 1241.7604.
Anal. Calcd for C67H107N3ol8: C~ 64-76; H~ 8-68; N~ 3-38
Found: C, 64.58; H, 9.01; N, 3.10
Example 13
Ravamycin-31.42-diester with N--Nbisr(1.1-dimethylethoxy)carbonvll-L-lvsine
Under a nitrogen atmosphere, a solution of Na,N~ bis-Boc-L-lysine (1.038 g,
3 mmole) and triethylarnine (0.42 mL, 3 mmmole) in 10 mL of anhydrous THF was
30 treated in one portion with 2,4,6-trichlorobenzoyl chloride (0.73 g, 3 mmole). After
stirring for 20 minutes at ambient temperature, the precipitated solid was collected and
the filtrate was concentrated in vacuo . The resulting mixed anhydride was dissolved in
5 mL of benzeme and added to a stirred solwtion of rapamycin (1 g, 1.09 mmole)
containing 4-dimethylamino pyridine (0.59 g,4.8 mmole) in 10 mL of benzene. After
35 stirring at ambient temperature overnight, the precipitated solid was collected and the
filtrate was evaporated to dryness (yellow foam). The crude product was purified by
AHP-9675/967S-1-N1
211~5~78 ~d
-
flæh chromatography ( on silica Merck 60, elution with hexane-ethylacetate 1:1) to
provide title compound (l.lS g, 67%). HPLC analysis shows that the diester,is a 9:1
n~xture of two conformers.
~H NMR (CDC13, 400 MHz): ~ 1.426 (m, 9H, COOBut), 1.438 (s, 9H, COOBut),
1.443 (s, 9H, COOBut), 1.446 (s, 9H, COOBut), 3.141 (s, 3H, CH30), 3.36 (s, 3H,
CH30), 3.378 (s, 3H, CH30), 4.68-4.76 (m, 2H, OH and 42-CHO); High res. MS
(neg. ion FAB): Calcd. for Cg3H13sNsO23 1569.9526, measured mass 1569.9537.
Anal. Calcd. for Cg3H13sNsO23: C, 63.46; H, 8.66; N, 4.46
Found: C, 63.06; H, 8.84; N, 4.09
.
.