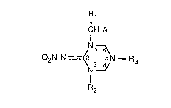Note: Descriptions are shown in the official language in which they were submitted.
~273~
- 1 -
PI/5-1 828]/A
Triazacvclohexane derivatives
The present invention relates to novel substitwted 2-nîtroimino-1,3,5-triazacyclohexane
derivatives, to processes for the preparation thereof, to pesticides that comprise those
compounds, and to their use in thc control of pests.
The triazacyclohexane derivatives according to the invention correspond to formula I
Rl
CH-A
N1~
02N-N =~2 3~N--R3 (I)
I
R2
wherein
Rl is hydrogen or C1-C4alkyl;
R2 is hydrogen, Cl-C6aLkyl, C3-C6cycloalkyl or a radical -CH2B;
R3 is hydrogen; C1-ClOalkyl; C3-C6cycloalkyl; Cl-ClOalkyl substituted by f}om 1 to 12
radicals from the group halogen, hydroxy, Cl-C4alkoxy, Cl-C4haloalkoxy having
from 1 to 9 halogen atoms, di-(Cl-C4alkyl)amino and Cl-Csalkoxycarbonyl;
C3-C6cycloalkyl substituted by from 1 to 4 Cl-C4alkyl radicals or halogen atoms;C2-C8alkenyl or C2-C8alkynyl; C2-C8alkenyl or C2-C8alkynyl each of which is
substituted by from 1 to 6 halogen atoms; phenyl; benzyl; or phenyl or benzyl each of
which is substituted by from l to 3 ring substituents ~rom the group halogen,
Cl-C4alkyl, Cl-C4haloalkyl having from l to 9 halogen atoms, C~-C4alkoxy,
Cl-C4haloalkoxy having from 1 to 9 halogen atoms, Cl-C4alkylthio, nitro alld cyallo;
A is an unsubstituted or mono- to tetra-substitllted arom;ltic or noll-arom.ltic,
monocyclic or bicyclic he~erocyclic ra(lic;ll tll;lt can have one or two substituents from
the group Cl-C3haloalkyl having from 1 to 7 halogen atoms, cyclopropyl,
halocyclopropyl having from 1 to 3 halogen atoms, C2-C3il1kenyl, C2-C3alkynyl,
2~273 ~
C2-C3haloalkenyl and C2-C3haloalkynyl each having from 1 to 4 halogen atoms,
Cl-C3haloalkoxy having from 1 to 7 halogen atoms, Cl-C3alkylthio,
Cl-C3haloalkylthio having from t to 7 halogen atoms, allyloxy, propargyloxy,
allylthio, propargylthio, haloallyloxy, haloallylthio, cyano and nitro, and from one to
four substituents from the group Cl-C3alkyl, Cl-C3alkoxy and halogen; and
B is phenyl; eyanophenyl; nitrophenyl; halophenyl having from I to 3 halogen atoms;
phenyl substituted by Cl-C3alkyl, Cl-C3haloalkyl having from 1 to 7 halogen atoms,
Cl-C3alkoxy or by Cl-C3haloalkoxy having from 1 to 7 halogen atoms; 3-pyridyl;
5-thiazolyl; 5-thiazolyl substituted by one or two substituents from the group
Cl-C3alkyl, Cl-C3haloalkyl having from 1 to 7 halogen atoms, cyclopropyl,
halocyclopropyl, C2-C3alkenyl, C2-C3alkynyl, Cl-C3alkoxy, C2-C3haloalkenyl and
C2-C3haloalkynyl each having from 1 to 4 halogen atoms, Cl-C3haloalkoxy having
from 1 to 7 halogen atoms, Cl-C3alkylthio, Cl-C3haloalkylthio having ~rom 1 to 7halogen atoms, allyloxy, propargyloxy, allylthio, propargylthio, haloallyloxy, halo-
allylthio, halogen, cyano and nitro; or 3-pyridyl substituted by one or two radicals
from the group Cl-C3haloalkyl having from 1 to 7 halogen atoms, cyclopropyl,
halocyelopropyl, C2-C3alkenyl, C2-C3alkynyl, C2-C3haloalkenyl and C2-C3-
haloalkynyl each having from 1 to 4 halogen atoms, Cl-C3haloalkoxy having from 1to 7 halogen atoms, Cl-C3alkylthio, Cl-C3haloalkylthio having from 1 ts) 7 halogen
atoms, allyloxy, propargyloxy, allylthio, propargylthio, haloallyloxy, haloallylthio,
eyano and nitro, or by from one to four radieals from the group Cl-C3alkyl,
Cl-C3alkoxy and halogen;
and salts thereof with inorganic acids.
The compounds of formula I aeeording to the invention also include the salts thereof with
agrochemically tolerable inorganic acids. Examples of such acids are hydrochloric aci(l,
hydrobromic aeid, sulfurie aeid, phosphorie aeid and nitric acid, and also acids hllving the
same central atom and higher or lower degrees of oxidation, such as perchloric aci(l,
nitrous acid or phosphorous acid.
The compounds of forrnula I can occur in talltomeric forms la or Ib whell the l-ldic.ll R2 is
hydrogen:
~a273~
- 3 -
R1
CH-A
02N-N =< ~N--R3 (la)
X
1 1
CH-A
N ~
02N-NH~\ ~N--R3 (Ib).
N
The compounds of formula I can also occur as double-bond isomers with respect toN=C(2).
Formula I according to the invention is therefs)re to be understood as including formulae Ia
and Ib and the double-bond isomers.
In the definition of forrnula I according to the invention, the individual generic terms are
to be understood as having the following meanings:
The halogen atoms that come into consideration as substituents are fluorine and chlorine
and also bromine and iodine, with fluorine, chlorine and bromine being preferred.
Halogen is here to be understood as being an independent substituent or part of a
substituent, such as in haloalkyl, haloalkylthio, haloalkoxy, halocycloalkyl, haloalkenyl,
haloalkynyl, haloallyloxy or haloallylthio. 'I'he alkyl, alkylthio, alkenyl, alkynyl and
alkoxy radicals that come into consideration as substit~lents can be straight-chain or
branched. There may be mentioned as examples of such alkyl radicals methyl, ethyl,
propyl, isopropyl, butyl, isobutyl, sec-butyl and tert-b~ltyl. Suitable alkoxy radicllls are
inter alia: methoxy, ethoxy, propoxy, isopropoxy, or butoxy and its isomers. Alkylthio is,
for example, methylthio, ethylthio, isopropylthio, propylthio or the isomers of butyltllio.
If the alkyl, alkoxy, alkenyl, alkynyl or cycloalkyl groups that come hlto consider,ltion as
substituents are substituted by halogen, they may be only parti.llly h.llogenace(l or altern,l-
tively per-halogenated. Halogen, alkyl and alicoxy here have the definitions given above.
Examples of the alkyl elements of those groups are methyl substitute(l fiom one to three
7 3 ~
- 4 -
times by fluorine, chlorine and/or by bromine, for example CHr2 or CF3; ethyl substiluted
from one to five times by fluorine, chlorine and/or by bromine, for example CH2CF3,
CF2CF3, CF2CCI3, CF2CHC12, CF2CHF2, CF2CFC12, CF2CHBr2, CF2CHCl~, CF2CHBrF
or CCIFCHCIF; propyl or isopropyl substituted from one to seven times by fluorine,
chlorine ancVor by bromine, for example CH2CHBrCH2Br, CF2CHFCF3, CH2CF2CF3 or
CH(CF3)2; butyl or one of its isomers substituted from one to nine times by fluorine,
chlorine and/or by bromine, for example CF(CF3)C~IFCF3 or CH2(CF2)2CF3; 2-chloro-
cyclopropyl or 2,2-difluorocyclopropyl; 2,2-difluorovinyl, 2,2-dichlorovinyl,
2-chloroalkyl, 2,3-dichlorovinyl or 2,3-dibromovinyl.
If the alkyl, alkoxy or cycloalkyl groups defined are substituted by other substituents, they
may be mono- or poly-substituted by identical or different substituents selected from those
listed. Preferably, one or two other substituents are present in the substituted groups. The
cycloalkyl radicals that come into consideration as substituents are, for example,
cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl. Alkenyl and alkynyl groups contain
one unsaturated carbon-carbon bond. Typical examples are allyl, methallyl or prop~Lrgyl,
but also vinyl and ethynyl. The double or triple bonds in allyloxy, propargyloxy, allylthio
or propargylthio are separated from the point of linkage to the hetero atom (O or S)
preferably by a saturated carbon atom.
Of the compounds of formula I defined above, prominence is to be given to those wherein
the radical
R3 is Cs-CIOalkyl; C3-C6cycloalkyl; C1-ClOalkyl substituted by from 1 to 12 radicals
from the group halogen, hydroxy, C1-C4alkoxy, C1-C4haloalkoxy having from 1 to 9halogen atoms, di-(C1-C4alkyl)amino and Cl-Csalkoxycarbonyl; C3-C6cycloalkyl
substituted by from 1 to 4 Cl-C4alkyl radicals or halogen atoms; C2-C8alkenyl orC2-C8alkynyl; C2-C8alkenyl or C2-C8alkynyl each of which is substituted by from 1
to 6 halogen atoms; phenyl; benzyl; or phenyl or benzyl each of which is substituted
by from 1 to 3 ring substituents from the group halogen, Cl-('4alkyl, Cl-C4hil10alkyl
having from 1 to 9 halogen atoms, Cl-C4ialkoxy, Cl-C4haloalkoxy hiaving from 1 to 9
halogen atoms, Cl-C4alkylthio, nitro and cyiano; an(l Rl, R2 and A are as defil-e(l
above.
Of special importance according to the invention are also those compouncls of formuhl I
wherein the heterocyclic radical A is unsat-lrated, is bonded v a a carbon atom to the
radical of the molecule of the compound of formula I and contains at least one nitrogen
3 ~
atom; those compounds wherein the heterocyclic radical A is unsaturated, is bonded via a
carbon alom to the radical of the molecule of the compound of formula I and contains
from one to three hetero atoms from the group oxygen, sulfur and nitrogen, not more than
one oxygen or sulfur atom being present; and those compounds wherein the heterocyclic
radical A contains from one to three hetero atoms from the group oxygen, sulfllr and
nitrogen, of which one hetero atom is always nitrogen, not more than one oxygen atom or
sulfur atom being present.
The ring systems covered by the derlnition of the heterocyclic radical A are signific.lrlt as
regards the biological activity of the compounds of formula I according to the invention.
These ring systems contain at least one hetero atom as ring member, that is to say at least
one of the atoms forming the basic cyclic structure is other than carbon. In principle, all
atoms of the periodic system of the elements are capable of acting as ring members,
provided they are able to form at least two covalent bonds. The heterocyclic radical is
preferably unsaturated and bonded to the basic structure of formula I via a carbon atom as
ring member. Unsaturated ring systems of the definition A contain one or more double
bonds; such ring systems are preferably polyunsaturated and are generally of aromatic
nature. Preference is given to ring systems that contain at least one nitrogen atom as hetero
atom. Such rings of the definition A usually contain from one to three hetero atoms from
the group oxygen, sulfur and nitrogen, not more than one oxygen or sulfur atom being
present. Preference is given to ring systems of the definition of A wherein the heterocyclic
radical A contains from one to three hetero atoms from the group oxygen, sulfur and
nitrogen, of which one hetero atom is always nitrogen, not more than one oxygen atom or
sulfur atom being present. Examples of heterocycles of definition A according to the
invention are to be found especially in the following group of basic structures:
- 6 -
o~ o~ s 3 o~ s ~ S ~ 5 3 S d~ N
E E
O ~ S~o3 S~J o 3 o~ 3 '
0~ S Jl S ~3 o~N 3 o~ o i s ~;3 S ~ N 3
E E
N~\N N~ 3, D~ ~N ~ N~ 3
sd~oJ o~o o oo o-- o~s
~ ~N S~ N~ 3 ~ ~N ~
E E E
s ~ o3 s ~o J s ~o-- s J\ s 3 s ~ s J
S ~ S ~S ~1 N 3 S ~ N S ~ N
E E E
2 ~ ~ 2 1 3 :l
N ~3 N~;;3 [~3 ~3
1~3 ,N0~3 ' N~3 , Nf~3 .
N ~ ~N ~3 ~ N ~ N . ~ N
N 1~ N N
~N~ ~N~ ~N ~ N N . N~ ~N ~ N~ J
ND--IN~
O O O Y S
~N ~~N . ~, ~3 ~,
S S Y
N~ N~ N--~ 1 ~N~
I~NJ~N JJ O J~N ~ I~ S J~`N ~ N J~N
- 8 -
~S /~N~I Nrl~3
N~N ~3 N~N ~ N~N ~ N~N
o~N O~NlS O~N I S~N
N~\N N~\N
S ~Nl~l S ~\N~ ~3
In the above formulae, E is C1-C3alkyl and Y is hydrogen, C1-C3alkyl or cyclopropyl.
The heterocycles A listed as examples above can be unsubstituted or, depending on the
number of substituents possible in the ring sys~em, can carry up to four of the substituents
indicated under formula I. Preferab]y, these heterocycles carry from one to three
substituents fr~m the group halogen, Ci-C3alkyl, Cl-C3haloalkyl and Cl-C3haloalkoxy
each having from 1 to 7 halogen atoms, and Cl-C3aLkoxy. Especially preferred hetero-
cycles A are pyridyl radicals or thiazolyl radicals, for example 3-pyridyl, 2-halopyrid-5-yl,
2,3-dihalopyrid-5-yl, 2-halothiazol-4-yl, 1-oxopyrid-3-yl, 1-oxo-2-halopyrid-5-yl and
1 -oxo-2,3-dihalopyrid-5-yl.
In the compounds of formula I, the radical B is preferably a phenyl, pyridyl or thiazolyl
radical that can be unsubstituted or substituted by one or two radicals from the group
halogen, Cl-C3alkyl, Cl-C3haloalkyl and Cl-C3h,lloalkoxy each having from I to 7halogen atoms, and Cl-C3alkoxy.
Of the compounds of fonnula 1, prominence is to be given, on account of their biological
properties, to those compounds wherein Rl is hydrogen, R2 is mcthyl, ethyl or
cyclopropyl, and A is pyridyl, l-oxopyridyl or thiazolyl, or is pyridyl. l-oxopyri(lyl or
thiazolyl each of which is substituted by frs)m one to three substituents from tl-e group
halogen, Cl-C3alkyl, Cl-C3haloalkyl and Cl-C31-aloalkoxy e.lch having from 1 to 7
~5~7cl~
halogen atoms, and Cl-C3alkoxy. Within this meaning, also of interest are those
compounds of formula I wherein
a) Rlis hydrogen; and/or
b) R2 is methyl; and/or
c) R3is cl-c3alkyl~ cyclopropyl, cyclohexyl, phenyl, benzyl or the radical
-CH2-COO-CH3.
Also of intercst in accordance with the invention are those classes of compo-1nd of
formula I wherein
R3 is benzyl or phenyl each of which is substituted by from I to 3 ring substituents from
the group fluorine, chlorine, bromine, Cl-C2alkyl, Cl-C2haloalkyl, Cl-C2alkoxy,
Cl-C2alkylthio, nitro and cyano;
R3 is Cl-C6alkyl substituted by a hydroxy group;
R3 is Cl-C6alkyl substituted by a Cl-Csalkoxycarbonyl group;
R3 is-CH2CH2F,-CH2CH2Br,-CH2CH2CH2Cl,-CH2CH2CH2Br or
-CH2CHClCH2CH2CH2Cl;
R3 is-CH2CH20 CH3,-CH2CH2CH2-O-CH2CH3,-CH(CH3)CH2-O-CH3,
-CH2CH(OCH3)2, -cH2cH2-N(cH2cH3)2~ -CH2-CI~2CH2-N(CH3)2 or
-CH2CH2CH2-N(CH2CH3)2;
R3 is c4-c6cycloalkyl that is unsubstituted or substituted by one or two Cl-C4alkyl
radicals;
R3 is cyclopentyl or cyclohexyl;
R3 is c3-c6cycloalkyl substituted by one or two methyl groups;
A is 2-chlorothiazol-4-yl, 2,3-dichloropyrid-S-yl, l-oxopyrid-3-yl or
l-oxo-2-chloropyrid-5-yl; R2 is methyl and R3iscyclopropyl~-cH
-CH2CH(OCH3)2 or -CH2CH2N(CH3)2;
A is 2-chlorothiazol-4-yl;
A is 2-chloropyrid-S-yl; or
A is 2-chloropyrid-S-yl, 2,3-dichloropyrid-S-yl, 2-chlorothiazol-4-yl, 1-oxopyrid-3-yl or
l-oxo-2-chloropyrid-5-yl; Rlis hydrogen; R2 is methyl; and R3 is n-propyl.
The compounds of formula I according to the invention can be preparc~l by, ior examplc,
a) reacting a compound of formula Il
~5~73~
~o -
Rl
I
NH--CH--A
02N--N =< (II)
12
with forrnaldehyde, or paraformaldehyde, and a compound of forrnula Ill
H2N-R3 (III);
or
b) reacting a compound of formula IV
02N-N =~ ~N--R3 ~IV)
N
R2
with a compound of formula V
l1
X--CH--A (V);
or
c) for the preparation of a compound of ~ormula I wherein R2 is other than hydrogen,
reacting a resulting compound of formul.l I wherein R2 is hydrogen with il compou
of forrmula VI
Y-R2 (Vl);
and, if desired, converting a resultillg compoulld of fomlula I into a salt thereof in a
7'~ ~
- 11
manner known ~ se; R1, R2, R3 and A in formulae II to VI being as defined above, X
being halogen and Y being a leaving group. There may come into consideration as
leaving groups X and Y, for example: halogen, preferably chlorine, bromine or iodine, or
sulfonic acid radicals, such as alkanesulfonic acid radicals, mesylate or tosylate.
Variant a) of the above process according to the invention is advantageously carried out
under normal pressure, but may also be carried owt under clevatcd pressure, in an inert
solvent and at temperatures of from 0C to +140C, especially from +20C to +12()C.
Suitable solvents are especially alcohols, such as mcthanol, ethanol and yropanol, ~mcl also
water. Other suitable solvents are, for example, aromatic hydrocarbons, such as benzene,
toluene and xylene; ethers, such as tetrahydrofuran, dioxane and diethyl ether; halogenatecl
hydrocarbons, such as methylene chloride, chloroforrn, carbon tetrachloride and
chlorobenzene, and other solvents that do not impair the reaction. The solvents may also
be used as mixtures. The reaction may be carried out with the addition of an acid catalyst,
such as HCl, H2SO4, or a sulfonic acid, such as p-toluenesulfonic acid. The resulting
water of reaction can, where necessary, be removed by means of a water separator or by
the addition of a molecular sieve.
The above-mentioned process variants b) and c) can preferably be carried out under
normal or slightly elevated pressure znd in the presence of preferably aprotic solvents or
diluents. Suitable solvents or diluents are, for example, ethers and ethereai compounds,
such as diethyl ether, dipropyl ether, dibutyl ether~ dioxane, dimethoxyethane and
tetrahydrofuran; aliphatic, aromatic and halogenated hydrocarbons, especially benzene,
toluene, xylene, chloroform, methylene chloride, carbon tetrachloride and chlorobenzene;
nitriles, such as acetonitrile or propionitrile; dimethyl sulfoxide and dimethylformamide.
The processes are generally carried out at a temperature of from -20 to +140C, preferably
from 0 to +120"C, preferably in the presence of a base. Examples of suitable bases arc
carbonates, such as sodium and potassium carbonate. Hydridcs, such as sodiurn hydridc,
potassium hydride and calcium hydride, can also be used as bascs.
The starting materials of formulae II, III, V and Vl arc kllown or cal- bc plcp;llcd
analogously to known processes.
The 2-nitroguanidine derivatives uscd as starting m;lteri;lls of formul;l Il, all(l the
preparation thereof, are known from EP Patent Applications 375 9()7 an~l 376 279. The
primary amines of forrnula III are products that are readily avail;lbîe commercially.
2 ~ ~ ~ rt 3 ~
- 12-
The 2-nitroimino- 1,3,5-eriazoles of folmula IV, to which the present invention also relates,
are obtainable by reacting a 2-nitroguanidine of formula VII
NH2
O2N N=< (VII)
NH
l2
with formaldehyde, or paraformaldehyde, and a compound of formula III
H2N-R3 (III),
R2 and R3 in forrnulae VII and III being as defined above. The reaction conditions for this
process are the same as those for process variant a) above for the preparation of the
compounds of formula I. The compounds of formula IV are novel with the exception of
2-nitroimino-5-methyl-1,3,5-eriazacyclohexane (EP Patent Application 0 386 565) and
2-nitroimino-1,3,5-triazacyclohexane (US-PS 4 937 340). The nitroguanidines of
formula VII are known (see US-PS 4 804 780 and 4 221 802) or can be prepared in
analogous manner.
A large number of compounds of formula V are known (see, for example, EP Patent
Applications 375 907 and 376 279). There are preferred as starting materials those
compounds of formula V wherein X is chlorine.
Similarly, a large number of compounds of forrnula VI are known. They are products thae
are commercially available or that are readily obtainable analogously to known processes.
The leaving group Y in those compounds is preferably a halogen atom, especially
chlorine.
It is already known that some open-chained 2-nitroguani(line dcrivatives have pesticidal
properties (see, for example, EP Patent Applications 0 375 907 and 0 376 279). I-lowever,
pesticidal heterocyclic compounds based on a nieroguallidine stmcture are also known.
For example, EP Patent Applicatiol1s 0 192 060 and 0 259 738 dcscribe
2-nieroiminopyrimidine derivatives having insecticidal activity. Furtllermore, in
US-PS 4 937 340, 2-nitroimino-1,3,5-triazacyclohexane and other corresponding
~ ~3 ~i ~ ,Y 3
- 13 -
derivatives containing nitro groups are proposed as additives for explosives. Insecticidal
compounds of the type according to the invention are propose~ in EP Patent
Application 0 386 565, the compounds of formula I according to the invention being
partially covered by the broad scope of the claims of this EP Patent Application.
Surprisingly, it has been found that the compouncls of formula I according to the invention
are valuable active ingredients in pest control while being well tolerated by warm-blooded
animals, rlsh and plants. The compollnds according to the invention can be uscd
especially against insects that occur on us~ful plants and ornamentals in agriculture, espe-
cially in cotton, vegetable and fruit crops, in forestry, in the protection of stored ~oods and
material stocks, and also in the hygiene sector, especially on domestic animals and
productive livestock. The compounds are effective especially against plant-destructive
sucking insects, especially against aphids and cicadas. They are effective against all or
individual development stages of normally sensitive and also resistant species. Their
action may manifest itself in the death of the pests immediately or only at a later date, for
example at moulting, or in reduced oviposition and/or a reduced hatching rate. The
above-mentioned pests include:
of the order Lepidoptera, for example
Acleris spp., Adoxophyes spp., Aegeria spp., Agrotis spp., Alabama argillaceae, Amylois
spp., Anticarsia gemmatalis, Archips spp., Argyrotaenia spp., Autographa spp., E~usseola
fusca, Cadra cautella, Carposina nipponensis, Chilo spp., Choristoneura spp., Clysia
ambiguella, Cnaphalocrocis spp., Cnephasia spp., Cochylis spp., Coleophora spp.,Crocidolomia binotalis, Cryptophlebia leucotreta, Cydia spp., Diatraea spp., Diparopsis
castanea, Earias spp., Ephestia spp., Eucosma spp., Eupoecilia ambiguella, Euproctis spp.,
Euxoa spp., Grapholita spp., Hedya nubiferana, Heliothis spp., Hellula undalis, Hyphantria
cunea, Keiferia Iycopersicella, Leucoptera scitella, Lithocollethis spp., Lobesia botrana,
I,ymantria spp., Lyonetia spp., Malacosoma spp., Mamestra brassic.le, Mallduca scxta,
Operophtera spp., Ostrinia nubilalis, Pamrnene spp., Pandemis spp., Pallolis nammea,
Pectillophora gossypiella, Phthorimaea operc-llclla, Pieris rap.le, Pieris spp., Plutcll.l
xylostella, Prays spp., Scirpophaga spp., Sesamia spp., Sparg.lnothis spp., Spodol)tera spp.,
Synanthedon spp., Thaumetopoea spp., Tortrix spp., Trichopl~lsi.l ni al-d Ypollolllcllta
spp.;
of the order Coleoptera, for examplc
Agriotes spp., Anthonomus spp., Atom;lria line~uris, Ch.lctocllema libialis, Cos-nopolitcs
spp., Curculio spp., Dermestes spp., Diabrotic.l spp., Epilaclln,l spp., Eremnus spp.,
Leptinotarsa decemlineata, Lissorhoptrlls spp., Melolontll,l spp., Orycaepllil~ls spp.,
7 ~ ~
- 14-
Otiorhynchus spp., Phlyctinus spp., Popillia spp., Psylliodes spp., Rhizopertha spp., Scara-
beidae, Sitophilus spp., Sitotroga spp., Tenebrio spp., Tribolium spp. and Trogoderma
spp.; of the order Orthoptera, for example Blatta spp., Blattella spp., Gryllotalpa spp.,
Leucophaea maderae, Locusta spp., Periplaneta spp. and Schistocerca spp.;
of the order Isoptera, for example
Reticulitermes spp.; of the order Psocoptera, for example Liposcelis spp.; of the order
Anoplura, for example Haematopinus spp., Linognathus spp., Pedictllus spp., Pemphigus
spp. an(l Phylloxera spp.; of the or(ler Mallophaga, ~or example Damalinea spp. and
Trichodectes spp.;
of the order Thysanoptera, for example
Frankliniella spp., Hercinothrips spp., Taeniothrips spp., T hrips palmi, Thrips tabaci and
Scirtothrips aurantii;
of the order Heteroptera, for example
Cimex spp., Distantiella theobroma, Dysdercus spp., Euchistus spp., Eurygaster spp.,
Leptocorisa spp., Nezara spp., Piesma spp., Rhodnius spp., Sahlbergella singularis,
Scotinophara spp. and Triatoma spp.;
of the order Homoptera, for example
Aleurothrixus floccosus, Aleyrodes brassicae, Aonidiella spp., Aphididae, Aphis spp.,
Aspidiotus spp., Bemisia tabaci, Ceroplaster spp., Chrysomphalus aonidium,
Chrysomphalus diclyospermi, Coccus hesperidum, Empoasca spp., Eriosoma larigerum,
Erythroneura spp., Gascardia spp., L.aodelphax spp., Lecanium corni, Lepidosaphes spp.,
Macrosiphus spp., Myzus spp., Nephotettix spp., Nilaparvata spp., Paratoria spp.,
Pemphigus spp., Planococcus spp., Pseudaulacaspis spp., Pseudococcus spp., Psylla spp.,
Pulvinaria aethiopica, Quadraspidiotus spp., Rhopalosiphum spp., Saissetia spp.,Scaphoideus spp., Schizaphis spp., Sitobion spp., Trialeurodes vaporariorum, Trioza
erytreae and Unaspis citri;
of the order Hymenoptera, for example
Acromyrmex, Atta spp., Cephus spp., Diprion spp., Diprionidae, Gilpini.l polytoma,
Hoplocampa spp., Lasius spp., Monomorium pharaonis, Neodiprion spp., Solenopsis sPI).
and Vespa spp.;
of the order Diptera, for example
Aedes spp., Antherigona soccata, Bibio hortllhll1lls~ Callipllor.l crytllroccpll.ll.l, Ccr.ltitis
spp., Chrysomyia spp., Culex spp., C~lterebra spp., Dacus spp., Drosopllila mel,lnog.lster,
Fannia spp., Gastropl1ilus spp., Glossina spp., llypodcrm.l spp., Ilyppobosc,l spp.,
Liriomyza spp., Lucilia spp., Melanagromyza spp., Musca spp., Oestrus spp., Orscolia
spp., Oscinella frit, Pegomyia hyoscyalni, Phorbi.l spp, Rh.lgoletis pomonella, Sciara spp.,
7 ~ ~
- 15-
Stomoxys spp., Tabanus spp., Tannia spp. and Tipula spp.;
of the order Siphonaptera, for example
Ceratophyllus spp., Xenopsylla cheopis;
of the order Acarina, for example
Acarus siro, Aceria sheldoni, Aculus schlechtendali, Amblyomma spp., Argas spp.,Boophilus spp., Brevipalpus spp., Bryobia praetiosa, Calipitrimerus spp., Chorioptes spp.,
Dermanyssus gallinae, Eotetranychus carpini, Eriophyes spp., Hyalomma spp., Ixodes
spp., Olygonychus pratensis, Ornithodoros spp., Panonychus spp., Phyllocoptruta oleivora,
Polyphagotarsonemus lat~ls, Psoroptes spp., Rhipicephalus spp., Rhizoglyphus spp.,
Sarcoptes spp., Tarsonemus spp. and Tetranychus spp.; and
of the order Thysanura, for example
Lepisma saccharina.
The good pesticidal activity of the compounds of formula I according to the invention
corresponds to a mortality of at least 50-60 % of the mentioned pests.
The activity of the compounds of the invention and of the compositis)ns comprising them
can be substantially broadened and adapted to prevailing circumstances by the addition of
other insecticides and/or acaricides. Examples of suitable additives include
representatives of the following classes of compounds: organophosphorus compounds,
nitrophenols and derivatives thereof, forrnamidines, ureas, carbamates, pyrethroids,
chlorinated hydrocarbons, and Bacillus thuringiensis preparations.
The compounds of forrnula I are used in unmodified form or, preferably, together with the
adjuvants conventionally employed in formulation technology, and can therefore be
formulated in known manner e.g. into emulsifiable concentrates, directly sprayable or
dilutable solutions, dilute emulsions, wcttable powders, soluble powders, dusts, granules,
and also encapsulations in polymer substances. As with the compositions, the methods of
application, such as spraying, atomising, dusting, scattering or pouring, are chosen in
accordance with the intended objectives and the prevailing circumstances. The
compounds of formula I are also suitable for use in the treatment of seed. For this purpose
it is possible either to treat or dress the seed with the active ingrediellt or with a formu-
lation comprising the active ingredient before sowing, or to apply the active ingredient
into the seed furrow at the time of sowing.
The formulations, i.e. the compositions, preparations or mixtures comprising the
2 ~ 3 ~
compound (active ingredient) of fomlula I, or combinations of those compounds wilh
other insec-icides or acaricides, and, where appropriate, a solid or liquid adjuvant, are
prepared in known manner, e.g. by homogeneously mixing and/or grinding the active
ingredients with extenders, e.g. solvents, solid carriers and, where appropriate, surface-
active compounds (surfactants).
Suitable solvents are: aromatic hydrocarbons, preferably the C8 to Cl2 fractions of
alkylbenzenes, e.g. xylene mixtures or alkylated naphthalenes, aliphatic or cycloaliphatic
hydrocarbons such as cyclohexane, paraffins or tetrahydronaphthalene, alcohols such as
ethanol, propanol or butanol, and glycols and their ethers and esters, such as propylene
glycol, dipropylene glycol ether, ethylene glycol, ethylene glycol monomethyl ormonoethyl ether, ketones such as cyclohexanone, isophorone or diacetone alcohol,strongly polar solvents such as N-methyl-2-pyrrolidone, dimethyl sulfoxide or
dimethylforrnamide, or water, vegetable oils such as rape oil, castor oil, coconut oil or
soybean oil; and, where appropriate, silicone oils.
The solid carriers used, e.g. for dusts and dispersible powders, are normally natural
mineral ~Illers such as calcite, talcum, kaolin, montmorillonite or attapulgite. In order to
improve the physical properties it is also possible to add highly dispersed silicic acids or
highly dispersed absorbent polymers. Suitable granulated adsorptive carriers are porous
types, for example pumice, broken brick, sepiolite or bentonite; and suitable nonsorbent
carriers are, for example, calcite or sand. In addition, a great number of granulated
materials of inorganic or organic nature can be used, e.g. especially dolomite or pulverised
plant residues.
Depending on the nature of the compound of forrnula I to be formul.lted, or of the
combinations of those compounds with other insecticides or acaricides, suitable
surface-active compounds are non-ionic, cationic and/or anionic surfactants havillg good
emulsifying, dispersing and wetting properties. The term "surfactants" will also be
understood as comprising mixtures of surfact.mts.
Both so-called water-soluble soaps and water-solllble synthetic sulface-active co~ )0llnds
are suitable anionic surfactants.
Suitable soaps are the alkali metal salts, alk.lline earth metal salts or unsllbstitllte~l or
substituted ammonium salts of higher fatty acids (C1o-C22), e.g. the sodillm or potassium
7 ~ :~
salts of oleic or stearic acid, or of natural fatty acid mixtures which can be obtained e.g.
from coconut oil or tall oil. Mention may also be made of fatty acid methyltaurin salts.
More frequently, however, so-called synthetic surfactants are used, especially fatty
sulfonates, fatty sulfates, sulfonated benzimidazole derivatives or alkylarylsulfonates.
The fatty sulfonates or sulfates are usually in the form of alkali metal salts, alkaline e~rth
metal salts or unsubstituted or substitute-1 arnmonium salts ancl gencrally contain a
C8-C22alkyl radical, which also includes the alkyl moiety of acyl raclicals, e.Lg the soclium
or calcium salt of lignosulfonic acicl, of dodecyl sulfate or of a mixture of fatty alcohol
sulfates obtained from natural fatty acids. These compounds also comprise the salts of
sulfated and sulfonated fatty alcohol/ethylene oxide adducts. The sulfonated benz-
imidazole derivatives preferably contain 2 sulfonic acid groups and one fatty acid radical
containing approximately 8 to 22 carbon atoms. Examples of alkylarylsulfonates are the
sodium, calcium or triethanolarnine salts of dodecylbenzenesulfonic acid,
dibutylnaphthalenesulfonic acid, or of a condensate of naphthalenesulfonic acid and
formaldehyde. Also suitable are corresponding phosphates, e.g. salts of the phosphoric
acid ester of an adduct of p-nonylphenol with 4 to 14 mol of ethylene oxide, or
phospholipids.
Non-ionic surfactants are preferably polyglycol ether derivatives of aliphatic or
cycloaliphatic alcohols, saturated or unsaturated fatty acids and alkylphenols, said
derivatives containing 3 to 30 glycol ether groups and 8 to 20 carbon atoms ir. the
(aliphatic) hydrocarbon moiety and 6 to 18 carbon atoms in the alkyl moiety of the
alkylphenols. Further suitable non-ionic surfactants are the water-soluble adducts of
polyethylene oxide with polypropylene glycol, ethylenediaminopolypropylene glycol and
alkylpolypropylene glycol containing 1 to 10 carbon atoms in the alkyl chain, which
adducts contain 20 to 250 ethylene glycol ether groups anci 10 to 10() propylene glycol
ether groups. These cornpounds usually contain 1 to 5 ethylene glycol units pcr propylene
glycol unit.
Representative examples of non-ionic surf.lct.lnts are nonylpllcnolpolyctlloxyetl~ lols~
castor oil polyglycol ethers, polypropylene/polycthylelle oxicle aciclllcts, tributylpilelloxy-
polyethoxyethanol, polyethylene glycol and octylphenoxypolyethoxycth;lnc)l. Fatty acid
esters of polyoxyethylene sorbitan, e.g. polyoxyethylcne sorbitan triolcate, are also
suitable non-ionic surfactants.
~273~
- 18-
Cationic surfactants are preferably quaternary ammonium salts which contain, as
N-substituent, at least one C8-C22alkyl radical and, as further substituents, unsubstituted or
halogenated lower alkyl, benzyl or hydroxy-lower alkyl radicals. The salts are preferably
in the form of halides, methyl sulfates or ethyl sulfates, e.g. stearyltrimethylammonium
chloride or benzyldi(2-chloroethyl)ethylammonium bromide.
The surfactants customarily employed in formulation technology are describcd, for
example, in the following publications:
"McCutcheon's Detergents and Emulsifiers Annual", MC Publishing Corp., (:~len Rock,
NJ, USA, 1988",
H. Stache, "I'ensid~Taschenbuch", 2nd edition, 1:~. Hanser Verlag, Munich, Vienna, 1981,
M. and J. Ash, "Encyclopedia of ~urfactants", Vol. I-III, Chemical Publishing Co., New
York, 1980-1981.
The pesticidal compositions usually comprise 0.1 to 99 %, preferably 0.1 to 95 %, of a
compound of formula I or combinations of that compound with other insecticides or
acaricides, 1 to 99.9 % of a solid or liquid adjuvant, and 0 to 25 %, preferably 0.1 to 25 %,
of a surfactant. Whereas commercial products will preferably be formulated as concen-
trates, the end user will norrnally employ dilute formulations comprising considerably
lower active ingredient concentrations. Typical application concentrations are from 0.1 to
1000 ppm, pre~erably from 0.1 to 500 ppm. The rates of application per hectare are
generally from 1 to 1000 g of active ingredient per hectare, preferably from 25 to
500 g/ha.
Preferred formulations have espccially the following compositions (tlllc)-lgllollt,
percentages are by weight), active ingrcdicnt being understood as mc,l~ g a compoun(l of
formula I:
Emulsifiable concentrates:
active ingredient: 1 to 90 %, prefer,lbly 5 to 20 /0
surface-active
agent: 1 to 3() %, preferably 10 lO 2() %
liquid carrier: 5 to 94 %, prefcrably 70 to X5 %
~27~
- 19-
Dusts:
active ingredient: 0.1 to 10 %, preferably 0.1 to 1 %
solid carrier: 9~.9 to 90 %, preferably 99.9 to 99 %
Suspension concentrates:
active ingredient: 5 to 75 %, preferably 10 to S() %
water: 94 to 24 %, preferably 88 to 30 %
surface-active
agent: 1 to ~0 %, preferably 2 to 30 %
Wettable powders:
active ingredient: 0.5 to 90 %, preferably 1 to 80 %
surface-active
agent: 0.5 to 20 %, preferably 1 to 15 %
solid carrier: 5 to 95 %, preferably 15 to 90 %
Granules:
active ingredient: ~.5 to 30 %, preferably 3 to 15 %
solid carrier: 99.5 to 70 %, preferably 97 to 85 %
The compositions may also comprise further auxiliaries such as stabilisers, e.g. vegetable
oils or epoxidised vegetable oils (epoxidised coconut oil, rape oil or soybean oil), anti-
foams, e.g. silicone oil, preservatives, viscosity regulators, binders, tackifiers as well as
fertilisers or other active ingredients for obtaining special effects.
The following Examples serve to illustrate the invention, but do not limit the invention.
Example l_(~aration of starting materials of formula IV):
a) Preparation of 2-nitroimino-S-methyl-1~3,5-tria~acyclohexane.
~ mixture of 26.0 g of 2-nitroguanidine, 31.1 ml of an 8M solution of methylamine in
ethanol, 38 ml of a 37 % solution of formaldehyde in water, and 1()() ml of ethallol is
heated at 50C for 2 hours and then filtered. The crystals whicll have been f;ltered off ale
washed three times with 20 ml of ethanol each time and then drie(l, yieIding the title
cornpound, m.p. 173-175C, of the formul.l
2 ~ ~ 2 ~ ~1
- 20 -
N~
02N-N=< N--CH3 ~compoundno. 2.001).
N
H
b) Preparation of 1-methyl-2-nitroimino-S-n-pr~y 1 ~3,5-triazacvclohexane:
A mixture of 17.1 g of 1-methyl-2-nitroguanidine, 12.0 ml of n-propylamine, 22.0 ml of a
37 % solution of formaldehyde in water, and 40 ml of ethanol is heate(l ae 50C for
4 hours. A further 7.0 ml of n-propylamine and 13.0 ml of a 37 % solution of
formaldehyde in water are then added. After stilTing at 50C for 2 hours, the reaction
mixture is concentrated by evaporation in vacuo and the crystals which have separated out
are stirred with ether, yielding 26.9 g of the title compound, m.p. 84-86C, of the formula
H
N ~
02N-N =( N--C3H7 (n) (compound no. 2.011).
CH3
c) Preparation of 1-methyl-2-nitroin-1ino-5-phem/1-1,3,5-~iazacyclohexane:
Three drops of concentrated hydrochloric acid are added to a mixture of 2.36 g of
1-methyl-2-nitroguanidine, 2.11 ml of aniline and 1.80 g of paraformaldehyde in 30 ml of
toluene, and the mixture is then boiled in a water separator for 6 hours. The reaction
mixture is then concentrated by evaporation in vacuo and the resulting crude product is
recrystallised from methanol, yielding the title compound, m.p. 169- 172C, Or the formula
N
N
02N-N :=~ N--~ (compo~lnd no. 2.()44).
C~13
The following compounds of formula IV c~m be prepared as inclicatc(l above:
7 3 ~
Comp. R2 R3 Phys. data
No.
2.001 H Cl-I3 m.p. 173-175C
2.002 H -C2E-ls m.p. 181-182C
2.003 H -C3H7(n)
2.004 H CH(CH3)2
2.005 H ~1 m.p. 225-227C
2.006 H ~3
2.007
2.008 H --CH2 ~
2.009 CH3 -CH3 m.p. 134-135C
2.010 ~H3 -C2Hs m.p. 112C
2.011 CH3 -C3H7(n) m.p. 84-86C
2.012 CH3 -CH2(C~I3)2 m.p. 154C
2.013 CH3 ~ m.p. 177C
2.014 CH3 {~3 m.p. 103-104C
2.015 S~H3 -Cfills m.p. 169-172C
2.016 CH3 --CH2 ~ m.p. 161-163C
2.017 -C2Hs -CH3
2.018 -C2l-Is -C2H5 m.p. 95-96C
2.019 -C2Hs -C3H7(n)
2.020 -C2Hs -CH(CH3)2
7 3 :~
Comp. No. R2 R3 Phys. d~t~
-
2.021 -C2Hs -
2.022 -C2H
2.023 -C2H
2.024 -C2Hs - CH
2.025 ~ -C~13
2.026 ~ -C2Hs m.p.138-139C
2.027 ~ -C3H7~n)
2.028 ~ -CH(CH3)2
2.029
2.030
2.031
2.032 ~ -C~12 ~
2.033 - CH2 ~ -Ci 13 m.p. l()')-111C
2.034 - CH2 ~ -C2l-1s
7 ~ ~
- 23 -
Comp. No. R2 R3 Phys. data
2.035 --CH2 ~ -C3H7(n)
2.036 --CH2 ~ CH(CH3)2
2.037 --CH2 ~3 ~
2.038 --CH2 ~ {~>
2.039 --CH
2.040 --CH2 ~ --CH
2.041 H -CH2-COOCH3
2.042 -CH3 -CH2-COOCH3
2.043 ~ -CH2-COOCH3
2.044 -CH3 ~ m.p. 169-172C
2.045 -CH3 -CH2CF3
2.046 -CH3 -CH2CH2E~`
2.047 -CH3 -CH2CH2Br
2.048 -CH3 -CH2CH2C~-l2cl
2.049 -CH3 -CH2CH2CI 12Br
2.050 -Cl 13 -CH2CH2CI
2.051 -CH3 -Cl-12CH(CI)CH2CH2CI I~CI
2.0S2 -Cl-13 -CE-12CH2OH m.p. 121-123C
2.053 -CH3 -CH2CH2CI-I~OH
2.054 -CH3 -C~-12CH2CH2CH2OH m.p. X 1 -83C
2~27~
-24-
Comp.No. R2 R3 Phys.d~ta
2.055 -C~13 -CH2CH2Cl-I2c~-l2cH2OH
2.056 -CH3 -CH(CH3)CH30H
2.057 -CH3 -CH(C211s)CH2OH
2.058 -CH3 -CH2CH(CH3)OH
2.059 -CH3 -CH2CH(OH)CI-12OH
2.060 -CH3 -CH(CH2OH)2
2.061 -C~I3 -CH2CH2OCH3
2.062 -CH3 -CH2CH2CH2OC2Hs
2.063 -CH3 -CH(CH3)CH2OCH3
2.064 -CH3 -CH2CH(OCH3)2
2.065 -CH3 -CH2CH(OC2Hs)2
2.066 -CH3 -CH2CH2N(CH3)2
2.067 -c~3 -CH2CH2N(C2Hs)2
2.068 -CH3 -CH2CH2CH2N(CH3)2
2.069 -CH3 -CH2CH2CH2N(C2Hs)2
2.070 -CH3 -CH2COOC2Hs
2.071 -CH3 CH2CH2COOC2Hs m.p.llO-112C
2.072 -CH3 -CH(CH3)CH2C~OC2~s
2.073 -CH3 -CH(CH2OH)COOCH3
2.074 -CH3 ~
2.075 -CH3 ~ CH3 m.p.lsl-ls3oc(cisi~o~ r)
m.p.l38-14()~C(trl~sisomer)
2.076 -CH3 ~ CH3
CH3
CH3
>~
2.077 -CH3 ~ H>
- 25 -
Comp. No. R2 _R3 Phys. data
2.078 -C~13 -CH2-CH=CH2 m.p. S3-55C
2.079 -CH3 ~ - Cl
2.080 -CH3 ~F m.p. 170-173C
2.081 -CH3 ~OCH3 m.p. 174-176C
2.082 ~3 m.p.195-197C
2.083 -CH3 ~NO2 m.p.230C
2.084 -CH3 ~ CN m.p.222-226C
2.085 -CH3 ~CF3 m.p. 163-166C
NO2
2.086 -CH3 ~
SC~13
2.087 -CH
2.088 -CH
2~273~
- 26 -
Comp. No. R2 R3 Phys. data
_
NC
2.089 -CH3 ~3
2.090 -CH3-CH2 ~ No2 m.p. 235-238C
2.091 -CH3-CH2 ~ F m.p. 143-145C
2.092 -CH3-CH2 ~3OCH3 m.p. 132-134C
2.093 -CH3-CH2 ~ Cl m.p. 160- 162C
2.094 -CH3-CH2 ~ CH3 m.p. 161 - 163 C
2.095 -C~3-CH2 ~ CF3 m.p. 160- 162C
N02
2.096 CH3-CH2 ~3
2.097 -CH3-CH2 ~3
F
2.098 -Cl13 -CH2 ~ F
20~ii2r~3~
- 27 -
Comp. No. R2 R3 Phys. data
CN
2.099 -C~13 -C~l
Example 2:
a) Preparation of 1-(2-chloropyrid-5-ylmethyl)-2-nitroimino-S-ethyl-1~3 5-
triazacvclohexane:
A mixture of 1.15 g of 1-(2-chloropyrid-5-ylmethyl)-2-nitroguanidine, 0.75 ml of a 37 %
solution of formaldehyde in water, 0.32 ml of a 70 % solution of ethylamine in water, and
5 ml of ethanol is heated at 50C for 4 hours. The reaction mixture is then concentrated
by evaporation in vacuo, the residue is suspended in 20 ml of ethanol, and the resulting
crystals are filtered off, yielding the title compound, m.p. 125-126C, of the fs)rmula
CH2~ N
~--CI
02N-N=~ N--C2H5 (compoundno. 1.001).
H
b) Preparation of 1-(2-chloropyricl~lmethyl)-2-nitroimino-5-cyclop
1.3~S- riazacyclohexane:
A mixture of 2.96 g of 2-nitroimino-S-cyclopropyl-1,3,5-triazacyclohexanc7 2.59 g of
2-chloro-5-chloromethylpyridine and 2.43 g of potassium carbonate in 60 ml of
acetonitrile is heated under reflux for 16 hours. Thc resulting rcaction mixture is filtercd,
the filtrate is concentrated by evayoratioll in vacuo and the residue that forms is
chromatographed on silica gel with dichlorometh.me/ethyl acetate (1:1), yieldin,g the title
compound, m.p. 125-127C, of the formula
- 28 -
CH2~ N
N~ ~CI
02N-N=< ~ ~1 (compound no. 1.003).
H
c) Prepalation of 1-~chloropvrid-5-ylrnethyl)-2-nitroimino-3-n ethYI-5-n-proPy -1 3,5-
triazacyclohexane:
A mixture of 20.1 g of 1-methyl-2-nitroimino-5-n-propyl-1,3,5-triazacyclohexane, 16.2 g
of 2-chloro-5-chloromethylpyridine, 0.17 g of caesiwn chloride and 27.7 g of potassium
carbonate in 150 ml of DMF*) is heated at 110C for 9 hours and then filtered over Celite.
The filtrate is concentrated by evaporation in vacuo. The resulting crude product is
dissolved in 200 ml of dichloromethane and washed with 100 ml of water and 100 ml of
saturated sodium chloride solution, dried over magnesium suifate and then concentrated
by evaporation. The residue is recrystallised from ethyl acetate, yielding the title
compound, m.p. 137-138C, of the formula
CH2 ~ N
~CI
N ~
02N-N =~ N--C3H7(n) (compound no. 1.009).
N
CH3
~)dimethylformamide
d) Preparation of 1-(2-chloropyrid-5-y~ ethyl)-2-nitroimino-3,5-_(~
1 ~3,5-triazacvclohexane:
0.30 g of sodium hydride (80 % in white oil) is adcled to a solution of 3.12 g of
1-(2-chloropyrid-5-ylmethyl)-2-nitroimino-5-1l-propyl-1,3,5-tri.lz.lcyclollcx.lne in 5() ml of
acetonitrile. After stirring the reaction mixture at room tempcratllrc for 3 hours, 1.8 ml of
n-propyl iodide are added, and the reaction mixture is thel- stirrcd at room temperat-lre for
16 hours and at 80C for 2 hours. The residue obtained after concentr.l~ion by evllpor.ltior
in vacuo is taken up in 100 ml of ethyl acet.lte~ washed with 50 ml of saturatecl sodilln-
chloride solution, dried over magnesium sulf.lte and ag.lin concentrated by evapor.ltion.
2~5273~
- 29 -
The crystals obtained as residue are recrystallised at 0C from ethyl acetate, yielding the
title compound, m.p. 112-113C, of the formula
CH2 ~ N
~CI
02N-Na~ N--C3H7(n) (compound no. 1.025).
C3H7(n)
The following compounds of formula I can be prepared as indicated above:
2~ J31
- 30-
Comp. A Rl R2 R3 Phys. data
No.
1.001 ~ ~ H H -C2~Is m.p. 125-126C
1.002 ,¢~1' H H -C3H7(n) m.p. 115-117C
1.003 ,¢~1' H H ~ m.p. 125-127C
1.004 ,¢~1' H H {3 m.p. 150-151C
1.005 ,¢~J~ H H ~ m.p. 143-145C
1.006 ,¢;~ H H -CH2~ m.p. 108-110C
1.007 ,¢~/ H CH3 -c~3 amorphous mass
1.008 ,¢;~ H CH3 -C2lIs m.p. 124-125C
1.009 ,¢~1' H CH3 -C3H7(n) m.p. 137-138C
1.010 ,¢~1~ H CH3 -Cl-I(C~I3)2
1.011 ,¢~1' H -CH3 ~ m.p. 1()4-1()6C
1.012 ,¢~/ H -CH3 {~ > m.p. 146- 147~C
2 ~ 3 1
- 31 -
Comp. A Rl R2 R3 Phys. data
No.
1.013 ~/ H -CH3 ~ m.p. 146-149C
1.014 ,¢~1~ H -CH3 --CH2 ~ m.p. 116-118C
1.015 ,¢~1' H -C2Hs -CH3
1.016 ,¢~1~ H -C2Hs -CH2CH3 m.p. 113-114C
1.017 ,~/ H -C2Hs -C3H7(n)
1.018 ,¢~ H -C2Hs -CH(CH3)2
1.019 ~/ H -C2Hs ~1
1.020 ,¢;~1' H -C2Hs {~3
1.021 ,¢;~/ H -C2}Ts
1.022 ,¢~/ H -C2Hs --CH
1.023 ,¢~/ H -C3H7(n) -CH3
1.024 ,¢~1~ E-I -C3H7(") -C2Hs
~ ~ 5 ~
- 32 -
Comp. A Rl R2 R3 Phys. data
No.
1.025 ,¢~/ H -C3H7(n) -C3H7(n) m.p. 112-113C
1.026 ,¢;~/ H -C3H7(n) -Cll(CH3)2
1.027 ,¢~/ H -C3H7(n) ~
1.028 ,¢;~1' H -C3H7(n) {~3
1.029 ,¢;~)/ H -C3H7(n)
1.030 ,¢~1~ H -C3H7(n) _CH
1.031 ,¢;~/ H -CH(CH3)2 -CH3
1.032 l!~ J H -CH(CH3)2 _~2Hs
1.033 J!~ J H -CH(CH3)2 -C3H7(n)
1.034 ,¢;~ H -CH(CH3)2 -CH(CI-I3)2
1.035 ,¢~ H -CH(CI-I3)2 <I
1.036 J~ H -CH(CH3)2 ~3
2~527~
- 33 -
Comp. A Rl R2 ~3 Phys. data
No.
1.037 ,¢~1' H-CH(C~I3)
1.038 ,¢~ -CH(CH3)2 --CH
1.039 ,¢~1/ H <1 CH3
1.040 ,¢~J~ H <1 -C2Hs m.p. 115-116C
1.041 ,¢~J~ H ~ -C3H7(n~
1.042 ,¢~/ H ~ -CH(CH3)2
1.043 ,¢~ H <1 ~
1.044 ,¢~1' H ~ {~3
1.045 ,¢~1' H
1.046 ,¢~1' H--~ --Cl1
1.047 ,¢;~1' 11 H -CH3
~27
- 34 -
Comp. A Rl R2 R3 Phys. data
No.
_ . _ _ . . .. _
1.048 ,~ H H -C2Hs
Cl N
1.049 J~ H H -C3H7(n)
o
1.050J~ H H -CH(CH3)~
Cl N
1.051,¢~ H H
o
1.û52 ,¢~ H H {~3
o
1.053 ,¢~ H H
1.054J~ H H --CH
o
2 ~
Comp. A Rl R2 R3 Phys. data
No.
Cl J~ H CH3 -CH3
1.056,¢~ E-I CH3 -C2fls m.p. 152-155C
1.057,1~ H CH3 -C3H7(n) m.p. 117-121C
1.058,¢;~ H CH3 -CH(CH3)2 m.p. 138C
o
1.059 ,¢~ H CH
o
1.060 ,¢;~ H CH3 {~3 m.p. 153C
1.061 ,1~ H CH3 ~
7 3 ~
- 36 -
Comp. A Rl R2 R3 Phys. data
No.
1.062 ,¢~ H CH3 --CH
o
1.063 ,¢~ H ~ -CH3
1.064 ,¢~ H ~ -C2H5
o
1.065 ,¢~ H<1 -C3H7(n)
1.066 ,¢;~ H ~ -CH(CH3)2
1.067 ,¢~ H<1 <I
o
1.068 ,¢~¦~ H ~ { 3
o
2~2~3~
Comp. A Rl R2 R3 Phys. data
No.
_ _ _
1.0~9 ~ H ~ ~
r
1.070 ~ H ~ - CH
1.071 ~ H H -CH3
1.072 ~ H H ~2Hs
1.073 ~ H H -C3H7(n)
1.074 ~ H H -CH(CH3)2
1.075 ~ H H
1.076 ~ H H
1.077 ~ H H
3 ~
- 38 -
Comp. A ~ R2 ~3 Phys. data
No.
_ _
1.078 ~ H II --CH
1.079 )~/ H CH3 -CH3
1.080 )~/ H CH3 -C2Hs
1.081 ~/ H CH3 -C3H7(n)
1.082 ~ H CH3 -CH(CH3)2
1.083 )~/ H CH3 --<
1.084 ~/ H CH3
1.085 ~/ H CH
1.086 )~/ H CH3 --CH
1.087 ~/ H ~ -CH3
3 :~
- 39-
Comp. A Rl R2 R3 Phys. data
No.
1.088 ~/ H~ -C2Hs
1.089 ~ H~ -C3H7(n)
1.090 ~/ H~ -CH(CH3)2
1.091 ~/ H~ ~
1.092 ~ H~ { 3
1.093 ~ H<
1.094 ~/ H<1 --CH
1.095 CIJ~3~ H H -C2Hs
N
1.096 Cl~ H H -C3H7(n)
N
1.097 Cl~ H H -CH(CH3)2
1.098 Cl~ H H --
1.099 CIJ~ H H {~
3 ~
-40 -
Comp. A Rl R2 R3 Phys. data
No.
1.100 Cl~l~ H H
1.101 Cl~3~ H H --CH
1.102 Cl1~3~ H CH3 -CH3
1.103 Cl~ H CH3 -C2H5
1.104 Cl~ H CH3 -C3H7(n) amorphous
1.105 Cl~ H CH3 -CH(CH3)2
1.106 CIJ~ H CH3 ~1
1.107 Cl~3~ H CH3 {~3
1.108 CIJ~]~ H CH
1.109 CIJ~ H CH3 --CH
1.110 Cl~ H ~ -CH3
1.111 CIJ~3~ H ~ -C21 15
1.112 Cl~ H ~ -C3H7(n)
1.113 ClJ~ H ~ -CII(C~I3)2
1.114 Cl~l~ H ~ ~
1.115 Cl~]~ H ~ {~3
2~5~
- 41 -
Comp. A Rl R2 R3 Phys. data
No.
1.116 C~ ~ H ~ --
N
1.117Cl~ 3~ H ~ --C
1.118 ¢~ H H -CH3
b
1.119 ¢~ H H -C2Hs
o
1.120 ¢~ H H -C3H7(n)
o
1.121 ¢~ H H -CH(CH3)2
o
1.122 ¢~ H H
b
1.123 ¢~ H H {~>
1.124 ¢;~ H H
1.125 ¢~ H H --CH~
2~2~ ~
- 42 -
Comp. A Rl R2 R3 Phys. data
No.
_
1.126 ¢~ H H -CH3
1.127 ¢~ H CH3 -C2Hs
1.128 ¢~ H CH3 -C3H7(n) amorphous mass
1.129 ¢~ H CH3 -CH(CH3)2
1.130 ¢~ H CH3 ~ m.p. 18S~C
1.131 ¢;~ H CH3 {~3
1.132 ¢~ H CH
1.133 ¢~ H CH3 --CH
2~73~
- 43 -
Comp. A Rl R2 R3 Phys. data
No.
1.134 ¢~ CH3
1.135 ¢~ I I~ -C2115
1.136 ¢~ H <1 -C3H7(n)
1.137 ¢~ H ~ -CH(CH3)2
1.13~ H ~1 <1
1.139 ¢;~ H ~ ~3
1.140 ¢~
1.141 ¢~ H ~ ~C~12-~
- 44 -
Comp. A Rl R2 R3 Phys. data
No.
1.142 ¢~ H H -CH3 m.p. 142-144"C
1.143 ¢~ H -C~I3 -CH3
1.144 ¢;~ H -~ -CH3
1.145 ¢~ H H -C2Hs
1.146 ¢~ H -CH3 -C2H5
1.147 ¢;~ H <1 -C2Hs
1.148 ,¢~ H --CH2 ~ -C3H7(n) m.p. 127-129C
1.149 ,¢~ H - CH2~ -C3H7(n)
1.150 ,¢~ H - CH2~=~ Cl -C31-17(n)
1.151 ,¢~ H --CH2 ~ -C3H7(n)
NO2
1.152 ,¢;~ H --CH2 ~ ~
2 ~
- 4~ -
Comp. A Rl R2 R3 Phys. data
No.
_
1.153 ~¢~ H -CH2~CI <1 m.p. 190-192C
1 lSa, ,¢~ H -CH2~ --<1
1.155 ,¢~ H H -CH2COOCH3 m.p. 184-186C
1.156 ,~ H CH3 -CH2COOCH3 m.p. 185C
1.157 ,¢~ H ~ -CH2COOCH3
1.158 Cl~L H H -~H2COOCH3
N
1.159 CIJ~s~ H CH3 -CH2COOCH3
1.160 cl~L H ~ -CH2COOCH3
1.161 ,1~ H H -CH2COOCH3
1.162 ,¢~ H CH3 -Cl-12COOCI-13
r
~2~
- 46 -
Comp. A Rl R~ R3 Phys. d~ta
No.
_ _ _
1.163 ,¢~ H ~ -CT I2COO('H3
~/ H 11 -CIIzCOOCHl
1.165 ¢;~ H CH3 -CH2COOCH3
1.166 ¢~ H ~ -CH2COOCH3
1.167 ~ H H -CH2COOCH3
1.168 ~ H CH3 -Cl12COC)CI-1
1.169 ~ ~ Cl-12COOCfl
1.170 ,¢~ H CH3 -C112C~3
2 ~ 3 ~
- 47 -
Comp. A Rl R2 R3 Phys. data
No.
1.171 J~ H CH3 -CH2CF3
1.172 ~ H CH3 -CH2CF3
1.173 C~ L H CH3 -CH2CF3
1.174 ¢~ H CH3 -CH2CF3
1.175 ,¢~ H CH3 -CH2CH2F
1.176 ,¢;~ H CH3 -CH2CH2F
1.177 ~ H CH3 -CII2CII2F'
1.178 CIJ~L H CH3 -cH2clI2F
1.179 ¢~ H CH3 -Cl12CH2F
2~273 ~
- 48 -
Comp. A Rl R2 R3 Phys. data
No.
1.180 ,¢~ H CH3 -CH2CH2Br
1.181 ,¢~ H CH3 -CH2CH2Br
1.182 ~ H CH3 -CH2CH2Br
1.183 C~ L H CH3 -CH2CH2Br
1.184 ¢~ H CH3 -CH2CH2Br
1.185 J~ H CH3 -CH2CH2CH2cl
1.186 ,1~1' H CH3 -CH2CH2CH2CI
1.187 ~ H CH3 -CI-12CH2Cl-l2
1.188 ,¢~ H CH3 -CH2('l-l2Cl-
2~273~
- 49 -
Comp. A Rl R2 R3 Phys. data
No.
.. . . _
1.189¢~ H CH3 -CH2CH2C~I2cl
1.190,¢~ H CH3 -CH2CH2CH2Br
1.191,¢~ H CH3 -CH2CH2CH2Br
Cl~
1.192 J~ J H CH3 -CH2CH2CH2E~r
N
1.193CI~L H CH3 -CH2CH2CH2Br
1.194¢;~ H CH3 -CH2CH2CH2Br
1.195,¢~ H CH3 -CH2CH2CI
1.196,¢;~ H CH3 -CH2CH2CI
Cl~
1.197 H CH3 -CH2CH2CI
~ ~ rV ~ 7 3 :L
- 50 - .
Comp. A Rl R2 R3 Phys. data
No.
-
1.198 C~ L H CH3 -CH2CH2CJ
1.199¢~ H CH3 -CH2CH2CI
1.200,¢~ H CH3 -CH2CH(Cl)CH2CH2CH2Cl
1.201,¢;~ H CH3 -CH2CH(CI)CH2CH2CH2Cl
o
1.202~ H CH3 -CH2CH(Cl)CH2CH2CH2CI
1.203CIJ~L H CH3 -CH2CH(Cl)CH2CH2CH2Cl
1.204¢~ H CH3 -CH2CH(Cl)CH2CI-I2CH2CI
1.205,¢~ H CH3 -CH2CH20E:I amvrpho~ls mass
1.206,¢~ H CH3 -CH2CI-12OH
20~731
- 51 -
Comp. A Rl R2 R3 Phys. data
No.
1.207~ H CH3 -CH2CH2OH
1.208C~ L H Cl13 -CH2CH2OH
1.209¢~ H CH3 -CH2CH20H
1.210,~ H CH3 -CH2CH2CH2OH amorphous mass
1.211,¢~ H CH3 -CH2CH2CH2OH
1.212~ H CH3 -CH2CH2CH2OH
1.213CIJ~L H CH3 -CII2CH2CH20H
1.214¢~ H CH3 -Cl-I2CI 12CH2OI 1
1.215,¢~ H CH3 -Cl12(cH2)2cI-I2O~l m.p. 108-110C
2~273~
- 52 -
Comp. A Rl R2 R3 Phys. data
No.
_ _
1.216 ,¢~ H CH3 -CH2(CH2)2CH2OIl
1.217 ~ H CH3 -CH2(CH2)2CH2OH
1.218 C~ L H CH3 -CH2(CH2)2CH20H
1.219 ¢~ H CH3 -CH2(cH2)2cH2OH
N
1.220 ,¢~ H CH3 -CH2(CH2)3CH20H
1.221 ,¢~ H CH3 -CH2(CH2)3CH20H
1.222 ~ H CH3 -CH2(cH2)3c~I2O
1.223 Cl~ H CH3 -CH2(CH2)3~ 2O~
1.224 ¢~ H CH3 -CE-I2(CE-I2)3CI-12o~ 1
2 0 .~ 2 7 3 ~
Comp. A Rl R2 R3 Phys. data
No.
____
1.225 ,¢~ H CH3 -CH(CH3)CH2OH amorphous mass
1.226 ,¢~ H CH3 -CH(CH3)CE~20H
g
1.227 ~ H CH3 -CH(CH3)C~ 12CH
1.228 C~ L H CH3 -CH(CH3)CH20H
1.229 ¢ ~ H CH3 -CH(CH3)CH20H
N
1.230 ,1~ H CH3 -CH(C2Hs)CH20H
1.231 ,¢;~ H CH3 -CH(C2Hs)CH2QH
1.232 ~ H CH3 -CH(C2Hs)CH2Oll
1.233 Cl~ H CH3 -Cl-l(C2T-Is)CH201 I
2~27~1
- 54 -
Comp. A Rl R2 R3 Phys. data
._ _ ___
1.234 ¢~ H CH3 -CH(C2Hs)CH2OH
1.235 J~ H CH3 -CH2CH(CH3)0H
1.23G ,¢~ H CH3 -CH2CH(CH3)0H
1.237 ~ H CH3 -CH2CH(CH3)OH
1.238 C~ L H CH3 -CH2CH(CH3)OH
1.239 ¢~ H CH3 -CH2CH(CH3)0H
1.240 ,¢~ H CH3 -CH2CH(OH)CH2OH
1.241 ,¢;~ H CH3 -CH2CII(OI-I)CH2OH
1.242 ~ H CH3 -CH2C~-I(OI-I)CH2OH
Comp. A R~ R2 R3 Phys. data
No.
... _ _ .... . ... _ . .. _ . .
1.243 C~ L H CH3 -CH2CH(OH)CH2OI I
1.244 ¢~ H CH3 -CH2CI l(OI-l)CH2OI l
1.245 ,¢~ H CH3 -CH(CH2OI-I)2
1.246 ,¢;~1' H CH3 -CH(CH20H)2
1.247 ~ H CH3 -CH(CH2OH)2
1.248 Cl~ H CH3 -CH(CH2OH)2
1.249 ¢~ H CH3 -CH(CH20H)2
1.250 ,¢~ H CH3 -CH2CH20CH3
1.251 ,¢;~1~ H CH3 -Cl 12C~-120CI-13
~2~
- 56 -
Comp. A Rl ~2 R3 Phys. data
No.
_ _ _
1.252 ~ H CH3 -Cl-12CI-I2OCH3
1.253Cll~ H CH3 -Cl~2CH20CI-I3
1.254 ~ H CH3 -CH2CH2OCH3
1.255 ~ H CH3 -CH2CH2CH2OC2Hs
1.256 ~ H CH3 -CH2CH2CH2OC2H5
1.257 ~ H CH3 -CH2CH2cH2OC2Hs
1.258Cll~ H CH3 -CH2CH2CH20C2H5
1.259 ~ H CH3 -CE-I2CH2c~l2OC2I-15
1.260 ~ H CH3 -Cl-I(CI-13)CI12OC H3
~2~3~
Comp. A Rl R2 R3 Phys. data
No.
1.261 ~ H CH3 -CH(CH3)CH20CH3
1.262 ~ H CH3 -CH(CH3)CH20CH3
1.263 Cll~ H CH3 -CH(CH3)CI 120CH3
1.264 ~ H CH3 -CH(CH3)CH20CH3
1.265 ~ H CH3 -CH2CH(OCH3)2
1.266 ~ H CH3 -CH2CH(OCH3)2
Cl N
1.267 ~ H CH3 -Cl-12CH(OCH3)2
1.268 Cll~ H CH3 -CH2CI-I(OCI-13)2
1.269 ~ H CH3 -Cl l2CI l(OC113)2
N
2 ~ ~' 2 r7 ~ ~
Comp. A Rl R2 R3 Phys. data
No.
. . .
1.270~ H CH3 -CH2CH(OC2~ls)2
1.271~ H CH3 -CH2CH(OC2~ls)2
1.272~ H CH3 -CH2CH(OC2lIs)2
1.273Cll~ H CH3 -CH2CH(OC2Hs)2
1.274~ H CH3 -CH2CH(OC21I5)2
1.27~~ H CH3 -CH2CH2N(CH3)2
1.276~ H CH3 -CH2CH2N(CH3)2
1.277~ H CH3 -CH2CH2N(Cl-l3)2
N
1.278 Cll~ H CH3 -Cl-12Cl 12N(CI 13)2
2 ~ ~ 2 ~ 3
- 59 -
Comp. A Rl R2 R3 Phys. data
No.
1.279 ~ H CH3 -CH2CH2N(CH3)2
1.280 ~ H CH3 -CH2CH2N(C2Hs)2
1.281 ~ H CH3 -CH2CH2N(C2Hs)2
1.282 ~ H CH3 -CH2CH2N(C2Hs)2
1.283Cll~ H CH3 -CH2CH2N(C2Hs)2
1.28~ ~ H CH3 -CH2CH2N(C2~Is)2
N
1.285 ~ H CH3 -CH2CH2CH2N(CH3)2
1.286 ~ H CH3 -CH2CH2CH2N(CH3)2
o
1.287 ~ H CH3 -CH2CH2CH2N(CH3)2
2~5~731
- 60-
Comp. A Rl R2 R3 Phys. data
No.
N
1.288CIJ~L H CH3 -CH2CH2CH2N(cH3)2
1.289 ¢~ H CH3 -Cf-12CH2Cf I2N(CH3)2
1.290,¢~ H CH3 -CH2CH2CH2N(C2Hs)2
1.291,¢~1' H CH3 -CH2CH2CH2N(C2Hs)2
~r .
Cl~
1.292)~ J H CH3 -CH2CH2CH2N(C2Hs)2
1.293Ci~L H CH3 -CH2CH2CH2N(C2Hs)2
1.294 ¢~ H CH3 -CH2CH2CH2N(C2Hs)2
1.295,~ H CH3 -~H2COOC2Hs alIIorpllous mass
1.296,¢~ H CH3 -CH2COOC2Hs
2~2t~3,~
- 61 -
Comp~ A Rl R2 R3 Phys. data
No.
1.297~ H Cl-13 -cH2cooc2Hs
1.298Cll~ 11 CH3 -Cl-12cOOc2f-ls
1.299~ H CH3 -CH2COOC2l ls
1.300~ H CH3 -CH2CH2COOC2Hs m.p.78-80C
1.301~ H CH3 -CH2CH2COOC2H5
1.302~ H CH3 -CH2CH2COOC2Hs
1.303Cll~ H CH3 -CH2CH2COOC2H5
1.304~ H CH3 -CH2cH2cOoc2~s
1.305~,~ H CH3 -Cll(CH3)C1-l2cOOc2l-l5
20~273~
- 62 -
Comp. A Rl R2 R3 Phys. data
No.
1.306 ,1~ H CH3 -Cll(CI-I3)CH2COOC2Hs
Cl~
1.307 J~ ~J H CH3 -CH(CH3)CH2COoc2~ls
N
1.308CI~L H CH3 -CH(CH3)CH2COOC2H5
1.309 ¢~ H CH3 -CH(CH3)CH2cOoc2Hs
1.310,~ H CH3 -CH(CH2OH)COOCH3
1.311,¢~1' H CH3 -CH(CH2OH)COOCH3
Cl~
1.312,,l~ ,J ll CH3 -CH(CH2OH)COOCII3
1.313CIJ~ H CH3 -Cl-l(CH2OH)COOCH3
1.314 ¢~ H Cl 13 -Cl-l(CI-12OI-l)COOCI 13
N
2~73~
Comp. A Rl ~2 R3 Phys~ data
No.
. _
1.315~ H CH3 -
Cl ~N {~
1.317~ H CH
1.318 Cll~ H CH
1.319~ H CII3 ~
1.320Cl~ H CH3 ~ m.p.137-139C
(cis isomer)
m.p.170-172C
(trans isom~r)
1.321~ H CH3 - ~C~i3
o
1.322~ H CII3 ~CI~3
1.323Cll~ H CH3 ~ C~13
2 ~ ~ 2 7 ~ 1
- 64 -
Comp. A Rl R2 R3 Phys. data
No.
_
1.324 ~ H CH3 ~ CH3
1.325 ~ H CH3 CH3
1.326 ~ H CH3 CH3
1.327 ~ H CH3 CH3
1.328 Cll~ H CH3 CH3
1.329 ~ H CH3 CH3
1.330 ~ H CH
1.331 ~ H CH
2 ~ ~ ~ 7 3 ~
- 65 -
Comp. A Rl R2 R3 Phys. data
No.
1.332 ~ H CH3 ~>
1.333 CIJ~L H CH3 ~>
1.334 ¢;~ H CH3 ~>
1.335 ,¢~ - H CH3 -CH2CH=CH2 m.p. 75-77C
1.336 J~ lH CH3 -CH2CH=CH2
1.337 ~ H CH3 -CH2CH=CH2
1.33~ CIJ~L H CH3 -CH2CH=Cf 12
1.339 ¢~ H CH3 -CH2CH=Cl-l2
1.340 ,¢~ H CH3 ~ Cl
2 0 ~ 2 ~ 31
- 66-
Comp. A Rl R2 R3 Phys. data
No.
_ _ _ _
1.341 ,¢~ H CH3 ~ Cl
1.342 ~ H CH3 ~ Cl
1.343 CIJ~L H CH3 ~ Cl
1.344 ¢~ H CH3 ~ Cl
1.345 ,~ H CH3 ~ F amorphous mass
1.346 ,¢~f H CH3 ~ F
1.347 ~ H CH3 ~ F
1.348 CIJ~L H CH3 --~ F
3 ~
- 67 -
Comp. A Rl R2 R3 Phys. data
No.
1.349¢~ H CH3 ~ F
1.350,¢~ H CH3 ~OCH3 m.p. 204C
1.351,¢~ H CH3 ~ OCH3
o
1.352~ H CH3 ~ OCH3
1.353CI~L H CH3 ~ OCH3
1.354¢~f H CH3 ~ OCH3
1.355,¢~ H CH3 ~ CH3 amorphous m.lss
1.356,¢;~ H CH3 ~~ C~13
~0
1.357~ H CH3 ~ C~13
s~ j 3 ~
- 68 -
Comp. A ~l R2 R3 Phys. data
No.
-
1.358 C~ L H CH3 ~ CH3
1.359 ¢~ H CH3 --~ CH3
1.360 ,¢~ H CH3 ~N02 m.p. 219C
1.361 ,¢~ H CH3 ~ NO2
o
1.362 ~ H CH3 ~ NO2
1.363 C~ L H CH3 ~ NO2
1.364 ¢;~ H CH3 ~ N02
1.365 ,¢~ H CH3 ~ CN .Imorpholls mass
1.366 ,¢~ H CH3 ~ CN
2~2731
- 69 -
Comp. A Rl R2 R3 Phys. data
Ns~.
1.367 ~ H CH3 ~ CN
1.368 CI~L H CH3 ~ CN
1.369 ¢~f H CH3 ~ CN
1.370 J~ H CEI3 ~ CF3 amo~phous mass
1.371 ,1~ H CH3 ~CF3
1.372 ~ H CH3 ~ CF3
1.373 Cl~ H CH3 ~ CF3
1.374 ¢~ H CH3 ~ CF3
1.375 ,¢~f H CH3 ~
2 ~ ~ 2 7 3 ~
- 70 -
Comp. A Rl R2 R3 Phys. data
No.
1.376 J~ H CH3
1.377 ~ H CH
1.378 CIJ~L H CH
S
1.379 ¢~ HCH3 ~
1.380 ,¢;~ HCH3 SCH3
1.381 ,¢~1' HCH3 SCH3
Cl~ SC~13
Cl~ NJ 3 ~
N SCH3
1.383 CI~L H CH3 ~
~52~3~
Comp. A Rl R2 R3 Phys. data
No.
. . . _
1.384 ¢;~ H CH3 SC~3
1.385 ,¢~ H CH3 Cl
1.386 ,¢~ H CH3 Cl
o
Cl Cl
1.387 ~ H CH
1.388 C~ L H CH
1.389 ¢~ H CH
1.390 ,¢~ H CH3 CN
1~391 ,¢~ H Cl13 CN
~O~
~2~31
Comp. A Rl R2 R3 Phys. data
No.
.
1.392~ H CH3 CN
1.393 CI~L H CH
1.394¢~ H CH3 ~
1.395,~ H CH3 -CH2~ N2 amorphous mass
1.396,¢~ H CH3 -CH2~ N2
~0
1.397~ H CH3 -CH2~ No2
1.398CI~L II CH3 -CH2~ N2
1.399¢~ H CH3 -CH2~ N2
o
1.400,¢~ H CH3 -CH2~ F m.p. 162-164C
2~2~
- 73 -
Comp. A Rl R2 R3 Phys. data
No.
1.401J~ H CH3 -CH2~ F
~0
1.402 ~ H CH3 -CH2~ F
1.403c~ L H CH3 -CH2~ F
1.404¢;~ H CH3 -CH~ F
1.405,¢~ H CH3 -CH2~0CH3 m.p. 125-127C
1.406,¢;~ H CH3 -CH2~ OCH3
~0
1-407Cl~¢N~ H CH3 -CH2~ OCH3
1.408 C~ L H CH3 -CH2~ OCH3
7 3 ~
- 74 -
Comp. A Rl R2 R3 Phys. data
No.
1.409 ¢~ H CH3 -CH2~ oc~l3
1.410 ,~ H CH3-CH2~CI m.p. 147-149C
1.411 ,¢;~ H CH3-CH2~ Cl
o
1.412 ~ H CH3-CH2~ Cl
1.413 Cl l~L H CH3-CH2~ Cl
1.414 ¢~ H CH3-CH2~ Cl
1.415 ,¢~ H CH3-C112 ~CH3 m.p. 155-157C
1.416 '~?' H CH3 -CH2 ~C~13
7 3 ~
- 75 -
Comp. A Rl R2 R3 Phys. data
No.
1.417 ~ H CH3 -CH2 ~CH3
1.418 Cll~ H CH3 -CH2 ~CH3
1.419 ~ H CH3 -CH2 ~ CH3
1.420 ~ H CH3 -CH2 ~CF3 m.p. 167-169C
1.421 ~ H CH3 -CH2 ~ CF3
1.422 ~ H CH3 -CH2 ~CF3
1.423 Cll~ H CH3 -CH2 ~CF3
1.424 ~ H CH3 -C~12 ~CF3
~0
NO2
1.425 ~ H CH3 -CH2 ~
~ ~ ~ rJ 7 ~ ~
- 76 -
Comp. A Rl R2 R3 Phys. data
No.
.... . _ _ .
1.426 ,¢;~ H CH3 -CH
o
1.427 ~ H CH3 -CH
NO2
1.428 C~ L H CH3 -CH2 ~
NO2
1.429 ¢~ H CH3 -CH
1.430 ,¢~ H CH3 -CH
1.431 ,¢~1' H CH3 -CH
F
1.432 ~ H CH3 -CH
~a27~ lL
Comp. A Rl R2 R3 Phys. data
No.
1.433 CI~L H CH3 -CH
1.434 ¢~ H CH3 -CH2 ~
1.435 ,~ H CH3 -CH2 ~ F
1.436 ,¢~ H CH3 -CH2 ~ F
o
1.437 ~ H CH3 -CH~---~ F
1.438 C~ L H CH3 -CH2 ~ F
1.439 ¢~f H CH3 -CH2--~ F
2~ '3~
- 78 -
Comp. A Rl R2 R3 Phys. data
No.
.
CN
1 440 ,¢~ 11 CH3 -CH2 ~
CN
1.441 ,¢~ H CH3 -CH2 ~
o
CN
1.442 ~ H CH3 -CH2 ~
CN
N /~
1.443 C~ L H CH3 -CH2 ~
CN
1.444 ¢~ H CH3 -CH2 ~
1.445 ~ H CH3 -C3117(n) m.p. 157-158C
C N
o
~5~ ~'3:~
- 79 -
Example 3:
Formulations (throughout, percentages are by weight)
Example F1: Emul~sifiable concentrates a~ b~ c)
a compound of Example 2 25 %40 % 50 %
calcium dodecylbenzenesultonate 5 %8 % 6 %
castor oil polyethylene glycol ether
(36 mol of ethylene oxide~ 5 %
tributylphenol polyethylene glycol
ether (30 mol of ethylene oxide) - 12 % 4 %
cyclohexanone - 15 % 20 %
xylene mixture 65 %25 % 20 %
Emulsions of any desired concentration can be produced from such concentrates bydilution with water.
ExampleF2: Solutions a) b) c) d)
a compound of Example 2 80 %10 %5 % 95 %
ethylene glycol monomethyl
ether 20 % - - -
polyethylene glycol
(mol. wt. 400) - 70 %
N-methyl-2-pyrrolidone - 20 %
epoxidised coconut oil - - I % 5 %
petroleum fraction
(boiling range 160-190C) - - 94 %
The solutions are suitable for application in the torm of micro-drops.
_ampleF3. Granules a) b) c) d)
a compound of Example 2 5 % 10 %X % 21 %
kaolin 94 % ~79 % 54 %
highly dispersed silicic acid I % - 13 % 7 %
attapulgite - 90% - 18 %
3 ~
- 8Q-
The active ingredient is dissolved in methylene chloride, the solution is sprayed onto the
carrier, and the solvent is subsequently evaporated off in vacuo.
ExampleF4. Dusts a)b)
a compound of Example 2 ~ %5 %
highlydispersedsilicic acid 1 %S %
talcum 97 %
kaolin - gO %
Ready-for-use dusts are obtained by intimatcly mixing the calTiers with the active
ingredient.
Example F5: Wettable powders a)b) c)
a compound of Example 2 25 %50 % 75 %
sodiumlignosulfonate 5 %5 %
sodium laurylsulfate 3 % - 5 %
sodium diisobutylnaphthalene-
sulfonate - 6 % 10 %
octylphenol polyethylene glycol
ether (7-8 mol of ethylene
oxide) - 2 %
highly dispersed silicic acid 5 %10 % 10 %
kaolin 62 %27 %
The active ingredient or active ingredient combination is mixed with the adjuvants and the
mixture is thoroughly ground in a suitable mill, affording wettable powders which can be
diluted with water to give suspensions of any desired concentration.
Example F6: Emulsifiable concentrate
a compound of Example 2 1 () %
octylphenol polyethylene glycol
ether (4-5 mol of ethylene oxide) 3 %
calcium dodecylbenzenesulfon"te 3 %
castor oil polyglycol ether
(36 mol of ethylene oxide) 4 %
cyclohexanone 3() %
2~2~3 ~
- 81 -
xylene mixture 50 %
Emulsions of any desired concentration can be obtained from this concentrate by dilution
with water.
ExampleF7: Dusts a) b)
a compound of Example 2 S %8 %
talcum 95 %
kaolin - 92 %
Ready-for-use dusts are obtained by mixing the active ingredient with the carrier and
grinding the mixture in a suitable rnill.
Example F8: Extruder ~ranules
a compound of Example 2 10 %
sodium lignosulfonate 2 %
carboxymethylcellulose 1 %
kaolin 87 %
The active ingredient or active ingredient combination is mixed and ground witn the
adjuvants, and the mixture is moistened with water. The mixture is extruded, granulated
and then dried in a stream of air.
Example F9: Coated ~ranules
a compound of Example 2 3 %
polyethylene glycol (mol. wt. 200) 3 %
kaolin 94 %
The finely ground active ingredient or active ingreclient combin;ltion is unitormly applie(l,
in a mixer, to the kaolin moistened with polyethylenc glycol. Non-dusty coated gl,lllllles
are obtained in this manner.
Example F10: Suspension concentrate
a compound of Example 2 40 %
ethylene glycol 10 %
nonylphenol polyethylene glycol
- 82-
ether (15 mol of ethylene oxide) 6 %
sodium lignosulfonate 10 %
carboxymethylcellulose 1 %
silicone oil in the form of a 75 %
aqueous emulsion 1 %
water 32 %
The finely ground active ingredient or active ingredient combination is intimatcly mixed
with the adjuvants, giving a suspension concentrate from which suspensions of .my
desired concentration can be obtained by dilution with water.
Example 4: Action a~ainst Nilaparvata lu~ens
Rice plants are sprayed with an aqueous emulsion comprising 400 ppm of test compound.
After the spray coating has dried, the rice plants are populated with cicada larvae in the
2nd and 3rd stages. Evaluation is made 21 days later. The percentage reduction in the
population (% activity) is detennined by comparing the number of surviving cicadas on
the treated plants with that on untreated plants.
Compounds of Example 2 exhibit good activity against Nilaparvata lugens in this test. In
particular, compounds 1.007, 1.008, 1.009, l.S)11, 1.012, 1.013, 1.014, 1.056, 1.057, 1.058,
1.104, 1.128, 1.130, 1.156, 1.205, 1.215, 1.300, 1.320, 1.335 and 1.410 are more than
80-90 % effective.
Example 5: Action against Nephotettix cincticeps
Rice plants are sprayed with an aqueous emulsion comprising 400 ppm of test compound.
After the spray coating has dried, the rice plants are populated with cicada larvae in the
2nd and 3rd stages. Evaluation is made 21 days later. The percentage reduction in the
population (% activity) is determined by comparin~ the nurmber of surviving cicad.ls on
the treated plants with that on untreated phlnts.
Compounds of Example 2 exhibit good aclivity ag,linst Ncphotettix chlcticcps hl tllis ~est.
Inparticular,compounds 1.007, 1.0()8, 1.009, 1.()11, 1.()12, 1.013, 1.()14, 1.()56, 1.()57,
1.058, 1.060, 1.104, 1.128, 1.156, 1.205, 1.215, 1.30(), 1.320, 1.335 alld 1.410 are more
than 80 % effective.
2~ 2~3 L
- 83 -
Example 6: Action a~ainst Myzus persicae
Pea seedlings are infested with My~us persicae and then sprayed with a spray mixture
comprising 400 ppm of the test compound, and incubated at 20C. Evaluation is made 3
and 6 days later. The percentage reduction in the population (% activity) is determined by
comparing the number of dead aphids on the treated plants with that on wnlreated plants.
Compounds of Example 2 exhibit good activity against Myzus persicae in this test. In
particular, compounds 1.001, 1.002, 1.006, 1.007, 1.008, 1.009,1.0] 1, 1.()12, 1.104 and
1.156 are more than 80 % effective.
Example 7: Action a~ainst APhis craccivora
Pea seedlings are infested with Aphis craccivora and then sprayed with a spray mixture
comprising 400 ppm of the test compound, and incubated at 20C. Evaluation is made 3
and 6 days later. The percentage reduction in the population (% activity) is determined by
comparing the number of dead aphids on the treated plants with that on untreated plants.
.
Compounds of Example 2 exhibit good activity against Aphis craccivora in this test. In
particular,compounds 1.001, 1.002, 1.006, 1.007, 1.008, 1.009, 1.011, 1.012, 1.104, 1.155,
1.156, 1.205 and 1.300 are more than 80 % effective.
Example 8: Systemic acti n a~ainstNilaparv_talu~ens
Pots containing rice plants are placed in an aqueous emulsion solution comprisin~
400 ppm of the test compound. The rice plants are then populated with larvae in tlle 2nd
and 3rd stages. Evaluation is made 6 days later. The percentage reduction in thepopulation (% activity) is determined by comparing the number of cicadas on the treate(l
plants with that on untreated plants.
Compounds of Example 2 exhibit good acLivity against Nilap.lrvat.l lwgcns in this tcst. In
particul.u, compounds 1.001, 1.002, 1.0()3, 1.()04, 1.006, 1.007, 1.008, 1.009, 1.()11, 1.()12,
1.013, 1.014, 1.057, 1.058, 1.060, 1.104, 1.12X, 1.13(), 1.142, 1.156, 1.2()5, 1.215, 1.3()(),
1.320, 1.335, 1.410 and 1.445 are more th;ln ~() % effcctive.
Example 9: Systemic action a~ainst Nephotettix cincticeps
Pots containing rice plants are placed in an aqueoLIs emulsioll solution comprisin~
400 ppm of the test compownd. The rice plants are tllen popul.llcd with larvae in tlle 2nd
and 3rd stages. EvaluaLion is made 6 days latel. The pcrcent.lge reduction in the
2 ~ 3 ~
- 84-
population (% activity) is determined by comparing the number of cicadas on the treated
plants with that on untreated plants.
Compounds of Example 2 exhibit good activity against Nephotettix cincticeps in this test.
In particular, compounds 1.001, 1.002, 1.003, 1.004, 1.005, 1.006, 1.008, 1.009, 1.011,
1.012, 1.013, 1.014, 1.057, 1.058, 1.060, 1.104, 1.156 and 1.335 are more than ~() 'J/o
effective.
Example 10 Systemic action again~t Mvzus persica_
Pea seedlings are infested with My~us persicae and ~hen placed with their roots in a spray
mixture comprising 400 ppm of the ~est compound, and incubated at 20C. Evaluation is
made 3 and 6 days later. The percentage reduction in the population (% activity) is
determined by comparing the number of dead aphids on the treated plants with that on
untreated plants.
Compounds of Example 2 exhibit good activity against Myzus persicae in this test. In
particular, compounds 1.001, 1.002, 1.007, 1.008, 1.009, 1.011, 1.012, 1.025, 1.104 and
1.156 are more than ~0 % effective.
Example 11: Action a~ainst Bemisia tabaci
Dwarf bean plants are placed in gauze cages and populated with adults of Bemisia tabaci
(whitefly). When oviposition has taken place, all the adults are removed and 10 days later
the plants and the nymphs located thereon are sprayed with an aqueous emulsion of the
test compounds (concentration 400 ppm). Evaluation is made 14 days after application of
the test compound by deterrnining the % hatching rate in comparison with untreated
controls.
Compounds of Example 2 exhibit good activity against Bemisia tabaci in this test. In
particular,compounds 1.001, 1.002, 1.006, 1.007, 1.008, 1.()()9, l.011, 1.012, 1 013, 1.1)14,
1.016, 1.104, 1.156 and 1.335 are more than ~0 % cffective.
Example 12: Action a~ainst Ctenocepllal;ls felis
20 to 25 cat ilea eggs (Ctenocephalus felis) ~ure placed in each of a number of horizont.ll
50 ml cell culture bottles into which lS g of a flea larv.le nutrient medium comprising
100 ppm of the test compound have been introduced beforehand. The bottles .ure sealed
and placed in an incubator at 26-27C and 60-70 % humidity. After an incubation period
~273~
- 85 -
of 21 days, the development of adult fleas, unhatched pupae ~nd larvae is assessed.
Compounds of Example 2 exhibit good activity in this test. In particular, compounds
1.009 and 1.025 are more than 80 % effective.
Exam~ Action against Blattella ~ermanica
An amount of a 0.1 % solution of the test compound in acetone sufficient to produce a
concentration of 1 g/rrl2is introduced into a petri dish having a diameter of 1() cm. When
the solvent has evaporated, 10 Blattella germanica nymphs (final nymph stage) are placed
in the dish so prepared and subjeeted to the action of the test compound for 2 hours. The
nymphs are then narcotised with carbon dioxide, placed in a fresh petri dish and kept in
the dark at 25C and about 70 % humidity. The insecticidal action is evaluated 48 hours
later by determining the mortality rate.
Compounds of Example 2 exhibit good activity in the above test. In particular, compound
1.104 is more than 60 % effective.
Example 14: Action against Boophilus microplus
Adult female ticks which are replete with blood are affixed to a PVC plate and covered
with a cotton wool swab. For treatment, 10 rrll of an aqueous test solution comprising
125 ppm of the test compound are poured over ~he test insects. The cotton wool swab is
then removed and the ticks are incubated for 4 weeks until oviposition has taken place.
The action against Boophilus microplus manifests itself either as mortality or sterility of
the females or as ovicidal action in the eggs.
Compounds of Example 2 exhibit good activity against Boophilus microplus. In
particular, compounds 1.008, 1.009 and 1.025 are more thall 60 % effective in this test.