Note: Descriptions are shown in the official language in which they were submitted.
2~37J~ 1~r~
Device for performing a rapid single manual assay.
The invention relates to a device and the use of
the device for detecting and/or determining an analyte
in a test liquid and a method for detecting and/or
determining an analyte in said test llquid.
The field of specific binding assays has greatly
expanded as its importance in the diagnostic field has
become recognized. The ability to detect a spe~ific
compound and measure the compound quantitatively has
permitted the monitoring of the administration of a
wide variety of drugs, the determination of an
imbalance in a wide variety of hormones, the
quantitation of physiologically active proteins and
the diagnosis of the presence of a pathogen. Different
techniques have been distinguished in requiring or not
requiring separation steps, the nature of the signal
developed by the label, the development of the signal
. . . - , ,
in a solution or on a surface and the manner of
measurement for quantitative determination.
In developing an assay, there are a number of
considerations in devising the reagents and protocol.
One consideration is the degree of sophistication of
the individual performing the assay. There are a lot
of situations where it is desirable that a relatively
untrained or unexperienced individual should be able
to perform an assay and obtàin reasonably quantitative
results~ It is particularly desirable that the
untrained person be-~able to perform a quantitative
assay with a rapid simple test without the need for
sophisticated instruments.
; . ~
2 2~2~0!3
The last decade an enormous amount of so-called
dipsticks and filter assays has been developed varying
from all kinds of paper strips in different shapes,
promising a better result than a previous strip, to
plastic strips, coated with for instance an
immunochemical component.
For instance, European patent application EP
~,149,168 describes an immunoassay which can be
carried out by making use of Zl capillary glass tube.
At least 2 regions are packed with separate carrier
material. An immunoreactive component provided with a
labelling substance which, via an immunochemical
reaction, is capable of forming an immunocomplex with
a substance, whose pres~nce or concentration in the
test liquid is desired to determine, is bound to the
first carrier material. Subsequently, said complex is
transported by capillary action and ends up in the
second carrier material where it is immobilized after
binding to a second immunoreactive component which is
bound to the second carrier material. Thereafter the
quantity of immunocomplex, thus immobilized, can be
measured via known detection methods depending on the
labelling substance used. The labelling substances
used in the European application in question are
radioisotopes, enzymes or fluorescent substances.
A disadvantage of said immunoassays is that after
the immunochemical reaction, several op~rations always
have to be performed to separate solid phase bound
from non-reacted reactants, which action is known to
those skilled in the art as "bound/free separation"~
Additional operation steps are needed to add reagents
after bound/free separation, which is for instance the
case when a substance has to be added to detect a
solid phase bound labelled reactant. An additional
step is needed for certain when applying highly
sensitive assays requiring enhancement of the assay
signal.
Surprisingly, a device has now been found with
which the operations to be per.~ormed remain confined
to bringing a device into contact with a test liquid
and a wash fluid and determining the result - a~ter
some time - without impair:ing the accuracy and
reliability, making it possible also to perform
complex assays, e.g. ELISA, with only two operation
steps, irrespective the number o~ bound/~ree
separations and reagent additions and the sequence
thereo~.
The invention there~ore relates to a device ~or
per~orming an assay in order to detect and/or
determine an analyte in a test fluid, said device
comprising a track for liquid transport, at least
partly bordered by a semi-permeable layer, in said
track is transported during the assay and alongside
the semi-permeable layer a movable solid phase
material bearing a ligand capable to bind, directly
or indirectly, the analyte and/or to bind, directly or
indirectly, a reactant for the analyte, said semi-
permeable layer being incapable to let pass the
movable solid phase material.
The invention also relates to a method for
detecting and/or determining an analyt~ in a test
liquid by bringing the device according to the
invention into contact with said liquid and, when
appropriate, with a transporting liquid so that the
movable solid phase material provided with a ligand
comes into contact with said test liquid and,
simultaneously or subsequently, with a reactant
provided with a label and is transported by one or
both o~ said liquids through the track to a position
in the device where a detecting system for the label
is located and the label being bound, directly or
indirectly, to said solid phase material is detected,
in that the degree of binding is determined directly
or indirectly, which degree is a qualitative or
quantitative indication of the concentration of the
analyte to be determined or detected~
The invention also relates to a test kit which
may include a device according to the invention.
Optionally the test kit may contain, in or outside the
device, an immunochemical reagent coupled to a label
and a movable dispersed or dispersable solid phase
material be~ring a ligand e . g. an immunochemical
reagent. In another performance the test kit may
contain, in or outside the device, a nucleic acid
sequence coupled to a label and a movable, dispersed
or dispersable solid phase material bearing a ligand
e.g. a nucleic acid sequence.
The track in the device according to the
invention may contain provisions to control the
velocity of the liquid flux in said track. Such
provisions may be components which slowly dissolve
upon wetting e.g. like sucrose.
A particular embodiment of the device is a device
in which the track is a capillary canal e.g. a porous
hollow fibre of e.g. polyethersulfone, polyamide,
polyimide or regenerated cellulose, containing walls
that are semi-permeable and that is at least partly
surrounded by absorbent material and in which this
canal is used to forward, by capillary force, the
movable solid phase material e.g. a watery suspension
of particles coated with a ligand. The semi-permeable
wall is capable to let pass molecules e.g.
contaminations or particles smaller than said coated
particles, e.g. molecules, through its pores, however
not the said coated particles.
?, ~
The pores may be pr~sent in the semi-permeable
wall in e.g. a tortuous, parallel or at an approximate
perpendicular orientation.
The capillary canal may end in, or depart ~rom,
stations containing reagents, which reagents may be
said suspension, labelled or non-labelled speci~ic
reactants or a specific substrat2 to detect the
labelled reactant.
The capillary canal may contain such stations
itself. Said semi-permeable wall may be in contact
with reagents outside the capillary canal. Reagents
outside the canal may penetrate the canal across the
semi-permeable wall e.g. upon wetting. Reagents may be
dry reagents, which dissolve or resuspend upon contact
with the test liquid or a transporting fluid that is
transported through the canal.
For the benefit of bound/free separation the
semi-permeable wall of the capillary canal may be in
contact with an absorbent for liquid outside the
capillary canal.
However, the semi-permeable wall itself may have
a su~ficient absorbing capacity to make an absorbent
redundant.
Bundles of capillary canals rather than a single
capillary canal may be used.
Another embodiment according t~ the invention can
be constructed and function analogously to the above-
mentioned device by using a porous matrix, rather than
a capillary canal, provided this matrix is capable to
transport said coated particles and that this matrix
is, at least partly, alongside in contact with said
semi-permeable laver. The device according to the
invention may be used for bound/free separation in
immunological, nucleic acid hybridization or nucleic
acid amplification assay systems.
~32~
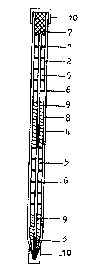
A particular device is described with re~erence
to figure 1. The device (1) (see figure 1) consists o~
a tubular semi-permeable membrane e.g. a porous hollow
fibre (2) ~illed with dry dispersable movable solid
phase material e.g. lyophylized polystyrene latex
particles (3) coated with ligand for the analyte.
Further upstream the fibre capillary has been filled
with a dry, e.g. lyophilized, labelled reactant (4)
for the analyte. The porous hollow fibre is at least
partly surrounded bv absorbent material (5), separated
by polymer layers (6) which do not let pass watery
liquids.
Depending on the label used, dry substrate (7)
reactive for the label is located at the end of the
device. The detection and/or determination o~ the
assay performed takes place at said end of the device.
Optionally the porous hollow fibre, surrounded
with absorbent material, has been provided with a
casing (8) such as an oval or round tube, or a square
or rectangular casing, which casing at the casing open
ends ~ and B may be closed with a cap (10).
The casing is, at least at the position of the
determination or detection place, manu~actured from
transparent material. Suitable materials for a casing
and the caps are glass or plastics, such as
polystyrene, polypropylene, nylon, polycarbonate or
polyvinylchloride.
Rather than using polystyrene latex particles
(3), as a movable solid phase material, any particle
capable to be transported by a liquid flux and to be
coated with a ligand can be used as long as these
particles can exist as a dispersion in said liquid and
provided these particles are incapable to pass the
semi-permeable membrane. Said movable solid phase
material according to the invention is a dispersed or
dispersable solid phase material; the dispersable
2~
solid phase material becoming dispersed upon contact
with a liquid. Said particles may be micro-crystalline
cellulose, polyacrylamide spheres, stabilized blood
cells, blood cells from the sample itself, metal sols
etc. Such particles may also be coloured as a built-in
visible proper control on the transport of the movable
solid phase material e.g. to observe arrival at the
site where the substrate is present (7) in order to be
sure that the test is completed.
In the embodiment of figure 1 e.g. latex
particles may be coloured to observe arrival of the
movable solid phase material at the site where the
substrate is present (7). Said ligands, either
antigens or fragments thereof, antibodies or fragments
thereof or nucleic acid sequences can be coated to the
particles either by physical adsorption or chemical
binding e.g. covalent binding~
The lyophilized labelled reactant (~) can be
labelled antigens or fragments thereof, antibodies or
~ragments thereof or nucleic acid seguences. These
reactants can be located in or outside the capillary
canal. Reactants outside the canal are localized that
way that these reactants dissolve upon wetting and
enter the canal across said semi-permeable membrane.
The term "label" should be understood to mean an
enz~me, a dyestuff sol particle, a metal sol particle
or other coloured disperse particles as long as above-
mentioned labelled reactant is capable to pass through
the pores of the semi-permeable membrane.
2 ~ ;r3 ~
The dyestuff sol particle, the metal sol particle
or other coloured disperse particles function as a
directly detectable labelling substance. When an
enzyme has been chosen as label a dry substrate (7)
reactiva for the enzyme is present at the detection or
determination site at the end of the device. Rather
than or in addition to a substrate an electronical
sensor, directly or indirect:Ly, sensitive for the
presence of the label, may be present.
In case one uses a metal sol particle as a
labelling substance it is possible to ~ill the end of
the device (7) with for instance an intensifying
substance for said metal sol capable to enhance the
sensitivity of the result, yielding a signal visible
by eye or readable colourimetrically or
reflectometrically~
Methods for coupling said labels to
immunochemically active substances are known per se
and do not form part of the present invention. The
coupling may be direct or indirect, chemical, e.g.
covalently, and non-chemical, e.g. adsorptively.
The enzyme Horse Radish Peroxidase (HRP) is often
used as a suitable label. Substrate for this H~P is
hydrogen peroxide, which has to be mixed with a
chromogenic co-substrate e.g. 3,3',5,5'-tetramethyl-
benzidine (TM~).
Absorbent material (5) may be composed of
materials such as cellulose, cotton, wool, silk, glass
fibres, nylon fibres, acrylic fibres, polyethylene
fibres, polyester fibres or ceramics or hardening
materials such as gypsum. Absorbent materials may be
composed of powders or granulates, such as chalk,
norit or silicagel.
9 2 ~
Optionally the absorbent material may contain
active, e.g. immunochemically active, substances with
affinity for contaminating components in the test
liquid.
Using the device according to the invention
various kind of analytes can be determined with said
device: antigens or fragments thereof, antibodies or
fragments thereof and haptens as well as nucleic acid
sequences.
The device is of use in determining analytes in
test liquids, such as urine, serum or whole blood. The
device is suitable for performing a so-called sandwich
reaction, an inhibition or competition or blocking
reaction.
The use of a prefered embodiment of the device
according to the invention for performing an
immunoassay detecting an immunochemically active
substance in a test liquid will be explained in more
detail.
The device accarding to the invention is brought into
contact with a test liquid by placing one end of its
capillary canal in a test liquid, or by dipping this
end in a test liquid and subsequently placing it in a
transporting liquid. By this an assay e.g. ELISA
(enzyme linked immuno sorbent assay) is performed
autonomously. A typical assay mechanism is:
- the liquid resuspends the movable solid phase
material e.g. lyophilized particles coated with
ligand,
- the test liquid and/or a transporting liquid
forward(s) the movable solid phase material through
the capillary canal,
- either simultaneously or subsequently said liquid
enables a labelled reactant to come into contact
with said coated particles,
2 ~
- as a consequence of this the labelled reactant
binds to the particles in case the analyte being
present in the test liquid,
- unbound substances, like unbound analyte, unbound
labelled reactant and contaminants, are removed
from the canal and thus separated from the
particles by a liquid stream through the semi-
permeable membrane, which hereto is in contac~ with
absorptive matter outside the canal,
- upon further transport through the capillary, the
liquid transporting the particles dissolves, in
case of an ELISA, the components of a substrate, as
a consequence of which this substrate comes into
contact with the particles,
- label, bound onto this particles, converts the
substrate to yield a reaction product that is
visible by eye or that can be measured
colourimetrically or by reflectometry.
Said typical assay mechanism may be function
analogously with a metal sol or dye stuff sol as label
instead of an enzyme as label. Using a metal sol or
dye stuff 501 as label a substrate may be superfluous.
The device has enormous advantages as compared
with conventional dipsticks and filter assays. The
disperse solid phase material, e.g. latex particles
coated with antibodies, remains in suspension so that
always an optimal interaction between the reaction
components is obtained and by this a higher
sensitivity is obtained and/or shorter incubation time
is achieved.
Using conventional solid phase filter techniques
non-specific interactions occur with the solid phase
filter material. Using the device according to the
invention, contaminating components interactive with
the semi~permeable membrane are not able to inter.ere
with the eventual detection process.
11 2~?,~
The device can be used as an easy manual, xapid
assay for all kind of liquid biological samples of
human or animal origin. The samples can be body
fluids, excrements, tissue preparations, saliva etc.
or liquid extracts thereof.
The invention is explained with reference to the
following examples.
Exam~le I
Onto the surface of 800 nm polystyrene latex particles
anti-HBs (antibodies against hepatitis B surface
antigen (HBsAg) was coupled through physical
adsorption according to the method as described by
Fritz and Rivers (1972 ~. Immunology 108, 108-111).
HRP was conjugated to anti-HBs according to Wilson and
Na]cane (1978). A 10 cm piece o~ a polyamide porous
hollow fibre (PHF), with an inner diameter of 310 ~m
and composed of a 75 ~m thick tubular polyamide
membrane with 200 up to 500 nm pores was filled at one
end (A~ over a l~ngth of 1 cm with a suspension o~ the
above produced anti-HBs loaded latex particles and
approximately 5 up to ~ cm from the end (A) the
capillary was filled over a length of approx 1 cm with
the anti-HBs/HRP conjugate. This was achieved by
cutting the 10 cm piece in two halves, filling one end
of each half over a length of 1 cm with liquid
reagents, by subsequent lyophilization of the fibres
containing the liquid reagents and by coupling the two
halves by means o~ a socket.
12 2~5~9
To construct a test device for HBsAg, said fibre was
subsequently brought into approximately the centre of
a tubular transparent holder with an inner diameter of
0.5 cm and a length of approx. 11 cm. At one end,
corresponding with the end A of the hollow fibre, the
tubular holder was conical over a length of 1 cm and
had a hole through which the ho:Llow fibre ends. The
space around the fibre in the p:lastic holder was
filled with layers of powdered cellulose, separated by
thin polymer layers which do not let pass watery
liquids accordiny to the below scheme (number between
dashes refer to fig. 1):
Subsequent materials Subsequent materials
inside the ca~lllary inslde the holder
around the capillary
starting from lower end A starting at lower end A
_________________________ ____________.__________
1 cm lyophilized latex (3) 2,0 cm polymer (9)
4 cm empty 6 x 0,45 cm powdered
cellulose (5)
0,05 cm polymer (6)
1 cm lyophilized conjugate (4) 2,0 cm polymer (9)
4 cm empty 6 x 0,45 cm powdered
cellulose (5)
0,05 cm polymer ~6)
inside the holder
on top of the
capillary near end B:
_______________________
1 cm substrata
station (7)
~3
The substrate station was constructed by impregnating
a piece of ceramics with a mixture of hydrogen
peroxide and T~B in an appropriate buffer system and
by subsequent dryiny the piece under vacuum at
18-25 C.
At the end (B), opposite to the end (A), the open end
of the fibre is covered with this substrate sta~ion.
The ceramics allow the latex spheres to pass.
On the end (A) and on the end (B) the device was
closed with a plastic cap (10).
Tests, with a total test durat:ion of 10 minutes, were
per~ormed using devices constructed as above. Caps
(10) were removed from the device.
In an approximately vertical position the device was
dipped with the end A in a serum sample for 30
seconds, which allowed the sample to penetrate into
the canal over a length of approx 2 cm.
Subsequently the device was placed at the end A in
demineralized water at an angle of 15 degrees
(deviating from horizontal) and kept so for 10
minutes. Subsequently the colour produced in the
substrate station was read by eye. A dilution series
of HBsAg in serum was tested with the above device.
Results as produced by the device:
.. _ _ .. . . _
HBsAg dilution Read by eye
series
. . _
1000 ng/ml: strong blue colour
100 ng/ml: strong blue colour
10 ng/ml: blue colour
0 ng/ml: no blue colour
This proves the system is valid as a rapid single
manual assay for HBsAg.
2~?~909
14
Example II
Analogously to the methods as described in Example I,
anti-hCG (antibody against human chorion gonadotrophin
(hCG) was coupled to 800 nm latex particles and HRP
was conjugated to anti-~hCG (antibody that reacts with
an epitope located on the ~ suhunit of hCG). Devices
were constructed as described in Example I,
implementing the above hCG speci~ic reagents instead
of the HBsAg specific reagents.
A dilution series of hCG was made, using a diluent
consisting of a mixture of urine specimen of fertile
non-pregnant women. This dilution series was tested
analogously to the procedure for testing as described
in Example I, using the above mentioned devices.
Results as produced by the device:
hCG dilution in urine Read by eye
series
.. . . . . _ _ . _ _
2500 U/l strong blue colour
250 U/l strong blue colour
25 U/l blue colour
0 U/l no blue colour
This demonstrates that the device can be used to
perform a rapid single manual assay for the detection
of hCG in urine samples and is consequentlv very
suitable for pregnancy testing.