Note: Descriptions are shown in the official language in which they were submitted.
2 ~
LOW NOX BURNER
TECHNICAL FIELD
The present invention relates" in general, to
combustion apparatus and, more particularly, to a combustion
technique that produces an extremely low level of NOX
emissions.
BACKGROIJND ART
Recently, there has been a great deal of concern over
the problem of air pollution. This problem is particularly
acute in the urban areas of the country. There are many
sources of air pollution such as the internal combustion
engine, chemical processing plants, power generating
facilities, etc. One of the more serious pollutants is the
oxides of nitrogen, such as NO and NO2, which are
collectively known as NOX and which contribute to air
pollution by the formation of smog.
In fuel burning facilities, such as power generating
stations, there are various sources of NOX emissions. One
source of NOX emissions, refer~ed to as th~rmal NO, results
from the oxidation of the nitrogen (N2) component of the
combustion process air. Thermochemistry requires
temperatures in the order of 2800~F. for the formation of NO
in this ~nn~r, The diatomic nitrogen (N2) component must
first be dissociated into atomic nitrogen (N) prior to the
formation of NO. Another source of NOX emissions, referred
to as fuel NO, results from the fact that many fuels contain
the single atomic nitrogen species, for example, ammonia
~NH3). In this case, N2 bond splitting is not a
prerequisite to NO formation thereby allowing conversion of
fuel-bound N to NO at temperature significantly below
2800~F. Conversion of fuel-bound nitrogen to NO can occur
at temperatures as 1QW as 1300 F. A still another source of
--2--
NOx emissions, referred to as prompt NO, results from high-
speed reactions. Formation of NO by high speed reactions
within the flame front have been report:ed and is the subject
of ongoing research. No widely accept:ed mechanism for this
mechanism has been developed. In those geographic areas
where stringent air quality control regulations have been
enacted, such as in Southern California, it has become
extremely difficult to reach the standards established for
NOx emissions by utilizing presently available burners
and/or methods of operating same.
Various approaches have been developed for reducing
~~x emiss~ons, however, the resulting reduction is not
sufficient in many cases to satisfy the foregoing stringent
air quality standards. Some of these approaches are based
on reducing NOx emissions by multi-stage combustion. For
example, such ~ulti-stage combustion might involve burning a
first fuel as a "lean mixture" and subsequently burning the
resulting combustion products with a second fuel to form an
atmosphere ~ which causes a reduction in NOx emissions.
Alternatively, fuel and air can be introduced into a burner
so as to form two separate streams each having different
ratios of fuel to air, i.e., one stream would have an
excess of air while the other stream would have an excess of
fuel. One of the streams is then ignited effecting a first
stage of combustion which then ignites the second stream
effecting ~a second stage of combustion. A third stage of
combustion is provided by mixing and burning the excess
fuel in one of the streams with the excess air in the other
of the streams. A still another approach to reduce NOx
emissions requires a plurality of burners disposed in a
series connection with respect to the direction of flow of
combustion air. In this case, the last burner in the
series of burners utili2es a fuel having lower NO~ producing
properties.
2~0~
Decreasing the temperature of combustion can also
result in a reduction in NOx emissions. The combustion
temperature can be reduced by direct flame cooling through
water injection of the combustion gases or by adding a
cooling gas to the air-gas mixture~ Flame temperature can
also be reduced by utilizing radiant burners which are
essentially surface burners often employing ceramic fibers,
metallic fibers or reticulated ceramic foams as the radiant
surface. A major disadvantage of most surface combustors is
that because of their large size, a substantiaI volume of
air/gas mixture is trapped within the burner. In the event
of flashback, which is a distinct possibility, the
deflagration created may be of explosive proportions.
Another disadvantage of surface combustors is that to
achieve optimal radiant output for a given input (radiant
efficiency), the surface temperature must remain extremely
high. Surface combustion temperatures are very sensitive
to air/fuel ratio~ velocity, and ~low uniformity. A
reduction in surface temperature diminishes the radiant
output by the fourth power wh1ch would likely result in
higher N~x emissions levels, via higher flame temperatures.
NOx emissions can also be reduced by recirculating the
flue gases within the combustion chamber. In this approach,
a portion of the flue gases can either be mixed with the
combustion air prior to combustion, or delivered into the
combustion zone separately. The recirculated flue gas acts
as a diluent to lower the overall oxygen concentration and
flame temperature. In essence, the combustion air supply is
vitiated, thus reducing NOx, however, carbon monoxide (CO)
emissions might increase.
--4--
Another approach for reducing the production of NOx
involves changing the composition of the air-gas mixture.
For example, if a mixture of oxygen and an inert gas, other
than nitrogen~ is utilized as the combustion atmosphere, NOx
emissions are reduced. Alternatively, an additive can be
introduced into the combustion chamber to form reducing
agents which react with the nitrogen oxides to produce
nitrogen, thus reducing the production of NOx. Thus, there
are many approaches for reducing NOx emissions.
All of the foregoing approaches for reducing NOx
emissions have certain inherent disadvantages with respect
to cost, reliability, performance, etc. For example,
reducing the combustion temperature to reduce the production
of NOx may result in a reduction in the heat flux produced
by the burner. Multi-stage combustion requires a
significant amount of equipment and associated controls, all
of which can become quite costly. Similarlyj flue gas
recirculation techniques require additional equipment and
might increase the production of carbon monoxide (CO),
whereas the use of additives increases operating cost.
Radiant process ~ibrous materials are expensive, often
fragile, and sensitive to blockage from airborne dust, thus
requiring filtration equipment and associated maintenanceO
Such air filtration equipment will not prevent burner
plugging problems inherent in the combustion of numerous
fuel gases which contain contaminants, such as tar.
It is well established that thermal NO formation is the
predominant NOx producing mechanism in the combustion of
clean fuels, e.g., natural gas, and that the Zeldovich chain
reaction mechanism applies to thermal NO formation. The
chemical reaction kinetics of this analytical model predict
that NOx production increases with time and temperature.
These trends have been verified in practical combustion
2 ~
-5
systems with peak NOX formation rates occurring slightly to
the fuel lean side of stoichiometric~ Predictions of the
relative contributions of time and temperature in the
~ormation of NO using the Zeldovich chain reaction model are
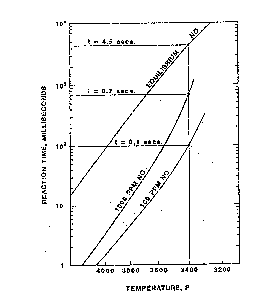
illustrated in Figure 1. This Figure also illustrates the
contribution of "residence time" to the formation of NOx,
i.e., the production of NOX takes a finite period of time.
Figure 1 illustrates the importance of "residence time" in
the formation of NOX as calculated using the Zeldovich chain
reaction model~ At a flame temperature of 3400~F.,
"residence times'l of 0.1, 0.7 and 4.5 seconds produce NOX
levels of 100 ppmv, 1000 ppmv and equilibrium levels,
respectively, all of which exceed proposed emissions
standards.
Reducing the combustion reaction (flame) temperature by
using an excess of combustion air can, in certain cases,
result in lower NOX formation. This effect can only be used
to significant advantage with a homogeneous pre-mix type
combustion apparatus; ln chemical parlance, a plug flow
reactor. In the plug flow method, the peak fuel to air
concentration equals the average concentration due to the
premixing. This results in the average flame temperature
being equal to the peak flame temperature. The NOX
emissions are then proportional to this temperature level.
In a nozzle mixing burner (stirred reactor), the mixing and
combustion reactions occur virtually simultaneouslyl and due
to mixing imperfections, wide variations in fuel to air
concentrations occur. This results in mixture
stratification with some localized peak fuel to air
concentrations siynificantly in excess of the overall
average value. Where the higher concentrations occur, high
temperatures result, with concurrent high levels of NOX
formation.
~4~
--6
Pre-mix combustion systems also offer the advantage of
a high heat release rate per un~t of combustion volume as
compared to nozzle mix systems. In other respects, they are
inferior to nozzle mixing systems; particularly with respect
to combustion stability limits. Beyond certain air to fuel
ratio valuas, combustion moves away from the burner
apparatus and the flame is extinguishea~ These effects are
apparent in Figure 2, in which it can be seen that pre-mix
;
burners have a limited stability range in the more useful
fuel lean non-polluting operating range. Also, for all
burner types, as the stability limits are approached, the
combustion efficiency decreases prior to flame extinction or
"blow-out". The reduction in combustion efficiency produces
large amounts of unburned combustible pollutants,
predominately CO in the case of natural gas combustion.
In addressing the NOX problem, it is necessary that NOX
and CO be considered simultaneously, because a reduction in
one pollutant may merely represent a compromise with regard
to emissions of the other. For most conventional burners,
CO and ~Ox emissions are generally produced in inverse
proportions. Whereas the elimination of carbonaceous
pollutants, e.g~, CO, etc., is amenable to relatively simple
techniques, the simultaneous control oE both NOX and CO has
presented problems using generally accepted control
techniques. The foregoing occurs since CO requires time and
a relatively high temperature, typically of the order of
2500~F., to oxidize such to carbon dioxide ~CO2).
Temperatures in excess of 2800~F. have been found to be
conducive to NOX formation. These factors can be understood
by referring to Figure 3 which is a graph of the NO versus
x
~ combustible~, such as CO, and illustrates the "emissions
- window" in which burners are considered to be operating at
acceptable emission levels.
:
2 ~
--7--
To sustain clean, efficient combustion, a ragion of
stable burning must be created~ In the a~sence of such,
flame extinction or "blow-out" wil]L occur~ Combustion
e~ficiency and flame stability are closely interrelated, the
"blow-out" condition representing the case of zero
combustion efficiency. Flame stabilization can be achieved
by the use of a flame holding device or bluff body in the
air/gas mixture stream. Typical flame stabilizing devices
include metal screens, rods, and flame inserts. It has been
found that these flame stabili~ing devices also reduce NOx
emissions. Radiant fiber and ceramic sur~ace burners have
also been used for similar reasons. In the foregoing
cases, the rods or surfaces provide a heat absorbing
mechanism capable of re-radiating the absorbed heat to an
absorbing surface beyond the flame region. By such means
the flame t~mperature is reduced with concurrent reductions
in NOx formation. A key element in this approach is the
ability of the radiant emitter surface to remove a
substantial proportion of the heat generated, thereby
controlling flame temperature. Experimental evidence of
this phenomena sho~s an increase in NOx emissions as the
heat flux to the emitter is increased. This since, for a
fixed emitter geometry, i.e., surface area, the amount of
heat radiation from the reaction zone is essentially
constant, thereby impairing its ability to control the
reaction temperature at the higher heat flux rates. Surface
burners change from radiant to a blue flame mode as the heat
flux (BTU/hr ins ) is increased. In general, at heat fluxes
in excess of 1000 BTU/hr inS2~ the more common surface
burners "blow-out"; prior to this large quantities of CO are
also produced.
--8--
Because of the foregoing, it has become desirable to
develop a burner system which minimizes the production of
NOX and which produces low levels of CO. Referring again to
Figure 3 what is required is operation inside the "emissions
window". Furthermore, emissions should remain within the
window throughout the firing range ~rom low to high fire.
SUMMARY OF THE lNV~NllON
It is known that the use of excess air in pre-mixed
burners reduces NOX emissions since such excess air
decreases the temperature of combustion. In accordance with
the present invention, it has been found that increasing the
velocity of the air/gas mixture also reduces NOX emissions
since "residence timel' is decreased. Increasing the
velocity of the air/gas mixture does, however, create a
problem of flame "lift-off" from the burner. ~o prevent the
occurrence of flame 'ilift-off" while ~in;~izing NOX
production, flame stabilizing~devices may be employed. The
st bil~zers may be constructed from any suitable
configuration of heat resistant materials. Figures 4
through 6 and 8 through 10 show various types of pre-mix
burners that can utilize the methodology of the present
invention. Burners of the type shown have been operated
with port face loadings in the range of 5,000-100,000 BTU/hr
ins2. Flame stabilization can also be achieved by
aerodynamic means, e.g., opposed jet recirculation, wake
flow, etc., eliminating the need for mechanical stabilizers.
What was not recognized in the prior art was the
contribution of "residence time" in the formation of NOX.
By increasing the velocity of the air/gas mixture, the
"residence time" at the combustion reaction temperature is
reduced. Port face loadings in the 5,000 - 100,000 BTU/hr
ins range represent a ten to twentyfold reduction in
-9-
"residence" time as compared to prior art burners. It
should be recognized that the Poregoing port face loadings
are based upon the total port face area and not the open or
slot areas that form the air gas mixture passageways.
Experiments were conducted at the high heat flux rates
using ribbon, ported ceramic, and porous ceramic burner
types. soth ceramic rod and wire mesh flameholder types
were also used. In all cases, the combustion emissions of
both NOx and CO were very low; Figure 11 depicts typical
results obtained.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows the theoretical concentration of NOx
produced versus time and temperature as calculated using the
Zeldovich chain reaction model.
Figure 2 is a graph of Air/Fuel Ratio versus Blow-Off
Velocity for nozzle mix burners and premix burners.
Figure 3 is a graph of the Oxides of Nitrogen versus
Combustibles, such as CO, and illustrates the "emissions
window" in which burners are considered to be operating at
acceptable emission levels.
Figure 4 is a cross-sectional view of one type of pre-
mix burner utilizing external flame stabilization apparatus
and which can be operated using the methodology of the
present invention.
Figure 5 is a cross-sectional view of another type of
pre-mix burner utilizing externaI flame stabilization
apparatus and which can be operated using the methodology of
the present invention.
Figure 6 is a cross-sectional view of still another
type of pre-mix burner utilizing external flame
stabilization apparatus and which can be operated using the
methodology o~ the present invention.
- 1 0
Figure 7 is an enlarged partial cross-sectional view of
the distributor plate illustrated in Figure 6 and
illustrates the configuration of the ports therein.
Figure 8 is a cross-sectional view of one type of pre-
mix burner utilizing internal flame stabilization apparatus
and which can be operated using the methodology of ths
present invention.
Figure 9 is a cross-sectional view of another type of
pre-mix burner utilizing internal flame stabilization
apparatus and which can be operated using the methodology of
the present invention.
Figure 10 is a cross-sectional view of still another
type of pre--mix burner utilizing internal ~1ame
stabili ation apparatus and which can be operated using the
methodology of the present invention.
Figure 11 is a graph of NOx Emissions versus Percent
xcess Air.
DESCRIPTION OF THE P~EFERRED EMBODIMENT
The production of NO is a function ~f combustion
temperature and the time required to complete combustion.
In addition to the previously mentioned prior art methods
for reducing NOx emissions, it is known that the use of
excess air in the air/gas mixture also decreases the
production of NOx- The reduction in NOx production in this
case can be attri~uted to a decrease in the temperature oE
combustion as a result of the excess air. Alternatively, an
increase in the velocity of the air/gas mixture can be
utilized to reduce NOx emissions. Such an increase in
velocity can be achieved by reducing the size of the
orifices through which the air/gas mixture flows or by
increasing the port face loadings. By increasing the
velocity of the air/gas mixture, the "residence time"
associated with the formation of a flame is decreased, i.e.,
the combustion gases are in the reaction zone of the flame
for a significantly shorter period of time which, in turn,
reduces the production of N9x. The velocity of the air/gas
mixture can only be increased to a level where the flame
begins to "lift-off" the burner. An increase in the
velocity of the air/gas mixture beyond the foregoing level
results in the flame being blown out. In order to increase
the velocity of the air/gas mixture beyond the velocity
where flame "lift-o~f" occurs, a flame stabilizing device
must be utilized.
Referring again to the drawings, Flgure 4 is a view of
one of a number o~ burner units 10 which utilizes a ~1ame
stabilizing device and which can be operated using the
methodology of the present invention to produce a very low
level of NOX emissions. The burner unit 10 includes a
plenum 12 with a distribution plate 14 extending across its
upper surface forming the outlet of the burner~ The
distribution plate 14 has a plurality of orifices or ports
16 passing therethrough. A flame arrester/distributor
matrix 18 is positioned adjacent the upper surface o~ the
distribution plate 14. Another embodiment o~ a burner unit
which utilizes a flame stabilizing device and which can
utilize the methodology of the present invention so as to
produce a very low level of NOX emissions is burner unit 20
illustrated in Figure 5. Burner unit 20 includes a burner
body 22 and a plurality ~ of parallel flame
arrester/distributor ribbons 24 adjacent its upper surface
forming ports 26 therebetween. A still another embodiment
of a burner unit which utilizes a flame stabilizing device
and which can utilize the methodology of the present
invention so as to produce a vexy low level of NOX emissions
is burner unit 30 illustrated in Figure 6. Burner unit 30
2~
-12-
includes a ceramic tile distributor plate 32 having a
plurality of ports 34 therein as shown in Figure 7. Each
port 34 has a through portion 36 o~ substantially constant
diameter or may incorporate a tapered portion 38 of
increasing diameter from its junction with through portion
36 to the outer surface 40 of the distributor plate 32. The
foregoing burner units are merely examples of some types of
burners that can utilize the methodology of the present
invention so as to produce very low lavels of NOX
emissions. Many other types of burners can be utilized with
similar results and there are no restrictions as to burner
size, shape, porting configuration, method of fabrLcation,
or materials utilized for same. Regardless oE the type of
burner utilized, the plenum or burner body is connected to
an air-gas supply. In this manner, a combustibla mixture of
air and gas is supplied to the plenum or burner body from
the air-gas supply. In ~any event, one or more flame
stabilizing devices are positioned a short ~istance above
the ports in the burner units utilized. The flame
stabilizing devices may include one or more ceramic flame
rods, wire mesh flame screens, or any combination thereof,
in order to stabili~e the flame above the ports provided in
the burner utilized. It should be noted that in addition to
stabilizing the flame above the ports, the flame stabilizing
devices may also produce radiant heat which further serves
to suppress NOX formation.
Experimentally, flame screens formed from 0.092 in.
Nichrome or Inconel wire have been used successfully with
various types of burners. ~he optimum distance between the
flame stabilizing means an~ the top of the burner to
; ni ~i ze the production of NO can be determined empirically
or by experimentation.
Alternatively~ if the burner has a single or a
relatively small number of outlet ports, a bluff body 60 can
-13-
be located within the outlet 62 of the burner, shown
generally by the numeral 64, in Figure 8~ The bluff body 60
can be formed from any of a variety of geometries, e.g., a
weld cap having a generally semi-spherical configuration, or
the like, which is held within the outlet 62 of the burner
by means of set screws 66 which are threadably received
through the bluff body 60 so that their ends contact the
inner surface of the burner 64. ~luff body 60 is
positioned within the outlet 62 so that the flow of the
air/gas mixture contacts the convex surface of same. In
this manner, the bluff body 60 presents a contoured
obstruction to the flow of the air/gas mixture. In Figure 8
a separate pilot (not shown) is utilized to ignite the
air/gas mixture and the velocity of the air/gas mixture
approaches the velocity at which the flame begins to "lift-
off'l the surface defining the outlet 62 of the burner 64~
It should be noted that flow of the air/gas mixture impinges
upon the upstream face of the bluff body 60, and then
recirculates counter to the air/gas flow direction in a zone
on the downstream side of the bluff body creating a region
which supports co~bustion before passing outwardly therefrom
to the outlet 62 of the burner 64.
Another burner structure which incorporates flame
stabili ation is shown in Figure 9 and includes a bluff body
attached to the end of a pilot tube 72.~ Here again, the
bluff body 70 can be formed from any of a variety of
geometries, e.g., a weld cap having a generally semi-
spherical configuration, or the like. Alternatively, the
pilot tube 72 and the bluff body 70 oan be formed from a
pipe and a reducing coupling. The pilot tube 72 and bluff
body 70 are received within the outlet 74 of the burner,
shown generally by the numeral 76, and are held within same
by means of set screws 78 which are threadably received
2~
-14-
through the bluff body 70 so that their ends contact the
inner surface of the burner 76. The pilot tube 72 and the
bluff body 70 are positioned within t~he burner 76 so as to
be substantially concentric therein. The aix/gas mixture
passes through a passageway 80 between the outer surface of
the pilot tube 72 and the inner surface of burner 76 and
the mi~ture impinges upon the upstream face of the bluff
body 70, and then recirculates counter to the air/gas flow
direction in a zone on the downstream side of the bluff body
creating a region which supports combustion. After
ig~ition of the air/gas mixture by the pilot flame within
th~, pilot tube 72, the resulting combustion gases pass to
the outlet 74 of the burner 76. As in the burner structure
illustrated in Figure 8, the velocity of the air/gas mixture
approaches the velocity at which the flame begins to "lift-
off" the surface forming the outlet 74 of the burner 76.
It has been found that the foreyoing bluff ~odies in Figures
8 and 9 provide flame stabilization, permitting the
velocity of the air/gas mixture to be increased beyond the
velocity at which flame "lift-off" would occur if a flame
stabilizing device had not been used. It has also been
found that the use of such bluff bodies negates the need for
a flame stabilizing device exterior to the outlet of the
burner.
A still another burner structure which incorporates
flame stabilization is shown in Figure 10 and includes a
flameholder 90 attached to the end of a pilot tube 92. The
flameholder 90 can be cup-shaped and acts as a bluff body,
as in the structure shown in Figures 8 and 9~ The pilot
tube 92 is positioned within a pipe 94 so as to be
substantially concentric therein. The circumferential end
96 of pipe 94 abuts a refractory diffuser 98 having a
tapered opening 100 therein. The diameter of the tapered
-15-
opening 100 increases from the inner surface 102 of the
refractory diffuser 98 to the outer surface 104 thereof.
The inner diameter of pipe 94 is approximately the same as
the diameter of the tapered opening 100 at the inner surface
102 of the refractory diffuser 98. The pipe 94 is aligned
with the tapered opening 100 so that no discontinuities
exist between thé surface defining the inner diameter of the
pipe 94 and the surface defining the tapered opening 100 in
the refractory di~fuser 98. A swirl vane assembly ~06 is
positioned ad~acent the outlet 108 of the flameholder 90 and
is interposed between the flameholder 90 and the surface
defining the tapered opening 100 in the refractory diffuser
98. Air and fuel are provided through apertures 110 and
112, respectively, in the burner housing 114 and pass
through a plurality of mixing venturis 116 into a chamber
118 before passing into pipe 94 through end 120 thereof.
The air/gas mixture passes through a passageway 122 between
the inner surface of the pipe 94 and the outer surface of
the pilot tube 92 into a passageway 124 between the surface
defining the tapered opening 100 in the refractory diffuser
98 and the outer surface of the flameholder 90. As the
air/gas mixture passes through the swirl vane assembly 106,
the mixture recirculates counter to the aix/gas flow
direction in a zone on the downstream side of the
flameholder 90 creating a region which supports combustion.
After ignition of the air/gas mixture by the pilot flame
within the pilot tube 92, the resulting combustion gases
pass outwardly therefrom to the outlet 126 of the burner~
The velocity of the air/gas mixture approaches the velocity
at which the flame begins to l'lift-off" the surface forming
the outlet 12S of the burner. As in the previous burner
-16-
structures, the flameholder 90 permits the velocity of the
air/gas mixture to be increased beyond the velocity at which
flame "lift-off'l would occur if a flameholder had not been
used.
Regardless of whether a flame stabilizing device is
utilized, it has been found that NOX emissions can be held
to acceptable levels by operating the burner unit with high
velocity excess air to keep the combustion temperature
slightly below the temperature at which a significant amount
of NOX is produced and to minimize the "residence time"
associated with the formation of a flame~ In the method of
the presen-t invention, a high velocity premixed air and gas
stream in combination with high heat flux rates, together
with suitable proportions of excess air, has been shown to
control the "residence time" and temperature thereby
minimizing NOX emissions. However, because of the high
velocity of the excess air, flame stabilizing devices in the
form of flame rods, flame screens or bluff bodies might be
required to ensure that the flame does not "lift-off" the
burner. The use of a fl ame stabili~ing device increases
the maximum flame extinction or "blow-out"'i velocity of the
air-gas mixture. The device may also act as a radiator of
heat thus keeping the resulting temperature from exceeding
the temperature at which a significant amount of NOX is
produced. It should be noted, however, that flame
stabilization can also be achieved by aerodynamic means,
e.g., opposed jet recirculation, wake flows, etc.,
eliminating the need for a stabilizing device. It has been
found with foregoing operating conditions that a very high
heat flux of approximately 5,000-100,000 BTU/hr in.2 can be
achieved; the former heat flux of approximately 5,000 BTU/hr
in~2 heing without the utilization of a flame stabilizing
-17~
device, the latter heat flux of approximately 100,000 BTU/hr
in.2 being achieved with the utilization of a flame
stabilizing device. Referring now to the graph shown in
Figure 11, it is apparent that NOX emissions decrease as the
percent of excess air increases. If more than 20% excess
air is utilized, NOX emissions will be held within recently
proposed standards. Thus/ with the foregoing operating
parameters, viz., 3~00 degrees F~ nominal operating
temperature and high heat flux rates combined with suitable
proportions of excess air, acceptable NOX levels can be
achieved. It has been further found with the foregoing
operating parameters that as heat flux increases, the
production of NOX decreases if "residence time" is
n; i zed. This was not the case with prior art burner
systems wherein an increase in heat flux resulted in a
commensurate increase in NOX emissions. This latter
benefit, i.e., a decrease of NOX emissions with an increase
in heat flux, has not been previously taught.
It has been found in oxygen enriched applications,
~hich generally have higher flame temperature resulting in
increased NOX production, that an increase in the velocity
of the air/gas mixture decreases "residence time" which, in
turn, reduces NOX production. Similarly, in applications
where the air/gas mixture has been preheated, which
typically results in a higher flame temperature, pre-heating
increases the velocity of the air/gas mixture resulting in
decreased "residence time" and thus, reduced NOX production.
Another feature of the present invention is that the
resulting production of NOX and CO are within the "emissions
window" shown in Figure 3. As previously stated,
conventional burners typically produce NOX and CO in inverse
proportions since time and temperature, both o~ which are
conducive to NOX formation~ are required to reduce CO to
CO2. Test results using the methoclology of the present
invention, i.e., 20% and greater excess air at a high
velocity, reveal that even though extremely low levels of
NOX are produced, approximately 20 ppmv, the production of
CO is not excessive and is within the "emissions window".
Thus, the methodology of the present invention minimizes the
production of NOX while producing low levels of CO.
Certain modifications and improvements will occur to
those skilled in the art upon reading the foregoing. It
should be understood that all such modifications and
improvements have been deleted herein for the sake of
conciseness and readability, but are properly within the
scope of the following claims.