Note: Descriptions are shown in the official language in which they were submitted.
2054798
BEHRINGWERKE AKTIENGESELLSCHAFT HOE 90/B 032K - Ma 871
Dr. Pfe/Zi Ma 897
Ma 910
Ma 917
Description
HCV-specific peptides acients therefor and the aase
thereof
The invention relates to polypeptides for the immuno-
chemical determination of HCV-specific antibodies and HCV
antigens, and to agents suitable for this method and the
use thereof.
The invention also relates to an immunochemical method
for the simultaneous detection and/or for the simul-
taneous determination of a plurality of different anti-
body specificities against pathogens which are different
in each case in a single test.
Non A/non B hepatitis (NANBH) is, as transmissable
disease or group of clinical pictures, regarded as virus-
associated and distinguishable from other virus-induced
clinical pictures which are cau:sed by different hepatitis
viruses such as hepatitis A virus (HAV), hepatitis B
virus (HBV), hepatitis D virus (HDV) and hepatitis E
(HEV). Finally, it is also possible to diagnose hepatitis
caused by cytomegalovirus (CMV) or Epstein-Barr virus
(EBV).
It is possible on the basis of epidemiological investiga-
tions to define, in accordance with the transmission
route, at least two types of non A/non B hepatitis
(NANBH): epidemic hepatitis virus (enterically trans-
mitted NANBV), which is transmitted by water and food-
stuffs, and post-transfusion hepatitis virus (blood
transmitted NANBV), which is transmitted by blood, needle
sticks or similar routes. Besides these routes of infec-
tion there are also known to be transmissions which, as
- 2 -= 2054798
sporadically occurring NANBV ("community acquired
NANBV"), have no evident association with the two types
mentioned. Although the exact number of agen=ts or viruses
causing NANBH is unknown, so-called hepatitis C virus
(HCV) has recently been identified as a causative patho-
gen of this disease (WO 89/04 669).
Until recently, clinical diagnosis was based mainly on
serological determination of antigens and/or on anti-
bodies directed against them, the tests being specific
for parameters from the group of hepatitis pathogens HAV,
HBV, HDV, HEV, CMV or EBV. In this so-called exclusion
method, NANBH was diagnosed only when all the abovemen-
tioned determinations were negative.
Besides this, so-called surrogate markers have also been
used, such as, for example, GPT (glutamic-pyruvic trans-
aminase, also called ALT - alanine aminotransferase) or
anti HBc (hepatitis B core-specific antibodies). However,
these aids are neither sensitive nor specific enough to
be regarded as reliable. It is therefore possible to
avoid only a small part of the cases of post-transfusion
hepatitis, which occur in about 10% of transfused
patients, by investigating blood donors. The urgent need
for introduction of a specific test is underlined by the
fact that NANBV is regarded as responsible for about 90%
of cases of post-transfusic?n hepatitis. The main problem
with this disease is the fact that between 25 and 55% of
infected people suffer chronic liver damage.
The discovery of HCV has provided the basis for a
specific detection of HCV or HCV-specific antibodies. The
isolation and characterization of HCV and corresponding
cDNA replicates of parts of the HCV genome is the subject
of WO 89/04 669, where HCV is assigned to the family of
flavi-like viruses. There are also descriptions therein
of the use of HCV antigen for detecting HCV-specific
antibodies, and of the raising of antibodies for the
diagnostic determination of antigen in patient's blood.
2054710#8
- 3 -
However, use is made of genetically engineered proteins,
especially so-called non-structural proteins (NSP) from
the open reading frame region (ORF), which are used as
reactants in immunochemical detection methods.
An immunochemical detection comprises within theimeaning
of this invention all processes which, as homogeneous (in
solution) or heterogeneous (with solid phase) in vitro
methods, permit the determination of antigens and/or
antibodies of immunoglobulin classes A, D, E, G or M
(IgA, IgD, IgE, IgG or IgM) in body fluids such as serum,
plasma, saliva, cerebrospinal fluid or urine. Examples of
these methods, which are also called immunoassay, are
enzyme imxnunoassay (ELISA or EIA), radioimmunoassay
(RIA), immunofluorescence assay (IFA), radioimmunopre-
cipitation (RIPA), agar gel diffusion etc.
For example, WO 89/04 669 makes use of the antigen C-100-
3, mentioned therein, in the ELISA procedure for detect-
ing HCV-specific antibodies (anti-HCV). The construct C-
100-3, which is expressed by genetic engineering in yeast
cells, from the NSP 3/4 region comprises 363 amino acids,
whose sequence is depicted in Fig. 1, where the numbering
system corresponds to that in the abovementioned patent.
However, the maximum sensitivity which can be achieved
with the ELISA method for determining anti-HCV in samples
of human origin is assumed to be about 80% in the case of
chronic NANB patients and about 30% in the case of acute
NANB patients. These findings on blood from human
patients are supported by corresponding investigations on
chimpanzees infected with NANBV.
In them, positive anti-HCV detection on the basis of the
C-100-3 construct does not succeed until about 6 - 18
weeks after the ALT increase has taken place, which in
turn can be observed as sign of disease 3 - 10 weeks
after inoculation of NANBV. Thus, it emerges that 9 - 26
weeks after infection of a chimpanzee is the total time
- 4 - 2~~4798
before HCV-specific antibodies can be detected with the
immunoassay according to the state of the art using the
C-100-3 construct.
WO 90/11 089 describes further HCV amino acid sequences
which can be used to detect anti-HCV. However, no state-
ments are made about the diagnostic relevance of
particular protein sections, nor are there examples of
immunodominant epitopes.
The fundamental problem in the detection of anti-HCV with
the methods disclosed in the literature is that there are
still samples which react in a false-positive and false-
negative way. The false-positive samples may comprise up
to 40% in a group of healthy blood donors (WEINER A., et
al. Lancet 1990, Vol. 336, p. 695). The lack of a speci-
fic and sensitive HCV test therefore means that a not
inconsiderable proportion of samples which react falsely
are wrongly discarded in blood donation centers and, at
the same time, patients who are actually anti-HCV-posi-
tive are still not reliably identified.
To eliminate these disadvantages, other groups have
selected particular sequences of the ORF region of C-100-
3 (from WO 89/04 669, Fig. 1) and employed them in an
immunoassay (Peptides sp42, 117, 67 and 65, Fig. 2). On
investigation of NANB-infected chimpanzees it was not
possible to achieve an improvement in the diagnostic
value. On the contrary, the observations made therein led
to the conclusion that none of the described peptides is
suitable to serve as basis for a reliable IgG or IgM
determination, because the sensitivity is inadequate and
the diagnostic gap of at least 7 to 17 weeks until
antibodies are detected means that there is no reduction
in time compared with conventional detection methods.
Furthermore, it was found that sp42 in particular, with
a diagnostic gap lasting 20 to 40 weeks, is unsuitable
for a diagnostic test (SAFFORD J., et al. Int. Symp. on
Viral Hepatitis and Liver Disease 1990 Houston, USA).
- 5 - 205479$
However, the polypeptide called sp67 is said to be
immunodominant although only 86% identification of anti-
HCV-positive samples was possible with this peptide
(DAWSON, G.J., et al. Int. Congress of Virology 1990,
Berlin, FRG).
An improvement is said to have been achieved with synthe-
tically prepared core peptide (OKILMOTO H., et al. Japan.
J. Exp. Med. 1990, Vol 60, No. 4, p. 223 - 233). This
entailed selection of a sequence from the total of 3
potential antibody binding sites of the core amino-acid
sequence 1-120 (OKAMOTO, H. et al., Japan. J. Exp. Med.
1990, Vol. 60, No. 3, p. 167 - 177) with the aid of
physicochemical predictive criteria specified by HOPP and
WOOD (Proc. Nat. Acad. Sci. 1981, Vol. 78, p. 3824 -
3828). This peptide is depicted in Fig. 3 and comprises
36 amino acids numbered from No. 39 to No. 74.
In contrast to the anti-C-100 'test, it was possible to
detect HCV antibodies in some anti-HCV-positive sera with
an ELISA based on this core peptide. This finding was
confirmed by a comparative investigation using the
polymerase chain reaction (PCR) to detect HCV RNA.
However, it is evident from the 26 anti-core HCV-positive
samples found in an investigation on 606 blood donors
(4.3%) precisely that only 10 (1.65%) cases could be
confirmed by PCR. Conversely, 16 donors (2.65%) gave
false positive reactions with this peptide. Thus,
although the said core peptide is said to be more
suitable in some cases for an anti-HCV detection method
than the anti-C-100 test, the core peptide of the state
of the art is generally associated with considerable
susceptibility to interference.
The present invention was thus based on the object of
developing a test able to detect HCV infections as early
as possible and with high specificity.
- 6 - 2054798
It has now been found, surprisingly, that certain poly-
peptides from the area of the ORF region of C-100-3
and/or the amino-terminal region of the HCV core region
are particularly suitable for detecting HCV-specific
antibodies, and a distinct increase in sensitivity
compared with peptides of the state of the art with, at
the same time, significantly reduced susceptibility to
interference from unwanted false positive reactions can
be achieved.
It has furthermore been found that a high antigen concen-
tration, and thus a high epitope density, is achieved in
an immunochemical detection method with the peptides
according to the invention. This is possible particularly
when, owing to =the absence of interfering contaminations,
the investigation of highly concentrated patients'
samples is possible.
It was also completely surprising that the peptides
according to the invention carry immunodominant epitopes
for early and late HCV antibodies and thus can be
employed particularly advantageously for longitudinal
investigations.
Hence the invention relates to peptides or peptide
mixtures which react specifically with antibodies against
an HCV infection, the amino-acid sequences thereof
containing parts of the ORF region of C-100-3 and/or of
the amino-terminal HCV core region.
The terms peptides and polypeptides are used within the
scope of the invention as equivalent to peptides with up
to about 80 AF,.
The peptides according to the invention preferably
comprise amino-acid sequences from AA 121 to AA 175
(formula I) and from AA 337 to AA 363 (formula II) of the
HCV genome section described as C-100-3, with the follow-
ing sequences:
CA 02054798 2002-02-07
121 7 175
SGKPAI IPDREVLYREFDEMEECSQHLPYIEQGMMLAEQFKQKALGLLQTASRQA I
337 363
HVGPGEGAVQWMNRLIAFASRGNHVSP II
Preferred polypeptides are:
4083:
121 175
SGKPAIIPDREVLYREFDEMEECSQHLPYIEQGMMLAEQFKQKALGLLQTASRQA
4074:
121 160
SGKPAI I PDREVLYREFDEMEECSQHLPYIEQGMMLAE,QF
4073:
128 160
PDREVLYREFDEMEECSQHLPYIEQGMMLAEQF
4072:
136 160
EFDEMEECSQHLPYIEQGMLAEQF
4071:
144 160
SQHLPYIEQGMMLAEQF
4081:
161 175
KQKALGLLQTASRQA
4082:
144 175
SQHLPYIEQGMMLAEQFKQKALGLLQTASRQA
4090:
135 164
R,EFDEMEECSQHLPYIEQGMMLAEQFKQKA
- 8 - 2 0~ ~~x
4056:
121 135
SGKPAIIpDREV'LaR
-49-5 -5.*
129 143
DREVLYREFDEMEEC
4054t
137 151
FDEMEECSQHLPYIE
145 159
QHLPYXEQGMAiLA.EQ
40~
153 167
GMMLAEQFKQKALGL
4Q91:
345 359
VQ[+TMNRLIAFASRGN
Sequences from the carboxyl- and amino-terminal region of
formula I in particular have proven beneficial for
detecting late antibodies of an HCV infection, such as,
for example, the peptides 4081, 4056, 4055, and 4060
hereinafter:
- 9 - ~~~~~~~
AQ -O-L
121 139
S G KPA I I P DRE<7 LYRE F DE
Surprisingly, the carboxyl-terminal region of the ORF
region of C-100-3 has proven particularly suitable for
detecting late antibodies, containing amino acids of the
formula II or parts thereof, for example 4091.
It has also been found that the central region of formula
I is relevant for early identification of anti-HCV.
Preferred in this connection are the abovementioned
polypeptides 4071 and 4072, and peptides 4054, 4053 and
4052.
Furthermore 3 epitopes have been located on the peptide
of formula I, one of which recognises a late antibody (S)
and the others recognise early antibodies (F1 and F2).
Examples of preferred peptides have one of the following
amino-acid sequences:
AAsl-DREVLYR-BAbi ( S )
AABa-QHLPYIE-BAb2 ( F1)
AAB3-RQICALGL-BAb3, (F2 )
where AA and BA are any desired amino acid, and al - a3
and bi - b3 are each, independently of one another,
integers greater than or equal to zero.
It is advantageous to select the amino-terminal sequences
together with the carboxyl-terminal amino-acid sequences
of the formula I and the sequence of the formula II. The
central amino-acid sequence of the formula I from about
130 to 160 can be used separate therefrom.
2054798
-a0-
The peptides according to the invention furthermore
comprise parts of the amino-terminal HCV core region,
preferably that amino-acid sequence from AA 1 to AA 35 of
the HCV genome section described as core, with the
following sequence:
MSTNPKPQRKTKRNTNRRPQDVKFPGGGQIVGGVY (III)
Preferred peptides are:
1 30
P'iSTNPKPQRKTKRNTNRRPQDVKFPGGGQI SP 10
g 26
QRKTKRNTNRRPQDVKFPG SP 23
26
K'TKRNTNRRPQDVKFPG SP 30
12 26
KRNTNRRpQDVITpG SP 30 D
14 26
NTNRRPQDVKFPG SP 30 C
16 26
NRRPQDVKFPG SP 30 B
18 26
RPQDVKFPG SP 30 A
S 24
QRKTKRNTNRRPQDVKF SP 31
8 22
QRKTKRNTNRRPQDV SP 32
The peptides SP 10 and SP 23 are particularly preferred.
- 11 2054798
It is possible with the aid of the peptides according to
the invention of the core region to identify both early
antibodies from acute phases of infection and late
antibodies, which considerably improves the sensitivity
of the detections based on these peptides compared with
the state of the art. Another advantage is the high
specificity of the peptides, which generally results in
a minimal number of samples with a false positive reac-
tion.
It is generally advantageous to use peptides, linked
peptides or peptide mixtures which are specific for early
and late HCV antibodies, because it is,possible with both
types of binding site to identify both samples from early
and samples from late phases of infection. It is also
possible reliably to identify seroconversions caused by
HCV-specific IgG and/or IgM antibodies, and it is gener-
ally possible to discriminate between positive and
negative samples with extremely high accuracy. In
addition, there is generally no interference by the
expression system required for protein preparation by
genetic engineering, or other host cell contaminations
which are unavoidable for growing viruses. Reliable
determination of HCV-specific antibodies is also
generally ensured in sera, citrated, heparinized or EDTA
plasma of human origin, and inactivation of the samples
at about 55 C for about 60 minutes generally gives rise
to no false positive results. Finally, these peptides
according to the invention can be used to prepare
specific antibodies with whose aid, owing to the
immunochemical determination of corresponding antigens in
cell-free patient's blood, the diagnostic gap can be
further closed.
According to the subject-matter of the invention, a
series of novel peptides which preferably identify HCV-
specific antibodies from convalescent or chronically
infected patients and/or HCV antibodies from acute phases
of infection are described, and polypeptides and mixtures
- 12 - 2054'98
of polypeptides which are suitable as basis for screening
tests for the non-differentiating detection, which is,
however, highly sensitive and little susceptible to
interference, of anti-HCV antibodies are described.
The invention furthermore relates to antibodies which
have a biospecific affinity for at least one of the
peptides, described above, of the ORF region of C-100-3
or of the core region.
The immunochemical determination of corresponding anti-
gens with the antibodies according to the invention in
cell-free patient's blood makes it possible to establish
the presence of HCV even before endogenous antibodies
appear.
The invention furthermore relates to an immunochemical
method for the detection and/or for the determination of
HCV antibodies with the peptides according to the inven-
tion as antigen.
The invention also relates to a method for the purpose of
differential diagnosis between an early and late phase of
infection, where in each case one or more peptides which
react specifically to early antibodies and one or more
peptides which react specifically to late antibodies are
reacted with the sample in separate mixtures.
The invention furthermore relates to the use of the
peptides described above for raising antibodies in
mammals, especially in humans.
The invention also relates to the use of the antibodies
described above for diagnostic and therapeutic purposes.
The invention therefore further relates to agents which
contain at least one of the antibodies or peptides
according to the invention alone or in combination with
other peptides or antibodies.
- 13 - 2 05 4 79 $
The said immunoreactive peptides can be prepared by
synthesis or genetic engineering, preferably by synthesis
by the methods known to the person skilled in the art.
The chemical synthesis of the peptides can be carried
out, for example, as described by BARANI, G. and
MERRIFIELD, R.B. in "The Peptides, Analysis, Synthesis
and Biology", Vol. 2, Academic Press 1980, ed.
Erhard Gross, Johannes Meyenhofer, especially as a
polypeptide or as mixture of several sma11 peptides with
overlapping or non-overlapping amino-acid sequence.
The polypeptides prepared by genetic engineering include
fusion proteins whose fusion portion has subsequently
been eliminated. Also included are polypeptides which
have been modified where appropriate, for example by
glycosylation, acetylation or phosphorylation.
These amino-acid sequences can be synthesized both as a
polypeptide and as mixture of several small peptides with
overlapping or non-overlapping amino-acid sequence.
It has also been found that mixtures of individual
peptides may have better diagr-ostic properties for an
immunochemical anti-HCV detection than single peptides of
the said structures. It is particularly advantageous to
use mixtures of peptides of the ORF region of C-100-3 and
of the core region.
The invention therefore further relates to mixtures of
peptides containing one or more of the peptides according
to the invention.
In another embodiment, 2 or more of the said peptides,
preferably 2 to 10, especially 2 to 4 peptides are linked
with or without bridge. The length of the peptides is
preferably 6 to 15 amino acids. It is even possible for
polymeric forms of two or more peptides to be prepared by
methods known to the person skilled in the art and bound
to a carrier such as, for example, protein or latex
- 14 - ~0 5
particles. Thus, particularly suitable as carrier or
bridge are, for example, human serum albumin and/or
polylysine< It is likewise possible to modify the pep-
tides by an extension with 1 to 40, preferably 1 to 20,
in particular 1 to 10 amino acids. The additional regions
and structures may have a beneficial effect=on the
physicochemical behavior of the complete peptide, but the
immunoreactivity of the peptides or parts thereof ought
to be retained.
The invention therefore also relates to peptides which
are linked to one another with or without bridge, or can
also be bound to a carrier.
Modifications of this type generally alter the passive
adsorption or covalent bonding property to the solid
phase in a.beneficial manner, have an advantageous effect
on the coupling method or act more strongly as antigen
when raising polyclonal or monoclonal antibodies directed
against the peptides.
Thus, for example, the invention also relates to peptides
of the following formula
AAõ- QRKTKRNTNRRP QI7VK-BAs,
where AA and BA are any desired amino acid, and n and m
are each, independently of one another, integers from 0
to about 60, in particular from 1 to 40.
It is often advantageous for peptides to be derivatized
in a variety of ways, such as, for example, by amino-
terminal or carboxyl-terminal attachment of one or more
amino acids, preferably cystein, in order, for example,
to achieve the linkage of peptides with one another or to
a carrier, thioglycolic acid amidation, carboxyl-terminal
amidation such as, for example, with ammonium or methyl-
amine. Modifications of this type may alter the net
charge on the polypeptide and improve the physicochemical
a~~~7H
- 15 -
properties of the peptide or facilitate covalent bonding
of the peptide to a solid carrier, to carrier proteins or
to another peptide. The person skilled in the art is also
aware that the peptides according to the invention can
also be prepared among themselves or with themselves by
genetic engineering or synthesis in such a way' that a
plurality of immunorelevant epi=topes is located on one
peptide.
Thus the invention furthermore relates to peptides with
an amino-acid sequence according to the invention which
has been modified by replacement, addition or deletion of
one or more amino acids.
In general, modifications of this type do not result in
direct alterations of the immunoreactivity of a peptide,
but it is perfectly possible to achieve improved immuno-
logical properties of the peptides. Thus, for example,
methionine is prone to spontaneous oxidation, which can
be prevented by replacement by norleucin without essen-
tially changing the antigenic properties of the polypep-
tide.
The person skilled in the art is aware that given amino-
acid sequences can be subjected to a wide variety of
alterations such as, for example, deletions, insertions
or substitutions, which may be associated with various
advantages. Modifications of this type relate, for
example, to combinations such as Gly, Ala; Val, Ile, Leu;
Asp, Glu; Asn, Gln; Ser, Thr; Lys, Arg; Phe, Tyr; Ala,
Ser; Ala, Thr; Ala, Val; Ala, Pro; Ala, Glu; Leu, G3.n;
Gly, Phe; Ile, Ser and Ile, Met.
it may likewise be advantageous to improve the adsorption
properties of the polypeptide in the form of addition of
a hydrophobic sequence comprising about 2 to 20 hydro-
phobic amino acids, such as, for example, Phe Ala Phe Ala
Phe.
- 16 -
The invention furthermore relates to DNA sequences which
code for at least one of the peptides according to the
invention.
The invention also relates to an analytical method for
the detection and/or for the determination of HCV,
employing as specific step a hybridization reaction in
which at least one nucleic acid probe which is complemen-
tary in its specific part with at least one of the DNA
sequences according to the invention is used.
An immunochemical detection generally comprises processes
which, as homogeneous (in solution) or heterogeneous
(with solid phase) methods, permit the determination of
antigens and/or antibodies. Examples of these, which are
also called immunoassays, are enzyme immunoassay (ELISA
or EIA), radioimmunoassay (RIA), immunofluorescence assay
(IFA), radioimmunoprecipitation assay (RIPA) or agar gel
diffusion assay etc.
These numerous, very diverse methods differ in specific
embodiments in the marker used for detection or the
measurement principle (for example photometric, radio-
metric, visual or by the aggregaition, scattered light or
precipitation behavior) and in the solid phases. The
person skilled in the art is aware that separation of
bound and free sample antibodie:s or antigen is, although
widely used, not absolutely necessary, such as, for
example, in so-called homogeneous assays. Heterogeneous
immunoassays are preferred, especially heterogeneous
ELISA methods.
The person skilled in the art is likewise aware that the
term "confirmatory test" describes only the use of an
immunoassay, similar to the way that the tests called dot
methods only describe by name the way in which the
antigen is immobilized.
In the antibody detection, it is a prerequisite for an
immunochemical detection method that there is contact of
- 17 -- 2054798
the sample with the described peptide sequences during
the course, in order to form an antigen-antibody complex
in a particular step of the particular method or, in
competition and inhibition tests, to prevent the forma-
tion thereof by adding suitable labeled reagents.
In the direct method it is possible for the antibodies to
be contacted with peptides bound to solid phases or with
labeled peptides or with both, it being immaterial
whether the fundamental method is, as 1-, 2- or multi-
step method, based on the principle of the 2nd antibody
test or the immunometric test design (double antigen
sandwich) either with identical or different peptides (or
peptide mixtures) on the solid phase and as liquid
reagent for the detection and in combination with
specific so-called capture antibodies (for example anti-
IgM) or affinity reagents (for example protein A).
The peptides can be bound to the solid phase covalently,
by adsorption or using specific antibodies or similar
affinity methods, for example via the biotin/avidin
complex, but preferably by adsorption.
Suitable as carrier material for the solid phase are
plastics such as polystyrene, polyvinyl chloride, poly-
amide and other synthetic polymers, natural polymers such
as cellulose and derivatized natural polymers such as
cellulose acetate and nitrocellulose, as well as glass,
especially as glass fibers. Polystyrene is preferred as
carrier material.
The carriers can be in the form of beads, rods, tubes and
microtiter plates or in the form of suspensions such as,
for example, latex particles. Sheet-like structures such
as strips of paper, small plates and membranes are
likewise suitable. The surface of the carriers can be
both permeable and impermeable for aqueous solutions.
Preferred carriers are beads, tubes, wells, micro-
2054798
- 18 -
particles, strips of paper and membranes. Particularly
preferred carriers are microtiter plates, latex
particles, polystyrene beads or particles amenable to
magnetic attraction.
The peptide concentration for coating the carrier is
generally about 0.01 - 20 jug/ml, preferably 0.01 -
pg/ml, particularly preferably 2 - 10 pg/ml. It is
particularly advantageous to use synthetically prepared
polypeptides whose high purity and strong antigenicity
10 allow the use of tiny amounts of, for example, 0.01 -
2.0 mg/ml, preferably 0.1 - 0.5 pg/ml. The binding
capacity of the carrier, in particular when polystyrene
is used, is generally not saturated so that it is
normally possible to coat with a plurality of different
polypeptides, in particular with 2 - 5, especially with
3 - 4 different polypeptides, which is a particular
advantage.
When the peptides are used as labeled derivatives for -the
detection, suitable coupling techniques are all those
known to the person skilled in the art. It is also
possible to arrange multistage methods such as, for
example, preformed peptide-antibody complexes in which
the antibody carries the label, or high affinity systems
such as, for example, biotin/avidin with labeling of one
of these reactants.
Examples of markers which can be used are radioactive
isotopes, fluorescent or chemiluminescent dyes. It is
also possible to use enzymes which are detected, for
example, by chromogenic, luminogenic or fluorogenic
substrate systems, or by subsequent amplification systems
with a second enzyme which is activated by the first, as
markers.
Preferably used as markers are enzymes, especially
alkaline phosphatase and/or horseradish peroxidase or
chemoluminogens such as, for example, acridinium esters.
- 19 -. 2054798
The labeling is carried out by methods which are des-
cribed for the said markers in the state of the art.
Where the antibodies are labeled with peroxidase, the
periodate technique of NAKANE et al., 1974, J. Histochem.
Cytochem. 22, 1084 - 1090, can be used, or a method of
ISHIKAWA et al., 1983, J. Immunoassay 4, 209 - 327, in
which the partners are linked by a heterobifunctional
reagent.
Besides these methods, the peptides can also be used for
sensitization of suitable surfaces such as, for example,
latex or erythrocytes in order to measure, automatically
or visually, physicochemical changes induced by peptide-
specific antibodies, such as, for example, precipita-
tions, aggregation or light scattering. It is known that
the peptides can also be employed not derivatized for
inhibition of these measurement principles like also the
methods mentioned before.
It is possible to use for detection of the antigens
immunodiagnostic methods which make use of polyclonal or
monoclonal antibodies which are prepared with the aid of
the peptides or derivatives thereof according to the
invention. The embodimen=ts suitable for the detection
method are known to the persoxi skilled in the art and
comprise forming antibody-antigen complexes in a
particular step or inhibiting the complex formation in a
competition method by adding a labeled antigen.
Suitable as solid phases, markers or measurement prin-
ciple for establishing an antigen test are all the
possibilities described for the corresponding antibody
determination, with the competition principle and the
double antibody sandwich technique being particularly
preferred as immunochemical method. It is immaterial in
this context whether the methods are designed as 1-, 2-
or 3-step methods. Thus, multistep methods can be carried
out with unlabeled detecting antibodies which are
- 20 .- 2034798
determined with the aid of another antibody which is
directed against them and is appropriately labeled. It is
advantageous for the raising of the antibodies to modify
the peptides in such a way that their immunogenic
property is improved, as is possible, for example, by
coupling to serum albumin or keyhole limpet hemocyanin
(B.S. Schaffhausen in Hybridoma Technologie in the
Biosciences and Medicine, ed. T.A. Springer, Plenum Press
NY, London, 1985).
Finally, the present invention can also be applied when
using an immunodiagnostic element which contains the
solid phase and, in dry form, a part or even all the
reagents required, where in this case too the novel
peptides are contained either on the side of the solid
phase or in the detection reagent or in both, and an
antibody determination, an antigen detection or combina-
tions with other analytes is carried out.
An unexpected advantage of the novel peptides of the
present invention is that they allow reliable determina-
tion of HCV antibodies. Furthermore, early antibodies
from acute phases of infection are also identified with
the aid of these peptides, which considerably improves
the sensitivity of the detections based on these peptides
compared with the state of the art and, moreover, allows
differentiation between acute phases on the one hand and
chronic or convalescent stages on the other hand when the
peptides with the appropriate binding sites for these
antibody types (early or late antibodies) are used
separately from one another in two different test
methods. Finally, another advantage which emerges is the
high specificity of the peptides of the present inven-
tion, which leads to a minimization of the number of
samples with a false positive reaction.
In view of the virus safety in blood donation centers on
the one hand and for reasons of cost on the other hand,
so-called combination tests have been developed and,
- 21 -
available since 1989, permit simultaneous non-differen-
tiating detection of anti-HIV 1 and/or anti-HIV 2
(K. Korner et al., Lab.Med. 14, 159 - 161, 1990). This
development was possible owing to the great similarity
which the two HIV sub-types display towards one another
and, moreover, they belong to the same virus class. Thus
the anti-HIV 1 determination in such anti-HIV 1/2 com-
bination tests is also based on a cross-reactivity of
anti-HIV 1 with HIV 2 antigens (M. Busch et al., Trans-
fusion 30/2, 184 - 187, 1990), as also conversely the
anti-HIV 2 detection is enhanced by the reactions which
take place between anti-HIV 2 and HIV 1 antigens. On this
basis the establishment of a combined detection of two
antibody specificities proves to be relatively straight-
forward when antigens which are related or structurally
analogous are used. The detection of a pathogen of non A
non B hepatitis, the so-called hepatitis C virus (HCV),
was the prerequisite for establishing an anti-HCV deter-
mination according to the present invention. Despite the
improvement, associated therewith, in the performance
features of screening tests, the problem again arises
also with modern anti-HCV tests that the investigation of
individual donors with anti-HIV combination tests on the
one hand and with anti-HCV single tests on the other hand
entails considerable effort and considerable additional
costs.
All commerci,al embodiments to date of the anti-HCV
determination and the majority of anti-HIV combination
tests are based on genetically engineered HCV or HIV
polypeptides. However, there has also been no success to
date in deterniining a plurality of different antibody
specificities in only a single test mixture containing a
plurality of different antigens simultaneously in one
immunochemical detection when it is not possible to use
similar antigens, since different virus class affilia-
tions are present as in the case of HCV (flavivirus) and
HIV (retrovirus). Within the meaning of the invention,
different antibody specificities mean antibodies which
- 22 .- ~0547rnlS
have very low or zero cross-reactivity with one another.
It was assumed that a test of this type for detecting a
total of three antibody specificities against a plurality
of different viral antigens tends to be worse in terms of
the sensitivity and in terms of the susceptibility to
interference (specificity) as a consequence of mutual
interfering interactions than the corresponding proper-
ties of the particular single test.
Surprisingly, it has now also been found that a plurality
of different antibodies or antibody specificities against
different pathogens in each case can be detected immuno-
chemically in a single test when different epitopes of
different pathogens in each case are immobilized on a
carrier.
Furthermore, the sensitivities found with the method
according to the invention at least correspond to the
sensitivities of single tests. Thus, for example, the
sensitivity feature.s determined for anti-HIV 1/2 and
anti-HCV for the method according to the invention at
least correspond to those of single tests.
It was therefore also completely surprising that the
susceptibility to interference by unwanted false positive
reactions was reduced with the method according to the
invention. Thus, for example, on testing the specificity
of an anti-HIV/anti-HCV test according to the invention
the specificity obtained was higher than would be given
by the total of the two single tests, with the conse-
quence that overall fewer blood donations may be wrongly
discarded.
The invention therefore also relates to an immunochemical
method for the detection and/or for the determination of
a plurality of different antibody specificities against
different pathogens in each case, which comprises one or
more epitopes of the particular pathogens being immobil-
ized on a carrier and the detection and/or the
- 23 - ~7 9 19
determination of the said pathogens being carried out in
a single test.
For certain problems such as, for example, longitudinal
investigations, it may be desirable to undertake non-
differentiating simultaneous determination of different
pathogens. This means that no distinction is made between
the various antibodies but only a yes response or no
response (negative for all antibody specificities) is
obtained.
Also desirable where appropriate is differentiating
determination of the simultaneous antibody detection.
This is directly possible within the scope of the inven-
tion by, for example, in an immunometric test, the
antigens which are used having different virus-specific
labels, such as, for example, HIV 1 antigens with peroxi-
dase, HIV 2 antigens with alkaline phosphatase and HCV
antigens with p-galactosidase. Successive or simultaneous
determination of the simultaneously bound antibody
specificities is then possible with different enzyme
substrates.
An alternative form of differentiation is the inhibition
of one antibody specificity by the specific addition of
the corresponding antigen to the sample.
The invention thus further relates to an immunochemical
method for the simultaneous detection and/or simultaneous
determination of different antibody specificities, where
the detection and/or the determination is carried out in
a differentiating or non-differentiating, preferably non-
differentiating, manner.
Different pathogens according to the invention mean
pathogens against which antibodies of different
specificity and, in general, very low or zero cross-
reactivity are directed. Examples of these are HIV 1 + 2,
HCV, HTLV I + II, HBV or Treponema pallidum, preferably
HIV and HCV.
- 24 _. 2074798
It is particularly advantageous to immobilize on a
carrier antigens of the said pathogens, especially of HIV
1, HIV 2 and HCV, which have a high density or concentra-
tion of binding sites (epitopes) for the corresponding
antibodies. It is possible in this way, for example, to
identify seroconversions, a discrimination of positive
and negative samples is possible with an accuracy which
is generally high, interference by the expression system
which is necessary for preparation of proteins by genetic
engineering cannot generally occur, other host cell
contaminations which are unavoidable in growing viruses
are not generally present, in general reliable determina-
tion of HIV- and HCV-specific antibodies with different
specificity is ensured in sera, citrated, heparinized and
EDTA plasmas of human origin, and inactivation of the
samples at 56 C for 60 min normally results in no false-
positive results.
Preferably used are single epitopes and/or combinations
thereof, especially of polypeptides of HIV 1 and/or HIV 2
and HCV, which are suitable for a highly sensitive anti-
HIV/anti-HCV detection, and, furthermore, suitable
polypeptide mixtures as basis for a screening test for a
combined antibody detection.
The invention thus also relates to polypeptide mixtures
of HIV 1 and/or HIV 2 and HCV.
The following polypeptides are particularly preferred:
1. HIV 1(numberinq system of Ratner et al., Nature
1985, 313, 277 - 284):
IV transmembrane protein (gp 41): AA 580 - AA 630
V envelope protein (gp 120): AA 490 - AA 540
VI core protein (p 24): AA 240 - AA 390
2. HIV 2(numbering system of Gyader et al., Nature
1987, 326, 662 - 669):
VII transmembrane protein (gp 36): AA 570 - AA 620
- 25 .- 2054798
VIII envelope protein (gp 110): AA 480 -.AA 530
IX core protein (p 26): AA 230 -.AA 380
3. HCV (numbering system of WO 89/04669 and
WO 90/11089):
X non-structural protein 4 (NSP 4): AA 121-AA 175
XI non-structural protein 3 (NSP 3): .AF, 1-AA. 265
XII structural protein (core): AA 1-AA 80
Especially preferred are mixtures of the said polypep-
tides, especially for a non-differentiating anti-HIV/
anti-HCV screening test, some being described by way of
example without confining the conceivable possibilities
thereto:
XIII gp 41 HIV 1
gp 36 HIV 2
NSP 3 HCV
XIv gp 41 HIV 1
gp 36 HIV 2
NSP 4 HCV
core HCV
Xv gp 41 HIV l
gp 36 HIV 2
NSP 3 HCV
NSP 4 HCV
core HCV
XVI gp 41 HIV 1
p 24 HIV 1
gp 36 HIV 2
NSP 3 HCV
core HCV
or
XVII gp 41 HIV 1
p 24 HIV 1
_ 26
gp 36 HIV 2
p 24 HIV 2
NSP 3 HCV
NSP 4 HCV
core
In general, better immunological properties for diagnos-
tic use are achieved with the said peptide mixtures.
The following peptides of HIV 1, HIV 2 and HCV have
proven particularly suitable:
XVIII SPH 9 (HIV 1, gp 41):
586 620
RILAVERYLICDQQLLGIWGCSGKLICTTAVPWNAS
XIX SPH 20 (HIV 2, gp 36):
578 613
RVTAIEKYLQDQARLNSWGCAFRQVCHTTVPWVNDS
XX SP 4083 (HCV, NSP 4):
121 175
SGKPAIIPDREVLYREFDEMEECSQHLPYIEQGMMLAEQFKQKALGLLQTASRQA
or parts thereof, for example
XXI a mixture of SP 4060 and SP 4082 (HCV, NSP 4):
127, 139
SGI{PAIIPDREVLYREFDE SP 4060
144 175
SQHLPYIEQGPgiLF,EQFKQEALGLLQTASFiQA SP 4082
XXII SP 10 (HCV, core):
1 30
MS TNPKPQRIC'!'KRNTNRRPQDVKFPGGGQI
and/or
- 27 .. 2054798
XXIII SP 31 (HCV, core):
$ 24
QRKTKRNTNRRPQAVKF-NH2
It is likewise possible to integrate further peptides
from different protein regions of HIV 1, HIV 2 or HCV, as
long as these polypeptides are immunorelevant. Con-
versely, it may also be advantageous, for example for
epidemiological problems, to exclude a specific antibody
in the non-differentiating detection by omitting corres-
ponding peptides in order, for example, to determine with
a mixture of HIV I and HCV peptides only anti-HIV 1/anti-
HCV simultaneously and not, for example, anti-HBV/anti-
HIV 1/anti-HCV. It is also possible for the peptides
which are not identical to HCV, such as, for example,
precisely HIV, to be derivatized and modified in a manner
analogous to that described above for the HCV peptides.
A considerable advantage of the method according to the
invention is that a plurality of antibodies against dif-
ferent pathogens can be detected in a single test and,
moreover, a specificity or sensitivity which is still as
high as that which can be achieved with single tests is
achieved.
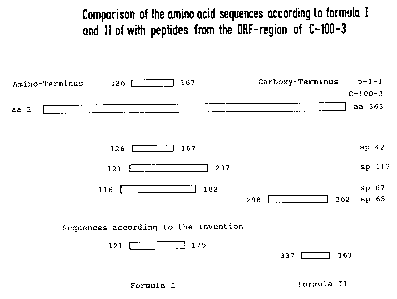
Fig. 1 Primary sequence of the amino acids of the ORF
region of C-100-3 (from WO/89/04669)
Fig. 2 Comparison of the sequences, according to the
invention, of the formula I and II with known peptides
from the ORF region of C-100-3
Fig. 3 Comparison of the sequences, according to the
invention, of the HCV core protein with known peptides
The examples described hereinafter represent embodiments
of the invention without, however, confining it to them.
CA 02054798 2002-02-07
- 28 -
Example 1
Preparation of peptide solutions and coating of micro-
titer plates with these peptides or peptide mixtures
Serial 2-fold dilutions in 0.10 M sodium bicarbonate
pH 9.6 were made up from stock solutions of the peptides
according to the invention in 50% acetic acid in dis-
tilled water containing 1 to 10 mg of peptide/ml, that
is to say a series with the concentrations 50, 25, 12.5,
6.25, 3.12, 1.56, 0.78, 0.39, 0.2, 0.1, 0.05 and 0.01 g
i0 of peptide/ml was obtained. The procedure was analogous
for mixtures of single peptides, the stock solutions
additionally being mixed in various ratios, for example
: 1 or 1: 4, in order to obtain by dilution in 0.1 M
sodium bicarbonate the overall final concentrations which
are indicated above but which in the case of mixtures of
several peptides contain the latter in equal
concentrations (in the case of 1. 1 mixture) or in
various ratios to one another.
In each case, 100 ;.1 of each dilution were placed in 16
TM
wells of microtiter plates, type B supplied by Nunc,
Roskilde, Denmark. The test plates charged with the
di2. utions were left at 20 C for 18 hours and then the
solutions in the wells were removed by aspiration and the
wells were washed 3 - 4 times with 300 p1 of a solution
of 10 g/l bovine serum albumin in phosphate-buffered
physi_ological saline (PBS, pH 7.4) by filling and removal
by aspiration, and the test plates were subsequently
dried over silica gel at 20 C.
Example 2
Preparation of a peroxidase-labeled antibody against
human immunoglobulin of IgG class (h-IgG) and T'!B
substrate for detection
Antibodies against h-IgG were raised by the method of
2054798
- 29 --
KOEHLER and MILSTEIN for preparing monoclonal antibodies
(Nature 256, 495, 1975), different anonoclonal antibodies
with the same antigen specificity being identified by the
method described by STkLI et al. (J. of Immunological
Methods 32, 297 - 304, 1980). After purification by gel
chromatography and dialysis against phosphate-buffered
saline (PBS, pH 7.4), the pool containing the monoclonal
antibody fraction (4 mg of protein/ml) was subsequently
reacted with N-gamma-maleimidobutyryloxysuccinimide
(GMBS), obtained from Behring Diagnostics, as described
by TANAMORI et al. (J. Immunol. Meth. 62, 123 - 131,
1983).
2-Iminothiolane hydrochloride (supplied by Sigma, Cat.
No. I 6256) was reacted with horseradish peroxidase
(POD), obtained from Boehringer Mannheisn, Cat. No.
413470, as described by KING et al. (Biochem. 17, 1499 -
1506, 1978). An IgG-POD conjugate was prepared from the
GMBS-IgG conjugate and the iminothiolane-POD conjugate as
described by TANAMORI et al. (supra).
The resulting solution of -the IgG-POD conjugate had a
protein content of 360 g/ml. The ratio of POD to IgG was
determined as 2.8. The solution was subsequently diluted
to 500 ng/ml IgG-POD with a solution of 50 ml/1 fetal
calf serum (FCS), 5 g/1 polyoxyethylene(20)sorbitan
monolaurate (RTween 20) in PBS and was called anti-IgG/
POD conjugate. For use in the ELISA, the subsequent
dilution in tris buffer pH 7.4, containing 0.5% "~Y'ween 20,
was varied (between 1 : 100 and 1 : 20,000 dilution) in
order to prepare uniformly a 1 : 26 final dilution in
conjugate buffer, containing 0.1 M 2-amino-2-(hydroxy-
methyl)-1,3-propanediol (tris), 0.1 M sodium chloride
(NaCl) and 0.1% RTween pH 8.4. Rabbit polyclonal anti-
bodies prepared according to the state of the art were
adjusted so that a dilution of 1+ 25 was likewise
achieved for use.
Used for the detection of anti-IgG/POD conjugate was a
- 30 _ 2054798
substrate system or a substrate preparation containing
hydrogen peroxide and tetramethylbenzidine (TMF3), which
was prepared from two stock solutions.
Stock solution 1: TMB dihydrochloride was dissolved while
stirring in a concentration of 5 g/l, i.e. of 16 mxnol/l,
in double-distilled water and adjusted to pH 1.5 with 5
normal hydrochloric acid. Penicillin G was added to this
solution while stirring in a final concentration of
200 mg/l, i.e. of 0.56 mmol/l.
Stock solution 2: 1.4 ml of glacial acetic acid, 1.5 ml
of 1 normal NaOH and 250 mg, i.e. 3 mmol, of H202 as urea/
hydrogen peroxide adduct were added to 900 ml of double-
distilled water. After dissolution was complete, the
mixture was made up to 1 1 with double-distilled water.
TMB substrate preparation: One part by volume of stock
solution 1 and 10 parts by volume of stock solution 2
were mixed together. =
Example 3
State of the art
Employed as example was a commercially available ELISA
test kit (HP1) in which the C-100-3 construct which is
described in WO 89/04669 and is prepared in yeast by
genetic engineering is used as antigen. The procedure for
investigating human sera and plasmas was that indicated
in the manufacturer's pack insert, for example 1 : 11
sample dilution and 1 h incubation of serum, 1 h incuba-
tion of anti-human IgG/POD conjugate and the enzyme
substrate system with o-phenylenediamine (OPD) as sub-
strate, photometric measurement at 492 nm and establish-
ment of limits (a threshold of 0.40 E is added to the
mean of the negative controls).
An entirely analogous procedure was used with another
2051798
- 31 -
commercially available ELISA (HP 2) which, besides the
C-100 construct, contains additional epitopes from the
core region and from the NSP 3 region (c33c), that is to
say the original test kit was used, observing all the
statements about procedure stated by the manufacturer -
as described above, for investigating human samples.
By contrast, the procedure for determining HCV-specific
antibodies in chimpanzee serum or plasma with the two
commercial products was such that a polyclonal antibody
raised in rabbits against human IgG was used for detec-
tion. For this purpose, the IgG fraction of rabbit
antiserum was, as described in Example 2, purified,
dialyzed and labeled with peroxidase (POD). The final
concentration was adjusted to about 4 times the concen-
tration of the monoclonal anti-IgG/POD conjugate in order
reliably to fashion over the cross-reaction, validated in
preliminary tests, of the antibodies against human IgG
for chimpanzee IgG.
It was necessary to modify the establishment of limits as
described in Tab. 10 A - C because the initial values of
all the chimpanzees already exhibited elevated values
(see Tab. 10 A - C).
The comparative anti-HIV 1+ 2 combination test for the
non-differentiating determination of HIV 1 and HIV 2
antibodies is a commercially available product (HP 3)
which is based on synthetically prepared peptides of HIV
1 and HIV 2. The procedure for this test was also that of
the manufacturer's pack insert, for example sample
dilution 1: 2, 30 min incubation of serum, 30 min
incubation of conjugate (anti-human IgG/POD) and the
enzyme substrate system with tetramethylbenzidine (TMB)
as substrate, photometric measurement at 450 nm and
establishment of limits (a threshold of 0.250 was added
to the mean of the negative controls).
- 32 - 2054798
Example 4
Determination of human antibodies of immunoglobulin class
G against HCV in the ELISA with the peptides according to
the invention
50 l of serum or plasma were added to 50 l of sample
buffer containing 0.3 M tris, 0.3 M NaC1, 20% boviserine
and 0.1% RTween 20 in wells of microtiter plates which
were coated as described with peptides or peptide mix-
tures. After incubation at 37 C for 30 min, the content
of the wells was removed by aspiration, and the wells
were washed five times with washing buffer containing 1
g/1 RTween 20 in PBS. Then 100 l of conjugate in the
final dilution were added to the wells, preferably using
a preliminary dilution of 1: 3000 in tris, 0.5% Tween 20
and a final dilution of 1: 26 in conjugate buffer. After
incubation at 37 C for 30 min, the content of the wells
was removed by aspiration and again washed five times.
Subsequently 100 l of TMB substrate preparation were
added to each well, incubated at 20 - 22 C for 30 min,
and incubation was stopped by addition of 100 l of 1
normal sulfuric acid. The extinction of the colored
solution was measured at a wavelength of 450 nm (E450)
with a PBS blank as reference.
Samples classified as anti-HCV positive were those which
produced an E450 greater than 0.10, samples classified as
anti-HCV marginal were those whose E450 was in the range
from 0.05 to 0.10, and samples classified as anti-HCV
negative were those which produced an E450 below 0.05.
Tab. 1 summarizes results obtained from the determina-
tion, described in Example 1, 2 and 4, of human samples
with the peptide 4083 according to the invention and with
a mixture of smaller sequences of the formula I. The data
in Tab. 2 were obtained analogously with the peptide SP
10 according to the invention, the results of both ELISAs
being compared with those of HP 1 (Example 3).
2054798
- 33
Tab. 1
Results of the deternnination of anti-HCV with an ELISA
containing the peptide 4083 and with a mixture of smaller
peptides on the solid phase with human samples
Positive with peptide ELISA
(2 pg 4083/m1) (2 Ug 4060/mi
and 2 ug 4082/ml)
initial retest retest
positive positive positive
anti-HCV
positive samples
from France n=15 15 15 15
from Austria n=17 17 17 17
from Germany n=20 20 20 20
from USA n=49 49 49 49
total n=101 1011, 1011) 1012)
paired sera/plasma
from healthy blood
donors
n = 259 sera 2 1 n.d.
n = 259 plasmas 1 0 n.d.
n = 97 samples showed extinctions >> 2.5 E which
resulted in ratios > 25 at a limit of 0.10 E
n = 94 samples showed extinctions > 2.5 E.
Only 4 and 7 samples, respectively, reacted more
weakly, but with E450 values between 0.8 and 2.5 and
a limit of 0.10 E still rather strongly.
2054798
- 34 -
Tab. 2
Results of the determination of anti-HCV with an ELISA
containing the novel peptide SP 10 on the solid phase
with human samples
Positive with peptide
ELISA (2 pg SP 10 /ml)
Anti-HCV positive initial retest
samples positive positive
from France n = 35 35 35
from Austria n = 26 26 26
from Germany n = 29 29 29
from USA n = 53 53 53
total 143 1431) 143"
Paired sera/plasmas (see distribution plot of healthy
blood donors in Fig. 2)
n= 500 sera 0 0
n = 500 plasmas 0 0
1) n = 142 samples showed extinctions > 2.5 E, which
results in ratios >> 25 at a limit of 0.1 E; only one
sample reacted more weakly with a value of 1.2 E, but
still considerably more strongly than with the commercial
ELISA (HP 1).
All of the tested human samples which were classified as
anti-HCV positive with a commercial test (HPI) were
likewise found to be positive with all ELISAs based on
the peptides according to the invention. Besides the very
good agreement with the results of the commercial test
(HP1), the very strong signal formation in the peptide
ELISAs is also noticeable. At the same time, the results
of the longitudinal investigation of healthy blood donors
makes it clear that the susceptibility of the test to
interference is extremely low. Thus, on testing of
CA 02054798 2002-02-07
- 35 -
healthy donors of serum and plasma there was found to be
only extremely minimal non-specific binding to the
peptides according to the invention, i.e. only a few
false positive results were obtained.
Tab. 3 presents further results obtained on testing of
humar- samples with ELISAs based on the use of smaller
sequences (15 amino acids) of the formula (I) according to
Examples 1,2 and 4. It is clear that the peptides are
suitable for epitope definition of HCV antibodies and as
mixture of several peptides, preferably containing 3 to 6
peptides, for the determination of anti-HCV.
Tab. 3
Reactivities of human anti-HCV-positive samples in ELISAs
with smaller peptides comprising 15 amino acids on the
surface of microtiter plates according to Example 1; the
results are reported as extinctions at 450 nm (E,so)
{- = negative finding below 0.10 E).
4056 4055 4053 4052 4081 4091 Mixture
4056
Sample 4055
4053
No. 4052
4081
4091
jug/m1 2 2 2 2 2 2 0.5 each
BC90-89 >2.50 0,20 - - - - 1,40
252 - - 0.20 - >2.50 - 2.10
90 0.80 - - - 1.60 - 2.00
268 >2,50 - 0.20 - >2.50 >2,50 >2.50
84 - 1.20 1.60 - 1,70 >2.50 >2,50
225 >2.50 >2.50 - - >2,50 >2,50 >2.50
270 2.00 1.70 - - 2,20 >2.50 >2.50
229 0.20 - - 1.10 1,00 - 1.50
288 >2.50 >2.50 - 0.50 >2,50 >2.50 >2,50
290 >2.50 >2.50 - 0.20 >2050 >2.50 >2.50
242 - 0.50 - - 1,20 1.40 >2.50
137 - - - - 0.40 0,35 0,70
286 - - 0.60 0.15 0,45 0.15 0.90
192 0.20 >2.50 - 1100 - - >2,50
235 0.15 - 0.40 - 0,20 - 0.30
85 - - - 0,75 - - 0,40
2054798
- 36 --
The reactivities obtained with a mixture of peptides
according to the invention are shown. It is evident that,
owing to the very strong signal formation of the peptide
ELISA, a reliable discrimination between positive and
negative results is achieved. Analogous results were also
achieved with the following peptides or peptide mixtures
according to the invention.
4074/4081 (2 pg/ml each)
4074/4082 (0.5 and 0.125 pg/ml)
4060/4071/4081 (2 pg/m1 each), where mixtures of smaller
peptides comprising about 15 AA proved thoroughly suit-
able:
4056/4055 and 4052 (0.5 g/ml each).
No dependence of the reactivity of the samples on their
geographical origin was detectable in any case on testing
with all the peptides according to the invention.
Exaniple 5
Optimization of the ELIS.A.determination
The modifiable parameters of the ELISA determination
which were varied were mainly the peptide concentration
used for the coating, or in the case of mixtures of
peptides their total concentration and ratio to one
another, at a constant conjugate concentration of
1 : 3000 and 1 : 26 dilution. In addition, the conjugate
preliminary dilution was varied at fixed coating concen-
trations. Both modifiable variables were validated with
regard to specificity by testing blood donors' sera and
plasmas and with regard to sensitivity by determination
of anti-HCV in positive groups. Furthermore, the limiting
sensitivity was measured in the form of the analytical
sensitivity by serial dilution of anti-HCV-positive
samples (1 : 2, 1 : 4 etc. in anti-HCV-negative sera) and
compared with the data of a commercial test in just the
same way as with the results achieved using the peptide
described in the literature.
- 37 - 2054798
The results obtained with human samples are compiled in
Tab. 4 by way of example.
Tab. 4
Comparative titration of anti-HCV-positive sera and
plasmas in the peptide ELISA (4083) and a commercially
available test. The ratios are reported as quotients of
the specific signals to the limit, and values greater
than I indicate a positive result and values smaller than
1 indicate a negative result.
Peptide ELISA Commercial
Serum (4083) test (HP1)
Dilution 2 g/ml 4083
in neg. serum limit 0.10 E limit 0.454 E
HC 90-84 1: 1 > 25 > 6
1: 2 > 25 > 6
1: 4 > 25 > 6
1: 8 > 25 > 6
1: 16 24 > 6
1: 32 10.7 4,1
1: 64 6.1 2.0
1: 128 2.5 9-9 Imgz
1: 256 1.5 0.5
1: 512 1.1 0.5
1:1024 D_z,¾ nea, 0.2
HC 90-90 1: 1 > 25 > 6
1: 2 > 25 > 6
1: 4 > 25 > 6
1: 8 > 25 > 6
1: 16 > 25 > 6
1: 32 11.8 5,4
1: 64 5.9 3,7
1: 128 3.3 1.4
1: 256 1.8 ,Q.2
1: 512 1.08 0.4
1:1024 A.--I flea. 0.3
1:2048 0.4 0.2
HC 90-252 1: 1 > 25 > 6
1: 2 > 25 > 6
1: 4 > 25 4.5
1: 8 20 3.2
1: 16 12.2 2,7
1: 32 6.5 1.2
1: 64 4,2 P-1 npg
1: 128 2,4 1) 0.5
1: 256 9.17, marg.
1~ marg. = marginal
- 38 -
Continuation of Tab. 4
Peptide ELISA Commercial
Serum (4083) test (HP1)
Dilution 2 pg/ml 4083
in neg. plasma limit 0.10 E limit 0.454 E
HC 90-296 1: 1 > 25 > 6
1: 2 > 25 > 6
1: 4 20 > 6
1: 8 8.5 5.0
1: 16 3.7 2.9
1: 32 2,9 Q..ZnS-cj-.
1: 64 1..4 0.5
1:128 Q,,,.$ necz= 0.4
1:256 0.3 0.2
HC 90-83 1: 1 > 25 > 6
1: 10 20 2.1
1: 10 0 6.5 Q-A n2i
HC 90-240 1: 1 > 25 > 6
1: 10 8 1.5
1:100 1.4 1. 1 nea_
HC 90-239 1: 1 > 25 > 6
1: 10 7.8 2.-0
1:100 1.4 " ngg,
HC 90-89 1: 1 > 25 > 6
1: 10 > 25 > 6
1:100 9 3=5
HC 90-137 1: 1 > 25 > 6
1: 1 21 4..2
1:100 4 1.4
The results in Tab. 4 make it clear that the sensitivity
of the ELISA based onthe peptides according to the
invention, measured by the limiting sensitivity of serum
titrations, is at least as good as the commercial test
(HP1) which was tested comparatively with identical serum
dilutions. In fact, in many cases, diluted samples which
already gave repeated negative reactions in the
- 39 -= 2054798
commercial test (HP1) were still measured as signifi-
cantly positive with the peptide ELISA so that the
overall result was a better detection limit of HCV
antibodies with the peptides according to the invention.
Analogous results were likewise achieved with other
peptide concentrations, peptide sequences or mixtures of
the novel peptides; such as, for example,
4083 (0.25 pg/ml)
4074/4081 (0.25 g/ml each)
4074/4082 (0.5 g/ml each)
4074/4082 (0.5 and 0.125 pg/ml)
4060/4082 (2 pg/ml each)
Beyond the determination of the limiting sensitivity, in
the case of optimized systems the specificity was also
examined by means of non-HCV-specific antibodies and
other potential interfering factors, and it became clear
that the anti-HCV determination with the peptides accord-
ing to the invention is specific for HCV and is not
subject to any known interferences such as, for example,
cross-reactions of other antibodies, interference by heat
inactivation or the like. This also applies correspond-
ingly to other stated novel peptides or peptide mixtures,
with which uniformly a high seruin concentration (dilution
1 : 2 in sample buffer) was used in order to increase the
sensitivity, which was possible owing to the purity of
the peptides and the high antigen density on the solid
phase.
The comparisons summarized in Tab. 5 between another core
peptide according to the invention, SP10, and HP1 like-
wise make it clear that the peptide according to the
invention has, compared with the state of the art,
advantages which in turn comprise improved limiting
sensitivities, i.e. increased analytical sensitivity.
Thus, the 4 titrations in Tab. 5 were determined a factor
of 2 to 4 more sensitively than with the published
peptide, and the factor was even 8 to 32 compared with a
commercial test (HP1).
_ 40 - ~~54798
rn~ ~s x b
~ ="~ v Q~
N R! 04
~
s~ cna~b a a
ov~i~wa~~~ a
049 ctl 0 r w o N m O
tA ctl A U N =~ > u9 c~wf r~d Itf ORi
~x+, Q U U i a s=
b H~r-1 Q a~i o O cv N
=H rl yd 4J ~=-I A n n
(U+4 9 N ~ p 0 b x
04,N O c: rl
1 ,
O rl N
'JI =v U N p =~ rA
U N ,~ q - Cr
0 S1 ~,1õ~ =rl H ~ O e0 6s ~0 rl o 'O
O d-) N 4 ~ ~ ~ N 119 ~ ~ ~G Ln r)
O4.) 41 0-5 N O O O O ~V N
U) w.[ 3 b~ ~ U O 8 A A
R9 O +) 'p 0 w N
A O=rl ~ = Q) d+
- O 47~ N
V2 O 040 U N d, i ~-i O ~p qp O r~ O O O
H=~-I !!] F U U 'L~ O O e!' l~l O 6D O O
+J ~ o Uz = S-I \ it1 o t11 N ri O tf1 Ll1
UrC d-~ N M C) = = e = o = o,
fCS TJ N S-1 0 O a ~ N N rl O C1 Q C~ N N
A A A
p cvbm
orocvNro
~ ,~ .-= ~
~a~i~~~~
-I cn Tt N O N .-1
=> N tn R
04 b ~
N A Q 0~
O O O N e"1 ' N N v O O
N f 1=~-I 1=d 0 tdl d O ad' CO rl t'1 N M O O
N CC 4-3 tA U N 1n LL9 1`= 1- If1 N r1 0 Y1 lty
tA N Gd 0 cfi 1tl U 0 = = o = = = = = =
O Q O ~ ~,~ N N O O o O O O N N
f 10 ~. A A A A
b~r' S~r >~' ~
U = . I A *-a 4)
+~ f-I ~ U) =1-I .G N caa O
>4 CO 3~ vVi~ ~ r
r + i ~ =~ M ~1
O o~o N~o ca o0 0 0 0
O O dJ r"1 o N t6 O O
ro.N 5 +) .0 O -P 4J H ~ In cn Ln O ko eaf O sn tn
= e. = = = A
~4 a) V a' = e
~ N N r+ r+ O O O a N N
~ u D i~~--~ w ~ c~ n n A A
N 0'O 04 U+
44 4-3 oro.'d ~i~p,`C=r~+
o~~~c0a~ i~
cn N (D 4 o a) 0
rI 1* o
~~=~b ~io=.> ~ 0
RJ 4-3 =d ;3 -P 4-J N ~' tOV ~ ~ ~ 0 sn ,
a,.s~ ro s.~ a~ U = ~ In (n oc ri N sea ra N N.=a
1 es so 0o s= oe == ee 1 =e
~ v3~a,ba~ z ~
0 O
Ch H !`d i 4 P'd -1 7 4 9 I $~1 r~
rri
E (~ =s~i x
41 2054798
- -
' e e e ~ a e
o ~1 ~1 O N O N O ~e ~ O N O
o IL1 O O N o O co Lt9 h O tt1 o
itY !'% E"1 r9 !n 01 01 N M 6n e"8 N
= s = 0 b s te y m
N Pi O O O N r-0 O O O N O O
A A A
@% M V {C1 tL1 O N 4O QO %D rl O L4 N
m O e1 rl rl O N rd t- Oi eP O 0c N 0D
~ CO -n N rl In N O ~' O O It9 S'~ tt9 O
o o 6 o s o =
rl O O= O N o o+ o o a
P1 O O O O O N N O O O
A A
O Ln sr r l t0 O O O 60 e'1 N ri O .O A N Ln P LR9 N
N Ln rl CD V C0 O O i~ v N N LL"f O ri t0 6:0 10 ep t~
O 1O ep N r1 O tf1 t~ ~tN C~ t~1 a ~ O tt1 1 N t0 M rl O
o = e = o - s O o = = = = e e
N O O O O O N N rl O O O O N ri e^ii~ O O O O
A A A
u1 O O e1 e-1 e-1 O O O W N P9 U1 O O P1 t='1 O O O v
w N m t~ m CN O O O O O CO t~ O O P1 t~ rl e-I 11 CO
O ln P t"1 ~-I e-I 1f1 lf1 tf1 eP OD eM tn tC1 CO CO ~' M r~ d
= s = = s s = v + s e a = a
N+ i O O O O N N N~ I O O O N N rd O O O O O
A A A A
d 9~ rl rl !D un O O O N O !D O O O t"9 0 tV 1D h
O tG !v ~D O ~ O O O ri 0- e'1 O O O O a~l s0 N 6K1
1C1 l0 B- ~ et N U1 6tl lf1 t~1 ~f 6t1 1~ Il1 BC1 ia C1 6A C"9 ri
= ~ p ! s s o s e =
N ei O O O O N N N a-1 O O O N N N ~i O O d O
A A A A A A A
Ln
ro ~
E-4
w
p V RV d
CO !O N N O %0 O 1D N N ei'
4; t~7 ¾p N 1f1 r1 O 0 %D N v N tCf qr N I* N Ln e-1 O d
U C1 !D e-i N Lf1 r4 [- W rl F9 %0 ri N 1!1 i1 i kO rd N L41 r I N
=ri N ( 1
.P .. .. ,. .. .. .. e .. .. .. .. .. .. e .. .. .. .. .. .. ..
~ d o
0 ri rl W-4 a-t a-1 r-1 C, w! ~4 am1 ~i rd r4 M s-0 ri eA wl yd r4 r=I
41
r~:
0
U
- 42 - 2051798
Evaluation of another core peptide according to the
invention (SP 23) gave similar results. The comparative
results presented in Tab. 6 reveal that SP 23 is equiva-
lent to SP 10 with respect to sensitivity, and both
peptides are superior to the peptide described in the
]. iterature .
Although the literature-analogous peptide SP 12 (AA 47-
75) does not correspond exactly to the peptide described
by OKAMOTO et al. (AA 39-74), the results on the inter-
mediate sequence (SP 11, AA 24-53, Tab. 6) make it clear
that no immunorelevant epitopes are present in the
following sequence region (AA 39 - 47), and the very weak
reactivity of SP 11 which is still detectable is attribu-
table to the carboxyl-terminal region of SP 10 contained
therein (overlapping region AA 24-30 shown in Fig. 3).
- 43 - 2 0 5 47 19 UQ
Tab. 6:
Comparison of the i.mmunoreactivities in the ELISA between
2 peptides according to the invention and an amino-acid
sequence which is located between the published sequence
(AA 39-74, OKAMOTO et al.) and the novel structures
(SP 11, AA 24 - 53, see also Fig. 3). All data represent
extinctions (Epso ~~=
SP 23 SP 10 SP 11
Samples AA 8-26 AA 1-30 AA 24-53
2Ag/ml 2}cg/ml 2 gJml
pos. contr. > 2.500 > 2.500 0.405
neg. contr. 0.051 0.049 0.030
Dilutions
HC-90-225
1 16 2.203 > 2.500 0.428
1 64 1.329 1.367 Q.õ16,4_
1 256 0.454 0.386 0.024
1 : 1024 0i195 0.142
HC-90-354
1 16 > 2.500 > 2.500 0.164
1: 64 1.413 2.156 0.059
1 : 256 0.418 0.496
1 1024 Q.124 , Q,.-M
Anti-HCV-positive samples
(commercial test HP 1)
HC 90-371 ~ 2s-Q85 0,224
HC 90-336 > Z.L' OQ > 2s= Q2235
HC 90-453 > ,2.500 > 2.sL00 0.058
HC 90-570 1.827 2.222 0.088
This surprising advantage in sensitivity becomes parti-
cularly clear on testing undiluted samples. Thus, all 11
- 44 - 20547"M
of the 11 anti-HCV-positive samples shown in Tab. 7 were
found to be reactive with the peptide according to the
invention, whereas the peptide described in the litera-
ture finds only 6 samples positive, and in no case was
there a positive reaction with the commercial test (HP1),
With respect to the sampl s which agree in reacting
positively with both peptides, it is noticeable that the
peptide according to the invention reacts significantly
more strongly under identical test conditions, which
provides considerable advantages especially for positive/
negative discrimination.
- 45 -
N
re4-J ro~ a
0t!]=~+1 Ga
L, ~
0,
o a ow 1-, =
`~~I -A - ; t;t;~t;~,
0 Gl ~1 ~ W ~ 91 Q! ~ 41 a! 0
~ .., v Q z z z z z z z z z
+J 43
0 N la 0 o x 0 v 0 eo 6 r, eo N1 o ri m ~' O
N j ' ~'' ~ U r+ 0 c- o ~1 ao cn r1 co ~o 1-4 O
N ro a O N N t"1 O O rl O t~ w=1 Il~
r-{ = o s = s = o a o
0 ~ o O O O O O O O O N
=~ 60d SUd '=~d 0 A
.=e 0 0
tT 0
O
r-i 4R!=~ a .~
0
9 4 M >
O tn ,, ~ 0
a a) =~ tr+.,',~ H
-H tr1 44 -i~ ~ O Csa ~ ~ 4) Q) ww Rl
vi'nN~ ro ~0 o x z x r
~
W 0' H ai m1-1 o in r-+ ca ea o r+ ~ a~ r~ as o
N U U'~ 0 ~ ~ 0 l!1 N W O tn Co tl' U1 f' f = O
.. 0 d' N N tC1 O O O N N G U 1
bd N 4-) 4.1 =
U ~ = -i= ON a m Oa O o a Oa O
H 0 -11 ~ N O N N
~~p~k ~ ~ .N-i .N A A
aro a ~ u~ ~
i ci
co 0 00 w
4+it9>o
o =H a) =H
r-i 4J
oou
4 ~0~~ ~ -N o
=~ ~~~ 0 U N ~~ N t~ O O O O O O e0 O~+ O
V1 0+=~1 }~ 0 ="+ e 111 ri o o O o O Fe o
0 t~ f., rci r. tn si' 66! 111 1~1 !`9 l~1 tl9 N f~ N 1t!
Q1 Ql =r{ (p ~ =H :% .. = a s = o a a
U1 41 'O td W (L) W ai N N N N N N N N N ri N 10
.
44
0~ 010 6d ~i =~I .[ N 0 A A A A A A A
'ri 4-! ~(d la ~ q W c~I2 U
O~NUN0
r+viNt)
~ i =rl Q~ ttl
~ a
(a
=PI =rl Q) ~0 ~= ~' O~ O M r1 ~ N aei t0 +~= 119
1-I ~. O.!'C~ 4 "~ N O Co O N 1 O rl @'9 t"1 9% 0
RI ¾J =ei dJ 0 N N N M C 1 el' tn tn u1 N1 tl' tl'
C1i td 4.) 41 1 1 I I 1 ! I 1 1 I I 1
w C4 "i tA 0 O O O O O O O O O
t- U W 0 04 i ~ z 0 J1 ~Y1 ~tt 4J1 ~t 01 0- tA - 9- ~1
A 1-1
tU 0
E-i t!a
- 46
These findings (Tab. 7) derived from the titrations
(Tab. 5) and preselected native human sera with the core
peptides are confirmed by the results of the tests on
commercially available serum panel (LOT # PHV 101 and
LOT # PHV 201, Boston Biomedica, USA).
Specifically, the results of the so-called low-titer
anti-HCV panel are summarized in Tab. 8 and the results
of the mixed-titer anti-HCV panel are summarized in
Tab. 9. The results of the data obtained with the core
peptide according to the invention are compared to the
data obtained with commercial tests as state of the art.
Tab..8
Tab. 8 shows the comparison of the sensi=tivity of a
peptide ELISA (SP 10, 2 pg/ml) with the state of the art
for native anti-HCV-positive samples in the panel
PHV 101, comprising 15 low-titer well-characterized anti-
HCV-positive samples (material and comparative test
results marketed by Boston Biomedica, USA). All ELISA
data are ratio values, which describes the ratio of
sample extinction to cut-off. Values < 1.0 are regarded
as negative and > 1.0 are regardled as positive.
~
_ 47
.'.,
I.a .
0
~
~
~
=~
04 .~
~t F-4
=~ .~
N
.jJ ~ PJ $' O O 0
k-l ~ I c0 tt1 c d1 tfl tff U1 t1f u1 t1Y u1 ~!1 U1
W yJ v~ ri N N N N N N N N N N N
A A A A A A A A A A A
U~ In Np Co N t~ Upl 0 tpn V1 tpIJ L'I tPJ 0
A~ G~ QQi 6~ G~ W LL~ Gl~ R~+ PI P~ W
va H RV ao M N aP eo Oh oo O Ln O % 0
^la ~-l O e0 N P~0 V N c0 rl B1 N O P a Rr
~ ~ C~7 C9 9i N M O ai ~C t0 ti f rl O OD N P ~ N
= = = = = o = s s = = = m
r, U O O ri M N a-1 r 4 1-9 rl M r I N N O M
~4
Iy
=,~
i-I H P P O P 6A' O r+l 61 tP1 1 N OD Ln N 60
fti Ds7 O r! W C LL'f O O O tS1 ~= e0 v 1 O r4 W
~ C~ P H O P ~ 1o N O OD tp e0 O m S1 60
= o o m m m = = = m = m m
U'~' ~ M e-d ri M ~=i N t`l yd P9 O M tV a-1 ~"9
e=i N M V M %D P O M a4 N M iL9
O O O O O '~S O O P"~ 1 11 P' ~ ~ C'~ @ 1~
1 1 1 1 1 1 1 1 1 1 i 1 1 1 1
ri rd W-1 r^0 r4 ri rd e-i v-d aml w=1 r4 rd r4 r4
co f~ N ~ ~ ~ ~ ~ ~ ~ A -I t-4 P4 -1 '.4 ri 0-4
= ~ ~ ~ ~ ~ ~ ~ ~ '~ ~ ~ ~ ~
~r Q4 &4 iSa 8m R~ 6~
~ H 13e 6a 04 !S+ N aa N 04
~
2 0 5 4 7 49 $
48 -
Z Z Z Z Z Z.Z Z Z Z Z Z Z Z Z ~I 0)
Id r
N
Of-1
44
H O
O
Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z =N
s-I 'CJ
Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z 04
=ri
UI
L~ N =~ .
t! Z Z Z Z
rj 41 rU
1=I O
* I tU =rl
a a.a a a a'-a c~ a a a a a a a ~b~
(1) N 3 O
rl id =W =rl
t A C7 ~-1
Zz U 11 44 o
a.=H w
0 r-IHO
mu~
U N =s=~ N
I O tA
rV av I - e =~ =cr e ~, r~ r~ .r Z 0
=~-I
1
E- .II 44 =ri O
r-Ui 1 084
V ~1 1 .* I -4 -4 ~ w tV -a v ~= ~ rn Z +J r-~I
4 =Pi rl 4
O 1~ O b
ev e^^~ 'y =.1 44
co =~ ~ o =rl H b=I
-~j= 1 ~ I I I ~_. I + 1 1 ~ b ~ =I ~
+ I + +
H ~ ~0
O N O
o-a
O +~1 .w 1 ~ I \ 1 \ I =w -m N z m ~ 'P =
+ + + =~ + + N 4J W
0 0
=,j .=-1 N ry ~P tn 60 P % O a=i N N9 w U1
4J O O O O 0 0 0 O O W ~I nt vi 1 'J R9 =J (A
I I 1 1 I I I 1 1 1 1 1 I I I --14 tu 0
".9 y 3a .=i =N =~i ~=1 vi o.d <W ..d .=~ .~I m-4 .==1 .=1 .=i .-1 d, =N =I=-
'f .' Ca7 O O O O O O O O O O O O O 0 =11 t!I td
.=r ~ ..a -4 .-i -4 -4 -4 ~ --4 -4 tfa tA =N =IJ
0 ato H
tn il S=I
0 a E-i Q)
a* ~
2054798
- 49 -
It is evident from the data in Tab. 8 that, with one
exception (PHV 01-03), all anti-HCV-positive samples give
a correctly positive reaction with the novel peptide. It
is noticeable, by comparison with the commercial test,
that the signal strength, expressed by the ratio sample
signal: cut off, was found to be significantly stronger
with the peptide according to the invention than with the
assays tested for comparison, which expresses a
distinctly improved reliability of the novel peptide
ELISA.
According to these data (ratio > 25, which corresponds to
extinctions > 2.5), the samples are low-titer only
according to the definition of the state of the art and
are found to be, surprisingly, strongly reactive with the
novel peptide.
The one exception (sample 03) is explained by the data of
the comparative results on the confirmatory test which
demonstrates absence of core-specific antibodies in this
sample (C22c is the core-protein prepared in E. coli by
genetic engineering). According to this test, sample 03
ought in fact to be classified as negative.
The tests on the second panel surnmarized in Tab. 9 led to
similar observations: 19 of the total of 22 anti-HCV-
positive samples are found, surprisingly, to be extremely
strongly positive with extinctions greater than 2.5
(corresponds to ratio > 25). The three anti-HCV-negative
sera contained in the panel are correctly classified as
negative (ratios < 1.0). Finally, samples Nos. PHV 201-
08, -10 and -20 are also found to be correct within the
scope of the problem because these samples exhibit no
(-08 and -10) or few (-20) core-specific antibodies
according to the confirmatory test.
Tab. 9 shows the comparison of the sensitivity of a
peptide ELISA (SP 10, 2 g/m1) with the state of the art
for native anti-HCV-positive samples in the panel
~~~4798
-50-
PHV 201, comprising well-characterized anti-HCV-positive
samples with different anti-HCV titers. (Material and
comparative test results marketed by Boston Biomedica,
USA). All the ELISA data are given as ratios which
represent the quotients of sample extinction and cut-off
value. Values < 1.0 represent a negative result and
values > 1.0 represent a positive result.
_
~ 51
0
.,j
-P
a~ =~
w ~
Q+ O M
~rl f: N =ac N Ta N N
3~o C op o C
LP1 Lf1 LP1 7l1 L'1 LP9 t!'1 LP1 L=1 Ln Ln 6P1 0 0 {19 U1 119 61"f 3J9 LP1
O O N N N N N N N N N N N N N N Pl N N N N N
F-+UPaU nn n nn n nn n ns~nnn I~nnnnn
U tA,
W R1~V)
a
N U)
dJ H
a .
6f~ L^1 .^.. ~.^. L'~ =^~ Lr. 'C' 61 CS f9~ N^,,. N N P1 O! ~ L9 o N dl
c` 4 Z. %A `:. "` .-> %Z C %3 P'1 A9
. ~-I U C a C~ .~ 1t1 Q ~ N N!d 1 T+ V M !6~ O= tf1 f~1 s9 tZ 4~ !:~ %. x ~ .
= . . = . . . = . . . . . . . . . .
~+ M P`1 G> N'~ J ==~ ~1 N N T L'~ =C 1f9 N'f tr1 P9 ~a N N.~ 6P1 N tP1
~
4J
it P"5 .~l !=. ar
~-1 H tT :J LI1 'V L"1 O% L'f 6f1 tf1 C C> =a 0 -W tf1 o tf1
o`? C~ !== .. ... y"f w P1 M1 -.l:. - - ==w r1 C9~ .r r'1 N.a ^~ av
a W !e~ "V P"o N g 1~ N 6n Yf "~ C.7 V" ='~ 'd' =~ w~ wM !11 r a"a T!*= f
6P'f .~{
s s s =
rz > =~r1r~ ~ e~e ~~~aNNNf~.4- N9 =eQtn Nr=^w Nr
O U
.w N r'S ~ 1d1 s~ 1~ C~ ~~==a N P! ^~ 6H ~D P~ 9= C~ oma e Q e=y ~= !A . ..
d~ a ~ C ~ O O ~ ~ ~ ~ e~ +', .~c N ~ ~ ~ .~a .;v eV t ~d Pi ~ t r~ N
W W ..a =ar .w ~ IIIII I ~a .w .w .a .ot ~a . a .n .a .a ..+ .r w .ti ..=+ =~
.y .~t a
W At~ C~ o0 oCaoo C?oC ~:a~ OC c~o~ ~:~
õC~ ~," ='~ N N N N N N N N N N N N N N N N N N N N N N N N N
E-4 z
m w.=. a e '~. a. .. a.e m~. Ls m m. w ao Ge .m w. ~ ~ 'w a'n E
0
2054y~98
-
52
m
'ti
4J
a4J ~
o~ ,
4
4j 01 N 1 1 , t 1
~=~p a e~V ee e~+, n, . V
~i' W r-1 a~ = o o ' ~^i 1~
ti) O 0
tl9 td1 iP1 N1 L^1 6A 1l1 m L 1 L 1 L'1 Lf1 L!1 6,'1 Ln Lrl 6,=1 LPi {d9 tm
~y,i
1~ /~ \ N N N N h N N N N N N N N Caj N N N N N O
(
ya~-tn nn r~ nn n nns~r.nnnn Annnnn Ow
a 4-)
44 ~
ZZ: ZZioZ~Z8~~ZZ ZZZZZ ~2Z. ~^ 3~ o~t
=~1 ta
R
eI O $d (0
TI`I zzzZZZZZZZZZZ~zazzzzZzzZz .0
t~ =~1
>sf ~~b
ra, =~
i S]~ND
yI zzzzz r_zzxzzzzzmzzazxzzz~z
04 m
z4.)
0
di u No
U O .r=l
z~zzzzz;;~z~.z xx~~o
. $, .N -r
~ =.~ ~s
~ ' ~M_ =rM _^ _ a aru44 a
r ~q qoy
= M P1 == , M ~ N Yd PA IW I 0 le~ r = bM b! il W 44 m= =1 1~ ~ V
M1I ~ Y.^ w
0, r-I f=! A
e N 0
4J=0
`n tn mEn0
-1 o=~
1~ 1-1 O f=t
- , NN *a ~ vg 1 '~ = I I QY 1 e 1 M 4T ~ '~' a~ 7^, 1 ~ ~` ~` ~ s .~=) ri
41
O tC
;444
~ i N a I t. r 1 r~~Nr~N~~e~r ~=~ 1~ew a r~~ 4J ib1 rd~
O U ~= 0 04$4
z 04
~ wN 1 1 I 1 I 1 I t ii O tOAoOG
ro L=r O 1 N -+ 1 \ ~=1 1 "s 1 \ \ N ~ \ "~ ~ '~ N w o~ \ I P9 ~
E. e. ..a .r =~ 4. v Z
N i, 44
o
0
C N!V L 1 a N N PV Nv w t0-4 a.d \qv N@ to
Irl ~ 0 * y ) ~ ~ 4~,
co ..N~~ ~neAw~e~o ~Nt~1~at1,At~0e~o.rN~q~=u~
N N N =~ aj4J
0 0'6~ C> o o b o o ..a .~ +a! s~l e~ aw sv ~ W t~l N N
7 ~ i t 1 I t 1 1 1 1 1 1 I 1 I 1 1 I 1 I 1 I 1 9 I Oto 'I to
C ,q + as w - - - ..s - s.a mi P1 0) :3
~~=~~ OP~ O C~'ea 0 C OOOO G? OOOcoOOO Fa tAr;-4
2: 2t 2: 2: N N N N N N N i=V N N N N N N N N N N N N N N
rr u ~
Z ~
U~"",~ C+e.~ ce G~o ~: E:cobA E-+01
a* ~.0
2054798
- 53 -
Example 6
Determination of anti-HCV in chimpanzee sera with the
peptides according to the invention in the ELISA
Various peptides and peptide mixtures adsorbed in wells
of microtiter plates were investigated with sera from
chimpanzees which had been infected with various infec-
tious doses of NANBV. The sequential samples taken every
2 weeks after inoculation underwent measurement of not
only GOT but also GPT with commercial tests, and all
samples were determined in parallel and in the ELISA with
peptides according to the invention. Similar to the
commercial test (HP1) in Example 3, in the peptide ELISA
the polyclonal rabbit antibodies, labeled with POD, were
used concentrated 4-fold for the detection, using the
enzyme substrate TMB with photometric measurement at
450 nm. The test procedure corresponded to the determina-
tion of human anti-HCV described above, with 0.1 E as
fixed limit and presentation of the results of Tab. 10 as
ratio of the specific extinctions to this cut-off value
(ratios).
The results listed in Tab. 10A to 10C and 11A and 11B with
peptides from the NSP 4 region re:present so-called ratios
with which the quotient of specific signal cut off is
defined.
Tab. 10 A- C
Anti-HCV determination in chimpanzee samples with a
commercial test (HP1) and ELISAs with peptides or peptide
mixtures according to the invention from the 1NSP 4
region. The data for the first 3 time values are extinc-
tions which serve to fix the limits, from which the
subsequent ratios of specific signal and limit have been
formed.
_ 54 -
u
00 a~ www
O e~v~~ 0 melNmrlch ~
::r 000 .-1 OO OOO% hcnMM
C~ ~ 9 = = a a = = 9.
000 O 00000000000
0 to
N LI $1 $.i M 14 D4 W W W k 1d 0
=11
O
O a)
W w[s'I W 6~ d f16
O OOOOOOtflo a-h44' ~
m ~ = e
Q O uOMO= Oi =rl sri e =09
O Q O OOOrl0
s1~ O 3d bd ~1 ba ~J 74 $d }~l 7d kd ~1 0
4J
b
O
LO rl
Nhm G1iddWO
~ MNrI 0
~-iNa-INr~M r'
\
000 H 000000 t=9qw hh1S1
M Ol M A 9 9 9= a= O= f = w ~)
~ oao 0 00o00ott1h6i9Nr1 -4
cr N }d Xd Sd LI fd ~1 ~1 $d id 1.1 7d 04
U
rn
0
N V
~ =ri
~1i a w~ww
00o w
u~ o
oo~ s~
~ ![1 ~D m h o0 a9 t` C ln l4 h OD L19 !D %D
N . o s s = o e eOa==OO s a=Os V
0 ~ O O O O O O O O O O O
U+)
~4 34 b.13rkIS.1!=1W 34 kM
N
H tclt~t7 Q-OO~t10000N1t~90
N N N t'9tl1U1td1~h ~ e'NNC9 4-)
U
A QI
LI-4
0
=~
;t.
re
Sd
M 0 W "
N =i -
N ra N
~ 0 ~ () õH d.) .f.~
tC =r1
rl 3 0 Ci N tn
9 4-3 0
4-)4~a ~ 4V wONqw t OON:fi%0 U
N~rw0 Pa~~rtr4 N N N N
o
55 -
2054798
Q
un
W W W
W O O 0 0 O O O O 0 O O O 0 O O
cnot-
N Ilf f"1 O
q 0) 000 H %D O NNqie?l70d 0190M1116L16t11L9 p 0 = 07 07 Oo Oe ~ = N1 O 'D N
00 I~' C M=NANANA AN
N
.d4 C 1Nr4 s4 NNv-i riN
La W Sa !"d 6+ W 14 W W ld $4 14 W k+ 11 fa
O
!!1 ry .
~ W W W Ed
W O O 0 O d O d O O O O O O
~ ~
M 0) M%w M O LO
C- Z. O O O rd t0 0 01 N W W t` td1 P tl9 t8'f tt1 111 tt1 tSY
q m= m m a o m s a s e ao s s eN N N N
N 000 O OO~NhNrItt1M~-iri~= A A A A
rl ri r-1 ri r1 H ri N
~+ ~d ~d ~d ~i 7d 5.1 ~=1 S.1 ~A ~1 k b.i kl ~=I 3a
U
Op U)
q~\ W W W W ~~~ O O
co r rn
q 000 0
000 ri Nrle-INNrIriNNNNMOOr-!ln
zv (N
= m= m a e a= e e= e= s m
00 0 000 00000000r-dN NN
0 b i Sa LI Sr 44 LI 3d bd ba !d &d !=1 Ll !+
rI ~
~ W W W ool N V1 U! I!I dOd0 U1 N N N 1- O O ~~11 ~O O
r') N l, lC) 0
00 U1 O O O a-1 lf1 0 1 oD CO 1~ ~t N C 1L1 O 1S1 lC1
q . o = s = = + = = = = = m o = 0 e N
N
cM O 000 0 Or1~ MN~IL1l~1o LC1lDhNN A A
O id !4 ~=1 $d $4 ~1 ~1 L1 i=1 $1 ~d ~4 $4
Ifl
OH ko W O O O 0 0 0 0 O O O O 0 O 0
00 Z3. 000 rl MOl~Q!=N~O=7'C9COOO~ON~=elOtttl
0 == q e a m : e m m==== s= a N
d~ N 000 O Or Ir1P1~ODO0Q~OQ1P~tAt01~ A
r-I -= {=1 ~1 ~=I {=1 ~ I ~1 f=1 1 1d ~d ~=I ~=1 ~1 ~=I =1
00 tCS r-1 n
=~x ~ W ~s~ ~~ ton
N W ~1
W W d
alftl9ul h w v POc9co ao toao 00v rllT
O O m==
C) 4-3 O O Oo O= Om e eo 00 00 00 07 0P r1m NN = s Ne ~
O-10N
~a !d !.1 S.1 !4 ~=1 Sd 1.i bd 5d S.1 S=1 Sd 5i ~t =PI
E O
O Ic1 4=1 N O N O lL1 6~ t[1 M b O O It! tL1 OD em D ~~y
NNN t90~~MNMNNNNNNNNNN ,.i
e-I
00 O W ~ b~
to f0 t!)
W 0 O O 04 ~
z O U =A =~
CD 0 P ~= ~,
fC! v =rl ~i =a 4-)
(a
=H ',4
A ='i ~ II k' Q)=~
~ GlN~'mCDONaO'~00N=~ ~OSnO ,.,
H ~ H qN~ WTJr-1 v-+rI.~~a-1NNNNNMMMMM+T ~{
- 56 _ 2054798
U O ~
N 'y DO
ro
oo~ o
~ ~N~ d
c94\ N M N O
d CT 000 e-9 Me=11[90I rIt[fsr'o ld100
h ~ = = e = o 0 o a = = .
O 000 0 0000~1 MMt~~leollt'1
ei N rl e~
$d ~d ~i ~1 ~J bi $1 ~d ~d ~d ~d bd f=i
U O
ro u I
cor-i www W ~0 ~ad
o rg eoNLn
O r=1 r-1 0
O tT 000 H rirlNNHriNIW 1ICttil-0
~ t . o . O. O. a O= O. OO a . O . O .=
o O O O O O N N M
`f' N
$4 w 3~ $4 $4 !=+ Fa $a Sa }a ba Sa #a
O
Ln
~ Q1M w W d 0 01 1 0 1ad 6.Y 6.sf WW
C) ~ N M O
:a. 000 r^d NM W~01~OC-N 1r1l- t~t~1
= a s . = a~y! O a= o= o a= q (y
~ ~ 000 O 00 lf1 Mt0 V=t9~0ep01~ A
Co r1 r1 N
xr $d f-1 $4 51 k 34 $d $1 ~i ~=1
O
Ln
~~~ W ol1sI~~~~~p~~~
M m OOO H Ned~tff&Q10tl1~9101MS"0-C U1
~ o s s . =rp~ o a~ = e o s=. o aN
Q 000 O O 10r=dt~NOODrIMC1 O/~
et N r9 "^I rl rl yd r=i N
k 14 $d W 14 14 bd M $d
N
m WwW ~0
v 0 0 1C9 O
~p tt~ ^s4' t~ t~1 tt1 SO m~0 d' i[1 ~D ?~ W O
O O O o= O= s Oq O= Q= Oo O= O. Oa O= Oa Oo =~
4-) O O ~~ 4-)
3=1 ~d ;d S=1 bd rbl ~d ~1 ~=1 ~=1 ~=I S d =1 `rl
CO
0
H Oa
Ocno Nu1o ~nO~nNN~~~n~
MNM lnY`r4~=!~~ltlln~'a0"~'Mad'
~
.o
r.. ~=
=,-I
r ~
t~ 0
-i co
Pa ~
m (tt tA O
~ II
t~ 0 0) 0 0 4
z 4) U ==-I 4-)
0 0 ~~~
r-{ '--I -
0 =='{ Ci 0 +J R1
rE4 ~ II d~ 4-4 =
rtS i~ rl ~C ~==I tOOON~ 1DOON~'60oi1O
H rC H N=W w'd~ 9-4 p+NNNNNMMMMMV
- 5' 2054r198
The results presented in Tab. 10 A - C underline the
advantages of the peptides according to the invention,
especially on comparison with the corresponding results
from the commercial test (HP1) and the ALT course.
Thus, for example, animal No. 123 is not found to be
antibody-positive at all by commercial test (HP1)
although NANBH was detected by electron microscopy of
liver biopsies. By contrast, a reliable HCV antibody
detection is possible with the ELISA based on 4083 almost
at the same time as the ALT increase.
In particular, the chronological congruence of the
peptide ELISA results with the increased ALT levels is
evident for animal No. 048 where with all the described
peptides or peptide mixtures there is a very reliable, in
the sense of a sharp positive/negative discrimination,
anti-HCV detection almost at the same time as the ALT
increase and on average 18 weeks before the commercial
test (HP1). The comparisons among the peptides make it
particularly obvious that the cer-tral amino-acid sequence
makes a considerable contribution to the recognition of
the early HCV antibodies by the complete peptide of the
formula I(4083), as is made clear by the comparison of
the mixture of 4060 and 4081 (amino- and carboxyl-ter-
minal sequences) and 4090 which comprises the central
structure.
Similar results of an early identification of HCV anti-
bodies, almost simultaneously with ALT, were produced by
the investigations on animal No. 147 in which a reliable
HCV antibody detection is possible approximately simul-
taneously with the ALT increase, again about 16 - 18
weeks earlier than with the commercial test (HP1).
- 58 -
Tab. 11 A and B
Anti-HCV determination in serum samples from 2 chimpan-
zees infected with HCV. The results with the commercial
test (HP1) are presented in comparison with a syntheti-
cally prepared core peptide according to the invention
(SP 10, 2 g/ml), the results being presented as ratios
of specific signal and limit.
- 59 -=
Animal No. 048
ALT Commercial SP 10-ELISA
Time (weeks) HCV ELISA (HP1) 2 g/ml
0 = inoculation
20 0,5 E492 0 09 E450
25 0 5 E 0,21 E
23 0,5 E 0,13 E
Extinctions
(E) define the
limits:
0,70 E 0,10 E
8 XA_tjos (r) 34 r 0,4 0,50
32 r 0,6 r >20,0 12os.
12 100 r 0,4 r >20,0 pos.
14 112 r 0,7 r>20p0 Ros.
16 30 r 1 0 r >20,0 gos.
18 25 r 0,7 r >20,0 Ros.
30 r 0,8 r >20,0 pps.
22 25 r 0,8 r 19,0 p-os.
24 23 r 0,8 r 20,0 pos,
26 20 r 0,8 r 18,5 nos.
28 20 r 0,8 r 10,4 oos=
20 r 1,0 _ drgl) r 14,2 pps.
32 25 r 1,3 Izos. r 17,6 uos.
34 25 r 2g0 pos. r 15,4 R s.
36 28 r 2,4 320s. r >20,0 pos.
38 28 r 2,1 ngg- r>20,0 PQS.
: 26 r 2,9 pos. r >20,0 R2L&.
1) marg. = marginally positive
60 - 2 0 5) 4 7 9 8
Animal No. 147
ALT Commercial SP 10-ELISA
Time (weeks) HCV ELISA (HP1) 2 pg/m1
0 = inoculation
30 0,60 E492 0'013, E450
25 0,50 E 0,032
30 0,45 E 0,029
Extinctions
(E) define the
limits:
0,70 E 0,10 E
16 ratios (r) 52 r 0,5 r 0,2
18 75 r 0,5 r 0,1
20 110 r 0,8 r 0,2
22 40 r 0,6 r 0,4
24 75 r 0,6 r 0,3
26 70 r 0,4 r 0,2
28 55 r 0,5 r 0,3
30 52 r 0,6 r 0s2
32 42 r 0,7 r 0.4
34 48 r 0,8 r >20,0 nos
36 48 r 0 8 not tested
38 35 r~. as, not tested
40 45 rpos. r>20.0 p s.
1) marg. = marginally positive
- 61 - 205 1793
The results presented in Tab. 11 A and B with a novel
core peptide also underline the advantages of the pep-
tides according to the invention, especially by com-
parison with the corresponding results of the commercial
ELISA (HP1) and the ALT course.
In particular, the chronological congruence of the core
peptide ELISA results with the increased ALT levels is
clear for animal No. 048 where there is a very reliable,
in the sense of a sharp positive/negative discrimination,
anti-HCV detection almost at the same time as the ALT
increase and on average 20 weeks before the commercial
ELISA (HP1).
Similar results of an early identification, almost
simultaneous with ALT, of HCV antibodies were produced by
the investigations on animal No. 147 in which a reliable
HCV antibody detection is in turn possible about 4 weeks
earlier than with the commercial test (HP1).
Overall, the peptides according to the invention and the
use thereof in immunochemical detection methods prove to
be considerably more sensitive than all hitherto des-
cribed methods based on prote:i.ns prepared by genetic
engineering and other synthetic peptides of the state of
the art. With improved sensitivity in later phases of
infection, the novel peptides additionally provide
considerable advantages in that reliable determination of
early HCV antibodies is possible and thus the currently
existing diagnostic gap is significantly reduced.
Furthermore, the peptides according to the invention are
considerably less sensitive to non-specific bindings,
which is expressed not least by a drastic reduction in
the background compared with the commercial test (HP1)
(max. 0.10 E45D compared with max. 0.4 E4az with the
commercial test (HP1)), both for samples of animal and of
human origin so that a considerably sharper negative/
positive discrimination is possible. Finally, advantage-
ous aspects to be mentioned are the shorter overall
- 62 2054798
duration and the grea=ter precision of determination owing
to the sample volume of 50 pl which can be pipetted more
accurately.
Example 7:
Preparation of peptide solutions for producing a ffiizture
of NSP 4 peptide 4083 and core peptide SP 10, and coating
of microtiter plates with this peptide mixture
Serial 2-fold dilutions in 0.10 M sodium bicarbonate
pH 9.6 were made up from stock solutions of the peptides
SP 10 and 4083 in 50% acetic acid in distilled water each
containing 6 mg of peptide/ml, that is to say a series
with the concentrations 50, 25, 12.5, 6.25, 3.12, 1.56,
0.78, 0.39, 0.2, 0.1, 0.05 and 0.01 pg of peptide/ml was
obtained. The procedure was analogous for mixtures of
single peptides, the stock solutions additionally being
mixed in various ratios, for example 10 : 1 or 1 : 4, in
order to obtain by dilution in 0.1 M sodium bicarbonate
the overall final concentrations which are indicated
above but which in the case of mixtures of several
peptides contain the latter in equal concentrations (in
the case of 1: 1 mixture) or in various ratios to one
another.
In each case, 100 l of each dilution were placed in 16
wells of microtiter plates, type B supplied by Nunc,
Roskilde, Denmark. The test plates charged with the
dilutions were left at 20 C for 18 hours and then the
solutions in the wells were removed by aspiration and the
wells were washed 3 - 4 times with 300 pl of a solution
of 10 g/1 bovine serum albumin in phosphate-buffered
physiological saline (PBS, pH 7.4) by filling and removal
by aspiration, and the test plates were subsequently
dried over silica gel at 20 C.
A concentration of 1 pg of 4083/ml and 1mg of SP 10/ml
has proven suitable for coating the peptide mixture, this
- 63 - 2054798
concentration representing the basis for the results
which are summarized in Tab. 12 to 14 and were obtained
as described in Examples 4 and 5. In contrast to previous
comparisons with the state of the art, all subsequent
comparisons will relate to a commercial anti-HCV ELISA of
the 2nd generation (HP 2) as described in Example 3.
Tab. 12 and 13:
The data on the ELISA according to the invention and
commercial HP 2 represent so-called end point titers with
which the highest preliminary dilution, which was found
to be reproducibly positive in a given test, of a serum
in anti-HCV-negative serum is defined.
Tab. 12: BBI Low-titer anti-HCV PANEL PHV 101
MEMBER ELISA according to Commercial anti-
I.D. the invention HCV test
NUMBER SP 4083/SP 10 (HP2)
1 g/ml each
PHV101-01 1 16 1: 2
PHV101-02 negative negative
PHV101-03 1: 4 1 : 1
PHV101-04 1 : 512 1 : 64
PHV101-05 1: 2 1: 1
PHV101-06 1 : 128 1 : 32
PHV101-07 1: 128 1 a 128
PHV101-08 1 : 64 1 : 16
PHV101-09 1 : 64 1 : 16
PHV101-10 1: 256 1 : 128
PHV101-11 1: 32 1: 16
PHV101-12 1 : 512 1 : 128
PHV101-13 1 : 16 1 : 64
PHV101-14 1: 64 1 : 32
PHV101-15 1 : 128 1 : 64
- 64 - 2054798
Tab. 13: BBI Mixed-titer anti-EICV PANEL PHV 201
MEMBER ELISA according to Commercial anti-
I.D. the invention HCV test
NUMBER SP 4083/SP 10 (HP2)
1 g/ml each
PH'V201-01 1: 1000 1 : 128
PHV201-02 1: 1000 1 : 500
PHV201-03 1: 128 1 : 32
PHV201-04 negative negative
PHV201-05 1: 64 1 : 64
PHV201-06 1: 64 1 : 256
PHV201-07 negative negative
PHV201-08 1: 4 1 : 16
PHV201-09 1: 1000 1 : 512
PHt72 O 1-10 1: 4 1 : 16
PHV201-11 512 1 : 128
PHV201-12 1: 32 1 : 16
PHV201-13 1 : 256 1: 256
PHV201-14 1 : 64 1: 64
PHV201-15 1 : 512 1: 1000
PHV201-16 1 : 512 1: 512
PHZ7201-17 1 : 128 1: 256
PHV201-18 1 : 16 1 : 128
PHV201-19 negative negative
PHV201-20 1: 4 1: 4
PHV201-21 1: 500 1: 256
PHV201-22 1 i 16 1 a 256
PHV201-23 1: 256 512
PHV201-24 1: 256 512
PHV201-25 1: 1000 1: 512
65 -
Tab. 14:
Results of the tests on 930 healthy blood donors from
whom serum and plasma were obtained simultaneously.
Specificity Specificity
in % after in % after
initial tests repeats
n= 930 sera 99.5 99.7
n = 930 plasmas 99.6 99.7
Example 8
Preparation of peptide solutions for producing mixtures
according to formula XIV with peptides of the formulae
XVIII, XIX, XX and XXII, and coating of microtiter plates
with these peptide mixtures
The polypeptides SPH 9 (formula XVIII), SPH 20 (formula
XIX), 4083 (formula XX) and SP 10 (formula XXII) were
dissolved at a concentration of 6 mg/ml in 50% (v/v)
acetic acid.
These 4 stock solutions were mixed in various volume-
based ratios as described in Example 4 and diluted in
0.10 M sodium bicarbonate (pH 9.6) so that the total
concentration of the polypeptides was between 0.2 and
8 pg/ml.
In each case 100 l of each of the diluted solutions were
placed in 16 wells of microtiter plates, type B supplied
by Nunc, Roskilde, Denmark. The filled test plates were
incubated at 20 C for 18 hours. The solutions were then
removed by aspiration and the wells were washed 3 - 4
times with 300 pl of a solution of 10 g/l bovine serum
albumin in phosphate-buffered physiological saline (PBS,
pH 7.4), and the test plates were then dried over silica
gel at 20 C.
- 66 -
Example 9
Optimization of the concentration of HIV 1, HIV 2 and HVC
peptides for the coating mixture, and optimization of the
ELISA determination and evaluation criteria
Starting with a ratio of 1: 1: 1: 1(V/V) for the four
stock solutions described in Exairtple 8, all four modifi--
able contents were varied independently of one another
but keeping the three others constant in each case in
order to obtain the following final concentrations of the
individual peptides of HIV 1, HIV 2 and HCV in the
coating solution (in g/m1):
Pepticle Peptid e Peptide Peptide
xvTli xix xX xxll
2 2 2 2
1 2 2 2
0,5 2 2 2
0,2 2 2 2
0,1 2 2 2
0,05 2 2 2
Peptide Peptide Peptide Pept;ide
XVIII XIX XX XXII
2 1 2 2
2 0,5 2 2
2 0,2 2 2
2 0,]. 2 2
2 0.05 2 2
2 2 1 2
etc. to
0.05 0 05 0,05 0 05
- 67 - 20e9 4r! .0-18
These mixtures were immobilized as described in Example 8
on microtiter plates and evaluated in the ELISA as
described in Example 4 and 5, with the concentration of
the peroxidase-labeled antibody likewise being optimized
against human immunoglobulin G.
The samples used to evaluate the optimization were
composed of low-titer anti-HIV 1, anti-HIV 2 and anti-HCV
samples which were prepared by serial dilutions of
corresponding positive human sera in negative human
serum. In addition, several anti-HIV and anti-HCV-nega-
tive samples were likewise tested in order to be able to
define the background reaction (i.e. the non-specific
binding on a given coated microtiter plate).
Set up as selection criterion was a signal of maximum
specificity, i.e. high limiting sensitivity in the
determination of the titration series of human anti-HIV-
and anti-HCV-positive samples with, at the same time, a
low background in the tests on anti-HIV- and anti-HCV-
negative samples.
Coating mixtures which were usually favorable were found
on the basis of these criteria, from which the following
mixture was selected:
XXI SPH 9 0.500 g/ml
SPH 20 0.250 g/ml
SP 4083 0.500 og/ml
SP 10 0.125 pg/ml
A conjugate concentration of Example 2 of 1 : 3000 with
a uniform additional final dilution of 1: 26 was found
to be favorable, the test being carried out as in
Example 4. In contrast to the previous establishment of
limits, under these conditions samples having an
extinction at 450 nm which is greater than the mean of
the negative controls plus an addition of 0.250 O.D. were
classified as anti-HIV- and/or anti-HCV-positive (new
- 68 - ~~~4798
establishment of limits).
These establishments were applied uniformly and unchanged
to following Examples 10 to 15.
Example 10
Determination of human antibodies of the immunoglobulin
G class against i3iV 1, HIV 2 and HCV in the ELISA
The samples shown in Tab. 15 - 17 were tested as
described in Example 4 under optimized conditions of
Example 9 with the peptide mixture of the formula XIV.
With regard to anti-HIV 1 or 2, the reactivities of the
method according to the invention were compared with an
anti-HIV 1/2 combination test (EnzygnostR anti-HIV 1/2,
supplied by Behringwerke AG, HP 3). Commercially avail-
able Western blots (anti-HIV 1 and anti-HIV 2) supplied
by DuPont were used for control. HCV was detected with
the aid of two different ELISA methods (HP 1 and HP 2).
As is made clear by the data in Tab. 15 to 17, anti-HIV 1
and anti-HIV 2, as well as anti-HCV, are detected safely
and reliably in samples of human origin with the method
according to the invention.
- 69 -
Tab. 15 2054798
Determination of a.nti-HIiT 1with the method according to
the invention compared with an anti-HIV 1/2 combination
test (EnzygnostB anti HIV 1/2, HP 3)
Strongly reactive means extinctions > 2.5 in the photo-
metric evaluation; all 76 anti-HIV 1-positive samples are
HCV-negative
Method Cce[anercial anti-F3IV 1/2
Number of Origin of according combination
samples samples to the test
invention
n = 12 Europe n = 12 n = 12
(anti- strongly strongly
HIV 1 reactive reactive
positive)
n= 57 West n= 57 n= 57
(anti- Africa strongly strongly
HIV 1 reactive reactive
pos.)
n= 7 West n= 7 n= 7
(HIV 1/ Africa strongly strongly
HIV 2 reactive reactive
coinfec-
tions)
n= 76 n= 76 n= 76
positive positive
- 70 - 2054798
Tab. 16
Determination of anti-HIV 2 with the method according to
the invention compared with an anti-HIV 1/2 combination
test (Enzygnose anti HIV 1/2, HP 3)
Strongly reactive means extinctions > 2.5 in the photo-
metric evaluation; all 28 anti-HIV 2-positive samples are
HCV negative
Method Commercial
Number of Origin of according anti-HIV 1/2
samples samples to the combination
invention test
n= 21 West n= 21 n= 21
(anti- Africa strongly strongly
HIV 2 reactive reactive
positive)
n= 7 West n= 7 n= 7
(HIV 1/ Africa strongly strongly
HIV 2 reactive reactive
coinfec-
tions)
n= 28 n= 28 n= 28
positive positive
- 71 - ~~54793
Tab. 17
Detection of anti-HCV with the ELISA method according to
the invention
Samples were termed strongly reactive when they achieved
extinctions > 2.500. So-called ratios are stated for the
moderately strongly reactive samples, which means the
quotient of extinction of the sample to cut o.ff of a
given ELISA. Values below 1.0 are, by agreement, termed
negative. Values above 1.0 as positive, the reactivity
being stronger as the resulting ratio increases; all
anti-HCV-positive samples are HIV-negative
Number Origin Method Commercial anti-
of of the according to HCV ELISAs
samples samples the invention HP 1 HP 2
n = 61 Europe n= 57 n 57 n= 57
Anti HCV strongly strongly strongly
positivLo reactive reactive reactive
Europe n '- 4
HC 90-346 > 9.0 ++ 2j7 (+) > ,~i,.,_Q ++
- 3 51 ,A + ne ga t i v e ,;}õLa +
-516 g$ + negative J'Q +
-570 Aj + ILI (+) > 510 +
n= 53 USA n= 51 ng51 n- 51
Anti HCV strongly strongly strongly
positive reactive reactive reactive
USA n S 2
HC 90-511 $,,z + III (+) 3.99 +
-566 A,_1 + negative > ,5"9Q +
n = 114 n=114 n = 111 n- 124
Positive positive positive
- 72 -
Example 11:
Determination of the analytical sensitivity of the ELISA
according to the invention for anti-HIV and anti-HCV
compared with the state of the art
As a model for the assessment of the analytical sensitiv-
ity of the ELISA according to the invention, titrations
of positive samples were prepared and tested in the ELISA
described in Example 9. In total, dilutions of three each
of anti-HIV 1- and anti-HIV 2-positive human sera in
anti-HIV- and anti-HCV-negative serum were prepared and
the limiting sensitivity defined as the last preliminary
dilution still reactive in the particular test system
using the new limit (mean of the negative controls plus
0.250 threshold).
The results of these limiting sensitivity tests are
compiled in Tab. 18 (anti-HIV 1), Tab. 19 (anti-HIV 2)
and .and Tab. 20 (anti-HCV)_as extinctions.
- 73 -
~ ~~ 5 3 ~~ ~~ z~ ~~n
~~
Tab. 18
Determination of the limiting sensitivity of the ELISA
according to the invention for anti-HIV 1 comvared with
the anti-HIV 1/2 combination test (Enzygnost Anti-HIV
1/2).
Three confirmed anti-HIV 1-positive samples underwent
preliminary serial two-fold dilution with human anti-HIV
1- and anti-HCV-negative sera, and these dilutions were
tested as in Example 3 or in accordance with the pack
insert of the manufacturer of the comparative test. All
the data are extinctions (E450)= All anti-HIV-positive
samples had a negative reaction in HP 2.
Sample Preliminary Extinctions in Extinctions
dilution the ELISA in the anti-
according to the HIV 1/2 com-
invention bination test
(HP 3)
cut off=0.282 cut off=0.268
0.D. O.D.
8803/26 undiluted > 2,500 + > 2,500 +
1 : 128 > 2,500 + > 2,500 +
Anti 256 2,334 + 2,185 +
HIV 1- 512 1,408 + 1,412 +
positive 1024 0,825 + 0,837 +
2048 D,._4&7 + 0,457 +
4096 0,240 - 0,216 -
8000 0,137 - 0,135 -
8611/144 undiluted > 2õ500 + > 2.500 +
1 : 128 20162 + 2,014 +
Anti 256 1õ366 + 1,028 +
HIV 1- 512 0,806 + 0,648 +
positive 1024 Qc-42A + 0.454 +
2048 0,280 - 0 258 -
4096 0.153 - 0,136 -
8000 0,088 - 0,076 -
8803/24
Anti 1 : 400 ~ + 2458~ +
HIV 1-
positive
22281
Anti HCV- 1 256 2. 219 + 0,014
positive (negative)
- 74 -
Tab. 19
Determination of the limiting sensitivity of the ELISA
according to the invention for anti-SIV 2 comnvared with
the anti-HIV 1/2 combination test (Enzygnost Anti-HIV
1/2).
Three confirmed anti-HIV 2-positive samples underwent
preliminary serial two-fold dilution with human anti-HIV
1- and anti-HCV-negative sera, and these dilutions were
tested as in Example 3 or in accordance with the pack
insert of the manufacturer of the comparative test. All
the data are extinctions (E450). All anti-HIV-positive
samples had a negative reaction in HP 2.
Sample Preliminary Extinctions in Extinctions
dilution the ELISA in the anti-
according to the HIV 1/2 com-
invention bination test
(HP 3)
cut off=0.282 cut off=0.268
O.D. O.D.
8804/103 undiluted > 2,500 + > 2,500 +
1 : 128 > 2,500 + 2,500 +
Anti 256 2,420 + 2,045 +
HIV 2- 512 1,488 + 1,339 +
positive 1024 0,737 + 0,705 +
2048 Q,aaa65 + Qj $5 +
4000 0,196 - 0,190 -
8804/117 undiluted > 2,500 + > 2,500 +
1 : 128 > 2,500 + > 2,500 +
Anti 256 1,860 + 1,902 +
HIV 2- 512 1,233 + 1,100 +
positive 1024 0, Ei56 + 0,667 +
2048 P.O,;= + D_j 0 2 +
4000 0,166 - 0,172 -
8804/116
Anti 1 : 400 1,945 + +
HIV 2-
positive
22281
Ant,3..FICST- 1 256 2 219 + 0.014
positive (negative)
- 75 -
Tab. 20
Determination of the limiting sensitivity of the ELISA
according to the invention for anti-HCV.
Four confirmed anti-HCV-positive samples underwent
preliminary serial two-fold dilution with human anti-HIV-
and anti-HCV-negative sera, and these dilutions were
tested as in Example 3. All the data are extinctions
(E450 nM) =
Sample Preliminary Extinctions in Commercial
dilution the ELISA anti-HCV
according to ELISA (HP 2)
the invention cut off=0.481
cut off=0.270
HC 90-85 undiluted > 2 500 + > 2,500 +
1 : 16 > 2,500 + > 2,500 +
Anti 1 : 32 2,299 + > 2,500 +
HCV- 1 : 64 1,858 + > 2,500 +
positive 1 : 128 1,515 + > 2 500 +
1 : 256 1,045 + 1,707 +
1 : 512 0,648 + 0,871 +
1 : 1024 0 322 + 0.492 +
1 : 2048 0,207 - 0,255 -
HC 90-225 undiluted > 2,500 + > 2,500 +
1 : 16 > 2,500 + > 2,500 +
Anti 1 : 32 2,214 + > 2,500 +
HCV- 1 : 64 1,816 + > 2,500 +
positive 1 : 128 1,488 + 2,008 +
1 : 256 0,689 + 1,120 +
1 : 512 0,580 + D'= +
1 : 1024 Q`Z86 + 0,375 -
1 : 2048 0,148 - 0 111 -
Anti,HCV-
positive
HC 90-270 1 : 256 0222 + 1.544 +
HC 90-354 1 : 128 &,221 + 28291 +
Anti
HIV 1-
positive
8803/26 undiluted > ~~500 + 0,110 -
8811/144 undiluted > 2 00 + 0,098 -
8803/24 undiluted > 2"500 0m068 Anti = (negative)
HIV 2-
positive
8804/103 undiluted > 2.500 + 0,075 -
8804/117 undiluted > 2.500 + 0,081 -
8804/116 undiluted > 2.500 + 0 099 -,
(negative)
~ a r
- 76 - 2 t~'e~ r~ ~t ~(3
The procedure was analogous for anti-HCV in that serial
dilutions of four anti-HCV-positive samples were prepared
as described in Tab. 20, tested and evaluated in analogy
to anti-HIV and compared with the results with the
commercial anti-HCV ELISA of the 2nd generation (HP 2).
In addition, the specificity of the reactivities of
individual donations were also tested in the individual
tests by also investigating the anti-HIV 1- and anti-HIV
2-positive samples undiluted in the anti-HCV test and,
conversely, the anti-HCV-positive samples in anti-HIV 1/2
combination tests (EnzygnostR anti-HIV 1/2).
The results compiled in Tab. 18 to 20 make it clear that
the limiting sensitivity of the ELISA according to the
invention for both anti-HIV 1 and anti-HIV 2 corresponds
to the limiting sensitivity of the anti-HIV 1/2 combina-
tion test. The HCV limiting sensitivity of the ELISA
according to the invention also corresponded to the
limiting sensitivity of a commercially available anti-HCV
ELISA (HP 2). However, while a total of at least two
tests (anti-HIV 1/2 combination test and at least one
anti-HCV test) are necessary to detect these three
antibody specificities accordinci to the state of the art,
this detection is possible with the method according to
the invention just as reliably and with comparable
sensitivity using only one test mixture.
It is furthermore evident from the data in Tab. 18 to 20
that the strong reactivity of the anti-HIV- and anti-HCV-
positive samples in the novel ELISA is particularly
advantageous because the anti-HIV samples reacted nega-
tively in the specific anti-HCV test and the anti-HCV-
positive samples reacted negatively in the specific anti-
HIV test.
_ 77 -
2054798
Example 12
Determination of anti-HIV 1-positive samples from early
stages of infection (seroconversions) with the ELISA
according to the invention.
Tests were carried out on a total of 3 patients from whom
sequential blood samples were repeatedly obtained at
defined times during very early phases of an HIV 1
infection. These serum panels are commercially available
(Boston Biomedica Inc., USA). The results which were
obtained with the ELISA according to the ~nvention
compared with an anti-HIV 1/2 combination test have been
reproduced in Tab. 21.
Tab. 21:
All data are extinctions (E450 .) ~ and values above the
particular limit are to be regarded as positive.
- 78 - 2074798
Tab. 21
Sample ELISA Commercial anti- Commercial
code according to HIV 1/2 ELISA anti-HCV
the invention ELISA (HP2)
1 A 0,023 0,066 negative
2 A 0,020 0,055 A
3 A 0,055 0,193 4 A 1,163 + > 2,500 +
A > 2,500 + > 2,500 + n
6 A > 2,500 + > 2,500 + e1
7 A > 2,500 + > 2,500 + 91
8 A > 2,500 + > 2,500 + "
9 A > 2,500 + > 2,500 + 'e
1 C 0,014 0,022 negative
2 C 0õ015 0,054 "
3 C 0,054 0,145 t9
4 C 0,500 + 1,138 + 11
5 C 0,630 + 1,707 + "
6 C 0,889 + > 2,500 + "
7 C 0,942 + 10
8 C 1,010 + 11 '
9 C 1,081 + 10
i: 1,564 + t1 i
11 C > 2,500 + n u
12 C n oe n
13 C u u
14 C 1
C n ee
16 C u n n
17 C 1 ei
18 C n aa
G BBI
80 0,012 0,056 negative
81 0,013 0,053
82 0,018 0,059 83 0,478 + 1,112 +
84 0,733 + > 2,500 +
85 0,612 + > 2,500 + n
86 0,763 + > 2,500 +
87 1,296 + > 2,500 +
88 1,309 + > 2,500 +
89 1,544 + > 2,500 +
cut off 0,281 0,285 0,481
- 79 -
2054798
The results in Tab. 21 make it clear that the ELISA
according to the invention detects low-titer anti-HIV 1-
positive samples just as reliably as the anti-HIV 1/2
combination test (EnzygnostR anti-HIV 1/2). It is likewise
evident from comparison with the data in Tab. 21 that the
earliest time at which first detection of HIV 1-specific
antibodies is possible with the novel peptide ELISA is
also possible at least as early as with specific single
anti-HIV 1 tests as state of the art.
Example 13
Determination of low-titer anti-HIV 1-positive samples
with the ELISA according to the invention
Tab. 22 summarizes the results which were obtained in the
tests on a commercially available anti-HIV low-titer
panel with the novel HIV/HCV peptide ELISA compared with
an anti-HIV 1/2 combination test (EnzygnostR anti-HIV
1/2).
The sample panel supplied by Boston Bicanedica; Inc. , USA,
comprises a total of 15 samples which were classified by
means of anti-HIV 1 determination methods as low-titer
anti-HIV 1-positive.
Tab. 22
Results of the tests on the low-titer anti-HIV 1 panel
supplied by Boston Biomedica Inc., USA
All the data are extinctions at a wavelength of 450 nm.
Values above the stated limits are positive.
- 80 - 205d798
Tab. 22
ID No. ELISA according Commercial anti- Commercial
to the invention HIV 1/2 anti-HCV
ELISA HCV ELISA
BO 1-01 > 2,500 1,872 > 2,500 +
02 1,979 1,575 2.023 +
03 2,056 2,280 negative
04 1,751 1,960
05 1,781 2,044
06 1,428 1a728
07 1,183 1,564 08 0,641 1,152
09 0,984 1,499 "
"
0,682 0,940
11 1,302 1,650 12 2,032 0,540 1,833 +
13 10017 1,854 negative
14 1,079 1,484 "
> 2,500 2,090 > 2p500 +
cut off 0,279 0,265 0,479
- 81 - 2054798
The results in Tab. 22 reveal that, compared with the
anti-HIV 1/2 combination test (EnzygnostR anti-HIV 1/2),
all the samples are found to be comparably strongly
reactive with the HIV/HCV peptide ELISA according to the
invention.
It is interesting that four samples in the panel are in
fact found to be more strongly reactive with the novel
peptide ELISA than with the anti-HIV 1/2 combination test
(EnzygnostR anti-HIV 1/2) (No. 1, 2, 12 and 15). Addi-
tional tests on these samples revealed the simultaneous
presence of HCV antibodies, which again confirms the
reliability of a non-differentiating detection of anti-
HIV 1/-HIV 2 and -HCV by the method according to the
invention.
Example 14
Determination of low-titer anti-HCV-positive samples with
the ELISA according to the invention
Tab. 23 contains the results which were obtained with-the
novel HIV 1/HIV 2 and HCV peptide ELISA and the low-titer
anti-HCV panel supplied by Boston Biomedica Inc., USA.
This Tab. 23 also contains data which were obtained on
the same panel with a modern ant:i-HCV ELISA (HP 2).
The data on the ELISA according to the invention and
commercial represent so-called end point titers with
which the highest preliminary dilution, which was found
to be reproducibly positive in a given test, of a serum
in anti-HCV-negative serum is defined.
- 82 - 2054798
Tab. 23: BBI low-titer anti-HCV PANEL PHV 101
MEMBER ELISA according Commercial anti-
I.D. to the invention HCV test (HP 2)
NUMBER
PHV101-01 1: 16 1 2
PHV101-02 negative negative
PHV101-03 1: 4 1: 1
PHV101-04 1: 512 1 64
PHV101-05 1: 2 1: 1
PHV101-06 1 : 128 1 32
PHV101-07 1: 128 1 128
PHV101-08 1 : 64 1 16
PHV101-09 1 : 64 1 16
PHV101-10 1 : 256 1 : 128
PHV101-11 1 : 32 1 : 16
PHV101-12 1: 512 1 : 128
PHV101-13 1 : 16 1 : 64
PHV101-14 1: 64 1 : 32
PHV101-15 1 : 128 1 : 64
- 83 - 2 0 O, 17 9 8
The results in Tab. 23 make it clear that all 14 positive
samples were found reliably and unambiguously to be
positive with the ELISA according to the invention. It is
noteworthy in this connection that the signal strength is
very high, which is attributable to high reactivity.
This high reactivity is also reflected in the limiting
sensitivities found in Tab. 23. These detection limits
were defined by subjecting the native human samples to
preliminary serial two-fold dilutions in anti-HCV-nega-
tive serum and then to testing as in Example 4, where the
last preliminary dilution stage which is still found to
be reactive represents the so-called final titer.
On this basis, the data in Tab. 23 reveal that the novel
HIV 1/HIV 2/HCV peptide ELISA has in fact better detec-
tion limits for anti-HCV in the PHV 101 panel than the
state of the art.
Example 15
Determination of the rate of non-specific reactions of
the novel peptide ELISA in a group of healthy blood
donors
To determine the frequency of non-specific reactions,
which lead to false positive results in the ELISA, a
total of n = 512 healthy blood donors was used. Serum and
plasma were obtained simultaneously from these donors in
'25 order to be able also to investigate a possible interfer-
ing effect of the coagulation system on the method.
Samples which were reactive in one of these three tests
(ELISA according to the invention, anti-HCV ELISA HP 2
and anti-HIV 1/2 HP 3) (so-called initial tests) were
repeated in the same test (so-called retesting) and,
where the reactivity was reproducible, investigated in
the anti-HIV 1 Western blot and anti-HCV ELISA (HP 1) as
comparative methods.
- 84 - 2054793
It was not possible to confirm as anti-HIV 1- or as anti-
HCV-positive by means of this confirmatory method any of
the samples which emerged in this screening as reactive
in one of the three ELISAs. Thus, all the samples which
were rejected in the screening were consistently classi-
fied as false-positive in the particular methods.
Tab. 24:
Screening of n = 512 healthy blood donors from whom 512
sera and 512 so-called paired plasma were obtained
simultaneously. The findings after the initial tests are
indicated; see Tab. 25 for the retest results.
85
U9 N !~ ~
s s e~ ~
M M r1 r9 ~^I
(j) s = s \ !o \ \
O
=rl tti Ul
a +.)
x ro ro ~ . .
~ m 0+
w
U
=rl U
> U)
~ 0~
ro ~4 ~ \ \
z ~ ~ a o ~n
~
.
U2 = = s = + =
~
' (d
+) N Rf G ~ c0 C~~ 1C ~
pa
N
ri
~"/'
~ \ \ \
2 SU-i tUn 04 o .-d O
U M N Oi
= = o
U) a I ri C
s = = =
~ o ro ~ \ \ \ B+ tT tT ~+ ~ ~+
a~ ~a ro r+ o .t (1)
d+ ro a~ ~ . . m
~ M 04
..~
'CS
O
U
(d 0 a)
.4 p Lo
0 ~ N +J \ ~ N
~ P 9 ~ ~ ~ \ \
.~ Z S-I m LL M O
rd w~ t~l t"~ ~' BS1
M M H ~
a b ~
(0 ~ o rl sa
cn U ~` ~'
-
~ 86
2 0 5 4 71911
Itt
N
N
N N
vi N
_ d' =
~ U N '-1 C75 01
o a O1 1
w =~I ~
N N
`n o = N
N ~ ia d`
:n v Ch m
~ rn v,
~
.,.,,
4-1
~ ~ M o
~ -4 U) co
--ro .
> M ~ ~, O-
cn ~ Q, rn rn
~ x P4
~ ~ 9 o 0
~ ~
rt] )`I =
o a~ '
N
4-J
N
Q)
+,1
IC) F-1
A
04 W ~ O
Q) y ~c w
~ O ~ N = N =
ON Q1
O. o, o,
a
a w ~ ~I o
LO
~ cn
N
4-) O O ah arn
.,q a to O,
=~ 0
4-4
.,~
f)
O
04
N
Q)
N
~ ri
W N
O cG d" ^
... I dP
~
tA 04 U1 1 to to 1 to
a4-)-I O -P -i U ~ ) d
N -i a1
'C3 ~ ~O to . ~ ~
N L11 O r-I 0 N O 4=)
t!] (tl S 1 0 ed to 41 U CO U] ,F) -1
=
1~ N= ~ w ,1 44 rC O M r1 44 H ~ ) O -1 N U
N a~I O 0 44 N ~ =t) 0 N N ~ 44
=,I
~ ~ ~ ~ ~ ~ ~ ~ ~
u') Ln O A O4-) C: U) N d-) M Re
~ II II R9 .C ~=O C; (=-1U =O ~ d~
7r Pa =rl rl t[f tA
'-1 -0-)
H ~C 4~4 .L; .~ f-I
- 87 - 2 () 5 d '7 8
The results in the first series of tests (initial
results) are compiled in Tab. 24. Whereas three sera (and
two corresponding plasmas) were reactive in the method
according to the invention, 1 serum (and one correspond-
ing plasma) was found to be reactive with an anti-HIV 1/2
ELISA and five sera (and four corresponding plasmas) were
found to be reactive with the anti-HCV ELISA. The donors
reactive in the particular ELISA were not identical.
After repeat tests had been carried out on these nine
reactive sera, the picture which emerged was that depic-
ted in Tab. 25 that two sera (two paired plasmas) were
retest reactive in the peptide ELISA according to the
invention, one serum (one paired plasma) was reactive in
the anti-HIV 1/2 ELISA, and four sera (four paired
plasmas) were reactive in the anti-HCV ELISA.
On the basis that it was not possible to confirm any of
these samples as anti-HIV- and/or anti-HCV-positive, the
specificity data presented in Tab. 25 were determined for
each of the ELISAs investigated and for sera and plasmas
by calculating the percentage of samples found correctly
to be negative (in total 512 ser.a and 512 plasmas).
Based on the data in Tab. 25, according to the state of
the art - obligatory tests for anti-HIV and anti-HCV on
each donation, a total of five donors would have had to
be excluded:
One donor repeatably false-positive in the anti-HIV 1/2
test and four donors repeatably false-positive in the
anti-HCV test. By contrast, the number of donors possibly
found with a false reaction is only two in the ELISA
according to the invention.
in summary, it can be said that the novel peptide ELISA
for the simultaneous non-differentiating detection of
anti-HIV 1, anti-HIV 2 and anti-HCV at least corresponds
in terms of the currently valid criteria of sensitivity
to the in each case optimal performance features of the
- 88 - ~~54793
two single tests anti-HIV 1/2 on the one hand and anti-
HCV on the other hand: the data from the tests on panels,
i.e. native samples containing anti-HIV 1 and/or anti-HIV
2 and/or anti-HCV indicate comparable efficiency just
like the tests on the limiting sensitivities and time of
earliest recognition of low-titer anti-HIV 1 or anti-HIV
2 or anti-HCV samples. Whereas detection of these three
antibody specificities is possible according to the state
of the art in this manner only with three different or,
in view of the anti-HIV 1/2 combination tests, with two
different tests, the ELISA according to the invention has
the advantage that, while the efficiency is the same,
only one test need be carried out. Particularly for blood
banks which are obliged to carry out tests for anti-HIV
1, anti-HIV 2 and anti-HCV, the novel peptide ELISA means
a considerable reduction in effort and costs with at
least the same reliability and safety in the determina-
tion of anti-HIV 1, anti-HIV 2 and anti-HCV.
It was additionally found, completely surprisingly, when
determining the susceptibility of this novel test to
interference that, by reason of the very good specific-
ity, i.e. only low number of false-positive findings,
fewer retests and fewer confirmatory tests are necessary
and, moreover, fewer donations have to be rejected than
with the conventional screening procedure using an anti-
IHIV 1/2 test and, separate therefrom, an anti-HCV test.
This means that the method according to the invention has
economic and chemical advantages which are also offered
by other constellations of HIV and HCV peptides of the
fornlulae IV to XII and XIII to XVII.
The method according to the invention is also extremely
suitable for other problems in which maximal optimization
of limiting sensitivity is of most importance. Thus, it
is possible to show under other test conditions or, for
example, by calculation in Examples 10 to 14 that it is
perfectly possible to produce sensitivity features of the
method according to the invention which are significantly
- 89 - 2054798
better than those of the state of the art if somewhat
less favorable specificity data are accepted, although
these still correspond to the state of the art.
If, for example, the limit (Example 9) were to be reduced
to 0.1 extinction, all the limiting sensitivities for
anti-HIV 1, anti-HIV 2 and anti-HCV would be considerably
improved by a factor of at least 2. A total of five
donors would result as the rate of samples with a non-
specific, i.e. false-positive, reaction in the screening
of the healthy blood donors (Example 15), the donors
having been found to be repeatedly reactive, which
corresponds absolutely to the result of the two single
tests, although the sensitivity of the antibody detection
was considerably improved.