Note: Descriptions are shown in the official language in which they were submitted.
91~ 5~ 16~3~;TA~EDA PATENTS OSAKA Fetherst~n il ; 083006B01;$ 4/72
2 ~ iJ ~'~
- 1
~YC~OPBNT~NE D~RIV~TI~E~ AND THEI~ US~
FIE~D OF TH~ INVE~T~ON
~he pr~en~ invention relates to 4,5-
di(hydroxymethyl)-2~cy~10pen~nyl derivati.vl~ which ar~
l~w in to~i~ity, ~tx~ngly inhi~it the growt:ll o~
rotro~ixu~ or r~troid~iru~ Qnd u~ul ~ nti~ir~l
Age~æ .
BACK~ROUND OF ~HE INVEN~TC:IN
Silla~ th~ df3~elopment o~ ac~clovir, stlLdies on th~
~yntl~esi~ o~ alltl~lr~ enta or ~ucle~icie t~pe, and a
~u~Qb~r o~ u~ful ao~p~und~ hav~ ~o fa~ b~:n o~tai~d.
E~owe~v~r, a~3 ~he~:~ anti~riral ~gents o~ nuc: l~c~ide typ~
only inhib~Lt replic~tion o~ viruse~ but do ~ t
15 ~3~a~icate viru~es ~h~msel~r~s, virus~in~ect,s~:l p~tien~ -
are required to take ~u~1~ o~ thi~ tyE~ fo;~ a p~olonged
per~o~. Cil~c~næta~e~ bel~ ~uch, e~?loital.ion o~
orall~ admini~tr2ible antlretrovlr~l a~ents l:)f
nu~leo3ide t~pe i~ de~irable for vir~ infe~ !te~
~a p2ltien~. Pexivative~ oJ~ c~r3:ocycli~ nu~ lde,
pr~par~d ~ ~ub~tltutln~ oxyg~n atom~ c~ ~u~a~
portion of nucl~osi~e wlt:h ca~bon atom~ a:r~ ta~le
ag~inst aci~. If ~ ~he~efore, compound~ ~ho~,~ing
~t~on~ly antivi~al activity are obtained ~:rc~m the~
~5 cArbocy~ nucl~o~id~1 the~ c~ b~ u~d i~l! ~r~
adm~nistrabl~ an~iviral ~gen~s.
~ mong vixal $n~ec~iou~ di~ses, acqu;ixe~
immunode~iciency synCI~Onle ~AID8~ due ~ in:E~ ion O~
the human imm~ne~ ci~nay viru~ ~HIV) aaue e~. ~e~ious
depression c,~ nunît~ d i~ a lethal di6~ e.
Furthex,ep~clemic~ity of AIDS keeps c)n inc~rei~l ingl all
over the world, call~in~ ~ serious socia1 p;ct~lem.
Studie~ on the ~herapy o~ A~S h~e bfa6.n activ~ly
conducted all oveX the wc~rld, and several n~:cleo~ide
35 derivati~Te~ having ~C tlvi.ty to inhi~it propa gation of
MIV h~ve b~en 3cnown, for example/ ~ide~xynllc: 140~icl~at
91~ 16~36~;TAKEDA PATENTS OSA~A Father~n i 063006601;# 5/72
2 .
~uch as azi~.othymi~ine (~Z~) ~ 2' ,3~ dideo~ deno3ine,
2 ' ~ 3 ' ~ eox~i~o~ine, 2 ~ r 3 ~ ~di~ehydro~
did~oxythymidine, 2 ~, 3 ~ -didehyd~o-2 ', 3 ' - .
~ideoxycyto~ e, 2 ', 3 ' -dldeoxy-2 ~ f luoroadeno~ine,
5 ~' ,3'-clideoxy-2' ,~r-difllloroaderlosine, ~ncl, e~p~cially
azido~hymidine have been c:linically ~rovecl I;o ~how
life-prol~nging e~e~t 1n p~tl~nt~ ~uff~r~.n~;t ~rom AIDS~
which is only one d~ug ~pproved for the~apy o~ AIDS
[Elio~heln, Pha~n. 37, 3534-35~7,. ~1988~, an~ others].
~t has ~oeen ~la~ hclt the~e di~es~xynuc..eoside~
~;uppxeæ~ ~he growth of vf.~lls by illhi~i~ing, in ho~t
cell~, ~he aatlvi~y of ~v~r6e t~n~r~pta~ O~ H~V.
~d, it has al~o been recogniz~d tha~ they ~!lave the
~ctivit~r o~ inhi~iting the growth of re~roi~ ~ ~rlru~
15 havin~ ~lans~ip~e (~V), lil~e xe~xovlxu~ ~u~h a~
HIV .
With the aim at preparin~ an orall~ a.~rlini~tral~le
anti-~IV ~ge~nt, searc:h for an an~iret:rov.ira:'. ag~nt~
f ro~ rbocyclic: nuclevsi.de derivlativ~s ha g J:: een
20 conducted. A~ a re~ult, it h~ eell ~vu~d l.hat
~ax~o~ir~ (lR, 4R ~ - 4 - ( hydro~ylne thyl ) - ~ ~
cyclopenteny1 ~s~uanin~, has the ~c:ti~ity of I nhibiting
replicat~on o~ E~IV ~ 3iochem . Biophy~3 ~ Re~ . C'o~nun .,
15Ç, pp.1O4~Y1053, ~19~8~ 1 . Further, ik ha~ been
2~ cla~iied that nucl~o~ide!s havln~ ~~meniber~c cyclic
su~ar and car~ocyclic analogs of oxetano~ ~ as well
have antiretroviral acti~ity ~d a~ et~olc.vir~l
ac~ivity, ~ore ~pecifically, in 2,3-
di(hydroxyme~hyl~cyclo~ut~l deriva~ives ~ucl a~ 9- ~ 2, 3-
~i~h~d~ox~mothyl)ayolobutyl]adenine~ 9-[~l3
di(h~droxymeth~l)cy~lo~ut;yl~guanine, etc, ~;PA
H2(1ggO)-~4~7) and 3~(h~d~ox~methyl)cyclob7l1:
derivativeg ~uch aB 9-[CiS 3-
(hydroxymethyl)cyclobut~l.]a~enine, etc. (J7?P. H2~19~0)-
3~ 73072), an~ire~ro~iral aativity and antiret3:0id~1ra~
acti~ity have bee~ ~Qn~i~med.
9~ 5~ 16~36~;TAKEDA PATENTS OSAI~A Fether~ton ; OB3006LiOl;# 5/72
ThP ~id~o~ynucl~aosf-~es d~?scribed a~o~ are, in
general, very uns~able again~t acid ~ S~ nLI ,e ~ ~,
pp . ~12-415, ( 1~ 3 J, antl their ~herzLpeu~i.c e~c s c~n
hardly bs exlpe~k~3d by or~l admini~r~ion. And, whil~
5 AZT is lthe only clrug appxo~Ted a~ anti-AIr~S agent, it
ha~ heen known to ~ILO~ serious unde~ le si~e a~ c~s
suGh a~ y~3l0~uppre~ L o~ neu~rocy~ope!n~,! L - t~n. the
other hLantl~ r~rl~o~ir is s~ agains~ ac~ l and ~an be
adlrini~e~ed orall~, b~L~ s ~ntivi:r~l act...lrity is far
10 from ~eillg sati~3facto~ urth~rmore, c~ vir i~ very
po~rly 301uble in w~te~ i~nd 1~ i~ hardly ,~]: ~o~l~ed
o~al ~c~nini6~xa~10n t~n~imi~robi~l Ayen~ i!n~
~hemotherapy, 33, pp. 171.~175 ~ ( 19~g ) J . Fu:l~tllex, si~c~a
2 ~ 3-di ( hy~iroxylne~hyl ) c:yclobut~rl de~ivativ~. o~ 3-
15 (h~roxymethyl)cyclobu~y~l deriva~ives are ~ o ~able
against acid, the pos~ik~ y o~ their ora
adbninl~ration i~ ~ug~sti~e, but thei~ a~l;'-viral
acti~rity i6 nc:t sa~isfac~ory.
P~ying at~ention ~o the po~3ib~ lity ~i. using
21~ ~arbocyc~ ucleoside~ as or~ admini~t~ abl~
anti~rixal e~gsn~ the p~e~e:n~ i mr~nt~r:5 h~ cvnduc~qc~
re~a~c~ wo~k ~ox ~ynthe~izing ~vel car~oc:~clic
nucl~oside ~e~iva~ive~. A~ ~ xesul~ he ~:r~ent
. in~n~xs ha~e ~ound ~at 4,5-di~h~rox~met:hyl)-2-
~y~lopentenyl deri~ati~es a~ l~w ln to~icl~y an~~t~ongly i~hib~t ~he ~rowth D~ r~trovir
~etroi~l~u~
SUM~RY 0~ T~E IN~NTION
The prs~ent i~ve~tio~ relate~ ~o
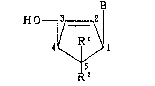
3~ (1) A ~pound r~p~ented b~ th~ ~OrmUlA
3r~ 2
4~ R' \ C13
~2
wh2~ein B i~ a puri~e ~e r~idue bon~ed a~ th~ ~-
., . '
91~ 6~ 16~36~;TAREDA PATENTS OSAKA Fethe~t~ ; 06300B601;~ ~72
2 ~ ~ 50~
4 _
position thereo~, or a py:rimidine b~ r6~ .d l~ bon~esd
at ~h~ l-po~l~lon ~A~3reo~, ~o ~he cy~;:lc7pent;e l~ rin~;
one of Rl and Ra is a hycl~og~n atom, ~,nd the! ~th6ar i~ a
methyl grc~up ha~ing an optionally p~o~ectecl. ;Iyd~ox~rl
S group; or a ~al~ ~hereo~, and
( 2 ) ~n an~iviral ~gent containing a com3?ousld c~f ~he
.~ormula [ I ] or a ~al~ the:ceo~ .
I:)ETAILE;D ~ESCRIPTION OP THE PREFERt~ DI~N~
q!he fonnula [1~ re~sarlt~ ~he o~tlcall ~ actlv~
~rmula 11 ' ~ B
H t~3~ Z ¦
1 ' ]
1~
R8
wherein 1~ ancl R2 a~e vf ~h~ sa~n~ meanin~ a~
men~ n~ad abo~; or ~he olp~ic*.ll~,ac~ re or:nula
B
~I
R2
~S whe:cein ~ nd R2 are o~ ~e sam~a meanincJ a
mentloned abo~Y~ r C~~ r~m.ics m~ x~u~ and ~
In l:he ~ompouTI~s ~p:resente~ by ~h~ foY~nula rl] r
- ~he confo~a~:Lon betw~en ~h~ }lyd:~oxymt~thyl g ~oup a~ the .
4-~o~i~ion ~ the cyclope.n~ne~ xing and the~ nethyl
~O g~oup havi~g a~ ~ption~lly protocted h~d~o~y l ~u~ a~
the 5~ ltion o~ th~ syclopenten~ rin~ is E ~ef~ably
the E-c~n~ox1n~ion.
In the compound~ ~p:reser~ted ~y the o.rnula [1~
tht~ conf o~Sa~iorl between the group ~p~es~nt ~d by B at
~5 l-po~ lon Qf S~h~ cy~lo~entense Lin~ ~nd ~he neth~l
g:roup having ~n Qptlonally pro~ec~e~ hydrox.y 1 g~up
.
,
2 ~ 73~ V ~24205-911
the 5-position of cyclopentene ring is desirably the E-
conformation.
In the compound of the formula [1], desirably B is
adenin-9-yl, guanin-9-yl or thymin-l-yl; one of Rl and R2 is a
hydrogen atom, and the other is a methyl group having an
optionally protected hydroxyl group; provided that, when B is
guanin-9-yl, Rl is a methyl group having a protected hydroxyl
group and R2 is a hydrogen atom, and, when B is thymin-l-yl, R]~
is a hydrogen atom and R is a methyl group having an optionally
protected hydroxyl group.
In the compound of the formula [1], preferably, B is a
purine base residue bonded at the 9-position thereof to the
cyclopentene ring.
Preferable examples of the purine base residue are
adenine and guanine residues, more preferablyr an adenine
residue.
Preferable examples of the pyrimidine base residue are
thymine, cytosine and uracil residues, more preferably, a thymlne
residue.
In the compound represented by the formula [1], the
purine base residue or pyrimidine base residue shown by s can
form a salt with an acid. Examples of the salt include pharma
ceutically acceptable acid addition salts. Examples of the acid
include an inorganic acid such as hydrochloric acid, hydrobromic
acid, sulfuric acid, phosphoric acid or nitric acid, and an
organic acid such as acetic acid, malic acid, citric acid,
ascorbic acid, mandelic acid or methanesulfonic acid.
- 5a -
2 4 2 ~ r~
The present invention also relates to a process for
producing the compounds represented by the formula [1] or salts
thereof. The process includes the following methods.
A cyclopentenyladenine derivative represented by the
formula [la]
2420!~ , i4 ._
-- 6 --
I
H 0~3~ 2
~ Ra,~ I ~la~
S R'~'
whe~ein B~ L~ ~. purina bas~ r~sidue bondecl It th~ 9-
pc~ition ~her~of, ~o ~}~e cyclop~3nton~ rin~, one o~
~nd ~4 i8 a hy~xogen aton~, and the o~he~ i~. a me~l~yl
~;~oup h~ving an opt~onall~y p~otect~d h~lrc~3~ ~OUp; o:~
0 ~1: 8~11; the~ pxodua~d by the me'cll~od ~;hich 1~
~h~ra~te~ized ~y xe~in~ ~ ~om~our~d ~3pxE3elented hy ~he
~o~nul~ ~ ~a ~ N H 8
~3~ 2
41W [~3
wh0~ein one of R3 and R~ ~tand~ ~or ~ hydrot; en atom and
th~ o~h~3r ia a me~yl gx~u~ h~v;Ln~ an o};~t;l.clnall~
20 pro~ectod hydroxyl gxoup, or ~ ~al~ ~h~30f' wlth 5
~mino-~, 6~dih~logenoE;~yr:Lmidln~3, then r~3A~: t.i. ng th~a
ul~a~t compound ~*lth ortho~ormic ~:e~r in th~3
pr~ nce o~ ~n ~cld ~o c~u~ ~orm~tlon o:E t; he pu~ine
~k~lo~on, then c:o~va:~ting th~ halogen on ~:h.~ ~u~llle.
25 rin~ n ~nlno s~roup, fGllOW6!C~ when n.~ s~ry,
~ub~o~ting th~3 amino grouE? to a el~proteat.i.c~n react~on,
~ ~y~:lop~an~a~-yl~ . d~arisratl~e xe~l~re6~nt~3d by .
th~ ~ormula ~ lb 3 B
~ [lb~
~' ,
wh~r~3in B2 i~s A .~urine bas~ ro~ due bc~nd~d e~t the 9-
35 . l?o~ltlon ~hareo~ ~o th~s cy~ penten0 r~nS~J one o~ R5'
~nd ~6 i~ a hyclro~n atom ~nd t.h~ oth~x 1~l a 3n~3~hyl
..
group havi~g e~n op~lonLall~r p~ ected hydro~lyl s~roup; o~
a ~:lt ~ 3x~ produ~e~ by ~h~ m~3thod w}llch i~
charac~3ri~d by re~c~ln~ 8 compound r~pr~a~len~ed by the
~rmul~ f 2b ~ N H 3
c
whexæin on~ o~ RJ ~nd ~e i~ ~ hydrogen a~o~ Rnd i;he
oth~x ~ ~ a me~hyl ~ up h~ving ~n. optl~r~ ,y p~:oto~;i.e,d~
hydrox~l ~roup, or ~ ~alt thereo~ wit:h ~ ino~
dih~logQr~opyrlmldine, th~n aminat ~ ng the c; pO~ ~ tion on
lS ~h~ pyrlmid~ne ring, and then reacting the resultant
compou~d with ortho~o~mlc e~ ~e~ in the ~r~ e of an
aaid ~o aau6~ rmation c~ a }~l3~in~ fikç31eton~ ~ollowad. -
by con~rtlng th~3 halogen on the pu~ine r~ g ~o
hydroxyl grou~.
20~ cyQlop~nt~nylth~mins d9rl~ v~ rl3p~sen~ed b~
the fo~mul~ ~ lo]
3~
HO~3~
2~ . 4~ 3
whereln B3 i~ 21 pyrimidin~3 ba3e re~ldue bo~ le~ a~ th~
l-po~ ion ~h~.reo~E, to the ~ lc-pentane~ ri.n ~, orle C~f
30 ~7 ~nd X~ a hydrogen atom and ~he o~c]le3: i~ a
mq~thy:L gxou~? hA~r~g a~ optionally psot~ d. hydroxyl
. groul?; dr ~ ~al~ th6~r~30~ iY pro~ucecl by th~ method
which i~ ~h4ralc~ex~ize~ by xea~ .ng a aom~ou
rtsp~e~n~ed by ~h~ ~onnul~ [~c]
91~5~ 16~736~;TAI~DA PAT~NTS OSAKA Fether3t~n . 063n06601;#11/72
H H
S ~5 ~ 2 ~ I
wherein X i~ a h~ro~n atom c)r a lo~er alk ~l grQup; Y
ii3 a lower allcoa~y group; on~ o~ R7 and Ra ;L~il a hydrogen
10 a~om anc~ ~he other is ~ methyl g~oup ha~rinsl an
op~ionally p~ote~d hy~x~xyl group~ o~ a ~ hereo;E
with ~I b~se~ ~ollowed by~ w~ell ne~:e~ iubj~ ing
the p~otecl;ecl group $o dep:rot~ç:tion.
Compounds ~ontaining the above-ment1c)n.sd formul,~e
15 C~a], C~b] ~nd ~Z~ or salt~ ~hereo ~re l?r~duc~d by
~he ~ol1owing reaction. M~e sp~ci~icz~
cyclopentes~ylarnin~ rivat~ v~ rep~3s~an~ y th~3
f o~ 2 ]
NH--Z'
H~ J C2 3
,~ ,
R~ .
wh~xe~n.Z~ is; a hydro~3n ~tom or an ac~ r~up, on~ ~ -
~3 and R4 i~ a hydr~en e~om and th~ oth~ a meth~l
~ou~ havinsT a protect~ h5,t~rox~rl group, e~r a ~alt
ther~o~ i8 p~nduced by th6~ m~h~d whl~h i~
30 ch~ract~ri~;~d l~y æu~ t.;~g z~ ~-n~ ic~clo~ 2 . ~ hept-
5-~n~3-one ~ riv~ti~e r~resen~ad hy thç~: i50 ~nula t 3 ~
J ~ J ~
9 _
~ -- --N ~ Z
3~ z
4 ~ C~
S lfi
wh~:raLn ~ n acyl ~roupt one o~ nd R ~nd~ ;eO:~
a hy~ gen ,~om and ~h~ o~her.ls ~ me~yl clroup h~vlng
a pro~a~ed hyrdroxy1 Çiroup ~ ~ a salt ther~o~ ~o
10 red~lc~ 3 rlng cleavAg~3 rea~til~n, .~ollow~cl by, whan
nec~ ry; ~b~as::~ing the reEIction m~ctu;~e to
daacyl~tion .
A~ th~s 8~l1t ~nention~ad abov~ UB~ ma:le 0~ the same salt
a~ ~h~ ~lt o~ compound ~
lS In tha compound~ o~ the ~ el9 E 1~, 11a ~ ~nd
~lbl, ox ~ ~alt th~r~ao:e~ ex~npl~ o~ th0 p~lrine ba~
re~nid~ he~wn by s, Bl or T32 inalu~ g~o~p~
r~p;r~3nt~d 13y ~h~ ~nula [4,]
~ 1 4 ]
N ~ s
.
25 wh~r~1n S~ 3 an amtno ~rQup, halog~n, ~ h~o~l
gr~upf Q 18 ~ h~d~o~e~ o.~om~ A;~ O ~r~ r
h~lo~n. Wh~n Q and Q in th~ ~o~ulA [~ J i~ halogen,
~h~ halogen ~ nalu~ luorine I chl~lne, ~b~ omln~3 a~
lodinQ.
In ~e~ compounds o~ ~he ~ormula~ [ 1 ] ~ nd t ~a
~5 C~ e thQ py~lmid$n~ ba~ r~3 id~ ~hcl ,m by B o~
B3 inc:lude S7roup~ repre~onted by ~hl3 f'O~ltUllL 1 5
~a
~X ' .
,
';
.
...
91~ 6~ lB~36~;TA~EDA PATENTS OSAKA Fether~ton ; OB3006601;#13/7~
- 10 - ' . %~r.~ i,
wherein Q3 i~ a h~droxyl gr~up or an amino çlr~upî X î~
a h~drogen ~tom, a low~r alkyl group or ~ lower ~lke~l
~roup. When X in th~ fo~ula ~ a lower alkyl
g~oup, the l~wsr alkyl group mean~ ~l5 alk~:l group,
S exemplified by methyl, e1;hyl, isopropyl,
cy~lopropylmethyl, cyc~loprop~lr bu~yl, l~ob~ yl, ~ec-
~ut~ te~t-but~l or pem:yl. And, w~e~ ~ a lower
alkenyl, th~ alkenyl group me~ns C2~4 al~en~:l group
opkionally sub~itu~ed with a halogen ~uch l!~S fiUOXi~h,
chlorine, ~romine ~x iodine, a3 ~pec~i~ically
exemplifi~d by v~nyl, ~r~ chloro~in~l, c~
chlorovlnyl, tran~~2-b~ovinyl, cis-2-~ m~;~vin~l, 1-
propenyl, l-~utenyl 0~ ~ethyl-l-prop~n~l..
The ~u~tltuent~ shown by X in the Gom]j~ound~
repres~nted ~y ~he for~u~.a [2c] are ~he ~aml l ~8 tho~Q
~hown by X in th~ comp~und~ xe~ee~ntod b~ l:h~ g~n~ral
$ormula 15~. Further, i~ the qen~ral forrhu:.a r~c3,
exampl~3s o~ the lower all;oxy group ~hown ~y Y include
20 me~hoxy~ ethoxyr pr~poxyr bu~oxy, sec-bu~o~, tert~
}:u~oxy or cl 5 alko~r group~ optlonall~ ~ub~;l;itu~e~ wi~h
a phenyl group ~uah a~ b~nzyl ~xy .
~ e amino group of t:he purin2 base res.ldue o~ ~he
pyr~midi~e ba~e residue of the ~oxmula~ and ~5~ may
~p~ion~lly ~e pro~ected. Exampl~ o~ ~he p;lotective
group Lnclu~e ~ho~ ~or pu~ino-ty~e ba~e o:r ~rimidin~-
type base in the chemi~try o nucleic aaid, mor~
speaifically alk~l-t~pe p~ot~ctive group~ 2lk~1-type
p~otective ~oups, ~llyl-t~p~ protective gri;lup~ an~
3~ ac~l-t~pe protective group~.
~ xampl~ f th~ alky~-typ~ prot~ti~ ~u~
include C~ low~r alkyl group~; ~ri- ub~ ed ~ilyl
groups ~ubJ3~ute~ with e.g. Cl s lower alky]. ~xoup~ or
~enzyl g~oup; and tetrahydropyranyl g~oup. ~vre
~p~ci~lcally~ methyl~ et~ propyl~ is~pr~ yl~ bu~yl~
sec-butyl, ter~but~ 6!ntyl: t~imethyl~ ,l, t.ert- .
91~ 16~6~;TAKEDA PATENTS OSAKA Fether3ton ; 063~0~601j~14~2
butyldime~hylsil~l~ tetr~lhyclx~pyran~l,
t~trahydro~ur~nyl, etcO are mentione~.
Aæ the aralkyl-t~pe pLo~ec~i~e groUpS r use is
advanta~eougly made o~, ~or examplet b~nzyl group o~
5 triphenylmethyl group opt:Lonally subs~itu~e~l with
h~logen, Cl_5 l~wer ~lkoxy groups an~ nitro- ~pe~i~ic
e~amples o~ ~he~ include benzyl, ~iphenylme~lhy~,
triphenyl~e~hyl, p-methw~yphenyldiphenylm~t]lyl, p-
ni~robenzyl, ~nd p-chlorobenzyl~
Exa~ple~ of the allyl-type protective ~;lroups
includra phen~l ~roups such a~ phenyl, p-m~ ox~phenyl
and p-c,hl~xophen~l9 ~ptic~nally ~u~stitu~ iri~h ~logen
or Cl5 lower alkoxy g~uE~
As a Schiff base-~ype prot~ctive grou;p, ~se i~
1~ made o~, fo~ ~xample, di~eth~lamino group~ ,!lUGh as
methylidene, ~,N-di.me~hyla~i~ome~h~liden~ nzylidene~
e~hylidene, pxopylidene~ b~tyll~e.n~ an~ nl;yll~e~
~nd Cl5 lowo~ ~lkylidene groups optlo~all~ l!ubstituted
with ph~n~l group.
~0 A~ the acyl-typ~ p~otective group6, t.~ se in the
prote~ive gr~up~ ~o~ hyclroxyl group of the
hydro~ym~thyl gr~p to b~ d~3saribed her~ina l't~r ~an he
~imilarly employed.
And, the hydroxyl gr~up on the ~urine ~i~a~e ~e~idu~
or pyrimirline ~ase re~idue shc~wn.b~ the ~on;lula~ ~4]
and C5] may alj~ vpti~ lr ~e protected. .IL~ th~
protective grou~ Q~ hydro~l grou~, u~e is ~imil~rly
ma~ o~ ether-~ype or acyl~type prote~tiv~ roup~ for
hydroxyl group o~ hydro~ym~thyl ~roup to he de cribed
hereinafter.
. In ~h~ ~oxmula~ [l]~ ~l~J~ llb~ [l~], t~ aJr
[2b;1, E2c~ ancl ~3Jr when the hyàrv~yl gro~p at the
methyl group, havin~ an optionall~ pxote~t~d hy~ro~yl
yroup, ~hown b~ Rl, R2~ R3, R4 R5 ~6 R7 R~ ~3~ R4
~5 kS ~ R6 / R7 ~r R6 i~ p;~vte~ct~d~ exQmple~ w~ the~
pr~te~tive gr~up o~ the hydroxyl ~roup incl~,lde
91~ 5~ 16~36~,TA~EDA PATENTS OSAKA Fethsrst~n . 063006601;#15/72
~ ``J.
- 12 -
prot~ctive groups u~ed a~ those the hydroxy:l. g~oup in
the ~i~ald c~f su~ar an~ n-lcleic ac;Ld che~misC:Iy~ ~ah as
et~er-typQ protective glrs)ups an~ acyl-type v ne~;, when
r~ece$~ary, aaet~l-t3!pe ones, ketal-~ypo onel: and
5 ~rtheester-type one~.
Examples of ether-t~Fpe prot~ti~r~ groul.~6 inalude
Cl 5 lower alkyl g~oup~ op~ionally 3iub~ u~ed with
halogen, C~.5 lower al~c~xy gxollp, ~enzyloxy t'roup or
phenyl grou~; C2 4 lower a.lkenyl group~; tX:i~ stib8tituted
10 ~ilyl groups he.ving, as t:he ~ub~ti~u~nt~, Cl 5 lower
alkyl ~roups, phenyl group, ~e~yl group, el;ç.; ~enzyl
S~roup optionally s~l~stitu~ed with Cl 5 lowex a1lco~y
gro~ps or nitro gxoup; Cl 5 lower alkox~ grot~p~;; and
tetrahydropyranyl group optionally ~ub~titul;ed wit~.t
15 haloyen,
~ xampl~ o ~he abov~-mentioned halo~ell~ includ~3
~luori3rlet ~hlorine, bromLne and iodine, examples o~ the
Cl~9 alkyl groups include m~thyl, ~h~l, prv~
iso~xopyl, butyl, isobukyl. se~-but~l, tert butyl,
pentyl~ i~opentyl and ne~pent~ nd exampl~5 of the
Cl5 alkoxy ~roup~ include methoxy optional~
substituted wi~h halo~enr ethoxy, propoxy, ;l~utoxy,
vinylo~ ~nd allyloxy. ~d, in th~ prote~..ve gxoup~
of the abovo-~en~ioned amino group, a~ 5 ~! lkyl ~roup,
C~s al~ox~ group and halogen, ~se i~ ma~e o~! ~ho~e
e~emplified a~ ether-type pro~ective group~ of the
hydroxyl g~oup~
Mor~ spe~ific example~ o~ ~he e~he~ e
protec~ive groups of hydxoxyl group inc~u~e m~hyl,
m~thoxym~thyl, benzyloxy~ethyl/ tert-~utox~lethyl, ~-
methoxyetho~methyl, 2 r 2,~~t~chlorometho~ ethyl, 1-
eth~xyethyl, l-m~thyl-l~m~th~xye~hyl, ~,2,2
txichloroethyl, propyl, i.sop~opyl, butyl, i.! ~o~utyl,
s~c-butyl, ~e~t~butyl, e1:ho~ethyl, ~riphe!nylm~hyl, p-
~etho~yph~nyldiphenylmethyli ~rim~thyl~ilyl te~t-
bu~yl~l~ethyl~ er~-bu~yldlphenyl 811~ ,3,3
91~ 16~5~;TAI~EDA PATENTS OSA~A Fether3t~n ; 083006~01;#16/~2
7, ~ i.; V
- 13 ~
tetraisopxopyl-l, 3-di~iloxane-dl-yl; ~en2~.1, p-
m~hoxy}~enzyl, p-nit~oben2yl, p~chlorobenz y;l;
tetrah~opyra~yl, 4-me~h4x~etr2lh~clLopyr~x~; l,
te~trahydrofu~nyl, e~c~
Examplea o~ the acyl-t~pe prote~tive g:l oups
in~lude CL 5 alkanoyl grou.~ option~ sub~J.~uted with
halo~n, Cl 5 lower alkoxy ~Txoup or pheno~y cl roup
op~ionally ha~ring haloçlen; ben20~1 group Op1l.ionally
sub~titu~ed. with nitro gXGup, phenyl ~roupt Cl 5 low2r
10 alk~l group optionally .;ubstituted with halogen
benzoyl group optionally sub~ituted wi~h C ! .6 lower
alkyloa~y~*.xbonyl group7 Ca 6 alkylo~ycarl~on~]; s~oup
optionally Ru~stituted ~,41th halo~en; C3 5
alken~,rloxycar~onyl grou~, ~en~yloxycarho~yl 6~rouP
15 optionally suk~stituted with Cl_5 lowex alkoxy group O~
nitro group; and phenoxyc:arbonyl g~oup su~sl.i~u~ed with
nitro grou~.
As the abov~s-me~ntio~led h~logen~ Cl 5 1~1er alkyl
gx:oup~, C~ 5 low~r alkoxy groups and C2~ alkenyl g~PUl;~8,
use i~ mad~ o~ thos~ ~xeMpllfl~d :Ln ~he cas/i~ o~ t~e
ether-type protective gr~up~.
More ~pecific example~ ~ the acyl-typ~:~ p~o~tive
gr~up i~cl~o ~o~myl, acetyl, ~hloxoacet~l,
dichloroacet~l, trichlorvac~tyl, t~i~luoroa~:~ yl,
m~thoxyace~yl, kri~heny].m~thoxyacetyl/ phe.nl:~xyac~yl,
p-chloropheno~yacetyl, pr.opionyl, i~opro~iol~yl, 3~
ph~n~lpropionyl, i~obutyl~l, pivaloyl; ben.z~;~yl, p-
nitrobenzoyl, o-(methoxyc~r~on~l)benzoyl;
methox~carbonyl, ethoxyc~rbonyl, t~rt-bu~o~ carbonyl,
~t4,~-~ri~hloroethoxYc~rb~n~ on~utylylo:!;yc~xbonyl;
~enzyloxy~arbon~l, p-met}lvxybsnzyloxycarbon~;yl r 3 t 4
dimethoxybenzylo~yc~rh~nyl~ p-nitro~en ylo;K~ rb~nyl~
p-nitrophono$~car~onyl, etc.
~nd, ~ ~a~tal-~ype, ke~al-type and o;rl;ho~ter-
type px~tectiv~ groupsr u~e i~ advantag~ou.~.:.y ma~e oftho~ h~ y 1 t~ 10 cQrbon ~tom~ whi~h ~r.!~
6~ 36~;TA~EDA P~TENTS OSAKA Fetherston ; 063006601;#17J72
r r ~ ~, .3
~ 14 -
speci~ically ex~?mpli~ie~ by m~thylen~e, l-~:elrt-
butylethyliden~ phenyl~3~hylid~n~, 2, 2, ~
~i chloroeth~rlidene; i sopropylidene "3uty.~i. den~,
c~yol~h~p~yli~n~ oynloh~ylid~n~, cyclbp~rltyll~ene, p~
5 methoxybenzylide~e, 2, 4-dilne~ho~:ybellzy~ c~rle, 3, 5-
~imeth~yb~n~lidene, p-dim~thyl~minobenzylid~ne t -
nit~ob~nzylid6ar~e; metho~ymeth~len~3, etho:~yn etl~ylene,
din~3thox~n6~thyl~3ne ~ 1 -methoxyethyliden~
dime~hoxy~ thylidene, e~ .
St~nnoxane-type prote?c~iv6! groups ~u~l a~
dibutyls~annyl or tributyls~annyl, cyclic c a~b~nate
type pr~tec~i~re groups and c~ cllc: ~orona~e type
prot~ativ~ grou~ c~n al~o ~ ~imilarly u~eld ~ep~nding
on kindx of those c: ompound~ .
lS In these prot~act~ ve groups o~ the h~l~ oxyl gr'oup,
~he kin~ls ~ protecti~ groups oi~ ~wo hyd.rc xyl groups
~nay be the ~ne or ~iff~ront. A~l~, like ~ tha ~:A3e
~uc:h p;rote!c!tive gr~o~lp~ a~ , 3 r 3-~etraiso~ ~pyl-l, 3-
~isilo~an~?di~rl, cyc~ic ace~ ype ~n~ cyc3.i::: ketal-
2 O 'c~?e, two hy~roxyl groups may be p~o~ec:tecl with on~
pro~ec:~ve gr~upA
Xn thq~ c~npound of th4 ~ons~ula ~ ydroxyl
g~oups of ~he hydroxymethyl ~roll~? shown l: y Rl a~d Fl~ o~
R may b~ phosph~d. A.s ~he p~ospha~e r~ idue in ~h~t
25 ~ase, mention is made /~f ~L group r~presenl:~ld by the
oxmula ~ ~1
~ R
30 . tll ~
wher~ n denote~; an in~ge~ o~ 1 to 3, R s~nd~ ~or a
hydrogen atom ~r a 1-14 C: hyclro~a~cbon ~e~; ;.cl ue, or a
gI~oup ~o~n~ b~ combination o~ hydroxyl g rc, up~ a~
3S hyd~o~sy;nethyl gro~p~ of ~ and E~ or ~, whic!h i~
represen~e~ by ~he :~ormula C6
9.
91~11,9 6~ lB~36~;TAKE~A PATENTS OSAKA Feth~r~ton , 063006601;#18/72
.3 ~
- 15 -
l '
~ R 7 ~ ~3
wherein ~7 is o~ the ~ame meanin~ as d~iEin~!~ above~
5 ~x~nple~ ~f h~droca:~on residuee sl~.ow~ by ~ 7 include
C 1-5 lo~qer alkyl group~ ~uch a~3 methyl, el~h.~ L, propy~l,
isopropyl, ~utyl, iso~u~yl, ~cn~u~yl, te:rt:-but~rl or
pentyl, ar~lk~l groups ~uch as 13~n~1, p-m6l thox~rbenzyl,
p-m~3~h~1ben~yl, etc. "phenyl ~roup, ~ ~.
~s ~he acyL grollp ~hown by Z in the ~clmpound
r~pr~sen~3d b~r the f ormula [ 3 ~, and , as t~ acyl ~roup
~n .the ca~e wher~ Z' in ~he c~mp~un~ x~pr~;ont~d b~
general fOI~nUla ~ cyl yroup, uæe ls ~ e of 2-6C
alkyloxycarb~nyl group~ or caxhaunoyl s~roup ~tlonally
15 substi~uted with a 2-~C alkellvyl ~.~oup op-t;i.~nally
~u~tituted wi~h a Cl 5 low~r alkoxy group..
9pec:if ic: ea;~mple~ o~ ~h~ c~cyl grou~ 3,u~
methc~xycar~vnyl, ethoxyca~on~l, t~art-bu~a: yc~ onyl S
~arbamoyl, N- ( 3-methoxyacryloyl ) c~xba~no~l, N ( 3-
20 m~3~hoxy-methylacryloyl)ca~bamo~l, N-~3 -~IthCI~y- 2-
vinylac~ylvy~ ) ca~a~o~rl, ra- ~ 3-me~hoxy- Z-
~o~l~yl)a~I~ylo~Jcaxbamoyl, ~t~.
In ~he com~o~nd ~eprf~sent~d by thg~ fc~I ~ula ~ 2~ l ~example~s of ~he lower ~lkoxy group ~how~ k~ include
~S ~1-5 alkoxy ~oup~ optionall~ su~sti~uted ~ h phonyl
yroup, such as m~3~hoxy, ethoxy, propc~xyr ~ t~r
butoxy, ~ec-butoxy or benzylox~ ~oU~, O~
Sp6aci ~i~ examples o~ th~s comp~un~ ~el;~r_sen~ec~ by
the ~ormula [ 1 ] ~e ~ ~0710~8
. ( 1 ) ( ~ ~ -9- [ 4~S, S~L-d.i ( hydroæym~ yclope~t; ~2~3n~
ade~s~in~
( 2 ) ( ~ - 9 - t 4~, S~-d.i ( hydroxyme~h~l ) cyclopent - ~-e;~ yl ]
a~e~ e
~3) ()~ s~-ben~:yloxym~hyl-4~ hydroxy3llelthyl3
cyclop~nt-2~ yl~G~enin~
( 4 ~ ( + ~ t S~;-ben2~10xym ~ 4~- (.hydroxy~ thyl 3
.. . . .
91~ 5~ 16~36~;TA~EDA PATENTS OSAI~A Feth~rst~n ; 06300~601;#19/~2
's~ $
~clopent~en~ yl]aden.ine
(5) (~)-9-[(lS,4R,5S)-5~ben~10xy~ethyl~4-
(hyd~ex~ethyl~cyclopent-2-en~ l]~deni~e
(6) ~ [(lS,4R,5~)-5-~enzylc~yme~hyl-4-
~hy~o~methyl)cyclopent-~-en~1-yl~3denine
(7) (~)-9-~(lS,4~,5S)-4/5-di(hydrox~methyl)cy~lopent-2~
en~ l]adenine
(8) (~ C(1R,4S.5R)-~,5-di(hy~oxym~thyl)tyclopen~
e~ L~adenine
(9) (~)-9-[4~,$~-di(hydrclxy~ethyl~cy~lopa~t-2-en-1~-
yl~adenin~
tlO) ~~)-9~~4,~f5,1~-di(h~dr~xyme~thyl~c:ycls:~pen1;~-2-en l,B-
yl ] adenillQ
t'll) (~)-9-~5,1~enzyloxymethyl~4,13-
(h~droxyme~hyl)~yclopen~-2-~n~ yl]~uanin~
(12) (~)-9-[~-benæy10xymeth~
thydro~ym~hy1)aya1Op~nt~2-on-1~-y1]g~ninl~
(13) (~ 4~,5a-di~hydroxymeth~ yaL~penl~ -en-1~-
y1]guanine
(14) (i)-9~ 5~-di(hydrox~methyl)cyclope~ 2-~n
y1]~uan1ne
( 15 ) ( + ) -~1 4B,5~-di(hydroxy~ethyl)c~clopenl: 2-en~
y1~uanine
9-[4~5~-di(~ydI~o~me~hyl~c~ pe~l-2-e~
~5 ~1~guanine
~17~ 9-t4~5~-di(h~dro~ym~thyl~y~
y1~uanine
(10~ 9-[~,5~-di(hydroxymethy1)~ya10p~nl,.-~-en-1~-
ylJ~uanine
~19~ 9-[5a-benzy10xyn~eth~1-4~-
(hydroxymethy1)cyc10p~nt-2-en-1~ yll-6-ch10:lopurine
(20) (~)-g-[5~-benzy10xy~ethy1-4~-
(hyd~ox~me~hyl)cyclopa~t~2-en-l,B-yl3-6-chlo:lop~rine
t 21 ~ ( + ) -1- [ 5c~-benzy10~cy~ethy1_4
3 5 ( hyclro~m~thyl ) ayo l~en~ n- 1 ,B-yl ~ - 5 -meth~
Z, 4 1 lH, 3~J ~py~imidinedione
91~ 3B~;TAK~DA PATENTS OSAKA Feth~r~ton ; 0~300B601;#20/~2
~ 17 --
(22) ~ r 4p,5~-di(h~droxymethyl)cyclopent: ~-en~
yl~ meth~1-2,4~1H,3~3-py~imi~lnedion~
~23~ ~+)~ 4~,$~-di(hy~roxymethyl~yclope~ en~
yll-5-me~hyl-2,4[lH,3H~ pyrimidinedione
(24) (~ 40,5a-di(hyd~ox~methyl~yclopen~2-e
ylj-5-me~hyl-~,4[1H,3HJ-~y~imidinedio~e
t25) t~ 4~ 5c~di(h~d~ox~tlethyl)cyc:lopellt~ n~
yl]-2,4[1H,3H]-pyrimidine.~ione
~26) ~+)~ 4~,$a~dl(hydrox~methyl)cy~10p~ 2-enwl~-
yl]-2,4~1H,3H]-pyximidine~ione
~2~ 4~,5~-di~hydroxyme~h~l)cycl~pen1~ en-1~-
yl ~ 4 1 lH ~ 3H ] -py~i~nidln~1~ion~
t28) (i)-4-amino-l-~4~5~ i(h~ roxymethyl)(;yclopent
en l~yl~-[lH~-pyrimi~in~!
1~ (2~) (+)-4~am~ [4~,5~ i(hy~rox~me~hyl)cy~lopent-2-
~n~l~-y~ ]-pyrimidine
~30) (-)-4-~mino~ 4~,5~,-dl(hydroxym~hyl~yclop~nt
-yl]-2[lHI-pyrimi~i~e
D~scr~ption i~ ~iven a~ follows ~ to 1he metho~
~ p~odu~in~ the ~ompound ~presellt~d by tl~. formula
~1] or a ~alt th~rao~.
At ir~t, a co~pound of ~he formula [~ n which
~ i~ a purine ba~e ~es~du2, i.e. a co~pound r0prese~ted
by the ~xmula ~la~, or ~ salt thereof can ~:e
~ynthes~zed by, for exampl~, ~h~ ~ollowin~ nethod
~arting f~ a aompound o~ th~ ~nmula r 2~
Namel~ ~ compound rep~e~ented ~y ~h~ loxmula [7a]
91~ 6~ 16~36~;TAr~EDA PATzNTS OSAE~A Fether~ton i 06~00660~ 2
Qi
H2N~N
NH
HO~ [7a]
10 whe~ein R3 ~n~ R4 are of ~he ~ame maani~g aL men~ione~
~bo~; and Q4 i~ halog~n ~uch as ~luori~ r ~, hlorin~,
~7romine or iodine, can ha p~od~ce~ by :roac:t.inS~ a
c~clopen~anylamina d~rivativ~ r~pres~n~e~ hy ~-h~
~ormula ~ 2a ~ with S~amino-4, 6-dihalo~e~o~y~ idine ~uch
15 as 5-amino-4~6-diahlorop~yrimidin~, 5-aminc~-~,6-
dibr~omo~yr~mi;line, 5-ami~.lo-4, 6-diiodopyximllline ox ~-
~ml~o-4-fluo~v~6-chlo~op~rimldine in th~ p~ enc~ ~ u
ba~e.
5~Amino-4,6-dih~logenop~rimidin~ ~o be reac~ed
~0 with a cy~lop~ntenyl~min~ d~rivativ~ ~epr~ nted by the
fo~mula ~2a~ i~ used a~ uivalen~ or mor~, u~uall~
l o S ko 5 ~3~ui~ valq~lnt~ ~ ~elati~ro ~o [ 2a ] .. A~3 th~ ba~ to
be employed for tho reac~ion~ m~ntlon i~ mall~ o~ an
~r~anic base ~uch as py~;idine, t~ie~hy~a~.nl3, ~,N-
25 dimethylaminopyridin~, 1,8-diazabi~yclo~5~4,0]-7
und~cene~ etc. and ~n lnorganlc ~a~ ~uch a,l so~ium
hy~xide, po~as~ium hy~oxl~e~ so~ium ~ar.bl~n~
po~a~lum car~on~te, sod:ium hydrogen~ar~o~ e, etc.,
and, pr~f~r~bly, an organLc bas~ su¢h ~ kr.!ethyl~mine
30 ~nd N,N-dimeth~l~minop~ridine
~he rea~tic~ aondua~cl ~leuall~ in a ;c:llv~n~,
and examples of pr~ra~;Le ~olv~t~ inclucl~ water,
alcohol~ (e.g. methanol, ethanol, propanol, butanol,
etc.), acetonitrile, N,N--dime~hyl~o~mamid~
3S dim~thyl~sul~c~ide, ~n~ ethe~ .g~ dic~x~rle,
~etrah~d~ofuran, ~tc.~. While the ~eaction tQmp~ra~r~
91~ 5~ 16~36~jTAKE~A PATENTS OSAKA Fetherston ; 063006601;#22/72
-- i9 --
varies wikh the kinds o~ compound~ repre~e!:nl ecl by the
:formul~L [ 2a], 5-amino-4, 6-dihalo~eIIopyximi.~ .ne and
bas~s employed fo;~ th~ r~!action, i~ u~u~lly ~ange~ ~rom
rooln temper~ture ~o about: 200C. ~hen it il~ nec~ary
5 t~ Con~ua~ the reac~ion e~t tempe~atu~es hi.~ r than ~he
boiling point of the sc~lvent, th~ r~aac~ion :l~y be
condu~t~d in a sieaLed ~ube. Whil~ ~h~ reacl:ion ~ime
~lso v~e9 with the kinds oi~ reagent~ empl~;~yed OI`
~e~c~ion temp~rature6, i1: ran~es ~rom ~OUIt 1 hour to
l ~ about 4 days .
~ hen/ a c~clopentenyl~?urine derivatlv~ apresented
by th~ ~ormula [ 8a ] ~2 ~
~N$
H ~ a~
~0 R''
wh~;~ein R3, R4 and Qq ~r~ o the ~nme m~nin~ :1 no~
~bc:ve, can bc2 produ~ecl ~y ;~e~ct.~n~ ~ comp~u~
represented by the ~onnula ~7a] wi~h c~::tho~unni~ es~e~
su~h as met~l or~ho~ormate, ethyl ortho;~or.~la~e ur
~5 butyl or~ho~o:~mate in ~h6~ pr~:sen~e c~f an ac.:.d.
While orth~or~i~ e;~ i~ u~ed ~ n la n)l~ of or e
e~ulvalellt ar more relat; ve to ~he com~ound represent~d
by the ~o~mula ~7a~ is usl~ally u~e~ in ,!l large
exce~ volume ~o be al00 used ag ~he reacti~ )n solven~.
3~ Examples o~ ~he acid to ~e em};)loyed ~or the reaction
in~:lud~ in~rg~nic: ac~icls2 s~u::h a~ pho~phc:ric! ,!Lc~d~
hydroc:hlc~ric acid, hyd~obromic ac:id zlnd ~ u~ic acid,
or organic sul~o~i~ a~id~ ~uch as benzen~u:.fonic ~cid,
p-tc: luene~ul~oni.c acid, methanesul~oni~ a~ l and
35 txi~luoxom~3t;han~ul~0nic aci~. Th~ id~ ar~ used
u~u~lly in an amount of 0.01 to 0.2 e~ui~ nt rel~
~.
9~ 6~ 16~36~;TAKEDA PATENTS OSAKA Fether3t~n ; 063006601;#23/72
20 -- ,
~o the compound represellt~d by ~he formula 1~ ia~
the ~ea~tlon ~lve!nt, th~ ortho~ t~ ?loy~d
for ~he r~3as~tlon is used al~o as the æol~nt" ~ut, when
necessa~y, by taking the ~:olu~ y of ~h~s c~:mpoux~d
5 repr63 sented by the ~or~ la. [ 7a ~ ~ n orthv;fc~rm i o ~e~
into con~ideration, a solv~nt such as al~oho:l s e . g .
meth~nol ox ethanol, eth~ . ç~ at.r~ahydro:El; x~n,
dioxane ~ or ethy le~her, and ~, N-dimethylf o:~lni! mide ~ The
reacti~n tempe~ature rang~ fr~m 0C ~o lOO~t:, usually
10 from 10C to 50C~ While the reaction tin~e ~; arie~ with
~he kln~s o~ r~agent~ u5~d and ~ tion te~CIX~tu~
th~ emplo~,red, i~ usually ran~e~ xom a};~out ~: ne hvur to
about 24 hour~.
Fur~he~, a cyclopentenyla~enine de~ivat:i.v~
15 repr~sent~ by ~h~ formula ~la~ can b~ p~od~ e~ ~y
allowing ~ çc)mpound shown by ~ ko r~ac~ w:l.th ammoni~
to th~re;l;~y subs~ituking tha halo~ell in ~he pl..rine
pnrtivn of ~a~ with an a~ino group, foll~ec. by, when
nece~ary, removing the pr~ot6~cti~a ~roup o~ ~.he
2 0 hydroxyl group in tht~ methyl g~c7~p h~ing ~ ]"rote~3ted
hydroxyl group ~ho~n by R3 ox ~4.
The reac~ion ~or ~u10~ ut~ns the halog~i~n on the
purine ri~ç~ is conducted }:~y allowing ~. lax~e exce
volum~ a~nonia ~o r~ct; u~u~ r in a pol~;l sol~ren~
~5 includin~ watRr ~ncl ~ c7hol~ ~3u~:h tA~ meth~no::. ar~d
qthanol. The reac:tion ~empexature rang~3~ u.s~ lly from
room temperature to l~O~Ct and ~he reaction ;i.
preferably ~on~ucted in a ~e~led tube ~or avclidin~
e~r~poration of ~mrnollla. ~hil~ ~he ra~ction I,ime tr~rie~
30 with th~3 ~eaa~lon t~3mperat;u~ usually ~.arlg~e! from. 5
hou~ tc> ~bout 50 hours.
A compound o~ t~ fo3~nula [lJ, ~her~in ~
purine base ~e~id~ae, i . e . a ao~pound ~hown ~ the
purine ~ormula ~ lh~, can b~ synthe~lzed ~y, ;I o~
35 example, the ~ollowing proce~æ, ~arting flo~l a
compound ~hown b~ the ~ormu~a ~ 2b 1 or a ~al t t:hereo~ .
gl~ 5~ 16~36~;TAKEDA PATENTS OSAKA Fetherq~on ; 063006601;#24/72
- 21 -
~ amel~, by allowing a ~ycl~pentenylam.il.e
cl~3rl~rative xepre~;en~ed })y the fox~ 2bJ ~r a sal~
tllereo~ tc~ ~ea~t wi~h a ~-amino-4 ~ ~-
dih~lG~nop~imld~ ~ 6~ah a~ 2-~m~ 4
dichloropyrimicline, 2-~ni no-4, ~-dibro~nopyri~ lidin~
amino-4 ~ 6-dii~dc~pyrimidirLe or 2-am3.n,o~~1 uoro-~-
chloropyrimidine in th~ pre~;nC~ of ~i ba~, a com~?ound
~ obtained represented b~ the ~ormula ~ 7b~
~N
N~I~N~a
H 0-~ [ 7 bl
whe::~in R5 and R5 ar~ of th~ same meaning clæ defin0d
a3~ove; an~ ~4 i~3 halogen su~ a~ l luo:rin~ c,hloI~ine,
~0 bromine and iodine . The r~action of ~he cox Ipound of
the formula [2a] or ~ ~}~ ~h~i3r~0f wi~ ino-4,6-
~ h~alog~nt~py~ mldines ~ ~ 8~1t th~ eol~ 1~ aonductad
under c:ondi~ions simil~ to tho~ 3 th~ z~f~:~re-
described reac~ion between t~ c~mpoun~ he f ormula
2~ ~ 2a ~ or ~ salt thereof and S-amino-4, 6-
~ih~loQIe~ ?py~imicline or ~1 ~al~ t~rec)~.
~hon~ a~t~r aunin~it~n o~ ~ho 5 poei~ on the
pyr~midine ring, orthofo~.~nic est~3r ~uqh a~ hyl
orthof o:l:mate~, e~h~l or~ho~ormat~ or butyl o . thoo~mate
30 is rea~t6a~ in kha pr~nce o~ an a~;ld to p~.)du~ a
a~alopenten~l~uri~e d~riva~ive re~r~nt~d :l~y tl~
~ormula ~ ~b ]
91i~11,q 5~ l6~q36s~;TAK~A PATENTS OSAKA Feth~r~tcn ; 063006601;#~.5/7~
2 ~
.
~N
~ Nl~J H 2
H0
R~
10 whe~ein R5/ R6 and Q are ~;f the same meaning a~ d~in~d
abo~a, or a sal~ thareo~
The ml n~tlon o the 5-po~L~ion on l--h~ ml~lne
rinS3 i~ conduct~d by allowing a coml?ound r~)r~sent~cl by
th~ ~onnula ~ 71~ ] or a sa~ hereof to reac ~ wi~h a
lS ~iazon~ wQ sal~ such as b~nzene dia20nium ch;:.o:ride or p-
chloxobenzen~ d~azoniuln chlorlde to th~ e~ caus~
RZOt~ZQtion O:e the 5-po~itios~ on the pyrim~i~;,i~e~ xLng,
followed by a }2~ s~a kno~m p~ocesl3, i.~3. hy~;.ro~enolysig
b~ ~he use o~ e.~. zinc-cl~et1c ac:id~ ~he ~!,action o~
~0 all~win~ i~. pur~.ne ~keletc)n to be ;Eorm~d by ,.he reac~ion
o~ ortho~or~lc ester ls :~ndu~te~ un~e~ con~.ltion~
61mlla~ to ~hoso ~or th~ r~a~tion bQtw~aæn tl.E~ o~cl
oi~ the ~o~mula [8a] or a salt ther~o~ and ~:lthofo~
acicl descri~ed above.
~5 Further, a cyclop~ntenylguanlne de~ 3 3hown
by t~ nulA rlb~ or R ~al~ ~hor~o~ prodused
l~y ~ ~er se known proce3~ which s:ompr~es a;l lowing a
compoun~ represent~d by t:he ~o;rmula ~b3 or a 5~l~
~hexeof ~o re~ wl~h ~ b~se ~u~h a~ sodiwn h~xoxide
30 or pot~ss:Lum ~droxide ~o th~r~by convç~ he
h~ gen on the puxlne x~in~ t~ hyflroxyl ~r~
A compound r~pre~ent,~d by ~he ~ormula 1 1~ whe~oin
B i~ a py~ ln~ ba~o r~!~idue, i.e., th~ coIIpound ~hown
~y ~he f ormula ~ 1c 1, or ~ ~alt ~hereoi~ can 1; 13
3S ~ynth~ by, for e,x~ 3, ~114w~ng a c~Q;n}, ~llnd ~:hao~
~ th~ general ~ormula [ ~c ] or a s~ he~c ~ to r~ct
... .
.(~,.,
91~ 6~ 16~3~;TA~E~A PATENTS OSAI~A Fetn~rston i C63006601;~87
~ 23 -
with a ~a~e, followed by, wklen neces~ary, r elnoving khe
protective grc~up o~ the h~ydroxyl g:roup ~ron;~ ;he me~hyl
~rouE;~ ha~ring prote~d h~roxyl Ç1~0Up ~hown :~y ~7 or
R~. B~so~ to be used for ~he ~ea~tion incluc.e
5 inorg~nic l~a~e~ suc:h as almnoni~, ~odi~n h~rax~d~,
pota~ium hydro~i~e, ~odium carhonate, po~e~ ~ium
~a~bonate alldl so~ium hydro~en~arbon~te, ~ncl ,~ganlc
basc~ ch ~8 pyridine and t~iekh~lamin~, p~ ~f era~ly
~e~k l:~a~e~ . u~h aæ ammoni~ and pyr~dine . Th,3 ~eactian
l5 u~ually ~onduc~ed in a sol~ren~, e, ~. wa1:e ~, alcohols
such a~ m~athanol, e~anol r propanol or but~nal, and
dimethy~rormamlde, c~ a mlx~ure Or ~ees~3 pola~ .
~olvenl;~. While the reaction tempe~ture va :ie~ with
th~ kind~ of ba~e~ then used, it usually ran J~ rom
15 ~oom temperAtur~ ~o l50~C. Wh~n ammonium i8 employsd
a~ the base~ preferable to con~uC~ th.e r~action
in A :gseelled tu~e) to pre~ent eYapo;~a~ion o~ a:nsnos~ a .
While the reac:tic:~ tilna ~r~rie~ wit.h the kind 3 O:e base6
and reaction temp~3ra~ure then employed, it x ~nge~
20 usually ~rom S hour~ to about S0 hour~.
Compound~ repr~senteCI by th~ ~ormula 121t
~naluding khos;o ~hown ~y :~orm~la~ [ 2a ~ ] and ~ 2c ]
o~ ~alts thereo~, are iln~o~tant intermediat;e ~ or
~thes~zins~ a cc~mpound repre~en~d ~y ~he for~ula
~5 ~l~or a ~lt ther~o~, and these co3cnpound~ ca:~ be
~y~t~e~aed by~ ~or ~x~mple~ a m~thod d~ ~d bolow.
~aniely, a cyclo~ente?nYlamine der~ v~tl~re ~hv~ l by the
~ormula ~2a~ or C2b] or ~ sal~ ~hexeo~ b~r ~3u:~je~tin~ a
compound shown b~ ~he ~ormula ~ 3 J ~x a ~alt ;here~f t~
3~ reduc~ rlng-openlng reaction, ~ollowe~l L~y
deac~ylati~n . ~h~ ~ ~educ:t.iv~ ring-op0ning ~e I ::kicln ca
be conciuated by using a metal boroh~drida ~3u~h a~
~ hium borohydridç, goclium ~orohy~ridç ~ p~ta~
borohydride, ~ Inetal cyanobors~hydride ~ oh a 3 sodiun~
~yano~orohydrLd~ o~ po~a~ium ~yano~orohy~a:i,i~, ~r a
metal trialkyl borohydrlde ~u~h a~ hium t~ieth~l
91~36~;TAI~EDA PATENTS OSAKA F~therst~n ; Q63006a01;#27~72
J (~
-- 24 --
.
bc~rohydride, pre~erably ~;odiu~ borohydri~. As th~
re~tion ~olvent, u~;e is mad~ o~ a polar so ~ nt ~u~h
a~ water, n~e~hanol, ethanol, propanol, N,N-
dim~thyl~ormamide or dimethylsul~ox.~ d~, o~ .!. mlxture
5 thereof, and alcc~hol~ suc:h a~ ml3~hanol , e~:hanol o~
p~opa~ xe ~v~tageou~ly employe~l. Whil~ ~ the
xe~c:tioi~ is ~o~ductecl ~ually at room temp~ ature~ it
m~y k~ ~on~uc~ed, depending on ca~es, und~;r cool ~n~
to -~0C or un~er heating up to the re~lux; I:em~era~ure
10 o~ the solvent. Nhile the r~action tl~ne va:l ies wit;h
the kinds o~ reduciny agen~s and ~e~C~ion k( ~pe~a~ur~,
it :canges ~m seve~ l mi.nute~i to ~bout 10 ~ ur~
Reactlon iEor removLng ~he ~cyl g~ollp i~ co:nc ucte~ by a
E2~E se known proces5, foI~ exampl~, allowin~ a ba~e such
15 as triethylamiI~e, sodium hydroxicl~ o~a~ n h~clroxide
oX ~odium me~hoxide ~o xe~ct for ~t~aining !he ~ur~o~e.
A ~o~npoL~Ild ~IOw~ l~y the~ form~lla t ~!a ] ,~li.n b~
producod by a E~ s knot~m method ~rom a co~llpound shoT,m
b~ the fo~mula ~ ~a] or a ~alt th~reo~ . Fo:r example, i~
20 c~n be p~c)d~c~ r allowi.ng a compound shor~. by ~he
~or~nula ~ ~a ~ to react wlt;h a compound 3~epr~ nted by
the ~orm~la ~ 9 ~
X
~ ~ C = i~ [ ! ~
whereln X and ~ re o~ ~he same meaning as s: .ef ined
abo~
30 .And~ a comlp~und shoT~n by the ~ mula ~:ic~ hl~O
b~ ~roduced by allowin~ a com~vlmd re~resen~ e~ b~ ~h~
fo~mul~ ~ 10
91~F11,9 5~ lB;~3a~,TA~EDA P~T~MTS OSAI~A Fether~tdn ; Q~300~601;l~2~/7
r ;~
. ~ ~5 --
~-- --NH
4~1 [10]
R~
~herein R7 anci R~ are o~ t:h~3 3ams meanln~ a~ de:~in~d
above ~o rE~ t wlt~ a cs~lpuun~ ~how~ by ~he 3 ormula ~ 9
to cause N-~cyla~ion, then by subiec!tl~g ~ r~ul~ant
~o r~ductive ring-openin4 r~c~ion. ~his rç!ductive
ring-opening reac~ion c~n be cvn~uated un~33 the
conditions de~cribed a~o~e ~or at~.ining th~! ob~ect.
In th~ me~ch~ ro~lp h~ g e~n opt~on~
pro~e~tive hyclroxyl ~roup, shown by Rl or R~, o~
çompound represen~ed by the ~ormula ~1 ] or cl ~alt
lS thereoP, when ~he hyd:co~yl ~rou;? i~ prc~ cl:~!d,
elimination of the prote~tive yroup can ~e c o~ducted b~
a ~pa~ B4;1 knowr~ l?roc~6~. Pro~c~a~lv~ g~oupl3 ~;hich ar~ ~
elimin ble wi~h an a~id, for exa~npl~, t,rityl ~roup, p-
me~hoxytri~yl group, t~r~. bukyl s~roup 0
~0 tetrahy~rop~anyl group, ca~ be xomQved by h~rol}r
wi th an ~ . o . g . hydrochlo~ c;;L~, ac:e~ s~:id,
~ri~luo~oacetic acid, ~-~olu~3ne~u~0nic acicl or
~ul onLc a~id type ion-3xchange resin, AGY3.-tYPe
p~ot~ .i ve gxoupe;, f or ~xam~ , an acetyl ~ oup or
z5 b~nzoyl group ~ can be ~6~mo~re~. by allowlng In ba3e e . ~ .
po~ l~n h~d3:0xide or ~dium m~hoxi~e ~o ~: f; act
therewith. ~ typ~ prot~c~ive.groups, ~c.r example,
a tert-~ui~ldilne~h~ ilyl group, can b~ el~lrin~ d 3
allowing te~r~buty~ an~nonium 1~oriide ~o r~ac~
therewl~:h. And, Benzyl-type prote~cti~re g:~c~ ps, ~o~
ex~npl~ ~ a benzyl ~roup or p-methoxyb~n~yl ~I roup, can
~e elimir~ated ~y allowin~ }~oron t~ichlo~ a~ to reac:~
~herewith, and, d~pending on CASes~ by h~dri.genolysi~
by mi~n~ o~ ~A~ly~ xeidui-tic7n.
Amon~ thi~ compounds :repr~ ntiad b~ this3 ;EQr~nUla.
ho~ in whl~h at liaask on~ o~ th~ hycl~: o~l groups
~.
91.1!11~1~ 5~ 36~;TA~;EI~A PATBNTS OSAKA Fetherston ; 06300~01;#2g/72
r
of hy~rc~x~ thyl gro~p~ shc~ by Rl or R2, or a ~al t
the~eof i~ phv~phated can l~e ol~taine~l by
pho~phoryla~i4n o~ ~he co:rrespo~din~ hydroxy L g~o~p or
gr~ups.
While th~3 phosphoric ~cicl compoun~ casl :~e produced
b~ a ~E se kno~n method ~3nerally conduct~d in ~h~
chemi~try o~ nucleoticle, .it i8 produced us~la Lly by
allowin~ a phosphorylatin~ agQnt 'co react, f ~Illow~d by,
10 wher~ r~quired, h~rolysis. ~xalnple~ o~ the
phospho~yl~ing agen t ~he~ emplo~ed include
pho~3phoryla~ing ~nt~ whieh mAy opt~n~lly ~r~duce:
oxidati.~n proc::ess, such a~ pho~phorus ~xy~hl ~ride,
pyrol?ho~phoryl~e~rachlo~i~e, phosphorus tri~:lllo~lda,
15 polypho~phoric ~cid~ metaphosphoric ~ic3, ph 3spho~ic
acid ~enz~rle~er.dich~ori~3, m~r~ollnopho~pll~ric a~id
dich~orld~ , phç~nylph~ pho;r~ aaid dich1oricle,
cyano e~hylpho~phoric a~ , dih~n~ylphosphor ic acid
chloride, O-~e2~zylpho~phorou~ acid-O,O-
~0 diphRnylpyxophos~horic ac.id, etc. The phos;p~ x~latin~
ag~nt 18 uaed ae~ on~ ulv~ nt or !nor~ r~ re to ~h~
aompound rel~res~nte~ b~ the ~aneral [ 1 3, pr~ E~rably 1 .
~o 10 a~ui~ralen~ he reaction i~ u~u~lly ~ dua~e~
in a polar ~olve~t i~cluding tri lkyl pho~ph lt~ su~h ~8
'criethyl phaspnate, trimethyl phosph~te, el:.c., phenols
~uah a~ ph~nol , n~or~a~ol , ~ta ., ~c~onltr~lo, N,N-
di~ethyl~orm~mid~, dimethyl ~ulfoxide, et~; a non-
pola~ s~l~rent ~uch ~s tol u~3ne, benæene,
dichloromath~ns, chlorofo.nn, etc.; o~ a mixt lre sol~rent
o~ tham~ The reactic:rl t~mper~ture~ r~n~ m -7~CC to
~he r~ uxins~ tempe~atur~ of ~he 601vent the 1 u~ed r
pre~erably - 20C to room temp63ratllre, Whil.~ the
reaction ~i~ne tle~aends on tha kind~ o:~ pho~ph ~rylating
~gen~ and ~eaction temp~rature~ then employ ad, i~
rang~s ~Erom 3.bo~ 10 mlr~ to aho~l~ 10 hou ~ .
Additionally to s~at~, a c11 pho~phori~ a ~id eæter
~ .
91~5~ lB~36~jTAKEDA PATENTS OSAKA Feth~r~t~n ; oa300~601;~30~72
2 ~
or ~ripho~phoric: aci~ ester can be produce~l by
allowing, ~or exan~ple, l-~luoro-2, 4~dinitrob ~n~ne or
~arbodiimid~zole to react with a mo~ pho3pho ri~ ~ci~
e~;te~, according to a ~er 3e 3;nown pro~e~sy ~o gi~e an
S ac~i~e l~ho~phoric acid e tex compound, whic~ is th~n
subj2~ed ~o ~l3action wit~ ~ ~alt of orthoph ~sphoric
acid or ~yroph~sphor1c ac.ld wlth an organio~ lmin~, ~uch
as l?yr~dine, tri~a~hylamine, etc,
Aativi~y o~ inhibiti:ng replication of H ~ 1 was
:lete~minecl ~ th~ follow~ ng method .
( 1~ Prepara~ion oi~ triru~ ~olution
HIV~ IIIB3 infeoted ~QLT 4 aQllB ~ l x 103
cells/ml ~ weX~ ina~b~ted for 3 ~ay~ . 'rhe ~0 Lls were
main~ained in RPMI1640 co.n~aini~g lP9~ f~ ovine
serum a~ 37 C in S% ~2~ A~er ~en~xi~uyatior. of the
~ell su~pen~ion, ~he supexnatant was f il~ere i b~r 0 . 4 5,um
~llt~r, ~nd w~ ~roz~:n Rt~ -70C U21'C~ 1 u~e. rhe tit~r
o~ ~he supe~n~t~nt wa~ c 10~ PFrt/~l (PFUs ~ ~laqu~
<:~xmin~ uni'c ) .
Z0 (2) Pr~paratl~n ~ ampl~ solut:ion
The ~ample compouncl was soluLbili~ed ln ~MSO
(di~ns3~hyl 63ul~oxids~ at ~0 mgJn~l, and ~hç~ Lu~ion was
cllluted with DMS~} to 2000, 200, 20, 2, 0.2~, ~.0~ ~g/ml.
Irhen t;he~e solution~ wer~3 dilut~d to 200, .~0, ~, û . 2,
0.0~, 0~00~, O.ûO0~ llg/ml with P~PMI1640 coI~t~in~ng 10~6
fetal bovine 5erum, Io~pec~i~roly, The ~exi.e ~ ~e the
~olu~ion~ wa~ used for antil~IV a~say.
( 3 ) AntiYlral aati~rity o~ sample ~ompound a~ ~inst HIV-l
~T ~ cells were exposed ~o HIV~ r IE~ ) at ~n
m . o . i . ~f 0 . 0~2 . ~hen 50 ,ul o:~ the cell st~.6 ~en~lon
(1~6 x 10~ ml) wa~ inoaul~t~d i~ ~-w~ .l U-bottom
plal;~ and 50 ,ul of ~he ~ample ~olutlon was a lded to the
cw~ll suspen~ion and ~ultu~d for 6 d~y~ e c~lls
were n~a~ ntain~d in RPMI1640 containing 10~ tal bo~r~ne
3S ~rum a~ 37~C in 5~ C~2. Go~rol cell~ wer~ tr~ted
Elimilarly bu~ no~ ~x~ ed to the ~i~u~. CellL
.
91~5~ la~3~;TAKEDA PATENTS OSA~A Feth~r~n ; OB300BB01;$31/7t~
2 !r~ F~
- 2B -
prolifer~ion was as~ssed by ~he X~ (2,3--b.ls~
me~hoxy-4-nitro-5~ulf~phenyl]-s~
~(phen~lamino~car~nyl~-~H-tetrazolium hyd~o~ide~
mathod. Then 50 ~1 of xrr solution (0.5 ~c1/1nl XTT, 3
~g~ml PMS (phenaæine methosul~ona~)) wa~ z~dlled to th~
~ell 6~spension an~ incub~t~d a~ 37C fo~
ab~orbanc~ at 450 nm w~ m~ur~. Th~ e~i.e~,ti~e do~e~
SO% (ED50~, rep~e~ent~ the concen~ra ion of c~mpound
tha~ ease~ ~bsorbance in the infec~d c:uL~ure~ to
50~ ~f untxea~e~, unin~ec~ed ~ell cont~ols~
Sin~e anti~rir~l agent~ o~t~n ~how to~ci.c. t~ ag~ins~
ho~3~ qolls t the ~o~i~ity ~f tas~ ~mple~ a,qa. n~t th~
ho~t cell~ wa~ examined ~y the followin~ m~tl~od.
The.cytotoxity of the ~ampl~ compound tl~ MT~4
cell~ was ~easured b~ ~he similar ~ethod de~s~ribed
a~ove. In a ~6~w~ U-bo~ttom platet 50 ~1 o:S the c~}l
~u&p~on (1.~ x 105 cQll~Jml) wa~ ulat~ld in a 96-
well U-~ot~om plate ~nd 50 ~1 of the ~ample ;3~1ution
wa~ added to th~ cell ~usp~nsion and cul~urelt for 6
days ~t 37~C in 5~ CO2. Cell proli~eration ~Oa9 as~ed
b~ XTq' a~ay and I~50 wa~ determi~ed. The ln~lbl~ory
doae, 50~ (IDso), r~pxe~n~ th~ toxi~ aon~nt~ti4n o~
~xu~ that reduc~s ab~orbance in uninfe~ed ~Illture~ to
5~
~he antl~i~l acti~it~ v~ 4,t3, 5a-
~i~hydro~yme~h~l)cyolopen~-2-~n-l~-yl]adeni.nl3 ~the
c:vmpound oi~ Example 3) d~t~rmin6~d by th~ m~tllod
de~cribed abov~ wa~ a~ fo:Llow~.
Anki-~IV : E~50 .3S5 ~g/mL
cytotoxlaity s ID50 66.4 ~g/mL
A~ the oom~o~ xepre~ented by the ~o~ la Cl~ i~
weak ~n cyto~oxici~y and ~how~ ~t~ong anti~i:~al
actl~ity, it can b~ ~ed ~or th~ therApy of.~ e~s
due to viral in~e~tion and ~or prote~tion f'rom the
3S in~cti~n~ Pu~ther, tho l~mp~u~d ~lJ did no: ~how
cytotoxici~y to vero cell~ at a concen~rati.ol~ o~ 100
91~ 5~ 16~36h`;TAl~E3A PATENTS OSAIIA Fethsr~ton ; 0a3008~01;#32/72
C~ J ~
~ 2g -- :
g/ml. In other words, the compound of thi~ kion
can be widely used for the thorapy And proph ~l~xi~ o~
d~ ~eases due ~o vi ral in~eation in m~mmal~ clucling
man. Especially, khe ~ompound o~ ~hi~ inv6~n~ion ox a
5 salt thereo~ i~ ef fective for the the~a~y an,l
prophylaxi~i o~ di~a~a~es such a~ AIDS or 8~ruln hepatitis
c~u~ed ~ i~f~c~ion o~ re~o~riru:s or r~xoi.dl~riru~s.
And, as ~he aoml?~und of ~his inven~ion or a ~alt .
thereof i8 stable again~t ~id, lt i~ u~e~ul for
10 p~ien~, like ~hose i~om ~I~S, xequiring
admini~tration of a~ vi~e~l a~en~ ~o;r a long period
tim~ .
Pharmacelltic: al~ coni~inin~ the colnpourld 11 ] or a
salt the~eo~ as th~ eff~ative ~olnponent can I)e prepared
15 into suitable dosage ~ 3 ~uch ~ ~ap~ule~, t~blet~,
ointm~n~s, in;ections, gr~mules, powder, solution,
suspe~sion or elixir, optionall~ mixed with ~!L suitabl~
aJnount c~f pharmaceutlcally accepta}: le ad ~u~ran~cs ~uch as
a c:arrier, dispar&~ant or e~ecipie!n,t/ ~hiah ca:lL be
20 administered uRually orally andl upon nece~s..t~
intrav~noue:ly or l~txamu~c~ularly.
Cap3ule~, pow~e~, ~r~nules and tabl~t~ I,o be used
$o~ oral admln~st~atio~ may include an 2.d~uve,nt such . s
~yrup, ç{um arabica ,, gelati.n, ~orbitol, tragar ~ an ~um or
25 polyvinyl pyxxolidorl~, a ~iller such as laceo~,
~ugars, corn ~tarch, calcium phosphat6~ or s~ ol
glycine~ ~ lubrican~ such~ a6 magnesium ~te~axe.te~ ~alc,
poly&thylene glycol or ~ilic~ ~ d.i6integrat;l~ agen~
~IlCh a~; po~a*o ~tarch, or a ukilizable lubric ~nt ~uch
30 a~ ~odium lauryl ~ at~. Coatin~ Oæ *~bletli .aarl be
conducted by a well-known meth~cl in th~ nt ~rt.
Liquld prepara~ions ~or or.al as:lministra~ion ll,a~ be~ in
the forln o~ aqueolls or oil sus~pension, solut:ion,
. q~u~ion, ayrup or elixLr, or a ~r~ produc~ l:o ~e
~issol~d in wat~ or any okher suita~lR so:L~tion
extemporaneou~ly, These l.iquid p~epa~AtiOn~3 may
91~ 5~ 16~36~;TAKEDA PATENTS OSAKA Fether3ton ; 0a3006~01;#33/72
-- 30 --
contain ~ ~u~p~3ndîn~ agent ~u~h as ~or~itol ~ yrup,
meth~ lulo~;ç, glucoseJ~ugar ~yrup, gslat:l n,
hydro~yeth~l callulo~e, carboxym~hyl c~ellulc ~e or
alumnium ~;t~arate gel, a hydrc)gena~ed e~llble oil ~uch
5 IS alrnoncl oll, ~Er~actionall~ di~tilled c~on~ o~il or
oily es~er, pxopy~ ene gl.yc:ol or ~3thyl alcoho:l, and a
pre~e~Yati~ ~uch as methyl or propyl p-
hyd~cc~xybenzoate, sorbi~ ~c:id, etc . The compoun~ ~ l ) or
a sal~ thereof ~an also b~ u~d a~ a suppo~ ..0~y ~it~ a
~uit~ble base ~uch as ~aaao butter or other ~ lyceride.
In~ect~ 3 pre~paxatlon~ m~y be ~?~e~entec. ln uni~
d~be cont~iner~ ~or ~x~n~pl~, ~p~le~ or ~ ainex~ to
which a pres~rva i~re i~ a~lded. ~he~ prepa:~.tion~ may
sui~aJ:~ly contaln an ad~uv~nt such a~ a susp~r.din~ agent
i~ t~e ol l or aqueou~ sol~v~3nt, a ~ al~ er c.ncl/or a
dispar~ing ag~n~. ~d, the acti~a compo~nt m~ ~o
p~p~rQd in~4 a p~wd~y ~or~n, which iS exteml;~oran~ou~ly
re~onstituted with ~ ~uit~lble ~ol~en~ J o~ ~a.! :ample,
sterile water containin~ ~lo p~ro~en.
And, the ~ompound ( 1 ) or a ~alt c~n be ~ eparedt
in ~cc;o~d~nc~ with ~ con~ntion~:L pro~ea~, i nto a
s~it~b1e form to ~e ~sorhed ~hrough a ~uit~ ~le route
inclu~ing nasal ~d phar~n~eal membrane or ~ l onchial
ti~sue, for example~ powde~r, a liquid ~pra~ r
zs inhalant, lo~enge~ or paint ~o be applie~ on pharyngeal
m~alnbrane .
~nd, in addition ~o t~he car~iexs ~ ~ny e-l.h6~r
cc~mponent~ such a~ a staLbilizer, binder, ant.l.-oxLdant,
pre~i~rvative, lubricanl:, t;hickening agant o:r flavc)ri~
age~. B~riid~ Qny oth~ ~c~i~e aomponen~ y be
allowe~l to be c:ontai~ed in guch preparation.~ bove
to give a broader meclicin~l use~.
Xn the~ preparationsi, ~he compound 1 l ~ or a ~alt
thereof may l:)e ie~llowed to be cc: ntained, in a liquid
~o~ id do~a~e ~nit, in an amount of 0 . l to 9~ wei~ht~,
pref erably about 10 to ~ O ~ ( weigh~ ) .
91~ 5~ 36~;TAKEDA PATENT~ OSAI~A Fe~h~r3t~n ; 0~3006601j#34/72
2 ~
-- 3~ ~
Th~ ~ntiviral agent o~ thi~ in~ention can be u~ed
i~or the ~h~rap~ and prophylaxi~ o~ h-lman
i~ununodeficlenc;:y, e~pecially AIDS, adult hum.. n ~-cell
leukocy~hemia, e~G~ ~ a~3 an agent o~ inhibl~ g th~
S ~rowth and lnf ection o~ retroviru~ . A ~uit;~
ef~ec~ do~s o~ ~h~ ~c)mE~ound~ o~ this in~-~ tion i3 in
th~3 ranqe of 20 to 2000 m~ (in term6 of th~3 ~ f:~ectiv~
~omponerlt) p63r day per a~ult person (body w~.ight al~out
60 lcg), p~eer~bly 50 to 100 mg, onc~3 or ~e~. ral ~ub~
10 do~e~ at appropria~e interv~ls . Prac~tical ~C ~e i~
d~te~nlnecl ~epe~ing on ~e age, ~o~y weigh~" ~ymptom
of ~he pati~3nt and th~3 dc~age o~n ~nd adminii ~ration
f requency .
[ Working Ex~mpla~ ]
The ~ollowing E~amplQæ ~nd R~feren~e Exclmples will
d~rlbe the p:roduction of the compound ~1 ] Il ore
3~eaif icall~,r,
R~3ference~ E~eample 1
1 -~enzylo~ne~hyl~2~aæ~bi~ hept~5~ n~,
20 7~nti benzylo~cymethyl-2-az~bicyclo~ ]h~ t~s~ 3~
one and 7-~yn-ben~yloxyme~hyl-2-A~abi~yal~ :a . 2 .1 3hep~-
s-en-3~o~e
. A ~u~p~n~ion of a~lopenta~ien$rl thallium (13.5 g,
50 mmol. ) in anhydrou~ ethyl ~3th~r (15 ml) ~ cooled
2~ ~o -~204C in argon stre2uns, to whlch wa~ add~E~cl ~ropwi~
a ~olu~ion of ben~yl(~hlo~e~nethyl~ether ~7. B g., 50
n~nol. ) in anhyd~ous ethyl ethe~ ~5 ~ The ~e~ ion
mix~ure was stirred ~or 7 hou~s at th6~ same
temperR~ure, ~hen precip~ta~e~ were separatli~cl ~y
30 ~iltrQtion un~er ~3u~king and wnshed with t~nlh~d~ou~
e~hyl e~her. Th~ fil~ra~e and the washing w~l~e
combined and khere was added p-toluenesulf on~ yanide
~9.0 g, SO rnmol. ) . q~his ~olution ~a~ aOncerlt.rat
under re~lu~ed preB~ure to ~ volume of abou~ ~5 InL,
35 whiah was left ~t~ndln~ for 12 hour~ ~t roo]n
temp~rature . ~o ~he c4n~entrat6~ were added e hloro:Eo~m
.
91~5~ 16~36~;TA~EDA PhTENTS OSAKA Fether3t~n ; 063006~01;~35/72
2 ~ v '~j ~, 3
_ 32 --
(100 ml ) and silica gel (:lO c~), an~l the ml~:t.llre waæ
s~ ed ~or 3 hours ~t room ~e~np~rature. Si:.ic~ gel
wa~ ~iltere~ of, anà the ~iltrate wa~ conc ~lltra~ed
unc~r reduced pre~3su~e~ ~h~ con~entra~e wa~ sub~ect~d
tc:~ a siliaa gel (400 g) ~ollllnn chromatograE)h~r. The
aolumn wa~ sub jec~ed to elution wLth hex~.ne I !th~l
~ce~at~ ( 4; 1, 2 . 5 I~ ), h~ane-e~hyl ~.çe~ate ~ 1, 3~ ),
h~3xane-ethyl acetate ( 1~1~ 3L ) and he~eane-0thyl aceta e
3, 3I. ), ~ucce~ ely to separate l-benz~ xyrne thyl r
2-azablc:yclc~t 2, ~ . 13he~pt 5--en-2-one, 7"-anti-
b~n~ylo~y~eth~l-2-azabic:yelo~.2.1~hep~-5-e~n 3-one and
7~n-bena~loxy~thyl-2-~7~Abi~ycl~2.~.l]helpl;-5-en-3-
one.
l-benzyoxyme~h~1-2-a~a~icyclo~ ,l]hept-5~e~ 2-one~
yiel~ 1.5 g (12~; c~lo~leæs oil; IR tC~Cl3) ~ 34S0,
1715 cmlslH~N~R (CDC13, 61) MHz)~ ; 2~20~2H, ~'lf 7 ~),
3.27~1EIt m, 4~ 3~83(2H~ nOCH~ 4.63 (2H~ e~
PhCH2~), S.28 to 5.71 (lH, broad ~, -NH- ~, 6 . 71
5~H ~nd.~H), 7.37 ~5H, 8~ C~Hg~).
MS s Calcd. ~or C14HlsNO2 (M ) M/Z~ 229. 1102~ Found
22~. lO9B.
7-anti~anzy1~x~me~hyl-2-~læa~y~1oc~.2.l~hel~t-5-en~3-
one: yîeld 2.8 g (2~ lo~le~ ~morphous s;u~tan~
~cry~tallized ~rom ethy~ ether-h~an~); m.p. 70 to
~5 75~. IR (CHC13) : 3450, 1710 ~ NMR ~C~Cl~, 500
MHz)~ s ~.92 (lH, m, 7~ 3~0~ (lH, broad g 4~
3.~ (lH~ dd~ J~lO, 6Hz3 ~d ~6g (lH~ dd~ J~ 9Hz)
(~nO~H2~ .2S (lH~ m~ 1-H)~ 4.50 (~Hf 8~ :I?hCH~
5.1~ (lH~ broad ~ -NH-), 6.71 (1~ 5-H)~ &.85 (lH~
dd~ J=6~ 2H~s~ 6-H), 7.30 t5H, m, CG~5_). .
MS : Cal~d. ~or ~14HlsNO2 ~ Z: ~2~. 1102~ Found ~tZ:
229. 1128.
7-3~n-b~nzyloxym~hyl-2-a~sabicy~1O~2.2.1~h~pl:-5-en-3-
one : yi~ld 2.0 y (17%); ~olorle~s oil. IR tC'~13) :
3450~ 1710 cm~lS 1H-NMR (C~Cla, 500 ~Hz)6 s 3.~8 (1~
broad, s, ~-~), 3.21 (1~ m/ 7-H~, 3.39 ~ ddr ~10,
91~ 5~ 16~36~;TAKEDA PATENTS OSA~A Fethsr3~n ; o63oo66ol;#~e/72
&
33
HZ) and 3,~3 (lH~ dd, J-10, 7 Hz) (~nOC~ , 4.~7
(lH~ m, 1-~)/ 4.44 (~H~ s, Ph~ 6.21 (l~ road
8, -NH-), 6 .49 (1~/ m, 5-H~, 6~5 ~lH, ~d, J ~5, ~Hz, ~-
H ) ~ 7, 3 0 ( S~ , C6H5- ) .
MS: ~alc~. f~ C~4~l5N~2(M ) M~z ~ 229~1102. ~uund N/Zt
~2g . 110~ .
P~eiEerenc~ Exampl~ 2
7-s~-Benzylo;R~nne~hyl-2-ethoxy~arbony~ z~t~ icyalo-
~ 2 . ~ ~1 ]hepk-5-en-3-one
A mlxtur~ o~ a ~oluti.on o~ 10~ hium t~ oprop~l
nide ~n hexane ( 1~ m~) and anhydrou~; te~trall~,dro;Euran
(20 mL) wa~ ~vol~d ~o 7~c and th~3re~ ~a~ lc.~
dxopwise~ slowly~ in Argon ~troam~ a ~oluticn OI 7-
~yn-benzylo~ymeth~ azablcyclo[2,2.1]h~pt~ en~3~one
(~25B mg, 5.4~ mmol.) in ~nhydrous tetr~hydrs:uran (10
mL), and the mixture w~ stirred ~ one hou~: at the
~me tempa~tur~ e reaoti4rl ~l~tu~a wa ~ added
ethyl chl~roca~on~te (0.7 ~L, ~.3~ mmol.), then th~
çooli~ bath wa~ removed, followecl by ~irril.g until
the re~ion temperatur~ w~t up to room tem~erature.
The r~a~tion mi~tura was stlrred ~o~ u~ther 12 hour~
a~ ~oom ~e~p~raturesv f~l low~d by c~ncentra~:ion under
reduc~d pre6~ure. To the ~onc~nt~a~e wa# adc.ed
chloro~c>xm, then ra~ultant lithiun~ c:hlor~ de l~as
f~l~e~ed o~, and the ~ilt~ate! was concentral:.ed uncle~
~duc~d p~ur~. The Gorl~ntr~t~ ~QJ ~ub~a~!t~d to a
~ilica ~el (50 ~ colu~n c:hroma~ography, ~ol:l.owed ~
elution with hexa~e eth~l açeta~e (3~ h~ f~tion
thu~ elut;ed wa concen~r~t,~d to dryne~s u~de:l re~u~ed
pre~ure to a~ord 7-~yn-benzyloxym~thyl-2-
ethoxyc:a~on~ as:abicyclo~,2.1~he~k~5-en.-;i-on~ as a
colorle~ oil (g83 mg, yie1d 59%~,
IR(CHC13) : 1790~ 17~1, 171~ cm ; l~_NMR (~ CL3, 60 MHz)
~i ~ 1.3~ (3H, t, J-7Hz), -CH2CH3), 3.13 ~1Hr 1~ 7 H)~
3.2~ (1H, m, 4 ~), 3.2 to 3.7 ~2~, m, snoc~:2-)~ 4~26
~2~I, q, ~-7 Hz, -C~kCH3), ~,4b 12H, s, PhCH~-:I, $ 00
91~ 5~ la~36~;TA~EDA PATENTS OSA~A F~thergt~n ; 063006601;~37~2
2 ~ ~ ~ ~ S ~
-- ~4 --
(1!H, In~ 1-H), 6.48 ~1HJ m~ 5-~1), Ç.73 (1~I, d~:l, J=6,
~HZ, 6 ~ 7 . 3 1 ( 5H, ~, CÇ~5_ ) -
M~; CA~ o~ ~13~14~ ~M -(~4H5NO3) ~q~Z ~ 18~ 4 . FOUnd~ 6 . 1 ~ ~ 7 .
Reference Example 3
7 anti-ben~ylo~methyl~-etho~ycarbonyl 2-
a~abic~clo~2.2~l]hep~-5weTl-3-one
7-an~i-henzylox~meth~1-2-a~a~icy~1~[~.2.13hept-S-
en~3-one (1~05 mg~ was p~oce~sed in the samle m~nner as
Re~ren~e ~am~le 2 to gi.~a 7-an~ enzyl~x,methyl-~-
~thoxyc~rbonyl~ zabicyclo[2.2.1]hep~-5-en-.i-one (1163
m~, yield ~ a~ a ~olorl~s~ oll.
IR(CHCl3) : 1792, 1770, 1715 cm1; lH-NMR (Cl:l~ L3, 60 NEI~
~ 32 (3~, t, ~7Hz, -CH~C~b), 2.81 (lH, m, 7~
3~29 (lH, m, 4 ~), 3.2 ~u 3.8 (2HI m~ BnOCH~-~, 4026
~2H, ~, J~7Hz), -C~C~3)r ~.4~ (2H, ~, PhCH2~'l, 4.g6
(l~, ~, l-~), 6.6~ (lH, dcld, J~5, 4, 2 Hæ, 5 H),
~.~4(1H, ~d, J=5, 3 Hz, 6-H~, 7.33 (S~ ,;H5~
MS : Calcd~ ~or Cl7HI~NO4 t~ 301. 1314. Found M~2:
~0 30~. 13~1.
Ref ex~nc~ E~mp~e 4
Bthyl (~ [S~-benz~lo~ym~thyl ~-(hydroxy~.~th~l)o~Glo
pent-2-en-1~-yl~caxbama~e
A ~olut~on o~ 7-~n~be~ylox~m~hyl-2-
ethoxycarbony~ abi~ycl~t2~ ]h~pt-5-~n~;~ one (404
mg/ 1.34 mmol~ anh~drC)u~ ~ekhanol (10 mL' w~s
cooled wlth lce-wate~, to whl~h w~s added oc.~um
borohydrido ~255 my, 6~71 ~mol.), ~ollowed ~j ~tirr~
for one hou~ a~ room ~mp~r~ture. T~e re~ct:lon mixture
was neutrallzed with acetlc acld - methanol ll;1), t~en
~he ~olven~ wa~ ~still~ of~ under redu~d ~,~re~sur~.
Tho ~ du~ wa~ ~ubj~ct~ to a ~ g~ oluunn
~h~o~atograph~, followed by elution wi~h he~ ne-eth
~cat~te (1~ he ~racti.on ~hus elu~ed wa~
concentr~ted to dryne~ undex xeduaed pr~Asu:le to
~ford e~ ~ 1 (i) N-~5a-b~nzyloxym~hyl-4
91~ 5~ 1&~6~;TAKE~A PATENTS OSAKA Feth~r~t~n ; 06300Ba01j~3g/72
- 35 - ~ ~ r~J ~; ~,",
(hy~oxym~thyl~yalopent-~-~3n~ l]car~lna~ (35~ l:ng,
yield ~5% ) ~s a colorles~
I~(C~ 34S0, 1715 c~ NMR (ClDC13, 60 MH~1.23 ~, t~ ~-7 E~ C~C;EI3), 2~3 to 3,0 (2E~ m, ~-H
and 5-H ), ~ . 2 to 3 . 9 ~ 5H, m, ~nOCH2~, ar~d -C'~ ~OH ~, 4 . 11
(2Hr S~l J=7 H~ CH3), ~.3 to 4.5 (lH, m, ;.-H~, 4.57
( 2H, s ~ PhC~ ), 4 ~ 8 to 5 . ~ ( lH, bro 3.d d, J 1 3 H~, -NH-
), 5.67 t~H, s, 2-~H and 3--H), 7.32 (SH, ~, C~jH5-)~
MS : Calcd. fo:c Cl7}i24N:)4 (~ +1) ~ 30~.1
10 M~Zs 30~.17S4.
R~îer~Pnc:e ~xamp~ e 5
Ethyl ( ~ ) -N- C 5.~ henzylo~ hyl- 4 ,B- ( hydroxyml. Ilkhyl ) -
c~clop~nt~2~en-l,B-yl]car~zlma~ 7-antl-Benzy~ x~neth
2-~hoxya~bonyl-2-azabL~y~lot2~2.l]h~pt-5-~r--3~
15 (1132 m~, 3~76 mmol.~ wa~ proces~ed in ~he ~e,me ~anner
a6 in ~efer~nce Examp1e 4 to ~fford ethyl (~ N-~5~-
b~nzyloxymethyl-4~-(hydr~xymethyl)cyclopent-'!-
en-1,~-yl~rbama~e (954 In5~,.yield 8~6) A~; a ,::olorle~
ui~ .
~0 ~CHCl3) ~ 3450, 1715 cm; lH-NlqR (~C13, 60 MH~
1~22 (3H, ~, J~7 H~, -CN2C~), 2.1 to ~.5 (lH, b~oad ~,
-OH~, 2.~ to ~0 ~2H~ m, 4-~ and !S-H)~ 3.5 ~ 4H,
m, BnOCHz- and -CH~,OH), 4.0~ (2~1, q~ J-7 H2r ~ C~3
4.S2 ~2~ ~ PhCH2-)/ 4.7 t4 5.0 ~1~, m, ~-:E~l.r 5.0 t~
25 5.55 (1~, b~oa~ ~, J=12 Hz, -NH~), 5.8~ , B, 2-H and
3-H)r 7.31 (5Hl sI C6Hs-).
MS ~ Calc~, for ~l7H24~ao4 (M +l) ~/Z s ~06 .170'i . ~ound
M~2: 3D6.1701
Re~eren~ ~:xample 6
30 (~ )-t5c4-Benzylo~ thyI-4l3~(hyd~o~ymethyl)~yl~loperlt-2
~n- 1~3-y~ 1 a~nlne
~rO ~ lutlon of eth~ N~[5c~-be~nz~l >xymethyl-
4J~-(hydroxymeth~l)cyclopeallt-2-~3n-l~B-yl]~ar~!amat~ (510
~ng, 1.~7 mmol. ) in meth~nol (lO ml) wa~ ad~Le~l a lO N
3~ ~olutiozl of potassium hydroxld~3 (lO n~)" ~r~d the
91~ 6F 16~36~;TAI~EDA PAT~NTS OSA~A Fether~ton ; 063006601j#39f~2
3 ~ $ ~
- 36
mix~u~3 was heated for 20 hours under reflux. The
r~action mix~ur~e wa~ concelltrated uncler redu~ d
pres3ure. To the conce~t~ ate wa~ added wat~s:l to gi~r~
an oily produc~ aIIcl was ~ t~acted wi~h ethyl acçtate.
5 The e~tract solution was clri~d over anh~rdrouli ~odium
~ulfa~e~ which wa~ then concent~ated unde~ r~lduced
pxe~ure. ~he ~oncentrat~3 was sub~ect.ed to ;!. ~llic2l
gel ~ 25 ~) column chxolnatography, follow~:d b,~r alution
wi~h e~hyl aceta~e-~nethanol (1~1) . The fI~a~i ion ~hu~s
10 eluted wa~ con~entrated ~o d~nes~3 unde:~ ro~llc~d
pressure to ai~for~ 5a-benzylo~yme~hyl- 41i;~
Ihyd~c~ neth~rl )Gyclopent-2-en~ yl]amine~ ~ 3:~ mg,
yield ~696) a~ a colorl~ oll .
IR(C:HCl3) : 3~0 ~m ; E~-N~ (CDC13~ 60 MHz) ~ : 3~ ~8 to
lS 3~87 (5Ht"n, -CH2- x 2 atld 4~ 4.5~ ~H, $~ :I?hCE~2-),
5 ~ 6 7 ~ 2H~ ~ S r ~ ~H zlnd 3-H ) ~ 7 . 3 3 ( ~H, ~, G6H~
MS ; ~alcd, ~or Cl4~ Oi(M~-l) M~; 23~138~ Poun~
~iJ$; 2~2 . 1333
~er~nc:s Exampl~ 7
(t)-~ $~ 3nzyloxyme~hyl-4~3- (h~droxymethyl ) c~ lopen~-2
en- l ~-yl ] amine
E~h~l (+)-~-~5~-bo~ylo~nethy~ hyd:~oxyqneth
cyclopent~2~en~ yl~carbalmate (324~ lng, 10.l;4 Irunol.
wa~ proc:es~ed in th~ same manner aa in RefeIn~nc~
2 5 l~a~n;ple 6 ~o ~ f ~ord ( ~ ) ~ 313-b~nzylox~methyl~
(hydroxyqnethyl)~yclopent-2-en~ yl~a~in~ lorle~s
~ri~m~ (cry~talli~ed ~rom ~thyl ether)O Yi.e;.d lg~O mg
( 79~ ) m.p .: 73 to 74 ~C;
IR (CHCl~) ~ 3380 ~ H-NMR (CDCl3, 60 MH~ 6 to
3.00 ~2~, m, ~-H ~nd 4-H~, 3.63 (2H, d, J=3 ll~, BnOCHz-
)~ 3.71 (2HI d~ 3~8 HZ~ O~)~ 3.36 (l~l~ dc!~ J-6~
) r 4 . 55 ~ PhCE~ ; . 91 t 2H~ ~3 ~ }I and
3~H)~ 7.34 (~H~ ~/ (6~5~)-
MS s Calcd. ~o~ Cl4H~8NOa(~ /Z: 232~1338~ ' Found
3~Z: 2~2.1341.
R~3~eren~:e .~;xample ;~
9~ I;EI 16~36~;TAKEDA PATENTS OSAI~A FeCherqt~ll ; 0~3300~601;#40~7~
~ 5~Amino-4-C5~-b~nzylo~.yme~hyl~ (h~drox~,m~thyl)
cyclop~n~ en~ yl~aminc--6~chloropyri~idin~i,
To ~ ~olu~ion o~ [5a-~enzyloxymethy:l-4~-
hydroXy-meth~l)cyclo~ent~ n~ l)amine (~2l'~ m~, ~.15
mmol.) in e~hanol (13 mL) were added 5-amino 4,6
dichloropyrimidine (378 ~c~, 2.3 mmol.) ~nd
~rle~hylamine (3~ mg, 3, e; mmol.)~ ~he mix:tl..re w~
h~ated for 48 hPur~ at 100C in a s~aled tu:be. The
solvent wa~ illed off under redu~ed ~e~;ur~, then
khe r~idue w~ s~bjected to a silic~ qel (1'l g) column
chroma~ogr~ph~, eluking wi~h he~e-ethyl a~ ta~
( 1 :1 ) . The .fr~c~lon th~ ~lut~d w~ cona~.t~ t~d ~o
dryne~ under redu~e~ pre~ure ~o af~ord (~) 5-amino-
4 ~S~-~enzyloxymethyl-4~-~hydroxymethyl)cy~ p~n~-2-e~-
lS l~-yl~a~ino-6~chloro~y~im~din~ (34~ mg, y~ 82%j ~ a
color~e~ oil.
IR (~HCl3~ s 3370, 1580 ~m ; l~_N~ ~c~Cl~, 6l1 MU12)~
2.16 (lH, m, 4-H 0~ 5~, 2.77 (lH~ m, 4-H 0l~ S_H), 3.
to 4.0 (7~ m, ~C~2-~2, -~H~ and -0~)~ 4.54 ~2~
PhCH2-), 5.03 ~1~, m, 1-~), S.73 3H, m~ 2-~I, 3-~ and -
NH )~ 7-29 t5H, ~ ~6~5-~, 7-9~ (lH, ~, ~yrJ.mL~ine ~-
H)
MS: Cal~d. ~o~ C~8H2lN402Cl ~ Z~ 360.1352. Found
~ 36~. 132; Cal~d. ~or Cl~M2lN602Cl* (M f)) M/Zt 362.
. 1322~ Found M~Z~ 362.~33:L.
~e f ~renc~ ~xampie 9
(+) g-~min~-4-t5~en~10xymethy7 ~-(hydrc-x~eth~l)
c~alopent~ n_l~_yl~amino-6-chloro~yri~idln
~ S~-ben~loxym~t]lyl-4~-(h~droxymeth~
cyclopent-2~en~ amine (42~ mg, ~.81 m~lo.~.) ~a~
pro~e~ed in tn~ ~am~ manner a~ ~efer~nc~ E;x;~mple 8 ~o
a~o~d (~)-5-~mln~-4~1~-h~n~yloxymethyl~
(h~droxymethyl)cyclopont~ en~ 13ami~o-6
chloropyrimi~ine (517 mg, yield 79~) aB a c:o;.orles~
oil.
IR (CHCl3) ~ i370, 15~0 a~ NM~ (C~19, ~l) MHz)
91~ 5~ 16B~36~jTA~.EDA PATENTS OSAKA Fether3t~n i 063osB6ol;#4l~72
- 38 - 2 ~
2.88(2H, m, 4-7~ and 5-~), 3.31 (3E~, broad 8~ -NH2 and -
~ 3 ~ ~ ~ t 4H, 3~, ~CH70H and BnOCH2- ), 4 . ~1 (: !II, ~,
PhCH2-), 5.3g (lH, m~ l-H), 5.~4 (3~, m, 2-1~1/ 3-H and
N~ 7-2B (5H~ ~ C6~s-)~ 8.O~ (lH, ~, pyrlm:.~in~ 2-
S ~)-
MS: C~ d. for ~l~H2lN402Cl ~) N~s 360. 135:!. Found
M~: 36~- 1335- S C~lcd. ior ClBH~lN402~1* (~+:~) M~Z:
~2. 1322. Fou~d M/~- 362. 1314,
Re~Eerence ~amp1~ 10
1 û ( ~ ) -9- [ 5~-ben~loxym~h~1 -4,6- ~ h~droxyn~ethyl ) I :yclopent-
2-enr 1~-yl]-6-chloropu~ine
( ~ ) -5-~nino~4- ~ 50~-bellzyloxym~thyl-4,~-
hydro~ymeth~yl ) cyc ~ opent~ en ~ yl ] amlno~
~hloropyrrimidin~ (~03 mg, 0.56 mmol~ ) was di!;~ol~ed in
15 ethyl orthofoxmatè ( 3 . 1 ~ To -the ~olu~i.on wa~
added, while ~.ir:ring under cooli3n~ wi~h ic~water~ 12N
hydroc:}llor~c acid (0.04 mL) . ~he reac~io~ ~m: xture wa~
s~lrred for ~2 hour~ at room temper~tur~3 and wa~ th~3n
conc~nt~a~d under xeduced pressure. The c!o:llcentr~te
w~s d~ol~ed in te~rahydro~uran (~ m~), t~ 17hiah ~a~
~ed O.SN hydrochloric acid ~12 mL)~ and t:hl~ mixtur~
wa~ ~tirred f~ 2 hours. Tne reac~lon ~ixt:u'~a wa~
neutrali~ed ~i~h lN solut;Lo~ of ~odiu~ hy~ro:~ide and
~ concen~ate~ ~nder reduced pre~sure. l'o ~h~
concentrRte wa~ ~ded water. The resultant;l~ily
pr~duc~ wa~s extra~ted ~ith ethyl acetato. Tl~ extract
~olu~lon was dri~ ~v~ al1hyd~ou~ ~diu~ ~ te~
~ollow~d ~ concentra~io~ under r~duG~ pres;sure. The
~ entrate was ~ubjected to a ~illca gel ~ ) colum~
chromatography~ eluting w.ith h~xane-ethyl ac,~tat~
~l~l). The ~ractlon thu~ elu~ed ~a~ concoJlt:~t~d
d~yn~ to af~ord (~ .S~-ben2ylox~methy~
(~ydrox~m~thyl)~yclopent~ e~ 1]-6-chlo~purine
(180 mg, yleld 86~) ~5 a colorle~s oil.
I~ (C~C13) 2 3450, 159~, 15~0 cm~~ ~NM~ (~'r,Cl3, ~0
MHz)~ : 2.6~ q~ 3-6 ~z~ 4-H or 5~ . 38 ~lH! m"
91~ 5~ 16~36~;TA~EDA PATENTS OSAKA Fethergt~n . 063006601;#~2Z72
~ ~ r~
~-H o~ S-H~, 3~23 (lE~, ~road s, -C~H), 3.72 ~;~H, d, ~6
Hz, :BnOCH ~ 3, 3 . 8 0 ( 2H, m t -C~20HI ) r 4 . 51 ( 2H:, 8, PhC~-
), 5.58 (lHI m, 1-~1), 5.77 (lH, ddd~ ~-5, lt l~l~, 3-~),
6 .0~ (l~, ddd, ;~-5, 2~ z, 2-~I) J 7 .2S (5H, s, C~H~
8.24 (1~, s, purir~e~ r 8.66 (lH, 5, pur~n~
MS > Calcti. ~or Cl9HlgM402~1 ~M ) M~; 370.11g~ Found
M/Z 370.1158. ; Cal~d. for ClgHl9N402Cl* (Mt~2 M/Z:
372.1165. Found ~/Z~ 372.1138.
~eference Ex~ le ll
~0 (~ S~-B~nzyloxy~e~hyl-4~-(h~drox~neth~l)t;yc~opent-
2-en- 1 0-yl ~ - 6 -chloropurin~!
( ~ ) -5-Amino-4- [ 5~-ben~:yloxymethyl-4,~-
(hydrox~methyl ~c:yclo pen~2-en-l,B-~l jamino-~ .
chloropyrimi~ine (517 ms~, 1.43 mmol. ~ ~ag p~ ces~ed in
15 th~3 ~ame manner as R~3fex~nce Example 10 to a:l or~i ~+)-
9-e5~ en~yloxym~hyl~4~-~h~roxyIoethyl)cyclc~pent-2-en-
l~-yl~-6-chloropurina as t:olorle~ p~l~ms ~c:lystallized
~om di~hl~romethane - hexane).
Yield ; 37~ m~ (70~); m.pt~ 120C~ IR (CHCl3) s 3450,
~O 1590, 1560 am~~; ~H-N~R (~13~ 60 M~ t 3... 85 (3H~ m,
4-H, 5-~ and -OH)J 3.51 (2H, ~, -CH2-~/ 3.83 (~H, m~ -
CH2-), 3.95 (2H, ~, PhC~), S.9~ (2H, mt l-~R and 3-H~,
6.36 ~l~, m~ 2-H), 6~92 to 7.23 (5H, s, C6N,~, 8.43
, purine~ 3 (lH, B, purine-~)-
~5 ~lemen~Al ~naly~i~ fo~ C1gHlgN4O~
~lad~(~)s C,~1~53; ~I,$.16s N,15tlO; C1,9.56
Found ~%); C~61,~; H,5.18; N,15.10; C1,9.54,
Re~exen~e ~am~l~ 12
~ 2-am~no-4-~5~-benæy~oxylmethyl-4~-~hy~ox~!methyl)-
cyclop~nt~2-en -1~-yl~amino-6-chloropyri~ldi.n~l~
~ o a ~olu~ion of ~+) r 5~-benzylox~nn~hy.:.-4~-
(h~droxynn~hyl)cyclop~nt-~-en~l~~yl~a~in~ (3"3 mg, l.~O
nunol.) ~n eth~nol (40 mL) were ~ed 2-amino~ 4,6~-
dichloropyri~ldine ~324 mc~, l.9~ nunol.) and
t~ieth~lamine ~85 m~ 4..8~ nunol.), and th~ rlixture ~a~
he~ted at lOO~C ~or 40 ho~lr~ in a ~i~aled t~:bli~. The
91~ 5~ 16~36~;TAKEDA PATENTS OSAKA Fether~t~n ; 063006601;~43~72
J~
40 ~
~ol~rent wa~ ~lstill~sd off undex reduc:ed pre~!ux6~, ~nd
the re~i~iue W~ uk ~0~ted to ~ ~ilica gel ( .~l: g) c:olumn
chromatocJraph~, eluting wi.th hexane-ethyl ac~ tate
(1~1) . ~h~ ~xac~ion thu~ e~lut~d w~s conc~llt3 ated to
S dr~,rness uncler r~duced pre~;~ure to afford
amino-4 - ~ 5,~-benzyloxy~n~thyl- 4 $~- ( hydr~ ; methyl )
ayclopent~-en~ yl]amino-6~c~10ropyr~idînl t526 mg~
yield 91~) a~ ~ colorle~s oil.
IR (CEICl;,) :3350, 34$0, 1610, 1580, 1570 cm ~ H~NMR
11~ (CDCls, ~0 MH~ 2 . 6 to 3 . 0 ~ 2H, m, 4-H ancl 5-H~,
3 . 1~ t 1~ road g, -OH ), 3 . 61 ~ 4~ C~ nd
CH2~Bn), 4.39 (~H, ~, PhC~2-), 4.g2 (1~, m, 1 :H), 5.27
(~H, ~road ~, -NH2), 5.74 (lH/ ~, ~y~imidin~ 5-H), 5.82
(~H, ~, 2-~I ~nd ~-~), 5.7 to ~.O ~lH, broad. ,~;, N~
~5 7-~5 (5H, 8, C6~
MS s Calcd. ~or Cl8H~N6O2Cl ~M ) M/Z: 3~0.1352. Found
M~Zs 360.2384. ; Calcd- ~or Cl~H2l~4O2Cl* ~M ~ /Z
362. 1322. ~ou~d N/Z: 36;~.1337
Re~er~n~e ~xampl~ 13
( ~ ) -2-}~nin~-4- [5~-~enzylo~cyqneth~ 4~ -
(hydroxy~nethyl)cyclopent-~-en-l~yl]amino-5-l p-
chlorophbn~l)azo-6-~hloro~y~imidine
In ~ mixture o~ ~e~i~ a~, wa~r ~nd r,lçthanol
(17:17:10, 44 ~iL) wer~ di~,~olved (~)-2-aminD ~4-t5~~
25 bengyloxg~nethyl 4~-(hydroxynnethyl)a~clopent-.!wen~
anlin4~6-chloropy~imidine ~1201 mg/ 3.33 nNnol 3 and
sodium acetate (6.6 g~/ an~ ~he ~olu~lon W~ stlrre~
under ice-~oo~inSI~ To ~he ~olution wa~ ~de,l a
cliazonium ~alt ~;olution cooled wi~h i~e ~prel)ared fxc~n
30 a 3N hydrochloric acid ( ~ mL) o:e p-chl~xoani .ine ~ 510
m~, 4 . O mmol . ) and ~ ~qu~30u~q solution t ~ mL, of ~odium
nitrit~ ~303 mg, ~.~ numol,)~ d the ml~turl~ w~
~:irred ~or 6 houx~ oorn temp~ra~uxe~ q~hl! reac~ion
mixtur~ was cencentrated under reduc:ed pxe~sllre. To
35 the collc~3n~r~a~e ~a~ added wate~ to gi~re (~ mi:no-4-
[ 5,~-ben~yloxymath~ ( hydr~ thyl ) c~clc,pl snt- 2 ~en-
91~ 6~ 16~36~jTA~EDA PATENTS OSAKA Fether~ton ; 063006601;#44/7~
~ V ,~
- 41 -
1~-yl~a~ino-5-(p~chloroph0nyl)~zo-6~
chloropyrimidine ~s ~ cry~talline p.roduct (:L;~36 m~,
~ield 80%, re~ry~alliz~d f~om diçhlorome~ha~e -
hexan~).
Yellow amorphou~; m.p.: 208 ~ C; IR (Nu~oll ~ 3450y
1570 cm~; lH-N~ (D~SO-d~, 60 MH~)~ ; 2.6 ~o .~.0 (2Ht
m, 4-H an~ 5-~), 3.t~ ~o 3.~ (4H, m, -C~OH ani -
C~OBn~, 4.1B (lH, d, ~-12 Hz) and 4.41 (lH/ ~t J=l~
~ Ph~2-3, 4.77 (lH, ~ro~d ~, -OH)~ 5O4 tc~ 5.8 llH,
m, 1-H), 5.~2 (~, m, 2-H ~nd ~-H~, ~.17 (5~ H3-
)~ 7.3 ~o 7.~ (~H, m, Clt~6}I4- and -~H23/ 1~.0(, (l~{,
broad ~ H-).
NS: ~lcd. ~or C24H24~6O~Cl2 (M ) ~Z~ 4~8. 13.3.l. Foun~
~ 9B. 1346.; C~la~. ~c)r ~4H~4N6O~ClCl* (]~l ~) M~-
500. 1307. F~uncl M~Z: 500. 12~5~ ; Calcd. for
C~4~24N6~2~12* ~ ~4~ M!~s 5~2- 1337. Found ~,~Z!g S0~.
1325~
;~e~er~nce Exampl~ 14
(I)-2~5-di~ino-4-~5~-bens:ylox~neth~l-4~-~hyelrox~-
me~hyl)~yc~o~ant-~-en-l~-yl~amino-6-chloropyrimidine
To a ~olution of ~ ^2-amino-4-[5~-b~nz1rloxy-
methyl-4~-~h~droxyme~h~)cyalopen~:-2-en~ amino~5-
~p-chloroph~nyl)~o-~-chloropyrimidine ~l3~6 m~, 2.03
m~ol.) in a mix~uxe of ~thanol ~nd watsr (l;:, 80 m~)
~5 wer~ added 7inc powder (1.74 g, 16.6 ~mol.) ,!lnd ~ce~ic
acid (0.~5 mI.). The ~ ture wa~ heatad for :I h~u~s
unde~ ~ef lUt~ . Insoluble~ were i~ ered o~, ~d the
filtrak~ wa~ ~o~cen~rated under reduced pres;!~uxe. ~h~
concen~rate ~a~ su~iecte~ to ~ ~lica gel ~711 g) ~olumn
a~romatograph~ eluting with hexane-ethyl ac~i~ta~e
(l:2). ~he r~c~i~n thu3 eluted wa~ concent:1ated to
drynes~ under r~!3duc;ed ~ ur~ o l:h~ C:o~llc:~ 9ntrfa t:e~ wa~
added ~ s~all ~olume o dichloromethane to g.:.ve
2,5-di~mino-4~[s~-b~n~ylo~methyl~4~-
(hy~o~yme~hyl)~yclope~t~-en~ yl]amino-6-
chloropyrimidine a~ aolori~ cal~s (76~ ~1g yield
91~ 6~3~;TA~EDA PATENTS OSA~A Feth~r~t~n ; 063006601;~45/7
~ 4
76~3. m.p~: 107 to 10g~C;
I~ (CHC13) 5 35~0, 1610r 1560 cm ~ 7MR(Cl~C:1.3, ~0 ~z)
ô~ 2.7 ~o 3.0 (2H, m, 4-~ and 5-H), 3.08 ~4~, bro~d
0, --~z x ~ 4 t4H, ~ C~OH ~nd --C~03311 1, 4.40
~H, ~, PhCH2~ 4.75 ~1~, bruac~ a ~ -OEI), ~.~' 4 (1~ m
l~I)r 5.82 ~2H, m~ 2~H ancl 3-~), 6.11 (1~ :b:load ~,
3-10 Hz, ~ ), 7 .24 ~5H, ~, C6Hg~) .
MS s Ca1ad. ~or Cl~H~2N5O2C1 (M+) M~Zs ~7S. 146.l. Found
~æ~ 375. 144~. ~ Ca1~d. ~r ~18HZZN~O~C1* (~+
377. 1~31. Found M/~; 377. 145~.
R~f ex~n~e EXA~P1e ~5
Amin~-9 c5~-ben~yloxyme~hyl-4~ hydrox!~ethyl)
~Y~1OPent-2-en~ 1]-6-Ch1OrOPUrine
( * ) - 2, s -Di~mLno- 4 - ~ 5,B-ber~ylQ~ th~l - 4 ,~
(hydro~ hyl )c~yclc:p~nt-2-en-1,B-srl]amlno-~-
chloropyr~nidine ( ~06 mg, 0 . 55 mmol . ) was ~ul;pen~ed i~
ethyl or~ho~ormat~ ~ 3 m~ o thle susp~3n~;.0ll . was
added, whil e ~irrln~ und~3r cooll;n~ with ic:o wi~ter, l~N
hydrochloric acid ~0~14 n~). ~rhe r~a~tion m:.x~ure was
~stirred ~or 14 hour~ a~ rt~om ~emp~Y~ature~ arld wa~ than
c~nc~ntra~ed und~3r ~e~u~ed pre~sure, The c:ol~cen~rake
was di~olve~, in tetrah~rorllr~n ( 3 m~) r ~0 ~Jnich was
wh$1e ~tirriIIg under coolin~ with ice . ~ . 5~7
hycl~ochloric a~id ~ 3 ~ , followed 1~y ~$irri~ f
hourg at rooTn ~;~mper~tllre. The r~ction mi.x :ure wa~
neutxali2~d with a ~N solution of ~odium hyd:~xide i~nd
w~ then concentra~ed ulld~3r ra~duc~sd pr~u~ he
aoncerltxato was sub jected to a ~ilica gel ( ~0 g ) column
chromatogr~aphy, elutin~ w:L~h chloroform met;hiulol
(50~ he fractlon thus elut~d w~ con~e~;xated to
dxyne~s undsr rodu~ed pres~urQ to ~ord
( :t ) -2-am~no-~~ t S~-benzylos~ ~h~ ,B-
(h~droxyme~hyl)c~clop~nt-~-en~ yl~6-chlc~repuxine
(1~3 mg, ~ie1d 53%) a~ ~ CO1Or1eS5 Oi1.
I~ (CHC~ 3450, 1610, 1570 C~ N~ C[X'13, 6~ krH~)
~; 2 . 8 to 4 . 3. ~ 7H ~ 4 -H, 5-H ~ 2~ t -C~12C~ 3n ~nd
91~ 5E lBli~3~;TAKEDA PATENTS OSAKA Féither~t~n ; 06300~601;#46~72
- ~3 -
OH), ~.0~ (2H, s, Ph~H2-), 5.2~ t2H, bxoad ~3, -N~
5.54 to 5.~0 (2~, m, 1-~ an~ 3-H), 6.22 (1~, ml 2-H),
~.85 to 7.36 ~SH, m, C6H5~), 8.0~ (lH~ ~, pu.r;l.ne 8 H).
M~ ~ Calad. ~or cl9~20~5o~Cl M/Z : 385. 1305. ~ound X~'Z
t 385. 1289, ; C~lad. for Cl9~20~502Cl* (M~2) ]'t/~ : 387.
1275, ~oun~ ~/Z3 387. lX42.
Re~e~anc~ ~x~mple 16
2~N-(3-methox~-2-n~ethylacrylo~ arba~oyl]~ n-
b~n~y1~xymeth~ azab~cyc,lo[~,~.l]hept-~-en-3-one
To a ~olutlon o~ 3-methoxy-~-m~thylacry:l.oyl
chlo~i~e tl7~ mg, 1.3~ mmc~1.) in anhyd~oux benz~ne (10
mL) wa~ ~d~d ~llver cyanAte ~ 293 mg~ 1 . 95 m;n~ol~)r and
~he mi~ture w~ ~tir~ed ~ 3 hou~ at 50~.
In~oluble~ were ~iltex~d o~. To the ~lt~ e wa6
add~ 7-~yn~en~yloxymeth~1-2-az~bi~clo~2.2.13hep~-5-
en-3-on~ (~00 m~, 0.87 ~mol~) obtaine~ in Re;l:erenc~
Example 1~ The mlx ure w~ tlrr~d ~or fu~ e~ 5 h~r~
at 50bC. rhe reactLon mixture was ¢once~tral;ed undex
reduaed pres~uro~ and ~h~ co~aen~ate was ~u!l-~c~e~ ~o
a ~ a gel (10 g) colu~ ch~omato~raphy, e:.utin~ wi~h
hex~ne~e~h~l ac~ate (2~1~. A~ the prec~delnl, ~ra~lon~
2-lN-(3-m~hox~-2-m~thylao~yloyl)-o~r~amoyl. ] 7 - ~yn-
~ehz~lox~me~hyl~2-azabicy~lo~2.2~l] hept-5-en 3~ono
(113 mg, ~ield 41~ was eluted, ~hen, a~ th~ 3ucceedin~
~ractlon, unreact~d 7-syn~enzylo~methyl~
azabicy~lo[2~ hep~ e~l-3~on~ (54 m~, re:c~:~v~r~
r~te:31~) W~$ ~lU~
2-fN-t3-m0~hoxy-~-lneth~lacryloyl)carbamoyl~ syn~
~en~y~oxyme~hy~ za~icyclol2.~l]hep~-5-en 3-one:
3~ colorlex~
IR (C~Cl3) o3300t 1755, 1730r 163~ C~l "
60 M~lz)~: 1.82 (3H, ~, -CH3~, 3.17 ~lH~ m~ , 3.4S
(3H~ m~ BnOC~Iz- a~ld ~ H3, 3.8S (3H, s, ~OCH~,3, 4.56
PhCH2-), 5.24 (lH, m, l~-H), 6.52 (lH" m, 5-~31
6.~3 (lHr m~ J-5, 2Hz~ 6-H), 7.27 (lH, ~, ~cryloyl
3~ 7.32 (~H~ ~, C61i5-), 10.50 ~ b~s~at ~ H-)~
9~ 5~ 1D~3a~;TAKEDA PAT~NTS OSAKA Feth~r~t~n ; 06300~601;~47/7
_ 44 ~ G V g
MS Ca1~ Or C20H22~25 (M ) Mt~: 370.1529. F~
D~Z: ~70 . ~497 .
R~ere~ce Exan~ple 17
5ac~ yL~xym~thyl 4)~- hydr~ymeth~ ' ( 3
S m~thOXY_2_~thOXYaCr~1OY1)UreidO]CYa1~Pent-~ ene
TO ~ ~O1UtiOn O~ 2~N-(3-methOXY-2~meth~,1aCr~1OY1)
CarbamOY1~-7-~Yn-b~nZY1OXYm~hY~
aZabiCYC1O[~,2.1~heP~_5_en_3_One (118 m~ 0.:'.1 ~mOl.)
in ~hYdrOUS me~hanO1 13 n~ W~ ~dded ~Odi~ll
~OrOh~Qri~e (60 mg, 1.$8 n~O1.) Under COO1~n!;- With iCe-
Wat~r, and ~h~ mi~tUre Wa; ~tirred fOr on~ :h~ r at ~oom
~empe~ature. lrhe reac~ion ~nix~ure wa~ n0u~r.!.1iz~d with
aaetic a.cld-methanol (lsl~ ar~d ~a~ then conlc~!!ntrated
~nd~r xeduced pres~u~e. ~he ~ncen ra~e ~a.s subjeated
~ ~ æilic~ gel ~10 g) ~olumn ~hromato~raphy, elutln~
with hex~n~-ethyl aaetat~ 1). The raction th~s
~lu ed w~a~ concent~tec~ under xe~du~ed prC:o~;ul.e. ~o th6
concen~rate wa3 add~d dichlorom~than~-hexane to afford
(+)-5a-be~z~lox~me~hyl~ h~droxymeth~ 11 ' ( 3-
metho~-2-mathoxy~cr~loyl)ureido]c~clopent-2-ene ~s
colorl~ n~edle~ (97 mg, yleld 81~). ~.p.: 113 ~
114 C ~ C~C! 13 ) : 3 4 5 0 ~ 3 3 0 () ~ l l; 5~ O r 11i 21:1 cn~ H-NMR
~CDC~13~ ~iO ~q~z~ 1. 7~ St -CH3) ~ ~ 12 ( LH~ m~ ~-
H), 2.76 ~1~, m, 4-~), 3.3 tO 3.8 ~5H~ m, -C,!I~OH and
~5 ~nOC~2-), 3.~1 ~3H~ S~ ~OCH3), 4OS7 t~H, ~ P;1CH2_),
4.77 (1H, m, 1-H)~ 5~7~ H, ~t 2-~ and 3-~). 7.31 (6~l,
~, C6H5- and ~Cr~1OY1 3-H)~ 8.~7 (1H, brOad ~. -N~
8.90 (1H, b~Oad dl J~10 HZ, -NH-).
MS ~a~d- fOX ~ZOHZ6~2O5 (M ) N/Z : 374.184X. POUnd
M/Z S 374,1825,
R~erenCe E~amP1e 18
( + ) ~ nino-4- 1 5~benz~ o~{yrne~hyl-4~5- ( hydrc~ rm~thyl )
. c~clopen~_2_en-1~-yl]amino~-chloropyrlmi~i.n
~ [Sa~Benzyloxymeth~l 4
(hydroxymethyl)c~yclopent~ n l~-yl~mine c~;alned i~
Re~erencl3 Example S t49~ y~ 1.3f~ mlnol) w~ ub~eot~d
91~ 5~ 16~36~;TAKEDA PATENTS OSAKA Fether~t~n ; 063006601;~8/72
2 ~ ~J ~ ~ ~ ~
- 45 -
su~tantially the same p~ocedure a~ ~efex~erl~
~ample 1~ ~v give ( ~ amino g ~ 5u-b~anzyl~ nethyl-
4,~- ( hydros~me~hyl ~ c~c lopent - 2 -en~
yllamin4 ~-~hloropyrimidine (552 In~, yi~ld l3~ %~ a~ a
5 colorle~ oily produc~
IR(CHC13~ : 355()~ ~450r 1610~ 1580, :1.570 ~m I; H-NMR
~CD~13, 60 ~E~æ) s 2.11 (I.H, m, S-H), 2.~4 ~:IH, m, 4-
X~, 3.35 (1}~, hroad ~, -OEI~, 3.4 ~o 3.7 ~4H, m, -CH~OH
and -~20Bn), 4.54 (2H, ~, PhCH2-), 4~65 (l~:I, m, l-H),
1~ 5.21 (2H, ~roa~ NH;2) ~ S.41 (1~, broad ~, JalO H~ -
), 5 . 7 0 ( 2H, 8 ~ 2 -~1 ~nfl 3 -H ~, ~ . 7 9 ( l}I, ;3
pyriln1d1ne ~ ), 7 . 28 ( 5H, ~, C6H5- )
~S ~ Calcd. M/Z ~or C~ 21N4~ (M ~: ~601; 52-
F~unà ~/2 o. 360 .1; ;23,;
1$ ~alcd. M/Z ~or CL8~21N4O~Cl* (~ ~ 2~ 362-1
Found M/2 362.1306.
Re~enc~ ~x~unpl~ lg
A~Lno-4~ ~5~-benzylox~eth~l 4~-(hyd~ox!~ethyl)
. a~clopent~2-en 1~-yl]amino-S-(p-chl~xophenyl;azo-6-
chlo.ropy~lmidine
~+)-2-Amin~4-cs~-b~I~zyloxym~hyl-4~-(h!~r
m~thyl)cyclop~nt-~-en-l~ yl)a~ino-6-~hl~roE~ .imidi~
(498 mg, 1.38 m~ol) wa~ subjected to sub~t~nl:ially ~he
~ame p~oc~dure as ~ef~r~nce ~xample 1~ to ~i~re (~)~2-
amino-4-~5~-~en~loxymethyl-~-thydro~m~thy~
cyclopent-2-en-l0-yl]~ino-5-(p-chlo~ophen~ ~æo- 6~
chloro~yrimidine a~ cry~ 55~ mg, yleld ~
~llow ~morphou~ c~stals ~recryæ~ ed ~rl~m
dichloromethane-hexane) ; ~.p.: 202 to 205~C
IR(Nu~ol) s 3450, 1570 cm 1; 1~_N~R ~V~o-cl6 60 ~H2)~
~ 2.~ , m, 5-H), ~.67 (l~, m~ 4-~, 3.4 ~o 4.1 (5~,
m, -CH20H, -C~20~n and -OH), ~51 (2H, ~, Phl~ , 5.20
~ m, 1-~), 5.82 ~HI m, ~-H ~nd 3~H), 7.26 (5H, s,
C6H5-), 7.3 to 8.0 ~6~, ~, ClC6H5- ~nd -NH,)), 10.33
~lH, bxoad ~ 8 ~z, ~
~S ~ Calcd. M/Z o~ C24H~4M62~12 (M ) ~ l98.1~7.
.
91~Fll,q 5~ 313~;TAXE~A PATENTS O~AI~A F~ther3t~n ; 063008601;#4g/72
~ ~ ~J
- 46 -
~ound M~Z ., 4~8.13~0.;
Calc~ 2 ~or ~4~24N6O2~1Cl (~ 2) ^
Found ~JZ ~ 500~1~10;
C~4~4N~02~ (M+~4~ ~ S02 1337
Found ~J~ ; S02.1324.
Re~er~nc~ Exampl~ 20
~+)~,5-Diamlno-4-[5a-benzylOx~meth~1-4~-
(hydr~xymeth~l~cy~lo~ent-~-en~ yl]~mino-6
ahloro~yrimidine
(~2-Amino-4-~5~-b~n~yloxyme~ 4
~h~droxymethyl)cyclop~nt-2-en-l~-yl]a~ino-5-lp-
chlorophe~yl)azo-~chlorolE~yrlmldin~3 ~520 mg~ 1.04 mm~l)
was.~u~; ct~d to sub~tanti.all~ th~ s~me proc/ldure as
Re~eren~e Example 14 to gi.~e (~ ,5-~iamin.o 4-~ga-
~nzyloxyme~yl-4~-~h~droxymethyl)~y~lopent~ en-l~-
yl]ami~o-6-chloropyrim$dille as cry~talæ ~231 mg, yield
53~).
Colorle~ n~edle~ (cxyhtalllzed from dL~hlorome~hane -
hexane) ; IR (CHC13) : ~520, 1~10, lS60 cm 1; H~NMR
(CD~13, ~0 MH~)6 : 2.1~ (:LH, m, S-H), 2.7~ , m~ 4-
H), 3.~ (3H, broad ~ and O~), 3.1 tv .l.9 (4~ m,
-CH20E~ and -C~OE~n), 4.5~ ~EI, 8, PhCE12 3, 4 68 (2H,
broad ~ -NH2), 4-74 (1~, m, 1 H), 5.70 (2~, ~, 2~ and
3-~), 5.74 tlH, b~o~d d, ~=9 H~, -NH-), 7.~7 (5
~5 )-
MS: C~llc:~. Mf3 ~ox C18H2~ 5 2 ~ ) s 3'l5.1461.
Poun~ ~Z : 3't5.1474;
Cal~. M/Z ~or Cl~H~N502Cl* (M +2) ~ 3'17-1431^
Foun~ M/Z : ~l7.1468
Reference ~xampla 21
( l )-2-Am~o 9~[5~-b4n~10~ymethyl ~4~-~hydr~e~hyl~
c~clopent~ n~ 13~-ahloro~urine
(i)-2,5-~a~ino-4-~5(x-benz~loxymethyl-4,3-thy~xo~
methyl)~yclopent-2-en~ ]amino~6-chloropy.^lmidine
~150 mg, 0.40 mmol) was ~ubjec~ad to ~uh~t~ iall~r the
~ame procedure a~ Re~erenae Example 15 to gi7e (~
91~ 5~ 16~36~;TAK~DA PATENTS OSAKA F~ther~t~n , 0~3~o66ol;#5Qi~2
- 47 ~ ~ v!"
a~ino-9~5a-ben2~10~methyl-4~-(h~droxymeth~
cyclopen~ en-l~-yl]-6-ch.loropurine (142 ~a, yleld
92~) as a c~lorl~s ~il.
IR(CHCl~) ; 345~ 1610, 1570 cm 1 1H-NM~ ICl3, 60
~Hz)S ~ 2.5 to 3.1 (~, ml 4-H and 5-H), ~7C (2~, d~
6 Hzt CH20H~, 3.B to 4.1 (3H, m, -CH~ORn ~ OH),
4~55 ~HI ~, PhCH~-), 5.27 (2~ br~ ~, NH;), 5~38
(lH, ~, 1-H)~ 6.0~ (lH, ~, 2-H), 7~32 (5H, IS,, ~H5-~
7.86 (1~, ~, purine ~H)
Reference E~ample 22
~lS, 5R, 6S, 7R)-6-t-~utyldimethylsi~loxym~31:hyl-7-
hydroxim~thyl-2-oxQ~i~yclo~.3,03Ootan-3-~n~
~1~, 5R~ 6S~ 7~)-7~B~!nzoyloxy~nethyl-6-h~;dro~2-
oxabicy~o~3.3~o]o~tan-3-one ~.76 C~i, lO ~m~:l..) wa~
gradually added to a 2N NaOH-~eOH solutlon ~'.0 ml)
while ~tirring . The mix~urq w~ stirred ~.r lS minut~
at r~o~ t~p~ratur~ ~d the~e wa.~ then a~de~
hy~xo~hlor~c a~id unde~ ic,e-cooli~g to mak& I.he
~lution acid. The ~i~ture wa~ ~ub~eated ~o
~0 ~istilla~i.on to dr~nes~ utlder redllce~ pr~su:l~e. The
~esidue was d~o~v~d in athyl ~ t~t~ - MeOLE ~Ssl~
and the 801ution was ~u~e3ct~d ~o a silica g~l column
~hromato~raphy. ~lution ~as per~o~med u~n~ th~ ~ame
solven~ sys~em t~ give 1.72 g ~uant~tati~e~ ) of (lS~
2~ SR, 6S, 7~)-7-hydroxy-6-hy~ro~y~eth~ ox~ .a~clo-
r 3-3 7 03oat~n-~-ons.
(1$~ 5R~ 6S1 7R~-7-hy~rox~-6-hydroxym~tl,lyl-2-
o~a~icyclo~3.3,0~oc~an~ one (1.72 g~ 1~ mm~ .) was
~ olved in D~F (30 ~1), To ~he ~oluk~on wl~e ~dded~
under i~e-~:ooling, t-butyl dimethylsilyl ch.lorlc~
(~BDSCl) ~1.80 ~ mmol~), th~n ~m~da~ole: l1.63 g~ 24
mmol~). Th~ rnlxt-lre wa~3 lef-t ~tandin~ ~03: 5 hour~ at
~oom tempex~tur~ and wa~ p~ured in~o ice-~.t~
~ollow~d by ~xtraction with e~har. The ethel~ layer ~a~
3~ dri;ed 4~r anh~rous ~odium ~ulfa~e, then ~tller wa~
~i#tilled off. The r~sidue was ~ub~ected t~ a silica
91~ 6~ 36~;TAKEDA PATE~TS OSA~A F~h~r~on ; 0~3006601;~51f~2
,,3, ~J
_ 48 ~
gel column chromatography, elutin~ wL~h ethy], ~cetate-
hexane (lsl), ~o giv~ 2.55 g ~89~ of (lS, S~i, 6S~ 7~)~
6-t-Butyldime~hyl~ilylo~y~l~thy~-7-h~droxym~yl 2
ox~bic~cl~t3,3,0~o~tan-~-one~ m.p. 58-59C.
~a]25~ 12.~9 (c-1.8, CHCl3)
Re~e~enc~ ~xample 23
~lS, SR, 6S) ~-t Butyldimethyl~lyloxymethy.L
o~abLcyclo~3~3.o]oct-7-en--3-ona
(1~/ 5R,. 6S, 7R~6-t~Butyldi~ethyl~ lc~x~me~hyl-
7~hydroxy-~ox~ yclo[~.~l.O]o~an-3-one (l.l~ g, 4
mmol.) ~nd O-n~tro~en~ne~i~le~o cy~nide (l.l. y, S
mmol.) we~e dl~solved ln ~nhydrvu~ THF (~0 m~ o the
~olutio~ war; addecl ~ropwis;e, in nitrogen 9t r~i~a~
tri~u~ylpho~phine ~l g, 5 mmol.) while ~tir~;l.n~ ~nder
ic~-cooling. The mi~ture w~s ~tirrod for 15 hour~ ~t
room temp~ratuxe and there w8~ ~hen add~3d 30'i: E~202 (3
ollo~red by ~irr~ng ~or ~urther o~e h.ou~ a~ room
~emperature, The reaction mixtur~ wa~ ~a~ ireakly
alkaline wi~h a ~atura~ed aq~eous ~olution o:l. NaHC03,
to which was then adde~ h~r (50 ml), ~oll.o~i~ed ~y
washin~ wi~h water. T~e organic layer wa~ d~ then
~h~ ~oiv~nt was d~ led of~ und~r reduaed l~r~suxe,
~nd t~e residu~ wa~ ~ubje~ed to a ~i1iaa ~e:. ~oluunn
chromat~graphy~ ~luti~g wl~h hexane - ethy1. ,l~a~tate
(3;1) to o~t~in 0.7 g t69~) O~ (lS~ SR~ 65~ t~
~u~y~i~o~hy1silyl~xynn~th~rl-2~ox~bi~y~lo~3.3 0lo~t-7-
en-3-one a~ a colorless o~l.
[~]u + 15g,4 ~c=l.O, ~HCl3)
Ref~rance Example 24
(lS, ~S, 6S)~ tyl~im~hyls~lyloxym~th~l 4-~y~r
2--ox~J~ a 1~ [ 3 . 3 . O ~ oct--7 -i~n - ~ - ono
(lS, ~ s)-~-lt-Bu~ imethyls~lyl~x~ml3~hyl-2-~
oxabiay~lo~3~3.o]oct-7 -~n--3-one ( 1. OD ~, 3 . 7 I mmol.)
was dissolvod in anhyd~ou~ ~HF ~40 ml). ~c) :he
~olutio~ wa~ ~dd~d ~ 7~C ~Ho~Ds ~ 0 . 5M he~a~
solut~on~ (15.9 m1, 7.94 mnol.). The mix~lrl~ w~s
91~ 16~36~;TAKEDA PAT~NTS OSAKA Fether3t~n ; 0~300B601;#52~72
$
4g -
~tirr~d for 15 ~inu~es ~ the same kempe~a~u~:e~ to
which wa~ ~dded~ a~ one ~roke, MoO5.~D~PA.P y (Z.58 ~
5.60 nunol~ ollow~d by ~tirring for 3 hourSI. To the
r~sultant ~ix~ure were added at -78C 10% HC]. (2 ml)
S and a sat~rated ~q~eous ~lution of Na~03 ('l~9 ml)~
which was ~h~n war~ed gradually to ~oom t~mp~rature.
To th~ reaction m~tur~ was ad~ed wa~err wh:Lch w~
~ub~c~d to ~x~xaation with ethyl ace~ate. ~he
e~t~ct wa~ driod and the ~olvent was diætiL:Ied ~f,
Th~ resld~e was ~ub~ec~ed ~o a sili~ gel co:l~mn
chromatography~ eluting wi.~h he~arle - ethyl ~! ce~ate .
t4;1) to r~ove~ ~15r 5RI c~
Butyldim~thyl~ilylo~ymethyl-~-oxabicy~1O[3.3,~oct-7-
en 3~one (42S my, 42~5~). Elution wa~ ~ur~hlxr
con~inu~d ~o obtaln 468 m~ (44~) o ~S, 5S, 6S)-6-t-
~ut~ldimethy~ lo~me~hyl-4-hyd:roxy-2-
o~ea~icyQlol3.3.0]oot-.7~ 3-on~.
(M~13Si~2NK
~,~, *
0xo~iperoxymoly~denum(p~ri.dine)~hexamethylp~h/l~phori~
tri~mid~
E. Vede~, D.~. En~ler, and ~. ~. T~l~chow
J. Org. Ch~., 43l 188(197~)
Re~erence Example 25
2S (15, SS, 6S~6-~ Butyl~im~thy~ yl~K~methyl~,4-
dihyd~o~y-2_~xabioyclo~3.3.0]oc~_7 ~ene
(lS, ~S, ~S)~ utyldimethylsily~ox~ hyl 4-
hydro~y~2-ox~bi~clo[3.3.0~oct-7-en-3-on~ (4l;8 mg~ 5
m~ol.~ wa~ di~ol~ed in anhyd~u~ ether (lO rll). To
the soluti~ was added ~du~lly ~iAl~4 ~6~ m~/ 1.66
mmol.) in ar~on ~r~am~ w~lile stirri~ und~r i~~
cooling, The mlx~-ur~ was s~ ed for 1. 5 hour and
th~re w~ ~hen added saturated NH4Cl (lO ml.) an~ lO~
HCl ~.5 ml)~ Th~ mixture ~as diluted with !~hyl
3 5 aceta~e, then washed wlth water. ~he or~ er wa~
d~ie~, then the ~olvent wa~ di~tilled o~ un~:ler reduced
91~ 5~ 16~36~;TA~EDA PATENTS OSAeA F~thergton ; 063006601;#53f7
- ~0 - . '
pres~ure to lea~e 447 mg o~ (lS, 5S, ~S1-6~
Butyld~me~hyl~ilyloxymethyl-3,4-dihydro~y-2-
ox~icyclo[3.3.0~oct=7~ens~, which wa~ u~ad ~:r the
~uccQ~ing rea~t~on with~.t re$inin~.
$ Reference Example ~fi
(lS, ~ 6-t-Butyl~ime~hyl~ilyiox~.m~lthyl-3,4-
dihy~ro~y-2-o~bicyclo~3~.3~o]oct-7-ene (321 mg, 1..l2
mmol.) ~nd K2C03 (~7~ ~, 2.01 m~ol.) w~re ~ pended in
anhydrou~ benzene (10 ml), ~o ~h~ ~uspensior. ~a~ adde~
P~0~)4 (51~ mg, 1.15 mmol.) in li~ d amol;nts, an~
th~ mi~ture was ~irred ~o~ one hour at room
t~mperature. T~ the: ~e~c~:iorl ml~ture~ wa~ ads:.ed
~atu~ated NaHOC3 to make render the pH 7, ~o:llowed by
extrActio~ with ~hyl ac~t~te. Th~ extrac~ as dried
o~er anhydrou~ Na~SO4, follow~d by distillin~; of~ the
solvent under reduced pr~sure to ob~ain 27B mg (87%)
o~ (lS, 4sl sRj-4-~-Butylcli~th~ yl~x~m~t~
form~loxy-5-~mylcycloperlt-2-ene.
Refer~nce ~ple ~7
~lS, 4~, SS)~4~ Butyldlm~hyl~ yloxyme~h~l l-hy~roxy-
5-~hy~xox~methyl3 çy~lopen1;-~-en~
(lS, ~S~ SR~-~-t-Butyldimethyl~ilyloxymethYl~
form~loxy-5_fo~ylc~c~operlt-~-en~ ~271 ~, 0.95 mmol.3
w~ di~solved ~n anhydrou~ eth~r (5 ml), ~'~ th~
~olution was adde~ LiAlH4 ~50.5 mg, 1~33 m~o.:.. 3 in
ted ~molln~3 wh~ ;ti~n~f undsi,r ic~-cclca~ g in
argon strea~3~ The mixtuxe wa~ ~tirred ~or ~:\ne houx at
oac, ~o which were added a ~atura~sd a~ueou~ ~olution
o~ N~14Cl ( 5 ml ) and 10~6 Ht~l ( 0 . 2 ml ) to make the
resul~arat ~olu~lon weaX~y ~cid, ~oll~wed ~ ~l~xk~ ion
wi~h ~Lh~l ~ae~te. Th~ ~3xtrac:~ wa~; dried, I:hen the
solv63nt- was di~illed of~ under red~lced p~ ure. ~he
re~idu~ ~a~ ~ub~ec:~eci to ~l ~illca ~el ~olumn
~hromatograph~, ~lu~ing with hRx~ne ~ ~thyl ,!~cetate
~ / to obka~n 155 mg ~63~) o~ (lS, 4S, SSI-4~t-
Butyldime~hyl~ loxymethyl-l-hydroxy~5-
91~ 5~ 36~;TAKEDA PATENTS OSAKA Fether3torl ; 06300B601;~64/~2
- 51
~hyd~ m~thyl)cyclop~nt-2-ene.
Re~r~nce ~xample 28
(lS, 4S, 5~)-4-t-Butyldim~hy~ yloxymethyl-5-t-
butyldiph~n~ yl~ime~hyl-l-hyd~oxycy~lop~l:t-2-ene
~1~, 4S, 5$)-~ ut~l~imethyl~ilylo~in~ yl-1-
hydro~y-5~(hydrox~methyl)~clopent_2-ene (1:3l m~, 0.507
m~ol.) and imida~ole (75 mg, 1.~0 mmol.) wer6. dl~olved
i~ ~MF (1 ml)~ Tv ~he ~ol,~ion was ~dd~d T~IP~Cl (148
m~, 0.542 ~ol~) dropwi~e gradu~lly while s~ ring at
0~ he mix~r~ wa~ then s~lrred for one hcur at 0C~
~ollowed by ex~action wlt;h ether~ The ethe~: layer was
dri~ nd ~h~ ~olvent wa~; dis~ e~ ~ff. ~rl.o re~idue
was ~ub~ected to a ~iliça gel column ch~omatc~raphy,
elu~ln~ wlth h~xane - ethyl a~eta~e ~10.1) tcl o~ain
14~ mg ~59~) of.(lS, 4S, 3S)~4-t-
Butyldi~eth~l~ilylo~ymeth~1-5~t-
bu~yl~iphenyl~ilyloxymethyl-l-hydxo~cyayalope~ ene.
Referen~e E~mple 29
g ~(lR, 4S, s~)-4~ utylcllmethy~ oxym~t~:~yl-5-(t
butyldiphenyl~ilyloxyme~hyl~cyclopent-2-en-1 yl]-6-
chloropurine
45! 5s)-4-t.-~utyldimeth~ ox~rm~ ;hs>l-S-t;~
btl~yld~p}~n~l~ilyloxymeth~l-l-hy~:rox~rcyclope;~ 2-~ne
( 138 mg, O . ~B mmol . ), 6-chloropurill~ t44 mg~, O . 2B
mmol~) and tripheny~pho~phine ~147 mg, 0.2~ ~ol~) ~er@
di~ol~r~cl in ~nhycl~ou~ ~HF ~5 ml'~ rO th~ OE~ ut~i~n ~a~
added 90~ diethYl azs:dicnrboxyla~e ~EAD) (;.0~.6 m~
O.S3 mmol~ dropwise at -30~C while ~ ring ~h0
mlxtur~ wa~ le~t ~t~nding ~vernigh~ at room ~e~pera~ure
an~ was ~hen ~ubjec~ed to a slli~a g~l cul~mll
ahro~a~og~aph~, ~luting with hexane. - ethy;. acetate
(4al) ~c~ o~tain 64 mg (36!~) ~ ~-r (lR~ 4S, 51~ 4-t-
Buthyldimethyl~ilyloxyme~]l~l S-(t-
b~t~ldiphenylsil~loY~ymethyl)c~clopent-2-en-1 yl]~6-
ahloxopur~n~.R~forence E~mple 30
g~ 5~ 1D~3~;TAKE~A PhTENTS OSAKA Feth~r~ton ; 06300a601;~55~7~
6~ 3 J~ ~
52
9 [ ( lR ~ 5R ~ 4, s-Di l hy~ro~ thyl ~ ay~lo]E~! n~ n
yl~adenin~
~-~(lR, 4S, SR)-4-t~utyldimeth~ls~lyl~ yme~hyl-5-
(t-~ut~ldiphenylsilylox~m~thyl)~yclopan 2-erl-1-yl~
c~loropurln~ (63 m~, 0.1 ~mol.) w~8 ~i solvecl in
methano~ (20 ml). ~o the ~olutlon wa introclu~d
ammonia ga~ ~or 5 mlnute~ under csoling with brine.
The resul~n~ ~olution wa~ hea~ed ~ ~O~C foI 11 hour~
in ~ sealed tube. FrQm ~he r~ac~ion ~ixtur~a, exces3
ammonia and the 801~ent we~e dist~lled of ullde~
r~duced pressure to obtain ~ t~ S, 5~ t-
sutyldimethy~ ylox~methyl-5-(t-
~uty~iph~n~yl~ilyloxymeth~l)cyclopent-2~n-:L-
yl]adenine. T~i~ wa~ ~is~4lvb~ in THP ~1 ~1'l. To he
lS solukion wa~ added 1 M BU4~F-THF ~olution (1)"3~ ml),
and the mix~ure was left standlng for 2 hour~ o the
r~s~ltant mix~r~ wa~ addq,d waterr whi~h wa~3 washed
with ~thyl a~e~a~e ~d ~uk~ec~d t~o evapora~i:on ~o
dryne~s und~r redu~ed p~e~ure. ~he reæi~ue wa~
~u~ected to a silica ~el ~olumn c,hro~atogri~hy,
elu~ing with CHC13~0~ to o~in ~ g (3~) o~
9-~lR, 4S~ 5~)-4~ 5-Di(hydroxyme~hyl~c~clo]?~.nt~ n-l-
~l]adenine.
m.p. 2n4-206OC ~eOH)
~D - 24.30 ~0.6, Me~H)
~orre:~ponding rclGemlG modi~lc~iorl eepara~ed b~r m~an6
of liquid ~hro~atography (~xample lS~:
m.p. 206-207C (MsOH~
~ D~ ~ 24~71 (~1.4, ~leo~I)
E~ampl~ 1
5~-Benz~loxyme hyl-4~~(hydroxyme~hyl)~:yclopent-
~-en-l~-yl]adenin~ and (~)-g-[5~-b~zyloxym-3lhyl-4~-
h~droxymethyl~cyolop~nt-2-0n~ yl]-6~methox!!pu~i~e
In~o A ~0 1 utlon o~ ( ~ ) -9 ~ 5~-ben~syLoxym~i ~hyl-4,~-
th~o~ hyl )cyclc~p~nt-2-er~ -y~ hlo~ purine
.
91~ 5~ lB~36~;TAKEDA PATENTS OSAKA F~the~ton ; OB300~601;$6B,~2
'
- 53 --
(lg~ m~, 0.53 mmi~1. ) o~t~ine~i in Re~er~3nce E~iampl0 10
in anhydrou~ me~hanol (20 mI ) wa~ int~oduced, while
cooling with ~odium ch~ori.de-ice, ga~3eou~ ~mrloniA for
one hour. Thiæ ~olutioll wa~ heatecl at 50C ~ a ~ealed
tubei for 20 hours. Thç xea~tion mi~tur~ wa~
concentxated ~n~le~ reduced p~essure, ~nd ~hl
concentr~t~ W~5 sub~ect~ o a ~llLca gel ~ ;I! g) colu~on
chroma~ograpll~y, elu~i~g with ~thyl ac~ate-m~ thanol
o ~epara~e ( i ) -~ - [ 5ct,-b~3nz~rloxymethyl- 4 ,3-
~h~roxymethyl)c~alopen~-2 en~ yl3
adenin~ and ( + ) 9- [ 5a-ben%~loxym~i~hyl-4B-
~hydroxymet~yl )cyclapent~2-en~ yl]-6-m~tho:i;ypurin63.,
( ~ ) - g - [ 5a-~Bexl~yloxyme~hyl- 4 ~- ( hydro~ne'chyl ) c yclopen~-
2-en~ yl)adenin~ rield 144 my (77%); colclrles~
needle~ (c~tallized fr~on~ dichloxome~hane); In.p,: 171
t~ 173~;
IR (Nujol~ so~ N~R (DMSO~-d6, 500 ~2)~ ~ 2.4
(lH, ddd, ,J ~5, 5, S, 5 HZr 5-EI), 2.72 (lH, m, 4-H),
3.S2 (lH, ddd, J-ll, 5,5H~:) ar~d. 3.~1 (lH, d.d~.l, J=llr
5,5 Hz) t-CHZOH), 3.66 (2~i, m, ~nOC'}~ta-), 4.46 ~lH, d,
J~12 Hz) and 4,~ (lH, d, J~.2 Hz) ~PhCII~-), 4.77 (lE~,
t~ JY5 Hz~ -~)t 5.S2 (l~r ddd, ~-~, 2, ~ H),
5.7~ cld, J-6, 2, 2 Hz, ~-H), 6.66 ~1~', dcld, J~
, 2 -~ ), 7 . 17 ( 2H, l~oad s, `-~H2 ~ r ~ ~ ;' 8 ~ 5~
C6~5-), 8.05 tl~I, s, purine~H), ~.13 (lH, ~, I?~rine-HI),
Elemental ~nal~is ~or Cl9~2lN5C)2:
~alcd. t%) : C, 64 .94; ~, ~;;02; N, 19~3.
Founcl (~ C, ~S.13t H, 6.07; N, 19.~3.
506-B~3nzylo~ylneth~1 4,B- (hyd~oxymethyl. ) ~::ycl~pent-
2-on~ yl3-~--m~hoxypurin~ ~i Yield 18 ~g (9i;);
aolorless c>11;
IR (C~Cl3)s 3450, 1600, l~8~ am~~ NMR (CllC'l~, 64 M~
~: ~.5-3.1 ~2~, m/ 4-H and 5-H), 3.71 (2H, d ~=~ }Iz,
~inOC~2-), 3.~iO (~H, mr -CH20H3, 4,17 (3~ CH3)l 4
~2H, ~, Ph~'-H2-~, 5.51 (l~ H), 5.75 ~lEI, ddd, J=6,
2~ ~ Hzr 3-Ht), ~6.07 (lH, ddd, ;~=6, 2,2 HZ, 2 -H), 7.27
91~ 5~ 16~36~;TAI~EDA PAT~NTS OSAKA Petherst~n ; 06300~601j~67~
2 ~
-- 54 _
(5H, s, C6H~-3, 7.94 (lH, ~ urine-H), ~.46 1lHt 8r
purine-H ~ .
MSs C~alc~. Ior C20H2~N403(M ~ M/Z: 3~ olind ~Jæ,
366~ 17~7.
S $x~m~1e 2
t. 5 ,B-B~n~yloxymethyl 4 ~- ( hydroxy~nethyl ) ~: yc 1 opent-
2~en-1~6-yl ~ adenine and ~ ~ ) 9- ~ 5,~-~enzylox~rmel: hyl-4,B-
(hy~ro~ymeth~rl)cy~:lopent-2-er~l,B-yl]-~ ?th~: ypurine
g- [ 5,~-~3er~zyloxYmethyl-4~B ~ (hyd~oxym~l: hyl ) -
cy~lopent 2~en~ 5-yl]-S~hloropurihe (244 m~, û.66 .
mmol. ) obtainec~ in Reference Example 11 was l,roces~eâ
in the~ ~am~a ma~nn~r as E~cample 1 to a~ord ( ~ ~ -9-lS,~-
ben zyloxymethyl .. ~,B- ( hydroxyme~hyl j ~yc lopent - .; -en~
~l]ad~anina t20~ mg, yi~d ~9~ n~ (~)-9-t5
15 benzyloxymethyl-4,~- ( hyd~o~ thyl ) ~yclopent-
2-en~ yl ~ -6 -me~hoxypu~lne ~ 2 6 m~g t yieid l l ~
n~yloxyln~thyl-~4~ (hyd~xy~nethyl)~ lop~t-
2-e~n~ yl]adelline~s colorle~ noe~ cry~t~..llized
from mqthanol); m.p.~ to 121~C; ~X~ (Nu~o;;.): 3450,
1710 c~ H-~MR (D~IS0-:16, 60MHz)~ 6 (1~, m, 4-H3,
3.06 (1~1, d~dd, J~g,9,9,9 Hz, 5-H), 3.27 (2:H, In,
BnOCH2-3~ 3~59 ~ ddd~ J~ll, 5~5~z) and 3.~;9 (lH,
ddd ~ ~-11, 5, 5 ~ CH70H ), ~ H, . d, .:r~ 12 Hz ) an~
4.09 (l~H, d, J~12 Hz) ~Ph(:H2-), 4~75 (1~ r ~=5 Hq~r -
2S OE~),.5.l3 (1~11 broad d, J ~Hz" 5.~3 (lH, ddcl, J~6, 2,
2 Hz~ 3~ 6.26 (1~1" dc~dlr Ja6~ ~r 2 H2:~ 2-~1 t 6-9B
~ 2H~ m~ and 7 . 22 t 3~,m) t~6H5- ), 7 .14 ~ 2H,
NH2), 8.07 (lH, ~, purine~I), ~.ll (1~, s, purlne~
Elemen~al An~l~8is ~Qr Cl9112lN5O2
30 C~lcd. (9¢): C, 6~.g4; }~ 0~; ~, 19.93.
~ound ~9~) s C, 65.12, ~II 6.11; N, 19.~4.
~ [ S~ Benzyloxynnethyl -4~ (hydrox~n~thyl.)l:~clop~nt-
2~en~ yl ] -6-metho~ypuril-e ~ oolorle~s oil.: I~ ( CHCl~ )
: 3450, 1~0~, 1580 c:m l; IH-NMR ~CDCl3, 6~ P~.lz~ 3.36
3 5 ( 3H, m ~ ~ -H ~ $ -H and -OH ) ~ 3 . 41 ( 2H, m, ~C~[2~ 7 ~
9~ 5~ 16~3~;TAKE~A PATENTS OSAKA Fetherston ; 063006801;#58/
~ 55 - 2
(~H, m, ~C~X~-~, 4.00 ~H, s~ PhC~2~ .21 (.3~
CH3), 5 . 89 ( 2H~ m, l-H and ~~H), 6.23 ~lH, ~t~ 2-H),
7~03 (2H~ m) a~d 7,23 (3H, ~) ~6~s~ 12 (!~ s~
purin~ . 51 ( 1~, ~, puxln~
MS~ Calcd~ .~or C2~H~N4O3(M~ M/Z: 36b.1~ 2. Fouc~d
3~. 1708.
~xampl~ 3
~ S~-D~(hydroxy~n~t:hyl~cyclop~nt-2-en~
yl]~d~ni~ .
A ~o1u~1on oï ~ 9-~Sc~-b~3nzylox~Tne~hyl 4,e-
~h~drox~nethyl)cyclopen~-2Qen~ yl3aderlLne I 10$ m
0.30 rnmol. ) in anhy~rou~ cli~hlnrorQ~ch~ne (24 mL) wa~
cooles~ to -78~C, To the elolutiorl wa~s add~d .xopwi~e
~l~wly, in argon ~re3am~, a ~olu~ion ( 1 M so;l.ution, 9
1 S mL ) of k oron ~richloride i.n d1chlorome~hane . The
reaction mi~tu:~ wa~ s~irxe~ for 3 hou~s at ll.he 6ama
temper~ture ~nd ~here was then ad~ed dropwl~ lowly
mixtllr~ a~lhyc~rou~ metharlc)l and anhydrou~
di~hlorome~-helne (1~ n~l,). 'rh~a cs~c~ling :bi~.th w~3
~elno~ed and the :~eaation mi~ture lwas ~ re~ until the
rea~tion tempe~ture w~nt up to room tempera l.ure . ~he
r~aac~on m~x~cure wa~; c~n~nt;~ und~:: r~d.u~ ed
p~e~ure and ~hex~e W2~# adcied anhy~rou~ metha~llol (5 mL),
and the mix~ure wa~ l~sf~ ~tarading fo~ lO mi.nn~es,
~5 followed ~y ~oncen~ra~io~ Imder redu::ed pre~;,;ure onae
more~ This proceduLe w~ repea~ed fC)ux ~me;l;~ th~qn ~h~
re~ult~nt ~onc~3n~rate wa~ dls~c:lv~ad i~ met~a:l~ol ~S mL
~o which was added Amberli~e I~ 5 ( OEI' typ~
then the mi~ture wa~ stirxed f or on~a hou~ ~t room
~en~p~:ratu~e, The ion-exchans~e x~e2~in wa~ ered off,
and the ~ rate was conc~antrA~ed und~ ul:ed
p~o~u~ h~ ~o~entrat~ was su~jected to !1 siliaa
gal (10 ~) col~m chromato~raphy, eluting wil:h
chloro~c)rm-methanol ( 10 ~ he ~xactlon t:hlls eluted
3S ~as corlc~ntral:~d unde:~ reduced pxe~ure, tc~ ilhl~h wa~
~dded a ~mall v~lum~ o m~thanol t~ a~o~d (~
91~ 5~ lB~36~;TAK~DA P.~TENTS OSAKA Fether~t~n ; 063006601;~59./7~
u~
- 5~ ~
9-t4~B,5c~di(hyd~o~ hyl)cyclopent-2-en-l~B-yl3ad~nine
as cry~ (6~ m~, yie~d ~39~).
Colorle~s amorphou~ product; :n.p. ~ 179 to 18(: C; W
(CH,O~ nrn (e)~ 260.9 (14200)~ D~!SO-d6~ 50O
NHz)~ ~ 2.23 ~lH, dcldd, J-5, ~, 5, 5 Hz, 5~1~1, 2.70
(1}~, m, 4-H), 3,51 (lH~ ddd, J-10, 5, 5Hz~ d 3,S7
(lH, .ddd, J=10, 5, 5 H~ 2OH)I 3.61 (2EIJ ~d, J=5, 5
H~, -CH70H)~ 4.78 ~, J=5 H~, -OH3, 4.8:~. (lH, t,
~=5 ~,-o~), 5,43 (lH, m, l-H), 5.79 (lH, In, 3-H), 6.V6
tlH, m, 2-H), 7.18 (~H, broad ~, -N~12), 8.05 (lH, ~,
pllrine-H), ~.13 (lH, s. purille-H~.
MS ~ Calc~ . f~r Cl2H15NgO~ (M ~ M~Z i 2~ 25 . Found
NfZ: . 261. 1212
~sample 4
15 ( ~ ) -9~ , 5,1~-D~ (hydro~net;hyl ) ~clopenl~-2-~n l,~- .
~11ad~nln~ ~
~ t~s~-Ben~yloxym~thyl-4~-(hyd~Qx~lel:h~
cy~lopent-2-en~ yl~adenln~ (1S4 m~, 0~4 ~ol.) was
pro~ ecl ln th~ same manner as Example 3 t.o af~rd
~0 ~ [4~,5~-di(hyd~oxy m~hyl)c~clopent-~-e
yl~ad~nlnR ~105 mg, yleld ~2~).
Colorle~ amorphou~ p~oduc~ ry~tal~ized ~ m
metha~ol), m.p.: 177 ~ 17~~; U~ (CH3~H) i~,xnm (~:
2~ 14700)~ NMR (D~SO-~6, 500 ~z)~ : 2.85 (lH,
~, 5-H)~ Z.g4 ~lH, m, 4-H~, 3.10 (1~, ddd, J~Sll~ 9, S
HZ) and 3.21 ~lH~ ddd, Ja:Ll, 6, 5 ~2~ ~-C~OE), ~,.57
~lHf d~d, J-~10, S, 5 ~z) and 3.65 ~1~, dddl J=lU, 5, S
~z) (~C~kOH), 4.4~ (lH, t, J~S ~ O~, 4.'7'i (1~
J-5 Hz, -O~, S.62 ~lH, d, ~ 9 Hæ, l-H), 5.g~ (lH, m,
3~ 6.31 ~1~, m, 2-H)~ 7.19 (2H/ bro~d g, N~2), 7.98
(lH, 8, purin~H), ~24 (lH, ~, purine-H).
~- Calcd~ 2~5N502 (~ M~z: Z61. 1225., Found
M/Z; 2~1. 1214.
Ex~ S
35 (~)~g-~5~-B~nzyloxym~hyl 4~-(hydroxy~ethyJ.~cy~lo~ent-
2 en-l~ gu~nin~
91~ 5~ 16~36~;TAREDA PATENTS OSA~A Feth~r~t~n ; 06300~B01;~B0/72
l~o a ~oluti~n o~ 2-amin~-9 [ 5~benæ~'lox~
n~ethyl-4 ~- ( hydro~3nne~hyl ) c~clopen t- 2 -~n~ y]. ~ ~ 6 -
chloro~ rin~ ( 123 mg, 0 . 32 mmol . ) in dioxanle ( 3 n~) was
add~ A O . S N ~;olu~ion of pota3~ium h~droxi~ 4ml ),
5 and ~he ~nixtur~3 wa~ ~e~luxed ~or 5 hours . ~'~he reac:t~ on
mixture wa~ concentrat~d undel~ reduce~ ~r~ xe. The
concen~r~te wa~3 ~ub jec:t~d ~ cs ~ilia~ ~el ~ l;i . g) ~olumn
ch~oma~og:raphy/ elu~ wi~h chloro~c~rm-metllclIl4l
(20-1), Thç~ frac~lon thus aluted was concent;rated
10 under reduced pre~ re . TO the con~en~rate ~i, a~ added a
~mall voluln~ o~ wa~er t~ ve t * ) ~9- C 51~-r
b~n~loxyln~hyl-4~-~hyd~ yqne~hyl)cyclop~nt~ en~
~rl]gu~nine ~ cry6tal~ (75 mgt yield 81~).
Colorles s amorphous pro~uat
l S ~-NMR ( DMSO-d~, j S 0 0 MHz ) ~ : 2 . 9 5 ~ aH, m ,. 4 - H and 5 -11 ~,
3.24 to 3v37 (~X, m, -CH2OH), 3.55 ~lH, dd, J=~l, 5 Hz)
a~d 3.~ dd~ ) (BnOCH2-3, 4.n~ (lH, dr
J=13 Hz) and 4.15 (lH, d/ ~=13 Hz) (I~h~:H~), 4.76 (lH,
t~ Ja5 HZ~ -OH~ 5~4~ t~ H~ 5,~7 (lH, m, 3-H~
20 6.~1 (lH~ m, 2-H)~ 6.~4 (2H~ broad s~ -N}12~ ~ 7.07 ~o
7.29 (5H, m, C6Hg~)/ 7.65 ~lH, ~, purlne 8-~:[) 10.50
~1~, Ç~, -NH--l ~
~xan:pl~ 6
( t ~ - r 5cx-B~l~zyloxym~9thy~ - ( h~srd~rmet;hy~ clop~int-
2S 2~en~ yl]-5-me~ 2,4~3.E,3E~-pyrimidinedic,ne
T:> a solu~ioll o~ en~yloxy:rnethyl~4~-h~rox~
methyl-lJ~ 3-metho~ -methoxyacr~loyl)u:l~eido3c!yclo
pen~ ne t6~ tng, 0.19 mn~ol. ) obt~ined in :RI.,fexence
E~ample 17 in ~ne~hanol (3 ~) was ~dded 25~ queou~
30 ~n~nonia ( 2 ~ . Th~3 mixture wa6 heated a~ 8:'i for ~0
hours in a ~e~led tube. ~h~ ~c~io~ ~ ~t~.r~l~ wa~
concentrat~d under xeclucecl ~ressu~e. The concentrate
wa~ ~ub~ ed to a ~ilica S~el ( ~ g ) colulnn
chromato~rE~phy, elu~ing with he~{ane-~hyl a.c,i 1tatia
35 ( 1:1 ) . Th~ :er~ctiOn ~hu~ eluted was ~nce~t.~ to
d~yne~ under red~lcad p~e~ure to afforcl ( ~ 5~
.
gl~ 5~ l6s~36~;TAKEDA PATENTS OSAKA Fetherston ; o63op6aol;#Bl/72
_ 5~ _
.
bç~næylox~ne~yl ~ (hycl~oxyme~h~l jcyc:lopen~ en~
yl ~ ~5-me~hyl-~, 4 [ 11~, 3H ~ -pyrimidin~dion~ ~ 6 Z n y, ylelc~
~8~ ~ a~ a colorles~ oil .
~E% (CH~ . 3430, 16~0 ~m l; lH-N~ ((:DCl3~ 6(l MH~
1.~0 (3~ s~ -C~3), 2.23 (lH, m, S-H~ 2.g~ (~H, n~
~nd -O~ ) ~ 3 4 tc~ d~ . O ( 4E, ~ CH~O~I and BnO~'H ~ ) ~ 4 . 5 6
( 2H, ~ ~ PhC~2~ . 56 ~ m, l~H and 3-H) ~ 5 . ~7 ( lH,
m, 2-H), 7.09 (lH~ m~ py~lmidine~H)~ 7.31 (i;F., s~ C6H5-
)t g-1;2 (lH~ broc:d ~ -N~
10MS 2 C~lcd ~ ~o:~ C,4H22~2~,s (M*) ~Z s .34~ . 15~0 ~ ~ound
M~Z: 342. 15~5.
E:~ampl0 7
4,B~Sa~ hydrox~neth~ yc!lopent~ 3n ~ yl]-5-
meth~l-2 ~ 4 C lE~ 3H~ _pyrl~nldi.ncid~ on~
A ~olution of ( ~ 5aL-ben~ylox~nnethy
( hydro~ymeth~l ) cyclopen~ -en~ yl ] -5-me~hy.l ~
2,4(lH,3H)-p3~imidinediorle (80 m~,t 0~2~ n~no,l, ) in
anhydrous dichlorome~hane ( 10 ~) wa~ ~oolecl to -78C
and there wa~3 ~;lowly ad~ecl dro~wi~e, whil~ ;sl..irri~ in
20 argon ~reams, a ~olutic)n of ~oro~ t~i~hlor.ic.e in
dichl~ro~ hane ~ lM ~c)lution, 7 mI. ) . The re~. .ction
mixture wa~ stirred for 3 hour~ at ~h~ same ~l~emp~rat~ e
and t:here w~ ~dded slowl~ ~ropw~e 2nh~rdrol.ls
methanol-~nhy~rous clichlo~omethane (1:1, lO l~). q~her~,
25 the coollng bath wa~ xemo~red, and the react.ion mixt~lxe
wa6 ~tirrecl un~11 th~ t~mper~ture went up t3 roo3n
~em~era~ure ~ The :reac~ TI mixtuxe w~ a~nae~ ~ated
und~ redu~ed ~pressllre. ~ro the col aentr~t~ ~ira~ added
anhy~lrou~ meth~nol (5 tnL) ~ whi~h ~;~8 le~t ~anding f~r
30 1~ minute~ at xoom temperæ~ture, ~ollowed by
c~na~ntra~ion u~der reducf3d pre~;ur~ once ~nor~ Thl3
proa~3du~ wa~ xopG~at~d f~ur t~me~. ~he conla~!ntrat~ was
~ub~cted ~o a ~iliaa gel ( ~ ~) c~lurn~ chromatogxaphy,
elutin~a with ahlo:coi~orm-methanol (30~1). q~hl3 fractivn
3S thu~ elut~d wa~i concentra1:ed under redu~ec~ p.e~ure.
~ ~h~ aons :~nt~a~e wa~; add~d a ~mall VO~ mel:hanol
,
914!11,~ 6~ 16~36h';TAKEDA PATENTS OSAKA F~sh~r~ton ; 063006601;#6~27
~ ~ r
to a$ford ~ 4~B/5c~ ~i(hydro~ethyl)c~c.lclpent-2-en-
l,B~yl J -S-me~hyl-~, 4 [ lH, 3~ -pyrimidinedione ia~l ~ol~rl2s 6
pri~lnæ (44 m~ ~ield 74~) . m.p. s ~2~ to ~:3''C,
I~ tCl~ 16~0 cm~~ l-NMR (~MSO~da, 500 M:E~ 1. 95
S (1~, ddd, J--5, 5, 5 Hz, 5-EI), ~.61 ~lH, m, 4~H)r 3~46
(lH, ~dd, J~10/ 5, 5 Hz), 3.51 (lH, ~dd, J=:Lt', S~ 5
H~)r 3.56 (lH! ddd~ J-10~ 5r 5 Hz) and 3.57 I;lH, dcld,
J--10~ 5~ 5 H2~ (-C~20H ~c 2~, 4.~;8 (lII~ t~ J:55 H2~ -OH),
4.76 (lH~ ~S Hæ~ -OH~, S. ~6 (l~lt m, l-M~,, 5.58 (lH,
m, 3~.01 (lH, m, 2-H) r 7 ~37 ~ 8~ pyximidine-H),
11020 (lH, ~, -N~
MS C~ o~ 16~2b ~ g~) M/Z: Zs~ .1110 . Found
~/Z s 252, 11 27 .
~xample 8
~ [5~-~snæylox~neth~1~4~-(hydroxymethyl)cyclop~nt-
~-en-l~-ylJguanine
2 Amlno-~- r s~-benzyloxymethyl~4~-
~hydrox~methyl)cyclo~nt-2~en-l~-yl]~-chlo:r~:p~rine
(105 mg, ~.~7 mmoll wa~ sub~ect~d ~o ~u~tanl;.iall~ ~he
~0 ~e pr~cedure a~ Ex~mpLe 5 to give (~-9~[Sc~
benæyl~x~m~thyl-4~-
(hydroxymoth~l)cycl~pont-2~e~ yl]~uanln~ ;!.~ o~y~tal~
(7~ ~, yi~ld 78%),
Colorle~ amorphou cry~tals ; 1H-NMR (DM80~ 00
MHz)~ s 2.30 ~lH, dddd, J=~, 6, 6, 6 ~z, 5-.H', 2.68
~lH, m, 4-~, 3.4~ (lH, d~d, J-10, 5~ S H2) ;!.~d ~.59
( lH ~ ddd , ,J~1 0 ~ 5 ~ 5 }~ CH ~O~ ), 3 . 6 1 ~ d , J- l O,
5 H~) and 3.64 (lH, dd, J~10, 4 H~3 ~BnOC~ , 4.48
~H, s, Ph~H~-), 4.76 tlH, tl J=5 ~z, -o~ 8 (lH,
m, l-H)t 5-72 (lH, m~ i.01 ~lH, m, 2-H , 6.39
(2H~ broad ~, -NM2), 7.22-7.33 (513:~ ml C~ 5- r 7-~
(lH, ~, pur~ne 8-H).
t+)-9-~4~5~-Di(hyd~oxym~thyl)cyclop~n~-2~!n 1~-
3 5 ~:L ] ~uanln~3
5!o a solut~on oi~ t~)~g-~s~ enzyloxyJne)t~l~yl-4
91~ 5~ 16e~3~h`;TAKEDA PATENTS OSAKA Fether~t~n ; 083006601;#83~72
roxylne~hy~cyclope~k-2-~3n~1s -yl~gllanine i n
anhydrou~ dichloromethane W~15 ~ded dropwi~E~ ~lowly,
while cooling ~ 8~C in argon stre~m6, a 1 mol boron
triahlorid~ - anhyd;ra~s dichlorometh~ne ~o] ution.
5 The mix~ure wzl~ ~tirred ~o~ 3 hou~ he ~ me
tempe~ature, . To ~che re?ction mixture wa~ a~cled
dropwise sl~wl~r anhyd~ou~ methanol - anhy~ o~s
dichloromethalle, then the cool~n~ bath was r~!moved,
followed by ~tirrin~ the ~nixture! un~il the r~.action
10 ~emp~rature ~sached rooln tempexature. ~ 3c lvent wa~
dis~illed o~f under r~duced pr~ ure, To the ~e~idue
was added m~athallol7 whLC~ w~ co~entrated a~ain urld~r
r~d~c~d pre~sure. This procedu~e wa~ repe~d ~ur
times, then ~h~ residue w~s di~olved in me~anol. To
the solution was added ~mherlite ~G-S0 (OH typ~)/ an~
~he mi~ture w~ irre~ ~o~ one hour at ro~3n
t~3mper~tur~ ~ Th~: ion-exc:h~n~a re~3in waE~ r~ of :~,
and the ~ rat~ wa~ qon~en~rQted und~r redu~e~
p~e~sure. The xe~idue wa~l ~ub~ected ~o a ~i:l.ica gel
column chromatogxaph~, ~lutlng with chloro~o:lm~
m~thanol. The fraction ~huæ elut~d wa~ conca~ntrated
un~er reduoe~ ~x~ur~. ~o th~ concentrat~ a8 ~dded a
small ~lum~ of wa~er to giv~ (+)-9-[4~,$~-
di(hydroxymethyl)cyclop~nt-~-en l~-yl~guaninlil as
~5 crystale (coLorle~s ~morphou~ cr~s~ls)~
Example 1 0
[ ~,~, 5O~i (h~,rdroxymet:hyl) cyc;:lo~ent-2-e;n~
~yuanine
~ 9-lSa-~en~ylox~mQthyl~
~hydroxyme,thyl)cyclopent-
2-~n~1~-yl~guanin~ ~76 mgr O.~ ol) ~a~ ~.u'l~je~ated to
~u~t~tanti~lly ~h~ sam~ pro~ed~re ~ E~a~pl~ o giv~
~ 4p,$a-di~h~dro~y~ethyl)cyclopen~-2-~)n~
yl]t3uan1ne a~ cry~tal~ S5:L mg, yield ~
35 Colorl~ei~s Am~rphou~ r~ttnl~tt 1H_~MR, (DMSO-dl;~ 50~ ~Ha:)
: 2.16 (lH, d~ldd, J~5~ 5, 5, 5 Hz~ 5-H)~ 2.6'~ (111, m~
91~ 5~ 16~38~,TAI~EDA PATENTS OSAKA F~th~r~t~n ; 06300~601;#~4/72
$
4-H), 3.4~ ~o 3.62 (~H, m, ~20H-~ 2), k~7A. ~
Jc5 Hz, -0~), 4.7~ ~lH, ~, ~=5 Hz, -O~), 5.:Ltl (lH~, m,
l-H)~ 5~71 ~r m, 3 - H), 6.00 (lH, m, 2-H)~ ~i.4~ t
broad 6~ -N~2), 7 62 (lH, 5, ~urine 8-H)/ 10.,5~ (lH~
5 hroad 5, -NH- ) .
~aml?1~ 1 1
,4R,5,5)-4-~ ( lS~ Camphanoylox~m~.thyl3-5-
(b~rlz~rlox~nel;h~l)cyclo~en~ en~l-~ 6 -~hlo~ opurine
an~i ( - ) - 9 - ~ t 1~, 4~, 5R ) ~ t lS ) - ( - ~ -C~mphanoy:Lc xyme~hyl ~ -
10 5- ( benzyloxymethyl ) ~yclol?0nt- ~ ~en-l-yl } ~ 6 -ch:l oropurlne
S~-B~nzylox~lethyl-4
(h~d:~oxymeth~rl )ayclopent~ n~ ]~ hlo:~c purin~
(~10 mg, 0~57 mmol~ ) wa~ dis~olved in anhy~rcus
t~a~rahydro~uran ( 5 ml ) . ~o the ~o9Lution werl~ added
lS (13)~(-)-~amphanic acid ~hloricl~ ~240 ~ng, 1.'; 2 ~nol.
ancl ~ri~thylamin~ t230 mg~ 2.28 mmol. ), ~he~ the
mixture WA~5 ~tirred ~r 1~ houxs ~t rs~om t~ atu~
The reat:tion mixtUx~ was poured irlto l~e-w~ir/ W~iCh
wa~ ~ub~ec~ed to caxtraCtion wi~h CH~13. The e~tra~t
~O wa~ dried ove~ anhydrous ~o~ium sulfa~ h.er. the
~olvent was di~i~illed o~ rhe~ ~esidue wa~ ~i;ub~ted
~o ~ ~iLiaa ~el col~nn ah~ om~togr~phy, ~he~ lut$on w~s
per~orm~ad wi~h ~ mlxture o:~ he~ane and e~hyl acetat~
( 1 s 1 ) ~o cb~in 244 mg (.78~6 ~ og a mi~ture of 1 and Z .
25 This mixture wa~ ~ub~e~ted to ~ high per~or.m,.lnce li~uid
ch~smatography ~Natera, p~r~lcil ~ he~ne~ O~ 0 ~1 ) ]
'I:Q ~;epara'ee 9~ 3,4R,5S) 4-[ ~lS)-[-)-
c~mE~h~no~rloxime~hyl ~ -5-benzyloxyn~ethylcyclop~!~nt-2-en
yl~-6-chloropurin~ (~) ancl ~{ (1~,4S,5R~4~[, 1
30 camphanoylo~y~a~h~l ] ~5-ben~yloxym~thylcyclc~pi:nt-2 en
6 ~hl~opurin~ IB).
~A): 24~S min.
column ~ poracil
1/4" x 1'~B) t ~4 min.
~olv~ntj hexAne-~tOH
(25~1) .
91~Fll,~ 5~ 16~36h`;TA~EDA PATENTS OSA~IA Feth~rgton ; 06300e~01;#65~72
$ ~
. ~ 6~ -
d~te~tor; UV 254 mm
Example l~
~ 9~t ~ 4R~5s)-5-Benz~l oxymeth~l-4-
s ~h~droxymet~l)cyclopent-2~en~1-yl3adenin~
9-{~lS,4R,5$)-4-C~lS~ 3~Camph~noylo~i methyl]-5-
(benzyloxymethyl)~yclopent-2-~n~ l}-6^~hl~opurin~
(70 mg~ 0.127 mmol,) w~ dis~olv~d in ~eOH ~:lO m~), to
which was in~rodu~ed ammo~la ga~ ~r S minu~ un~r
so~lin~ with ~alt-ice. The re~ul ant was he~iteA at 70-
80~ ~o~ 16 hours in a se~led tube. From ~h~ reac~l~n
mixt~r~t ex~os~ ~mmoni~ a~d M~0~ w~x~ di~til:l~d o~,
and ~he ~e~ldue wa~ ~ub~ected to a silica ~:l column
chroma~ogra~h~, Elu~ion wa~ ~er~ormed wi~h ~! mixkur~
lS o~ eth~l ace~e ~nd me~hanol (8:1). The re~ul~-an~
cry~al3 WQre recry8talli2~d ~rom a ~ixture c~ ethyl
a~t~t~ and mothan~ glve (~ (lS,4R,5~)-5~
b~nzyloxymeth~l-4-(hydr~xymeth~l~cyclopent-~ ~n-1-
y~]adenin~ a~ ~olorle~ needle~ (:35mg, yield 78%~.
2~ m.p. 1~6 ~ 1~7~C
[~D ~ 50.50 ~c~O.~ OH)
~pl~ 13
~ g-[~lR,4S~gR)-5-~ensyloxymeth~1-4-
lh~ro~ymeth~l)cyclopent~ n-l-yl]~d~ine
9{~1R~4S~5R)-4-[~18)~ Camphanoyloxym~l~thyl~ 5
(benzyloxylne~h~l~cyclopent-2~e~-1 y~ hlo:lopurin~
~0 mg, 0.145 mmol.~ was processed in ~u~tarltially ~he
~me ~anner a~ above to obtain ~ 9-~(lR,~S,5R)-5-
B0nzyloxym~hyl-~-(hyd~oxym~thyl)c~clopent~l en-1
yl]~d~nin~ (4~ m~, yi~ld ~%).
m.p. 186 to 137~C (colorles~ needle~)
lal26- 45.00 ~c-~.8, MeO~)
~x~ple 14
~)~9 r(lS~4~,5s) d~5-Di(.hydroxymethyl)cyc~o],~ent-2-en_
91~ 53 1~36~;TAKEDA PAT~NTS OSAKA Fetherston ; 06300B601j#a6f7
- 63
1 -yl ~ aclanln~a
9-[~lS,~R,5g)-5~-~enzyloxylne~hyl-4
(hydroxy~neth~l)cyclopent-~-en-1-yl~a~enirle (.'8 m~, 0,0
nu~ol. ) w~ ditE30lv~d in anhyc~r~us meth~rlene s:hloride
5 ( lO ml ) . ~o ~he f~ol~tion ~a~ added d:ropwise,. while
~tirrin~ q B(::13-(~H2~12 ( 2 ml ) at 78C . Tll~ mixtux~
wa~ ~tirred for 3 hour~ at ~his telnp~ar~t.ure ~! nd there
~a8 add~d ~H2~12~MeOH ( 1; 1 j d~ ml ) I then the 1: elnperatu~e
w~ reve~rted to room ~em~er~tur~. ~he? ~vlvel ~ was
10 di~tilled of under ~educed pre~sure. ~o tll~ re~idue
wa~ a~ded ~et)H ~ 5 ml ), whi.ch w~ a.gain dried ko
. Th~ ~ p~oce~ure wa~ condu~::ted fou~ t~ ~ l;hen
the residue wa~t cli~olv~d ill Me~H ( 5 ml ) . Ts: ~he
~olu~ion w~s addecl Amberli.te-IR-45 (W~kl~ b
15 resin) ~ th~n ~he ~n~x~ure wa~ ~tirred, Atet:r one ~oux~
the re~in wa~ filtered off., then ~he ~ol~nt w~
di~tilled o~ ~nd~ rçdua~-d pr~6~ureO q~ho ;r-læidu~ was
subjected t~ a silica ~el col~mn ~hro~atogral~hy,
followed ~y elutl~n with L'HCl3~MQOH ~5:1) to obtain
~0 (~)-3-[(lS,4Rt~S3-4,$-d$(hydxoxyme~hyl)~clo])~nt 2-en-
l~yl]aden~ne (l~ mg, yLelcl 7~
m.p. ~06~07C ~c~lorl~Y~ n~edle~ t MeOH)
t~3D ~ ~ 7~62 ~ 6, M~OH)
~5 ~x~m~le 15
(-) 9-[(1}~4S,5El~-4,!i-Di~h~drox~n~3thyl)ayal~l~)e,nt-:~-~n-
l~yl]ad~in~
subs~antially the xame pro~edur~ a~ bove,
~tar~in~ wi~h 24 mg of (-)-9~[(lR,4Sj~R~-5-
~enzyloxymethyl-4-(hydrox~methyl)cyclop~nt~ n~
yl]~denin~ (-)-9-[(lR~4s~5R)-4~5-dl~hyd~x~le~h~l)
c~alop~nt-2_en-l-yl~d~nine was ob~ained (1.~ mg, yield
78%3~ ~
m.p. 206 t~ 207C ~colo~l~s~ nee~le~, MeO~
3~ [a~JD -- ~4 . ~ C--1 . 4 / ~OH)
Exampl~ 16
91~ 5~ 166~36~;TAKEDA PATENTS OSA~A Fetherston ; 06300a~01;~B7/72
- 64 - ~135
9-~4,B,51;L-cli(hy~xoxymethyl)cyclope~nt-2~e:r~ yl]
ad~n;i.ne (~00 mg), la~to3e ~50 mg~, ~ellulo~,~ (250 m~)
and magn~silun ste~ra~e (~0 mg) w~re ~ufici~ntl~
b~ ded, whlch wE~3 c~lnpressed to mak~3 tablet,~!.
5 Exampl~ 17
9-C~,B~Sa-di~h~lro~ymet~hyl)cyclop~3nt;-2-e~ yl]
adenine ~20~ ms~) r ~4hite vaseline (40.O g), e1hanol
( 1~ . O g ), ~orbi~an ~esquiç,leat~3 ( S . 0 ç~ ), po:L~ oxylaury:l
alaoho~ e~her ( ~ . 4 g ), met.h~l parah ~ roxybenZ!oate (0.1
g) and refln~ wa~e~ (36.3 g) w~r~3 ~lellded 1tc prepare a
0. 2% olntment.
Example 1 ~
(+ )-9-~4,~,5a-di(hydrox~mQkhyl)c~rclopent-~-en l~-srl]
adenine (25 mg), lactose ~100 m~) O ~karch ~.~' 0 mg) and
15 magne~ earat~3 (5 my) ~re su~E~icien~ly ]~ended,
which wa~ ~hen filled intc~ a cap~ule.