Note: Descriptions are shown in the official language in which they were submitted.
~~~~~ ~.t
HOECHST AKTIENGESELLSCHAFT HOE 90/F 334 Dr.LO/PP
Description
Process for the preparation of a high molecular weight
olefin polymer
The invention relates to a process for the preparation of
olefin polymers having high isotacticity, a narroc~
molecular weight distribution and a high molecular
weight.
Polyolefins having a high molecular weight are par-
ticularly important far the production of films, sheets
1d or large hollow articles, such as, fox example, pipes or
moldings.
The literature discloses soluble metallocene compounds
based on bis(cyclopentadienyl)zirconiumalkyl or halide in
combination with oligomeric aluminoxanes. With these
systems, ethylene and propylene can be polymerized with
moderate activity, but isotactic polypropylene is not
obtained.
It is also known that the catalyst system bis(cyclopenta-
dienyl)titaniumdiphenyl/methylaluminoxane is capable of
converting propylene into stereo block polymers, i.e.
polypropylene having longer or shorter isotactic sequen-
ces (cf. U>S. Patent 4,522,952). Substantial advantages
of this catalyst system are the fact that the polymeriza-
tion temperatures ( 0°C to -60°C) are irrelevant on a large
industrial scale, and the completely unsatisfactory
catalyst activities.
Isotactic poly~,ropylene can be prepared with the aid of
ethylenebis(4,5,6,7-tetrahydro-1-indenyl)zirconium
dichloride together with an aluminoxane in a suspension
polymerization (cf. E1P-A 185 91~). The polymer has a
narrow molecular weight distribution, which i.s advan-
tageous for certain applications, for example for high-
performance in;jec~tion molding.
- 2
At the same time, the catalyst system has a number of
deficiences.
The polymerization is carried out in toluene, which has
to be purified by an expensive procedure and freed from
moisture and oxygen. Furthermore, the bulk density of
the polymer is too low and the particle morphology and
the particle size distribution are unsatisfactory.
7However, a particular disadvantage of the known process
is that, at polymerization temperatures of interest
industrially, it is possible to prepare only polymers
having an unacceptably low molecular weight.
A special preactivation method for the metallocene with
an aluminoxane was also proposed, which method leads to
a considerable increase in the activity of the catalyst
system and to a substantial improvement in the particle
morphology of the polymer (cf. DE 37 26 067). Although
the preactivation increases the molecular weight, no
substantial increase can be achieved.
A further, but still insufficient increase in the molecu-
lar weight can be realized by using specially hetero
atom-bridged metallocenes having high metallocene activ-
ity (EP-A 0 336 128).
Catalysts based on ethylenebisindenylhafnium dichloride
and ethyl~nebis(4,5,6,7-tetrahydro-1-indenyl)hafnium
dichloride and methylaluminoxane are also known, by means
of which relatively high molecular weight polypropylenes
can be prepared by suspension polymerization (cf. J.A.
Ewen et al., J. Am. Chem. Soc: 109 (1987), 6544). Among
industrially relevant polymerization conditions, however,
the particle morphology of the polymers thus produced is
unsatisfactory and the activity of the catalysts used is
comparatively low. In cox~~unction with the high catalyst
costs, economical polymerization is thus impossible with
these systems.
- 3 _
Tt was the object to find a catalyst which produces
polymers having good particle morphology and a high
molecular weight in a high yield.
It was found that this object can be achieved using
bridged metallocene systems substituted in a certain
manner in the ligand sphere.
The invention thus relates to a process for the prepara-
tion of olefin polymers by polymerization or copolymeriz-
ation of an olefin of the formula R°-CH~CH-Rb, wherein R~
and Rb are identical or different and are a hydrogen atom
or a hydrocarbon radical having 1 to 14 C atoms, or R° and
Rb, together with the atoms binding them, may form a ring,
at a temperature of _60 to 200°C, at a pressure of 0.5 to
100 bar, in solution, in suspension ~r in the gas phase,
in the presence of a catalyst which is eomposed of a
metallocene as the transition metal compound and an
aluminoxane of the formula (II)
R1~ R14 R
~, , ~4 ( )
~l - o A1 - o A1°~ II
R14 r p ~ R14
for the linear type andlor of the formula ZIT
ZO Rl~ (zTT)
Al - o
for the cyclic type,
wherein, in the formulae (II) and (III), the radicals R14
may be identical or different and are a Cl-GB-alkyl group,
a CB-C1~-aryl group or hydrogen, and p is an integer of
from 2 to 50, wherein the metallocene is a compound of
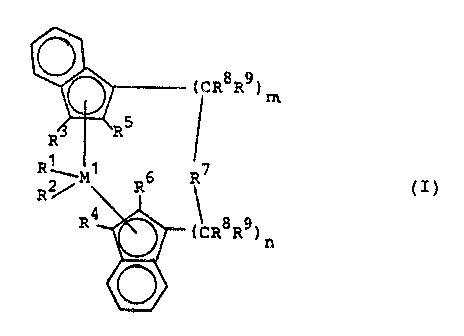
the formula I
- 4 -
d rR~R~ ) ~
fI)
~~~ M ° R6 R~
g'~ ~ 8 9
~rdcR ~ )~
wherein
Ml is a metal of group I~lb, ~1b or VIb of the Periodic
'able,
Rl and R2 are identical or different and are a hydreagen
atom, a Cl-Clo-alkyl group, a Cl-Coo-alkoxy group, a
C6-Coo-aryl group, a Cs-Clo-arylo~y group, a Cz_~C~o
alkenyl group, a C,-C4o-arylalkyl group, a C~-~C4o
alkylaryl group, a Ce-Caa-arylalkenyl group or a
halogen atom,
Ro and 12'' are identical or different and are a hydrogen
atom, a halogen atom, a Cz-Cl°-alkyl group wrhich may
be halogen~~ted, a CB-C~o-aryl group, an -NFt2lo, ~~
-OSiR3~a, -SiR3~a or -PR~lo radical, wherein Rl° as a
halogen atom, a Cl-Coo-al~y'1 group or a Cs-Clo--aryl
grOllp,
RS and RB are .~dentie:al or dif ferent and have the rn~araing
stated fog R3 and R", with the proviso that R5 anal RB
are riot hydrogen,
~~~a~~
-- 5 -
R7 i s
R11 Rll Rll Rll R11
_ ~q2 _ , _ M2 _ Dq2 _ , _ 1v~2 ( CR213) ! ~ ~, ,C ~ ~2
R12 X12 R12 R12' X12
R11 R11
s o2
.. C _ . _ 0 _ M
R12 X12
=BR~11 =AlRll, -Ge-, -Sn-, -~-, -S-, =SC7, =SOZ, =NR11, =CO,
=PRia or =~~p)Ry~
wherein
R'I, R12 and R~~ are identical or different and are a hydro-
qen atom, a halogen atom, a Cl-C~o-alkyl group, ~. ~
Clo-fluoroalkyl gxoup, a CB-Clo-aryl group, a Ce._Cxo-
flu~roaa:yl groaxp, a Cl-Clo-alkoxy group, a ~'Z-Clo-
alkenyl group, a C~-C4o-arylalkyl group, a Co~C,,o-
1~D arylalk~nyl group or a C7-C4o-alkylaryl group, or R11
and R~Z or Rl~ and R~3, together with the atoms binding
ahem, each form a ring,
MZ is silicon, germanium or tin,
Ra end R9 are identical or different and have the
~5 meaning stated for R11 and
m and n are id~nt3cal ox dif~arent and are zero, 1
or 2, m plus n b~~ing zero, 1 or 2.
Alkyl is straight:-chain ox branohad alkyl. Halogen
(halogenat~d) is fluorine, chlorine, bromine or
2d iodine, preferably fluorin~ or ohlorine.
The present invantion furthermore relates to the poly-
olefins prepared by t:~e process described.
«
~ ~~~
The catalyst to be used for the process according to the
invention is composed of an aluminoxane and a metallocene
of the formula I
CR8R9 D ~
R~
.R
,,
R ~ ~ 1 .7
R2r M R6 R
~1)
R4 ~ 8 9
' ( CR R ) n
In the formula I, M1 is a metal of group IVb, Vb or VIb
of the Periodic Table, for example titanium, zirconium,
hafnium, vanadium, niobium, tantalum, chromium, moly-
bdentun ar tungsten, preferably zirconium, hafnium or
titanium.
R~ and R~ are identical or different and are a hydrogen
atom, a Cl-Clo~alkyl group, preferably a Cl-.C3-alkyl group,
a Cl-C1°-al7cox~ grougs, preferably a C,-C~-~l~COxy group, a
Cs-C~o-a~'Yl group, Preferably a Cs-C~-aryl graup, a C6-Clo-
arYloxy group, preferably a Cs-C~-aryloxy group, a C2-Cl°-
alk~nyl group, preferably a C~-Ca~alkenyl group, a C~-Cao-
arYlalklrl group, Preferably a C~~Clo-arylalkyl group, a C~-
C4o-alkylaryl . group, preferably a Cy~Cl2-alkylaryl group,
a Cg-~~o-a~lalkenYl group, preferably a Cs-C12-arYlalkenyl
group, or a halogan atom, preferably chlorine.
R3 and R4 are i~3entical or different and are a hydrogen
atom, a halogen atom, preferably a fluorine, chlorine ar
bramine atom, a Cl-C1°-alkyl groug, preferably a C1-C4-
alkyl group, which may be halogenated, a Cs-Gl°-aryl
group, pref~rably a Cs-Cs-aryl .group, an -NRxI°, -SRi°,
~OoS1R310, -SiR~~° or -PRa~° radical, wherein Rl° is a
halogen
atom, preferably a chloxine atom, or ~ Cl-Clo-alkYl group,
~~~~~.~.r
_~_
preferably a C1-C3-alkyl group, or a CB-Cao-aryl groups
preferably a C6-Ce-aryl group. R3 and R4 are particularly
preferably hydrogen.
RS and R6 are identical or different, preferably identi-
cal, and have the meanings described for R3 and. R4, with
the proviso that RS and R6 may not be hydrogen. RS and Ro
are preferably (C1-C4)-alkyl which may be halogenated,
such as methyl, ethyl, propyl, isopropyl, butyl, isobutyl
or trifluoromethyl, in particular methyl.
R' i s
R11 R11 R11 R11 1I
M2 ' - M2 ' M2 - . - M2 - ( CR 1.3 ) _ . _ 0 _ M2 _ O _
Ra2 R12 ' R22 R12 2 ~ 12 '
R
~lI R11
_ C _ ' _ a . Nt2 _
R12 R12
=HR11, =A1R11, -Ge-, -Sn-, -0-, -S-, =S0, =SO2, =NRlx, =C0,
=PRl~ or =P ( 0 ) Rll, wherein Rli, Rl2 and Rl~ are identical or
different and are a hydrogen atom, a halogen atom, a C1-
Clo°alkyl group, preferably a C1-C4-alkyl group, ~.n par-
titular a methyl group, a Cl-Clo-fluoroalkyl group,
preferably a CF3 group, a CB-Clo-aryl group, preferably a
C6-C$--aryl group, a C6-Clo-fluoro~ryl group, preferably a
pentafluarophenyl groug, a Cl-Clo-alko~cy group, preferably
a Ci-~C~-alkoxy group, in particular a methoxy group, a C~-
Coo-alkenyl group, Preferably a C2-C~-alkenyl group, a C7
C4o-arylalkyl group, preferably a C~-Cio-axylalkyl group,
a Ce-Coo-arylalkenyl group, preferably a C8-C12-axylalkenyl
group, or a C~-C4o-alkylaryl group, preferably a C~-Clz
alkylaryl group, or Rll and R'~Z or Rll and RI3, together
with the atoms binding them, each form a ring.
M2 is silicon, germanium or tin, preferably silicon or
germanium.
R' is preferably =CR1''Rlz~ aSiRllRiz' =GeRllRxz, -C~,, .-g_,
=SC r =PRm ox _-P ~ O ) Rm .
Re and R9 are identical or different and hare the meaning
stated for Rli.
m tend n are identical or different and are zero, I or 2,
preferably zero or 1, m plus n being zero, 1 or 2,
preferably zero or 1.
The particularly preferred metallocenes are thus the
compounds of the formulae A, B and C
R5
R5 ~.
RgR9C i R1 ~,--'
M1\R2 (A) . R11\Si M1~R2 (B) .
R1 1 g12C i R12/ ~"~R
,".. R6 ~ R~
i
I
R5
i
R~ 1~ ' ,/R1
R 12s'C' ~''d ~ 2 ( C ) o
~ R
6
in which
Ml is Zr or Hf, R1 and RZ are methyl or chlorine, RS and Rs
are methyl, ethyl or trifluoromethyl and R°, R~, Rll and Rxa
have the abovementioned meanings, in particular the
compounds T mentioned in the Illustrative Rxamples.
The chiral metallocenes are used as a racemate for the
preparation of highly isotactic poly--1-olefins. However,
it is also possible to use the pure R or 8 form. Opti-
cally active polymers can be prepared with these pure
stereoisomeric forms. However, the meso form of the
metallocenes should be separated off, since the center
which is active in polymerization (the metal atom) is no
longer chiral in these compounds owing to mirror symmetry
at the central metal and therefore cannot produce the
highly isotactic polymer. If the meso form is not
sepaxated off, an atactic polymer is formed in addition
to isotactic polymers . ~'or certain applica.~tions - fox
example flexible moldings - this may be quite desirable.
The separation of stereoisomers is known in principle.
2p The metallocenes described above can be prepared accord-
ing to the following reaction schemes
HZRc + ButylLi -~ HRCLi X_(CRBRg)m-R~ (CR8R9) X
H2Rd + RutylLi --~----y~ HRdLi
HRC- ( CRBRg ) m- R~~ ( CRBR~ ) n RAH 2 8u~tyl L
LiRC-(CRBRg)m-R~-.(CR8R9)ri-RdLi M~C1
(RBRgC)m _ Rc (R8R9C)m _ Rc
~, C1 ~ r R1
R' Mle, R~ R7 M2\
Cl ~ Cl
(R8R9C)n _ Rd (R8R9C)n _ Rd
_,
(R~R~C)m _ Rc
R1
R2Li D R7 MZr
R~
(RBR~C)n - Rd
3
X = C1, Br, I, O-Tosyl; I32R° --
x
R4
H2R~ ~ ~ 6
The preparation processes are known from the literature;
cf. Journal of Organom~t~113.c Chem. 288 (1985) 63.-67,
EP-A 320 762 and the Illustrative Examples.
According to the invention, the cocatalyst used is an
5 aluminoxane of the. formula (Ix)
R14' R1~ Rlg
.~ pal _ o Al _ C ~l'' ( II )
Rig r 'R~~
for this linear type and/or of the formula (III)
R14
O ---- 111 p ( I I I
for the cyclic fiype, wherein, in the formu~,ae (II) and
(III), the radicals Rz~ may be identiaal or different and
are a Cl-~Ce-a7.ky1 group, a CB-C~~-ar~rl group or hydrogen,
arid p is an integer of from 2 to 50, preferably from ~.0
tp 35.
The radicals Rl4 ire preferably identical and are methyl,
- 11 -
isobutyl, phenyl ar benzyl, particularly preferably
methyl.
If the radicals R14 are different, they are preferably
methyl and hydrogen or alternatively methyl and isobutyl,
hydrogen or isobutyl preferably being present in an
amount of 0.01-40~ (number of radicals R1''j.
The aluminaxane can be prepared in various ways by known
processes. One of the methods comprises, for example,
reacting an aluminum-hydrocarbon compound and/or a
hydridoaluminum-hydrocarbon compound with water (gaseous,
solid, liquid or bound - fox example as water of crystal-
lization) in an inert solvent (such as, for example,
toluene). ~'ar the preparation of an aluminoxane having
different alkyl groups R14, two different aluminum~tri-
alkyls (A1R3 + A1R~3) are reacted with water, depending an
the desired composition (cf. S. Pasynkiewi.cz, Polyhedron
9 (1990) 429 and EP-A 302 424).
The exact structure of the aluminoxanes IT and III is not
known.
Regardless of the method of preparation, the common
feature of all alumi.nox~ne solutions is a chancing
content of unconverted aluminum starting compound, which
is present in free form ar as an adduct.
It is possible to preactivate the metallacene before use
a:n the polymerization reaction with an aluminoxane of the
formula (LI) and/or (III). This substantially increases
the polymer3:zatian aotivity and improves the particle
morphology.
The preactivatian of the transition metal compound is
carried cut in solution. The me~tallocene is preferably
dissolved in a solution of tae aluminoxane in an inert
hydrocarbon. A suitable inert hydrocarbo.l is an aliphat-
ic or aromatic hydrocarbon. Toluene is preferably used.
CA 02055216 2001-11-13
- 12 -
The concentration of the aluminoxane in the solution is
in the range from about 1% by weight to the saturation
limit, preferably from 5 to 30% by weight, based in each
case on the total solution. The metallocene may be used
in the same concentration, but is preferably employed in
an amount of 10-" - 1 mol per mol of aluminoxane. The
preactivation time is 5 minutes to 60 hours, preferably
from 5 to 60 minutes. It is carried out at a temperature
of -78°C to 100°C, preferably 0 to 70°C.
The metallocene can also be prepolymerized or applied to a
support. For prepolymerization, the olefin used in the
polymerization, or one of the olefins used in the
polymerization, is preferably used.
Suitable supports are, for example, silica gels, aluminas,
solid aluminoxane or other inorganic supports. Another
suitable support is a polyolefin powder in finely divided
form.
A further possible embodiment of the process according to
the invention comprises using a salt-like compound of the
formula R=NH,_xHR', or of the formula R3PHBR', as a cocatal-
yst, instead of or in addition to an aluminoxane. In the
formulae, x is 1, 2 or 3, the radicals R are identical or
different and are alkyl or aryl and R' is aryl which may
also be fluorinated or partially fluorinated. In this
case, the catalyst is composed of the reaction product of
a metallocene With one of the stated compounds (cf.
EP-A 277 004 and the Preparation Examples C and F).
To remove catalyst poisons present in the olefin, purifi-
cation with an aluminumalkyl, for example AlMe3 or AlEt3,
is advzntageous. This purification may be carried out in
the polymerization system itself, or the olefin is brought
into contact with the A1 compound prior to addition to the
polymerization system and is then separated off again.
The polymerization or copolymerization is carried out in
c~ ~ 3 .~ ~ .~ ~
- 13 -
a known manner in solution, in suspension or in the gas
phase, continuously or batchwise, in one or more stages,
at a temperature of 0 to 150°C, preferably 30 to 80°C.
olefins of the formula R~-CH=CH-Rb are polymerized or
copolymerized. In this formula, R° and R~ are identical
or different and are a hydrogen stem or an alkyl
radical having Z to 14 C atoms. However, Ra and Rb,
together with the C atoms binding them, may also farm a
ring. Examples of such olefins are ethylene, propylene,
ZO 1-butane, 1-hexane, 4-methyl-1-pentane, 3-octane,
norbornene or narbornadiene. In particular, propylene
and ethylene are polymerized.
If required, hydrogen is added as a molecular weight
regulator. The fatal pressure in the polymerization
system is 0.5 to 100 bar. Polymerization in the indus-
trially particularly interesting pressure range from 5 to
64 bar is preferred.
The metallocene is used here in a concentration, based on
the transition metal, of 10-3 to 10-e, preferably 10~° to
20-', mol of transition metal per dm3 of solvent ar per dm3
of reactor volume. The aluminoxane a.s used in a concent-
ration of 3.05 to 10-1 mal, preferably 10"° to ~.O~a mol, per
dm3 of solvent or per dm3 of reactor volume. In prin-
ciple, however, higher concent~atians are also possible.
Tf the polymerization is carried out as a suspension or
solution polymerization, an inert solvent conventionally
used far the Ziegler low pressure process is employed.
For example, the r~actian is carried out in an aligha~tic
or cyclaaliphatic hydrocarbono butane, pentane, hexame,
heptane, isooctane, cyclohexane and methylcyclohexane may
be mentioned as examples of these.
A gasoline or hydrogenated diesel ail fracti.an may
furthermore be used. Toluene can also b~ used., Polymer-
ization is preferably carried out in the liquid monomer.
- 1~ _ F ~~~~ f'3
If inert solvents are used, the monomers are metered in
as a gas or liquid.
The polymerization can be carried out far any desired
time, since the catalyst system to be used according to
the invention shows only a slight time-dependent decrease
in the polymerization activity.
Tn the process according to the invention, the metallo-
cenes described produce polymers having a high molecular
weight, high stereospecificity and good particle morphol-.
ogy in the industrially interesting temperature range
between 30 and S0°C.
In particular, the zirconocenes according to the inven-
tion provide a molecular weight range which, in the prior
art, was provided only by the hafnocenes. However, these
IS have the disadvantage of only low polyrn~rization activity
and very high catalyst costs, and the polymers prepared
therewith had a poor powder morphology.
'the Examples which follow ar~ intended to illustrate the
invention in more detail.
V.~1 - Viscosity number in cm3/g
M" = Weight avexage molecular Determined
by
weight in g/mol gel permeation
M%/Mn = Molecular weight dispersity chromatography
m.p. _~ Melting point determined DSC (20C/min
by
heating/cooling rate)
TI - Isotactic index (TT = mm+1/2 determined
mr) by
13'voNMR SpACtrOBCOpy
- Polymer bulk density in g/dm3
.
.MFI (23 0/5) = M~lt.flaw index, measuredaccording
to
DINV53,735, in g/10 min
Synthesis of the metallocenes used in the Exampless
~7~~~'i
- 15 - r
Starting substances:
The preparation of the indenyls HZR° and HZRd used as
starting compounds is carried out according to or analog-
ously to:
J. Org. Chem., 49 (1984) 4226-4237, J. Chem. Soc., Perkin
11,-1981, 403-408, J. Am. Chem. Soc., 106 (1984) 6702, J.
Am. Chem. Soc., 65 (1943) 567, J. Med. Chem., 30 (1987)
1303-1308, Chem. Her. 85 (1952) 78-85.
The preparation of the chalets ligands
I0 LiR°- ( CR~R9 ) ~-R'- ( CRBRa )n°F2'~Li iS described in
print iple in
Bull. Sot. Chim., 1967, 2954, J. Am. Chem. Sot., 1I2
(1990) 2030-2031, ibid. 110 (1988) 6255-6256, ibid. 109
( 1987 ) , 6544-6545, J. Organomet. chew. , 322 ( 1987 ) 65-70,
New. J. Chem. 14 (1990} 499-503.
I5 I) Synthesis of 2-M~-indene
110.45 g (0.836 mol) of 2-indanone were dissolved in
500 cm3 of diethyl ether, and 290 cm3 of 3 N (0.87 mol)
ethereal methylgrignard solution were added dropwise so
that gentle refluxing occurred. After boiling for 2
20 hours with a gentle refluxing, the mixture was poured
onto an ice/hydrochloric acid mixture and adjusted to
pH 2-3 with ammonium chloride. The organic phas~ was
separated off, washed with NaHC03 and sodium chloride
solution and~dried. 98 g of crude product (Z-hydroxy-2-
25 methyl3,ndane) were obtained, which was not further
purified.
This pxoduct was dissolved in 500 cm3 of toluene and
heated with 3 g of p-toluenesulfonic acid under a water
separator until the elimination of water was complete,
30 the mixture was evaporated doom, the residue was taken up
in dichloromethane, the solution was filtered over silica
gel and the filtrate was disti.lle~i in vacuo ( 80°C/10 mbar) .
Yield: 28.49 g (0.22 mol-26~).
r~~~~~3~ ~_O
-- 16 -
The synthesis of this compound is also described ins
C.F. Koelsch, P.R. Johnson, J. l~rn. Chem. Soc., 65 (1943)
56?-573
Iz} Synthesis of (2-Me-Indene)ZSiMe2
13 g (100 mol} of 2-Me-indene were dissolved in 400 cm3
of diethyl ether, and 62.5 cm3 of 1.6 N (100 mmol) of
n-butyllithium/n-hexane solution were added dropwise in
the course of 1 hour while cooling with ice, after which
stirring was continued for 1 hour at "35°C.
1O 6.1 cm3 (50 mmol) of dimethyldichlorosilane in 50 cm3 of
EtzO were initially ta3cen, and the lithio salt solution
was added dropwise in the course of 5 hours at 0°C,
stirring was carried out overnight at raom temperature
and the mixture was allowed to stand over the weekend.
The solid which had settled out was filtered off and the
filtrate was evaporated to dryness. After extraction
with small portions of n-hexane, filtration eras carried
out and the filtrate was evaporated down. 5.7 g
(18.00 mmol) of white crystals were obtained. The mother
liquor was evaporated down and then purified by column
chromatography ( n-hexane/HZCC12 9 . 1 parts by volume ) ,
2.5 g (7.9 mmol - 52~) of product being obtained (as an
isomer mixture).
r~ (Si02; n-hexane/HZCC12 9 r 1 parts by volume) = 0.37
The lH-NMR spectrum shows the signals to be expected fox
an isomer mixture, in shift and integration ratio.
III ) Synthesis of ( 2-Me-Ind) zCH2CH2
3 g (23 mmol) of 2-Me-indene were dissolved in 50 cm~ of
THF, 14.4 cm3 of 1.6 N (23.04 mmol) n-butyllithium/
n-hexane solution were added dropwise and stirring was
then carried out . for 1 hour at 65°C. Thereafter, 1 ml
~~~aa
_ 17 _
(11.5 mmol) of 1,2-dibromoethane was added at -78°C, and
the mixture was allowed to warm to room temperature and
was stirred for 5 hours. It was evaporated down and then
purified by column chromatography (SiOz; n-hexane/HZCC12
9 . 1 parts by volume).
The product-containing fractions were combined and
evaporated down, the residue was taken up in dry ether,
the solution was dried over .MgS04 and filtered and the
solvent was stripped off.
Yield: 1.6 g (5.59 mmol - 49~) of isomer mixture
rF ( Si02; n-hexane/HzGClz 9 . 1 parts by volume ) = 0 , 46
The 1H-NMR spectrum meets the expectation for an isomer
mixture in signal shift and integration.
A) Synthesis of rac-dimethylsilyl(2-Me-.1-indenyl)Z-
zircanium dichloride
1.68 g (5.31 mmol) of the chalets ligand dimethylsilyl(2-
methylindene)z were added to 50 cma of THF, and 6.63 cm3
of a 1.6 N (10.61 mmol) n-Bu~i/n-hexane solution were
added dropwise. The addition was carried out at ambient
temperature in the course of 0.5 hour. The mixture was
stirred for 2 hours at about 35°C, after which the solvent
was stripped off in vacuo, the residue was stirred with
n-pentane and the solid was filtered off and dried.
The dilithio salt thus obtained was added at -78°C to a
suspension of 1.24 g {5.32 mmol) of ZrCl4 in 50 cm3 of
CHzCl2 and the mixture was stirred for 3 hours at this
temperature. After warming up to room temperature
overnight, the mixture was evaporated down. The zH-N.MR
spectrum indicated a rac-meso mixture in addition to the
presence of a little ZrCl4(thf)2. After stirring with n-
pentane and drying, t)ie solid, yellow residue was suspen-
ded in THF, filtered off and investigated by NCR spec-
troscopy. These three operations were repeated several
s'~
!.n ,~ eJ' m_ ~,~
- 18 -
times; finally 0.35 g (0.73 mmol-1~~) of product was
obtained, in which, according to 1H-NMR, the rac form had
been concentrated to more than 17 a 1.
The compound gave a correct elemental analysis and the
following NMR signals (CDC13, 100 MHz)a 6 = 1.25 (s, 6H,
Si-Me); 2.18 (s, 6H, 2-Me), S.8 (s, 2H, 3~-H-Ind); 6.92-
7.'75 (m, 8H, ~-7-H-Ind).
B) Synthesis of rac-damethylsilyl(2-Me-1-iridenyl)2-
zirconiumdimethyl
1.3 cm3 of a 1.6 N (2.08 rnmol) ethereal Me7Gi solution were
added dropwise to 0.29 g (0.58 mmol) of rac-di.methylsilyl
(2-Me-1-iridenyl)ZZirconium dichloride in 40 cm3 of EtzO
at -50°C, and stirring was carried out for 2 hours at -
10°C. After exchanging the solvent for n-pentane, ~~tir-
ring was continued for a further 1.5 hours at room
temperature and the filtered residue was sublimed in
vacuo. 0.19 g (0.44 mmol-81~) of sublimate with a
correct elemental analysis was obtained.
C) Reaction of rac-dimethylsilyl(2-Me-1-indenyl)Z-
2 0 zirconiumdimethyl with [ Bu3NH ~ [ B ( CsHS ) a ]
0.17 g (0.39 mmol) of rac-dimethylsilyl(2-Me-1-indenyl)2-
zireoniumdimethyl was added at 0°C to 0.18 g (0.36 mmol)
of L Bu3NH ] f B ( C6H5 ) a 7 in 25 cm3 of toluene . The mixture was
warmed up to.ambient temperature while stirring for one
hour. The deeply coloured mixture was then evaporated to
dryness.
An aliquot part of the reaction mixture was used for 'the
polymerization.
D) Synthesis of rac-ethylene(2-Me-1-indenyl)ZZirconium
dichloride
14.2 cm3 of 2.5 N (35.4 mmol) n-BuLi/n-hexane Solution
- 19 -
were added dropwise to 5.07 g (17.7 mmol) of the ligand
ethylene(2-methylindene)2 in 200 cm3 of THF at room
temperature in the course of 1 hour, and stirring was
then carried out for 3 hours at about 50°C. A precipitate
which was formed in the meantime goes into solution
again. The solution was allowed to stand overnight.
6.68 g (17.7 mmol) of ZrCI,,(thf)z in 250 cm3 of THF were
added dropwise, simultaneously with 'the above dilithio
salt solution, to about 50 cm3 of THF at 50°C, and stir-
ring was then carried out for 20 hours at this tempera-
ture. The toluene extract of the evaporation residue was
evaporated down. After extraction of the residue with a
small amount of THF, recrystallization was effected from
toluene. 0.44 g (0.99 mmol-5.6~) of product was
obtained, the rac form having been concentrated to more
than 15 s 1.
The compound gave a correct elemental analysis and the
following NMR signals ( CDC13, 100 MHz ) : 8 - 2 . 08 ( 2s,
6H, 2-Me) ; 3 . 45-4 .18 (m, 4Fi, -CHZCHZ-) , 6 . 65 ( 2H, 3-H-Ind) ;
7.05-7.85 (m, 8H, 4-7-H-znd).
F) Synthesis of rac-ethylene(2-Me-1-indenyl)ZZirconium-
dimethyl
1.5 cm3 of a 1.6 N (2.4 mmol) et~aereal MeT~i solution were
added to 0.31 g (0.68 mmol) of rac-ethylene(2-Me-1-
indenyl)ZZirconium dichloride in 40 cm3 of Rt20, and
stirring was carried out for 2 hours at -40°C. After
exchanging the solvent for n-pentane, stirring was
carried out for 1.5 houxs at ambient temperature and the
mixture was filtered and the filtrate evaporated down.
0.22 g (0.54 mmol-80~) of sublimate with the correct
elemental analysis was obtained.
F) Reaction of rac-ethylene(2-Me-1-indenyl)zzirconium-
dimethyl with [ Bu33~1HH ] C B ( p-tolyl ) 41
2~i~~:~~t
- 20 _
0.13 g (0.32 mmol) of rac-ethylene(2-methyl-1-indenyl)z-
zirconiumda.methyl was added to 0.16 g (0.28 mmol) of
[ Bu3NH ] [ B ( p-tolyl ) y ] in 2 0 cm3 of toluene and stirring was
carried out for 1 hour at ambient temperature. The
solvent was stripped off and drying was then carried out
in vacuo.
An aliquot part of the reaction mixture was used for the
polymerization.
Example 1
A dry 24 dm3 reactor was flushed with nitrogen and filled
with 12 dm3 of liquid propylene.
35 cm3 of a solution of methylaluminoxane in toluene
(corresponding~to 52 mmol of AI, mean degree of oligomer
ization n = 17) were then added and the batch was stirred
at 30°C for 15 minutes.
At the same time, 6.9 mg (0.015 mmol) of rac-ethylene(2
Me-1-indenyl)zzirconium dichloride were dissolved in
13.5 cm3 of a solution of methylaluminoxane in toluene
(20 mmol of A1) and preactivated by allowing the solution
to stand for 15 minutes.
The solution was then introduced into the reactor and
heated to 70°C by supplying heat (10°C/min), and the
polymerization system was kept at 70°C for 1 hour by
cooling. ~'he polymerization was stopped by allowing the
excess gaseous monomer to escape. 1.56 kg of propylene
were obtained. The activity of the metallocene was thus
226 kg of PP per g of metallocene per h.
VN = 67 cm3/g; Mv, = 58, 900 g/mol; M,o/Mr, = 2. 0; IT = 95.9;
HD = 350 g/dm3
r ~-
~~~::~r~ ~ r.~
.-al-
Example 2
Example 1 was repeated, except that 10.1 mg (0.023 mmol)
of metallocene were used and polymerization was carried
out at 50°C.
0.51 kg of polymer powder were obtained, corresponding to
a metallocene activity of 50.5 kg of PP per g of metallo-
cene per h.
VN - 100 cm3/g; Rs,, = 10$,500 g/mol; ~i~/M" - 2.2; II -
96.4~~; MfT (230/5) = 210 gll0 min
Example 3
Example 1 was repeated, except that 10.5 mg (0.023 mmol)
of the metallocene were used and polymerization was
carried out at 30°C for 10 hours.
1.05 kg of polymer powder were obtained, corresponding to
a metallocene activity of 10.0 kg of PP per g of metallo
cane per h.
~1 - 124 cm~lg; 1~, = 157, 000 g/mol; ~,,,/1~, = 2 .2; IT -
96.3~5; MfI (230/5) = 104 g110 min
Comparative Examphs A - C
Polymerization was carried ~ut analogously to Examples 1
to 3, using tie metallocene roc-ethylenebisindenyl-
zirconium eliehloride. The viscosity numbers and molecu.-
lar weights of the resulting polymer products wars:
Comparative Polym. temp. VN iH~
Example LC] [cm3/gJ [g/mo1]
A 70 30 19,900
50 46 38,500
C 30 ' 60 48,700
'~~ ~~~~ ~~,
- 22 -
These Comgarative Examples show the effect of the sub-
stituent in the 2-position on the indenyl ligand in
respect of increasing the molecular weight.
Example 4
The procedure in Example 1 was followed, except that
4.0 mg (0.008 mmol) of roc-dimethylsilyl(2-methyl-1-
indenyl)Zxirconium dichloride were used. The metallacene
activity was 293 kg of PP per g of met~allocene per h.
VN - 171 cm3/g; Ma, = 197, 000 g/mol; Y~,/i~" - 2. 5; II
96.O~k; MFI (230/5) = 43.2 g/10 min; B~ = 460 g/dm3; m.p.
- 145°C
Example 5
The procedure in Example 1 was followed, except that
6.0 mg (0.013 moral) of roc-dimethylsilyl(2-methyl-1
indenyl)2zirconium dichloride were used.
The polymerization temperature was 60°C and the polymeri-
zation time was l hour. The metallocene activity was 178
kg of PP per g of metallocene per h.
VN - 217 cm3/g; M% _ 297, 000 g/mol; 1~/M=, = 2 . 3; II --
96.4; MFI (230/5) = 12.9 g/10 min; m.p. - 248°C
Example 6
The procedure in Example 1 was followed, except that
2.4 mg (0.0052 mmcrl) of roc-dime~thylsilyl(2-methyl-1-
indenyl)ZZirconium dichloride were used. The polymeri.za-
Lion temperature was 50°C and ~th~ polymerisation time was
3 hours. The metallocene activity was 89 kg of PP per g
of matallocene per h.
VN - 259 cm3/g; P~" = 342,500 ~g/mol; M~,/T~, = 2:1; II
96.8~; MFI (230/5) = 8.1 g/10 m~.n; m.p. 150°C
j'' !' ~ n
- 23 - ~~f~~~~~:
Example 7
The procedure in Example 1 was followed, except that
9.9 mg (0.021 mmol) of roc-dimethylsilyl(2-methyl-1-
indenyl)ZZirconium dichloride were used.
The polymerization temperature was 30°C and the poly-
merization time was 2 hours. The metallocene activity
was 26.5 kg of PP per g of metallocene per h.
VTV = 340 cm3/g; M,p = 457, 000 g/mol; 1~,/1~, = 2.4, II -
96.0~; MFI (230/5) = 2.5 g/10 min; m.p. - 153°C
20 Example 8
A dry 24 dm3 reactor was flushed with nitrogen and filled
with 6 dm3 of a gasoline cut from wha.ch aromatics had been
removed and which had a boiling range of 100-120°C and 6
dm3 of liquid propylene. 35 cm3 of a solution of methyl-
aluminoxane in toluene (corresponding to 52 moral of A1,
mean degree of oligomerization n = 17) were then added
and the batch was stirred at 30°C for 30 minutes.
At the same time, 14.7 mg (0.031 mmol) of roc-dimethyl-
silyl(2-methyl-1-indenyl)ZZirconium dichloride were
dissolved in 13.5 cm3 of a solution of methylaluminoxane
in toluene (20 mmol of A1) and preactivated by allowing
the solution to stand for 30 minutes.
The solution was then introduced into the reactor and the
polymerization system was kept at 50°C for 1 hour by
cooling. The polymerization was stopped by adding 50 cm3
of isopropanol. The metallocene activity was 159.2 kg
of PP per g of metallocene per h.
VN = 188 cm3/g; Mv, = 240, 000 g/mol; l.~,lMx, = 2 .1; TI -
96.0~; MFI (230/5) = 28.5 g/10 min; m.p. - 149°C
- 24 -
Example 9
Example 8 was repeated, except that 15.2 mg (0.032 mmol)
of the metallocene were used, the polymerizatian time was
2 hours and the polymerization temperature was 30°C.
The metallacene activity was 24.1 kg of PP per g of
metallocene per h.
VT~T - 309 cm3/g; Mw = 409, 000 g/mol; M~fl~, = 2 . 3; II
97.0~; MFI (230!5) = 3.5 g/10 min; m.p. - 153°C
Camparative Examples D - F
Polymerization was carried out analogously to Examples
4, 6 and 7 using the metallocene dimethylsilylbisindenyl-
zirconium dichloride. The viscosity numbers and molacu-
lar weights of the resulting polymer products weree
Comparative Polym. t~rrnp.VN 1~,,
Example [C] [cm3/g] [g/mol]
70 47 37,500
E 50 60 56,000
F 30 77 76,900
These Examples show the effect of the substituent in 'the
2-position on the indenyl ligand in respect of inGreas.ing
the molecular weight.
Example 10
A dry l6 dm~ reactor was flushed with nitrogen. 40 dm3
(corresponding to 2.5 bar) of hydrogen and finally 10 drn3
of liquid propylene and 29.2 cm~ of a salu~tion of methyl-
alumi:noxane in toluene (corresponding to 40 mmol of Al,
mean degree of oligom~srization l7) were then metered in,
and stirring was carried out at 30°C for 10 minutes.
At the same time, 2.? mg (0.006 mmol) of roc-dimethyl-
silyl(2-Me-1-indenyl)Zxirconium dichloride were dissolved
1 .~ J .:
'~ c.~ e~ id ~. ;.~
- 25 -
in 11.2 cm3 of a solution of rnethylaluminoxane in toluene
( 20 mmol ) and introduced into the reactor after 10 minutes .
Polymerization was carried out for 3 hours after heating
to 50°C. The polymerization was stopped by adding COZ gas,
and excess gaseous monomer was alowed to escape. The
metallocene activity was 102.9 kg of PP per g of metal--
locene per h.
VN = 25 cm'/g; Mp, = 8,500 g/mol; Ps~,/Mn = 2.4; no olefinic
chain ends according to ~3C-NMFL; II = 9'7.8; m.p. - 149°C
Example 11
Example 10 was repeated, except that 5.0 mg (0.011 mmol)
of rac-dimethylsilyl(2-Me-1-indenyl)ZZirconiumdimethyl
and 16 dm3 (corresponding to 1 bar) of hydrogen were used.
Polymerization was carried out at 60°C for 50 minutes.
The metallocene activity was 204 kg of PP per g of
metallocene per h.
VN = 47 cm3/g; M~,, = 41,100 g/mol; M~,/I~, = 2.2; no olefinic
chain ends according t0 '3C-NMR; TI = 96.9; m,p. -- 148°C
Example 12
Example 11 was repeated with 4.2 mg (0.01 mmol) of rac-
disnethyls ilyl ( 2-Tie-1-inde:~yl ) Zzirconiumdimethyl . However,
the polymerization temperature was 70°C and the poly-
merization time was 1 hour. The metallocene activity was
354 kg of PP per g of metallocene per h.
vN = 38 cm3/g; Mp, = 34, 900 g/mol; M"h'~, = Z .1; no olefinic
chain ends according to '3C-Nl~; II = 96.7; m.p. = 146°C
Examples 10 to 12 show the good regulatability of the
molecular weight with hydrogen when the polymerization
process according to the invention was used.
- 2s -
Example 13
Example 11 was repeated, except that no hydrogen was
used. The metallocene activity was 182.4 kg of PP per g
of metallocene per h.
STN - 210 cm3/g; MN, = 288, 000 g/mol; MN,/Mn = 2.2; TI -
96.2~.
Example 14
Example 11 was repeated, except that 4.2 mg (0.01 mmol)
of rac-ethylene(2-Me-1-ind~nyl)zzirconiumdi.methyl were
used. The metallocene activity was 144.3 kg of pP per g
of metallocene per h.
VN = 16 cm~lg; M~, - 8;900 g/mol; Mi"/M~, = 2.0; II m 96.0$;.
Example 15
A dry 24 dm~ reactor was flushed with nitrogen and filled
with 12 dm3 of liquid propylene and with 4.0 cm3 of a
solution of methylaluminoxane in toluene (corresponding
to 6 mmol of Al, mean degree of oligomerization 17), and
stirring was carried out at 30°C far 15 minutes. 2.5 cm~
of the reaction miacture of rac-dimethylsilyl(2-Men-1-
2 0 indenyl ) ~z irconiumdianethyl and [ Bu3NH ] [ B ( C6H3 ) a ] , v~rhich
mixture was described in Section C) of the metallocene
synthesis and corresponds to 17 mg. (0.04 mmol) of
me~allocen~, in toiuenca, were metered into the vessel:
Polymerization was carried out at 50°C for 1 hour: ~'he
me~all,ocene activity was 61.4 kg of ~P per g of
metallocene peg h:
VN = 238 cm~/g; ~k~'" = 328, 500 g/mol; Mo,/M" = 2 .2; IT --
96.O~k.
27 _ ~~~~~~J
Example 16
Example 15 was repeated, except that 2.5 cm3 of the
reaction mixture of rac-.ethylene ( 2-methyl-1-l.ndenyl ) 2-
z irconiumdi methyl and [ Bu3I~H ] [ B ( p-tolyl ) 4 ] , which mixture
was described in Section F) of the metallocene synthesis
and corresponds to 16.3 mg (0.04 mmol) of metallocene, in
toluene, were used. The metallocene activity was 42.9 kg
of PP per g of metallocene per h.
VN - 105 cm3/g; 1~,, = 110, 500 g/mol; i~~,,/Mn - 2 . 3; II -
96Ø
Example 17
Example 15 was repeated, except that a solution of tri-
methylaluminum in toluene (8 mmol of A1) was used instead
of the methylaluminoxane solution. The metalloc:ene
activity was 55.3 kg of PP per g of metallocene per h.
VN - 264 cm3/g; 1~., = 367, 000 glmol; M~"/I~, = 2 .3; II -
96.2~.
Example 18
Example 17 was repeated, except that no trimethylaluminum
was used in the polymerizat~.on.
The propylene used was purified with triethylaluminum
(1 mmol of AlEt3/dm3 of propylene) before being added to
the polymerization system, and the reaction products and
AlEt~ were separated off by distillation. The metallocene
activity was 56.9 kg of PP peg g of metallncene per h.
VN = 278 cm3/g; IMO = 3f2,000 glmol; Ma,/T~, = Z.3; II -
96.3~.
n,r .y.
Example 19
A dry 16 dm3 reactor was flushed with nitrogen and filled
at 20°C with 10 dm3 of a gasoline cut from which aromatics
had been removed and which had a boiling range of 100
120°C.
The gas space of the vessel was then flushed nitrogen-free
by forcing in 2 bar of ethylene and letting down the
pressure, these operations being carried out 5 times.
Thereafter, 30 cm3 of a solution of methylaluminoxane in
toluene (corresponding to 45 mmol of A1, molecular weight
according to cryoscopic determination 750 g/mol) were
added.
The reactor content was heated to 60°C in the course of
minutes while stirring, and the total pressure was
1S adjusted to 5 bar at a stirring speed of 250 xpm by
adding ethylene.
.fit the same 'tame, 2.3 mg (0.005 mmol) of rac-ethylene(2-
methyl-1-indenyl)Z~iroonium dichloride were dissolved in
cm3 of a solution of methylalum~.noxane in toluene and
20 preactivated by allowing the solution to stand for 15
minutes. The solution was then introduced into 'the
reactor, and tha polymerization system was :brought to a
temperature of 70°C and kept at this temperature for 1
hour by appropriate cooling. The total pressure was kept
at 5 bar dining this time by appropriate supply of
ethylene.
420 g of polyethylene were obtained, corresponding to a
metallocene activity of 182.6 kg of PE per g of metallo-
cene per h.~ The viscosity number was 300 cm3/g.
Camparative Example G
Polymerization was carried out with the metallocene
2g .-
rac-ethylene(1-indenyl)ZZirconium dichloride under
conditions analogous ~to Example 19. A polyethylene
having a viscosity number of 210 cm3/g was obtained.
The Comparative Example demonstrates the effect of
substitution in the 2-position of the indenyl ligand in
respect of increasing the molecular weight.
Example 20
Example 7 was repeated, except that the aluminoxane used
was isobutylmethylaluminoxane in the same A1 concentra-
tion and amount. The metallocene activity was 27.4 kg of
PP per g of metallocene per h, M" was 477,500 g/mol, the
V1~1 was 340 cm3/g an 1~"/ri° was 2.2. xsobutylrnethyl~-
aluminoxane was obtained by reacting a mixture of iso-
butyl-AlMe2 and AlMe3 with water in heptane and contained
9 mol ~ of isobutyl units and 91 mol ~ of methyl units.
Example 21
Example 7 was repeated, except that the aluminoxane used
was hydridomethylaluminoxane (prepared from Me2AlH and
water in toluene) in the same A1 concentration and
amount. The activity was 22.9 kg of PP per g of metallo-
cene per h, M~ was 469,500 g/mol, the VN was 339 cm3/g and
MW/Mn was 2Ø
Example 22
A dry 150 dm3 reactor was flushed with nitrogen and
filled, at 20°C, with 80 dm~ ~f a gasol3.ne cut from which
aromatics had been removed and which had a boiling range
of 100 - 120°C. Thereafter, the gas space way flushed
nitragen-free by forcing in 2 bar of propylene and
lett3.ng down the pressure, these operations being carried
out 5 times.
After the addition of 50 1 of liquid propylene, 64 cm3 of
_ 30 _ s~~~~~~.~3
a solution of methylaluminoxane in toluene (corresponding
to 100 mmol of Al, molecular weight according to cryo-
scopic determination 990 g/mol) were added and the
reactor content was heated to 30°C.
By metering in hydrogen, a hydrogen content in ~he gas
space of the reactor of 0.3~ was established and was
subsequently maintained during the entire polymerization
time by further metering (on-line monitoring by gas
chromatography).
Z0 24.3mg of rac«dimethylsilyl(2-methyl~1-indenyl)ZZirconium
dichloride (0.05 mmol) were dissolved in 32 ml of a solu-
tion of methylaluminoxane in toluene (corresponding to
50 mmol of A1), and the solution was introduced into the
reactor after 15 minutes.
Z5 The reactor was kept at the polymerization temperature of
30°C for 24 hours by cooling, and the polymerization was
then stopped by add~.ng 2 bar of Cn2 gas and the polymer
formed was isolated from the suspension medium over a
pressure falter. The product was dried for 24 hours at
20 80°/200 mbar. 10.5 kg of polymer powder, corresponding to
a metallocene activity of 18.0 kg of PP per g of metalla--
cene per h, were obtained.
VN -- 256 cm3/c~; M" = 340, 500 g/mol; 1~,/M° = 2 .2; II -
97.3~; 1S~'I (230!5) = 5.5 g/10 min; m.p. - 156°C
25 Example 23
Example 22 was repeated, except that 0.6~ of B2 was
established in the gas spaae,.20.6 mg (0.043 mmol) of the
metallocene were used and the polymerization temperature
was 50°C.
30 19.2 kg of polymer powder, corresponding to a metallocene
activity of 38.8 kg of PP pc~r g of metallocene per h,
were obtained.
-31_
Vt~1 - 149 cm3/g; M~ = 187, 500 g/mol; Mq,/Mr, - 2 . 3; II -
97.0~; MFI (230/5) = 82 g/10 min; m.p. - 150°C
Example 24
Example 23 was repeated, except that no hydrogen was
used, the weight of metallocene was 31.0 mg (0.065 mmol)
and the polymerization time was 4 hours.
8.0 kg of polymer powder, corresponding to a metallocene
activity of 64.5 kg of PP per g of metallocene per h,
were obtained.
VN - 175 cm3lg; M~, = 229, 000 g/mol; 1~,/1~, = 2.2; II -
97.1~; MFT (230/5) = 35 g/10 min; m.p. - 150°C
Example 25
Example l was repeated, except that 4.1 mg (0.008 mmol)
of the metallocene rac_phenyl(methyl)silyl(2-methyl_1
indenyl ) ZZrCl2 were used.
1.10 kg of polypropylene were obtained, corresponding to
an activity of the metallocene of 269 kg of PP per g of
metallocene per h.
VN = 202 cm~/g; M" = 230,000 glmol; I~,IM" = 2.3; II = 97~;
M~'I (23015) = 36 g/10 min; m.p. 147°C
Example 26'
Example 25 was repeated with 11.0 mg (0.02 mmol) of the
metallocene, but the polymerization temperature was 50°C.
1.05 kg of polypropylene were obtained. The activity of
the metallocene was 95.5 kg of PP per g of metallocene
per h.
VN - 347 cm3/g; ' M~ - 444, 000 g/mol; I~,/M" 2 .5; MFI
- 32 - ~~~ 8~~ ~J
(230!5) = 5.2 g/10 min; m.p. = 149°C
Example 27
Example 25 was repeated with 22.5 mg (0.04 mmol) of the
metallocene, but the polymerization temperature was 30°C.
0.57 kg of polyprapylene was obtained and the actieity of
the metallocene was thus 25.3 kg of PP per g of metallo-
cene per h.
VN - 494 cm3/g; M~,, -.. 666, 000 g/mol; 1~,,/MT, - 2.5; MfI
(230/5) = 1.3 8110 min; m.p. - 152°C
Example 28
Example 1 was repeated, except that 5.2 mg (0.009 mmol)
of the metallocene rac-diphenylsilyl(2-methyl-1--
indenyl)ZZrCl2 were used.
1.14 kg of polypropylene were obtained. The metallocene
activity was thus 219 kg of PP per g of metallocene per h.
VN - 298 am~/g; 1~,, ° 367, 000 g/mol; Mf,/Mr, - 2. 2; MFT
(230/5) = 7.1 g/10 min
Example 29
Example 28 was repeated with 12.6 ang (0.02 mmol) of the
metallocene but the polymerization temperature was 40°C.
0.44 kg of polypropylene was obtained and the metalloc~ene
activity was thus 34 :9 kg of PP per g of m~taJ.locene per h.
vN - 646 cm3/g; M" = 845,000 g/mol; 1~,/M~, - 2.4; MFT
(230/5) = O.l g/!0 min; m.p. - 155°C
S
- 33 -
Example 30
Example 1 was repeated, except that 17.4 mg (0.038 mmol)
of the metallocene rac-methylethylene(2-methyl-1-
indenyl } z2rClz were used.
2.89 kg of polypropylene were obtained. The metallocene
activity was thus 165.9 kg of PP per g of metallocene
per h.
VN = 238 cm3/g; M~ = 129,000 g/mol; M"/Mn = 2.2; m.p. -
150°C
Example 31
Example 30 was repeated with 1506 mg (0.034 mmol) of the
metallocene but the polymerization temperature was 50°C
and the polymerization time was 2 hears.
2.86 kg of polypropylene were obtained. The metallocene
I5 activity was thus 91. 7 kg of PP per g of metallocene per h.
V1V = 244 cm3lg; M", = 243,500 g/mol; M~,/M~, = 2.1; m.p. -
255°C
Example 32
Example 30 was repeated with 50.8 mg (0.120 mmol) of the
metallo~ene but the polymerization temperature was 30°C.
2.78 kg of polypropylene wore obtained and the metallo-
cene activity eras thus 27.5 kg of PP per g of metalloceue
per h.
VN - 409 cm~/g; 1~ ° 402, 000 g/mol; M"/M~, - 2 .2; 1~'I
(230/5) = 3.5 g/20 min; m.p. = 260°C
34
Example 33
Example 1 was repeated, except that 9.6 mg (0.02 mmol)
of the metallocene rac-dimethylsilyl(2-methyl-1-indenyl)z-
zirconium dichloride were used.
1.68 kg of polypropylene, corresponding to a metallocene
activity of 175.0 kg of PP per g of metallocene per h
were obtained.
VN = 143 cm3/g; 1~" = 132, 000 g/mol; N.~"/'MI, = 2. 3, m.p. -
14 0°C
Example 34
Example 33 was repeated, except that 10.4 mg (0.021 mmol)
of the metallocene were used and the polymerization
temperature was 50°C.
1.00 kg of polypropylene, corresponding to a metallocene
activity of 96.2 kg of PP per g of metallocene per h,
were obtained.
VN = 303 cm~/g; kiz" = 449,500 g/mol; Mw,/M" = 2.2; m.p. -
145°C
Example 35
Example 33 was repeated with 24.5 mg (0.049 mmol) of 'the
metallocene at a polymerization temperature of 30°C.
0.49 kg of polypropylene, corresponding to a metallocene
activity of 19.6 kg of PP per g of metallocene per h, was
obtained.
VPI = 442 cm3/g; I~,, = 564,000 glmol; TH~,/i~i" = 2.2; m.p. -
15 0°C
- 35 '
Example 36
A dry 24 dm3 reactor was flushed with nitrogen and filled
with 2.4 dm3 (S.T.P.) of hydrogen and 12 dm3 of liguid
propylene.
35 crn3 of a solution of methylaluminaxane in toluene
(corresponding to 52 moral of Al, mean degree of
oligomerization p = 17) were then added.
.At the same time, 8.5 mg (0.02 mmol) of roc-dimethyl-
silyl(2-methyl-1-indenyl)ZZirconium dichloride were
In dissolved in 13.5 cm3 of a solution of methylalumino7cane
in toluene (20 mmol of Al) and preactivated by allowing
the solution to stand far 5 minutes.
The solution was then introduced into the reactor.
Polymerization was carried out for 1 hour at 55°C with
continuous addition of 50 g of ethylene.
The metalloeene activity was 134 kg of C2/C3--copolymer per
g of metallocene per h.
The ethylene content of the copolymer was 4.3~.
VN - 2$9 cm3/g; M~, -- 402, 000 g/mol; ivi,~/Mn - 2. 0; 1~FI
(230/5) = 7.0 g/10 min
The ethylene was substantially incorporated as isolated
taiits ( 13C.-1~1MR, mean block length CZ < 1. 2 ) .
Example 37
A dry 150 dm~ reactor was prepared as described in Example
22 and charged with propylene and catalyst.
The polymerization was carried out in a first stage at
50°C for 10 hours.
36
In a second stage, 1 kg of ethylene was first rapidly
added and a further 2 kg of ethylene were metered in
continuously in the course of 4 hours.
21.5 kg of block copolymer powder were obtained.
VN - 326 cm3/g; M~,, - 407, 000 g/mol; P~"/~i,., - 3.1; MfI
(230/5) = 4.9 g/10 min
The block copolymer contained 12.5 of ethylene.
Fractionation gave a content of 24~ of ethylene/propylene
rubber in the copolymer. The mechanical data of the
copolymer were:
Ball indentation hardness (DIN 53,456, pressed sheets,
heated at 140°C for 3 h, 132 N) 60 Nmm Z, notched iatipact
strength (ax", injection molded specimens according to
DIN 53, 453 ) 23°C: na fracture, 0°C: 39 . a mJ'mtti Z,
~40°C: 20.1 mJmm 2.
The product is distinguished by an exceptional hardness/
impact strength relationship and can be used for struc-
tural components, for example in automotive construction
(e. g, bumpers), where high rigidity coupled with high
impact strength, in particular at low temperatures, is
required.
Abbreviations:
Me ~ Methyl, Et = Ethyl, Hu = Butyl, Fh = Fhenyl, THF =
Tetrahydrofuran, PE = Polyethylene, PP = Polypropylene.