Note: Descriptions are shown in the official language in which they were submitted.
2~5532~
PATENT
Patent Pro;ect No. 88A237
BOG 1-014.doc
26 November 1990
~YDROGEN A~D CA~BON MONOSIDE P~ODUCTION
BY ~yDR~GaaBON 8~A~-ByFQR~y_A~P
P~E8gURE ~R~NG AD80R~TION ~5~gaG~a~ZLQ~
BAC~GRO~ND OF ~ EN~IO~
1. ~ield of the Inven~ion
~he present invention is directed to a method
for producing merchant grade hydrogen and carbon monoxide
from a ~team reformed hydrocarbon feed mixture. More
particularly, the present invention is directed to a
method for producing hydrogen and carbon monoxide from a
feed mixture comprisinq hydrogen, carbon monoxide, carbon
dioxide, and methane.
~. De~cr~ption of the Prior A~t
Various methods are known for separating
gaseous mixtures produced by the steam reforming of
hydrocarbon-. Steam reforming to produce hydrogen
consists ot treatlng a hydrocarbon f-ed mixture with
-- 2 --
steam in a catalytic ~team reactor (reformer) which
consists of a number of tubes placed in a furnace at a
temperature in the range from about 1250 F. to about
1700 F. The reversible reforming reactions which occur
when methane i6 used as the hydrocarbon feed mixture are
set out below.
CH4 ~ H20 C0 ~ 3H2
CH4 + 2h20--C02 ~ 4H2
CO I H20 ~ C2 ~ H2
Carbon monoxide and~ carbon dioxide are
generally removed by 6hift conversion (reaction of carbon
monoxide with steam to form additional hydrogen and
carbon dioxide), absorption in ~mines or other alkaline
solvents (carbon dioxide removal), and methanation
(conversion of trace carbon monoxide ~nd carbon dioxide
to methane). When carbon monoxide is a desired product,
the 6hift conver6ion and methanation 6teps are not
employed.
The hydrogen-rich ga6 mixture exitinq the 6team
reformer consist6 of an eguilibrium mixture of hydrogen,
carbon monoxide, carbon dioxide, water vapor, and
unreacted methane. The reforming reactions are
endothermic and therefore hydrocarbons and process waste
gases are burned in the reformer furnace to provide the
endothermic heat.
Hydrocarbon steam reforming reaction6 and
hydrogen 6eparation proce6se~ are di6closed in more
detail in "A~monia ~nd Synthesis Gas: Recent and Energy
Saving Proces6es", Edited by F.J. Brykow~i, Chemical
Technology Review No. 193, Energy Technology Review No.
68, Published by Noyes Data Corporation, Park Ridge, New
Jersey, 1981.
2 ~ 2 ~
- 3 -
Conventional ~ethods for recovering hydrogen
and carbon monoxide from a hydrocarbon steam reformed
feed mixture have generally focused on cryogenic
di6tillation proce6se6 to ~eparate and purify hydrogen
and carbon monoxide in the ~ixture after carbon dioxide
i~ removed. Cryogenic separation proce~ses tend to have
a h$gh capital c06t e6pecially when more than one pure
product is reguired.
United State6 patent no. 4,778,670, i66ued to
Pin~o, disclo6e~ a pre~6ure swing ad60rption proce~s for
producing technical hydrogen which compri6es pa~6$ng a
raw gas containing a specific ratio of hydrogen,
nitrogen, and carbon oxides to a pressure swing adsorbent
and stopping the flow of feed ga6 in the cycle when the
integrated nitrogen content of the unadsorbed product gas
of the pressure ~wing adsorption stage ~6 in the range of
1% to 10% by volume.
~0 German patent application no. 3,427,804, to
Liade A.G., di6close6 a proces6 for reforming a
hydrocarbon with carbon dioxide to obtain a gas mixture
comprising hydrogen, carbon monoxide, and carbon dioxide
and separating the mixture into ~eparate 6treams of
hydrogen, carbon ~onoxide, and carbon dioxide. The
methods for purifying the hydrogen and carbon monoxide
stream6 are not di6closed.
Methods for separating hydrogen and carbon
monoxide by pressure ~wing adsorption proces6e6 are
di~closed in European patent application no. 317,235A2,
to Xrishnamurthy et al., and the reference~ cited
therein. Kri6hnamurthy et a~. di6close6 a method for
forming hydrogen and carbon monoxide from a feed mixture
exiting a hydrocarbon ~team reformer comprising hydrogen,
carbon ~onoxide, and carbon dioxide. The method
compri~es the 6teps of passing the feed mixture through a
sorptive 6eparation to separate a hydrogen product, a
carbon monoxide-rich product, and a carbon dioxide-rich
2 ~ 3 3 3 2 fJ
- 4 -
product. The carbon monoxide-rich product i6 further
purified in a two stage pressure 6wing adsorption system.
The first stage comprises an activated carbon adsorbent
which removes carbon monoxide and methane a6 the strongly
adsorbed waste ~tream. The second stage comprises a
zeolite adsorbent and produces a pure carbon monoxide
stream as an adsorbed product.
United States patent no. 4,917,711, is6ued to
~e et al., di6closes an adsorbent for carbon monoxide
and unsaturated hydrocarbon~ which comprises a high
6urface area support, 6uch as a zeolite, alumina, ~ilica
gel, aluminosilicate, or aluminophosphate, and cuprous or
cupric compound. The adsorbent may be used to ~eparate
carbon monoxide and unsaturated hydrocarbons from a
gaseous mixture containing hydrogen, nitrogen, argon,
helium, methane, ethane, propane, and carbon dioxide by
passing the ~ixture through the adsorbent and releasing
the adsorbed carbon monoxide by heating, or lowering the
pressure of, the adsorbent.
Japanese patent JP01203019 discloses a four
column pressure ~wing adsorption ~ystem for separating
carbon monoxide from a gaseous mixture. The columns
contain an adsorbent containing copper to adsorb carbon
monoxide gas.
United States patent no. 4,914,076, issued to
Tsuii et al., disclo~es a ~ethod for preparing an
adsorbent for carbon monoxide which compri6es contacting
an alumina or ~ilica-alumina 6upport with a mixed
solution or dispersion of a copper (II) ~alt and a
reducing agent, and then removing the 601vent.
United States patent no. 4,783,433, issued to
~aiima et al., di~closes an adsorbent for separating
carbon monoxide from a gaseous mixture containing carbon
dioxide which comprise~ a zeolite resin with a
silica/alumina ratio of not more than 10, in which not
2 ~ 5 ~ ~ h ~
- 5 - .
less than 50S of the cation exchange ~ites have been
replaced by Cu(I) ions, in the pores of which, one or
more 6alts of the metals Cu(I), Fe, Zn, Ni, and or Mg are
dispersed.
Japanese patent JF61242908 di6closes an
adsorbent for carbon ~onoxide which i6 prepared by
supporting a copper (I) compound on an activated carbon
support ~herein the volume of pore~ having a diameter of
~0 under 10 angstroms ~6 under 0.33ml/g.
United States patent no. 4,743,276, is6ued to
Nishida et al., discloses an adsorbent for carbon
monoxide which comprises a zeolite resin with a
~ilica/alumina ratio of not more than 10, in which not
less than 50% of the cation exchange 6ites have been
replaced by Cu(I) ions, in the pores of which, one or
more 6alts of the met~l6 Cu(I), Fe, Zn, Ni, and or Mg are
dispersed.
In a pressure ~wing adsorption 6ystem (~SA), a
gaseous mixture is passed at an elevated pressure through
a bed of an adsorbent material which 6electively adsorbs
one or more of the components of the gaseous mixture.
Product gas, enriched in the unadsorbed gaseous
component(s), is then withdrawn from the bed.
The term ~qaseous mixture", a6 used herein,
refer6 to a gaseous mixture, ~uch as air, primarily
comprised of two or more components having different
molecular 6ize. The term ~enriched gas" refers to a gas
comprised of the component(s) of the gaceou6 mixture
relatively unadsorbed after pa66age of the gaseous
~ixture through the adsorbent bed. The enriched gas
generally must meet a predetermined purity level, for
example, from about 90% to about 99%, in the unadsorbed
component(s). The term ~lean gas" refers to a gas
exiting from the adsorption bed that fail6 to meet the
predetermined purity level set for the enriched gas.
2~a~
When the 6trongly adsorbed component is the desired
product, a cocurrent depressurization 6tep and a
cocurrent purge step of the 6trongly adsorbed component
are added to the process.
The term ~adsorption bed" refers either to a
single bed or a serial arrangement of two beds. The
inlet end oS a ~ingle bed sy6tem is the inlet ond of the
single bed while the inlet end of the two bed ~y~tem
(arranged in series) i~ the inlet end of the f~r~t bed in
the 6ystem. The outlet end of a ~ingle bed 6ystem is the
outlet end of the 6ingle bed and the outlet end of the
two bed system (arranged in 6eries) is the outlet end of
the ~econd bed in the system. By using two adsorption
beds in parallel in a system and by cycling (alternating)
between the adsorption beds, product gas can be obtained
continuously.
As a g~6eous mixture travels through a bed of
adsorbent, the adsorbable gaseou6 co~ponents of the
mixture enter and fill the pore6 of the adsorbent. ASter
a period of time, the composition of the gas exiting the
bed of adsorbent i8 essentially the ~ame as the
composition entering the bed. This period of time is
known as the breakthrough point. At some time prior to
this breakthrough point, the adsorbent bed must be
regenerated. Regeneration involves stopping the flow of
gaseous ~ixture through the bed and purging the bed of
the ad60rbed components generally by venting the bed to
atmo6pheric or ~ubatmo6pheric pre6sure.
A pre6sure swing adsorption ~y~tem generally
employs two adsorbent beds operated on cycles which are
6equenced to be out of phase with one ~nother by 180 60
that when one bed i6 in the ad~orption or production
step, the other bed i6 in the regeneration ~tep. The two
adsorption beds ~ay be connected in series or in
parallel. In a ~erial arrangement, the ga6 exiting the
outlet end of the fir6t bed enters the inlet end of the
second bed. In a parallel arrangement, the ~a~eous
mixture enters the inlet end of all beds comprising the
system. Generally, a serial arrangement of beds is
preferred for obtaining a high purity gas product and a
parallel arrangement of beds i6 preferred for purifying a
large quantity of a gaseous mixture in a short time
cycle.
Between the adsorption step and the
regeneration ~tep, the pressure in the two ad60rption
beds is generally equalized by connecting the inlet ends
of the two beds together and the outlet ends of the two
beds together. During the pressure equalization step,
the gas within the pores of the adsorption bed which has
just completed its adsorption step (under high pressure)
flows into the adsorption bed which has ~ust completed
its regeneration step (under low pressure) because of the
pressure differential which exists between the two beds.
The adsorption bed which completed it~ adsorption ~tep is
depressurized and the adsorption bed which completed its
regeneration ~tep i~ repressurized. Thiæ pressure
egualization step improves the yield of the product gas
because the gas within the pores of the bed which has
just completed its adsorption step has already been
enriched. When more than two beds are employed in the
adsorption system, it is common to have a number of
pressure equalizations steps.
Gas ~eparation by tbe pressure swing adsorption
~ethod is more fully described in, for example, ~Gas
Separation by Adsorption Processes~, Ralph T. Yang, Ed.,
Chapter 7, "Pressure Swing Adsorption: Principles and
Processes" Butterworth 1987, and in United States patents
nos. 2,944,627, 3,801,513, - and 3,960,522.
Modifications and improvements in the pres~ure swing
adsorption process and apparatus are described in detail
in, for example, United States patents nos. 4,415,340 and
/~:
-- 8 --
4,340,398.
While the above methods disclose processes for
separating carbon monoxide, none of the methods disclose
~atisfactory processes for recovering both hydrogen and
carbon monoxide from a hydrocarbon ~team reformed feed
miYture economically and in high purity. Methods for
separating hydrogen and carbon monoxide from a
hydrocarbon steam reformed feed mixture reguire multi-
stage systemC to purify carbon ~onoxide. Methods for
separating carbon monoxide using copper exchanged sieves
have focused on the separation of waste gases from steel
mills which contain nitrogen, carbon monoxide, and carbon
dioxide but not hydrogen. Conventional cryogenic
separation processes tend to have a high capit~l cost
especially when more than one pure product is reguired.
The present invention provide6 an improved method for
b~' producing hydrogen and carbon monoxids from a hydrocarbon
6team re~ormed feed mixture employing a novel combination
of pressure swing adsorption methods which minimizes
capital cost requirements and increases the recovery of
carbon monoxide.
~MMARY OF ~ I~V~IO~
The present invention is directed to a method
for producing hydrogen and carbon monoxide from a feed
mixture compri6ing hydrogen, carbon monoxide, carbon
dioxide, and methane, which comprise6 the ~teps of (a)
passing the feed mixture through a first pres6ure 6wing
adsorption system containing an adsorption bed comprising
an adsorbent having a greater affinity for carbon
dioxide, methane, and carbon monoxide than for hydrogen
to 6eparate hydroqen as a pure non-adsorbed product and
carbon dioxide, methane, and carbon monoxide as an
_ adsorbed fraction, (b) desorbing carbon monoxide from the
2~3~2~
pressure swing adsorption ~ystem in step (a) to form a
carbon monoxide-rich fraction, (c) desorbing carbon
dioxide and methane from the pressure swing adsorption
system in step (a) to form a carbon dioxide-rich
fraction, (d) pa~sing the carbon ~onoxide-rich fraction
from step (b) to a second pressure swing adsorption
system containing an adsorption bed compri~ing an
adsorbent having a greater affinity for carbon monoxide
than for hydrogen, carbon dioxide, and methane to
separate carbon monoxide as an adsorbed fraction hnd
hydrogen, carbon dioxide, and methane as a non-adsorbed
fraction, and (e) desorbing carbon monoxide from the
pressure swing adsorption system in step (d) to form a
pure carbon monoxide product.
In a 6econd embodiment, the invention is
directed to a method for producing hydrogen and carbon
monoxide from a feed mixture comprising hydrogen, carbon
monoxide, carbon dioxide, and methane, which comprises
the ~teps of (a) providing a pressure swing adsorption
system having a first stage and a second stage, wherein
the first stage contains an adsorption bed comprising an
adsorbent having a greater affinity for carbon monoxide
than for hydrogen, carbon dioxide, and methane, the
6econd stage contains an adsorption bed comprising an
adsorbent having a greater affinity for carbon dioxide,
methane, and carbon monoxide than for hydrogen, and the
first and second 6tages are connected in 6eries and each
stage contains an inlet end and an outlet end, (b)
passing the feed mixture through the first stage of the
pressure swing adsorption ~ystem to separate carbon
monoxide as an adsorbed fraction and hydrogen, carbon
dioxide, and ~ethane as a non-adsorbed fraction, (c)
passing the non-adsorbed fraction from ~tep ~b) through
the second stage of the pressure ~wing adsorption system
to separate carbon dioxide and methane as an adsorbed
fraction and hydrogen as a non-adsorbed pure product, (d)
desorbing carbon dioxide and methane from the first and
second 6tages of the pressure swing adsorption system to
lo - 2 ~ 2 ~
form a carbon dioxide-rich fraction, and (e) desorbing
carbon monoxide from the first 6tage of the pressure
swing adsorption system to forD a pure carbon monoxide
product.
~RIFF D~CRIP~IO~ OF T~ ~IG~R~B
FIGURE 1 i~ a schematic process flow diagram
illustrating a novel combination of pre6sure 6wing
adsorption sy6tems according to the present invention to
separate hydrogen and carbon monoxide from a feed mixture
comprising hydrogen, carbon monoxide, carbon dioxide, and
methane.
FIGURE 2 i8 a ~chematic process flow diagram
illustrating a first pres~ure swinq adsorption method for
separating hydrogen a~ a non-adsorbed product and carbon
monoxide, carbon d~oxide, and methane as an adsorbed
fraction, according to the pre6ent invention.
FIGURE 3 is a 6chematic proce6s flow diagram
illustrating a second pressure swing adsorption method
for separating carbon monoxide as an adsorbed product and
hydrogen, carbon dioxide, and methane as a non-adsorbed
fraction, according to the present invention.
FIGURE 4 is a schematic process flow diagram
illustrating ~ novel two ~tage pressure ~wing adsorption
system according to the present invention to ~eparate
hydrogen ~nd carbon monoxide from a feed mixture
compri6ing hydrogen, carbon monoxide, carbon dioxide, and
methane.
FIGURE 5 i6 a schematic process flow diagram
illustrating a fir6t stage of a pressure swing adsorption
6ystem for separating carbon monoxide as an adsorbed
product and a second 6tage of a pressure swing adsorption
2 ~ 2 ~
-- 11 --
system for separating carbon dioxide as an adsorbed
fraction and hydrogen as a non-adsorbed product,
according to the present invention.
D~TAIL~D D~CRIPTIO~ 0~ T~E ~V~T~ON
Applicant has discovered a ~ethod for
sepAratinq hydrogen and carbon ~onoxide from a feed
mixture comprising hydrogen, carbon ~onoxide, carbon
dioxide, and methane. In a preferred embodiment, a novel
combination of non-cryogenic separation steps is utilized
which efficiently and economically yields tenriched
hydrogen and carbon monoxide in high purity and yield
from a feed mixture exiting a hydrocarbon steam reformer.
In a first preferred embodiment, the combination of non-
cryogenic steps comprises two pressure ~wing adsorption
systems. The first pressure ~wing adsorption sy6tem
separates hydrogen from the f0ed mixture a3 a pure non-
adsorbed product and carbon dioxide, methane, and carbon
monoxide as an adsorbed fraction. The adsorbed fraction
i8 then desorbed and passed to a second pressure swing
adsorption system. The second pressure swing adsorption
system separates carbon monoxide as an adsorbed fraction
and hydrogen, carbon dioxide, and methane as a non-
adsorbed fraction.
In ~ ~econd embodi~ent, a novel two stage
pressure swing adsorption system iB utilized which
efficiently and economically yields enriched hydrogen and
carbon monoxide. The first 6tage of the pressure swing
adsorption 6ystem eparate~ carbon monoxide as an
adsorbed fraction and hydrogen, car~on dioxide, and
methane as a non-adsorbed fraction. The second 6tage of
the pressure ~wing adsorption system separates carbon
dioxide and methane as an adsorbed fraction and hydrogen
as a non-adsorbed pure product.
- 12 ~
The novel co~bination of pressure swing
adsorption separation methods of the present invention
provides 6ignificant savings in capital and operating
expense over completely cryogenic methods. The steps in
the present method may be integrated into steps in the
hydrocarbon ~team reformer method to enhance the
reforming proce~. For example, the carbon d~oxide-rich
fraction from the fir6t pres6ure 6wing adsorption 6ystem
in the first embodiment or the ~econd stage in the second
embodiment may be recycled and used as fuel in the
hydrocarbon steam reformer, further increasing the
concentration of carbon monoxide in the feed mixture.
The hydrogen-rich fraction from the second pressure swing
adsorption system in the first embodiment may also be
recycled into the first pressure swing adsorption system
to separate additional carbon monoxide.
The feed mixture (exhaust gas, effluent gas,
exit gas, feed gas) in the present invention is a mixture
compri~ing hydrogen, carbon monoxide, carbon dioxide, and
methane. Preferably, the feed mixture is an effluent gas
from a hydrocarbon steam reformer. The feed mixture will
in general comprise hydrogen in an amount up to about
80%, carbon monoxide in an amount up to about 20%, carbon
dioxide in an amount up to about 30%, and methane in an
amount up to about 3%.
The feed mixture is typically available in a
saturated state and may be dried by passing the mixture
through a condenser (drier) containing a desiccant such
as alumina, 6ilica, or zeolite. Desorption of the water
from the desiccant may be accompli6hed by purging the
desiccant with a dry waste purge ga6 (~uch as the carbon
dioxide-rich fraction or nitrogen ga~). Any water
remaining in the feed mixture iB removed with the
strongly adsorbed stream ~carbon dioxide-rich fraction).
After being dried, the feed mixture may be compressed
prior to passage of the mixture into the pressure 6wing
adsorption 6ystem.
2 ~ 2 {3
- 13 -
The feed mixture from the hydrocarbon steam
reformer will first be passed through a process cooler to
cool the gas and condense and remove water vapor. To
maximize the carbon monoxide concentration and minimize
the carbon dioxide concentration in the feed ~ixture, the
hydrocarbon ~team reformed feed mixture will by-pass the
shift converter.
A typical feed mixture from a hydrocarbon steam
reformer will have a pres6ure in the range from about
150 psia to about 600 psia, preferably from about
150 psia to about 400 psia, and more preferably from
about 150 psia to about 300 psia. Generally the feed
mixture will be available at a pressure sufficiently high
to be used directly in the first pressure swing
adsorption system. Optionally, a compressor may be
employed to compress the feed mixture to the required
pressure for the pressure swing adsorption system.
The adsorbent material in the adsorbent bed in
the hydrogen pressure swing adsorption system (first
pres6ure swing adsorption ~y~tem in the first embodiment
and second stage in the pressure ~wing adsorption system
in the second embodiment~ is an adsorbent having a
greater affinity for carbon dioxide, methane, and carbon
monoxide than for hydrogen. m e adsorbent material may
be a molecular sieve or activated carbon, and preferably
i~ a combination of molecular sieve~ and activated
carbon. Both calcium and sodium aluminosilicate zeolites
may be employed. Carbon molecular sieves and ~ilica
molecular sieves are also u~eful. Suitable zeolite
sieve6 include, but are not limited to, the type 5A, lOX,
13X zeolite ~olecular sieves, and mordenitee. Preferred
zeolite sieve6 are the type 5A zeolite sieve6 and
molecular 6ieves with comparable pore size and molecular
attraction.
~a~2~
- 14 -
The adsorbent material in the adsorbent bed in
the carbon monoxide pressure swing adsorption system
(second pressure swing adsorption system in the first
embodiment and first stage in ths pressure ~wing
adsorption system in the second embodiment) is an
adsorbent having a greater affinity for carbon ~onoxide
than for hydrogen, carbon dioxide, and methane. In
general, suitable adsorbent materials ~re copper
exchanged substrate~ 6uch as those selectQd from the
group consi~ting of copper exchanged Y-type
aluminosilicate zeolite Dolecular sieves, copper
exchanged alumina, and copper exchanged activated carbon,
and mixtures thereof. In a preferred embodiment, the
adsorbent material is copper aluminosilicate zeolite
molecular sieve material, available under the tradename
NXX type adsorbent in a pac~age from Nippon RoXan X. X.,
Tokyo, Japan. Copper aluminosilicate zeolite molecular
sieves can be prepared by exchanging sodium in sodium
aluminosilicate zeolite molecular sieve~ with copper (2+)
followed by a heating and reducing treatment to enhance
the affinity of the adsorbent for carbon monoxide and
reduce the affinity of the adsorbent for carbon dioxide.
Copper exchanged 6upports, and methods for preparing such
supports, are described in detail in United States patent
no. 4,917,711, Japanese patent JP01203019, United States
patent no. 4,914,076, United States patent no. 4,783,433,
Japanese patent JP61242908, and United States patent
no. 4,743,276, which reference~ are described above and
are incorporated herein by reference.
In accord with the present invention,
hydrogen gas product can be prepared having a purity of
greater than about 99%, preferably greater than about
99.99%, and more preferably greater than about 99.999%.
A carbon monoxide gas product can be prepared having a
purity of greater than about 98%, preferably greater than
about 99%, and more preferably greater than about 99.85%.
2 ~
- 15 -
The method for producing hydrogen and carbon
monoxide from a feed mixture comprising hydrogen, carbon
monoxide, carbon dioxide, and ~ethane can be better
underetood by reference to the FIGURES in which like
numerals refer to like parts of the invention throughout
the FIGURES. Although the present invention i8 described
and illustrated in connection with preferred embodiments,
applicant intends that modifications and variations may
be used without departing from the spirit of the present
invention.
FIGURE 1 illustrates a preferred first
embodiment of the present invention for producing
hydrogen and carbon monoxide from a feed mixture
comprising hydrogen, carbon monoxide, carbon dioxide, and
methane by a combination of pressure Qwing a~sorption
steps. In FIGURE 1, gaseous feed mixture is fed through
feed conduits 1 and 2 to hydrogen (first) pressure swing
adsorption system A to separate the mixture. ~ypically
the feed mixture from the hydrocarbon ~team reformer will
enter hydrogen pre6sure swing adsorption sy~tem A at a
pressure in the range from about 150 psia ~o about
600 psia, preferably from about 150 psia to about
400 psia, and more preferably from about 150 psia to
about 300 psia. Optionally a compressor may be employed
to compress the feed mixture to the pressure swing
adsorption pressure. After being cooled, the feed
mixture sntering hydroqen pressure swing adsorption
system A will be at ambient temperature.
During the hydrogen product production step,
feed mixture iQ fed into and hydrogen product is
withdrawn from hydrogen pressure 6winq adsorption
system A. Hydrogen product i8 ~eparated as a pure non-
adsorbed product and carbon monoxide, carbon dioxide,methane, and water vapor are separated as an adsorbed
fraction. Hydrogen product (merchant grade, le~s than
about 10 vpm impurities) is withdrawn from hydrogen
- 16 -
pressure swing adsorption system A through feed conduit 3
and passed to hydrogen reservoir B.
After the hydrogen product production step,
hydrogen pressure ~wing adsorption system A undergoes a
pressure e~ualization step and an intermediate
depressurization step (carbon monoxide-rich fraction
production step). During the carbon monoxide-rich
fraction production step, a carbon monoxide-rich fraction
lo i6 desorbed and withdrawn from hydrogen pressure ~wing
adsorption sy6tem A via feed conduit 4 and passed to
carbon monoxide storage vessel C.
After the carbon monoxide-rich fraction
production step, hydrogen pressure swing adsorption
system A undergoes a depressurization 6tep and a hydrogen
product gas purge ~tep (carbon dioxide-rich fraction
production 6teps). In the fir6t carbon dioxide-rich
fraction production 6tep, a carbon dioxide-rich fraction
i6 withdrawn by depressurizing hydrogen pressure 6wing
adsorption system A. In the second carbon dioxide-rich
fraction production step, a carbon dioxide-rich fraction
is withdrawn by purging hydrogen pressure 6wing
adsorption system A with hydrogen product gas. The
carbon dioxide-rich fractions are withdrawn via feed
conduit 5 and passed to carbon dioxide compressor D.
Carbon dioxide compressor D compresse6 the carbon
dioxide-rich fractions to the 6team reforming pressure.
The compressed carbon dioxide-rich fractions from carbon
dioxide ~ompressor D are then pas6ed through feed
conduit 6 to the hydrocarbon steam reformer for recycle
a6 reformer feed ga6 to enrich the carbon monoxide
content of the reformer product gas. In general, carbon
dioxide compres60r D will compre6~ the carbon dioxide-
3S rich fraction to a pre6sure in the range from about150 psia to about 600 psia, preferably from about
150 psia to about 450 psia, and more preferably from
about 150 psia to about 350 p~ia.
2 ~
- 17 -
The carbon monoxide-rich fraction in carbon
monoxide storage vessel C i8 passed to carbon monoxide
compressor E via feed conduit 7. Carbon monoxide
compressor E compresses the carbon monoxide-rich fraction
S to the separation pressure. In general, carbon monoxide
compressor E will compres~ the carbon monoxide-rich
fraction to a pressure in the range from nbout 20 psia to
about 600 psia, preferably from about 20 psia to about
200 psia, and more preferably from about 20 psia to about
100 psia. Compre6sed carbon monoxide-rich fraction is
then pas6ed from carbon monoxide compre~sor E to carbon
monoxide (second) pres~ure ~wing adsorption system F via
feed conduit 8 to further separate the mixture. The feed
gas in feed conduit 8 may be available at temperatures
ranging from about ambient to about 150 F.
During the carbon monoxide adsorption step,
carbon monoxide-rich feed mixture is fed into, and carbon
dioxide, methane, water vapor, and any remaining hydrogen
is ~ithdrawn from, carbon monoxide pressure ~wing
adsorption 6y~tem F. Carbon monoxide is ~eparated as an
adsorbed product and carbon dioxide, methane, any water
vapor, and any hydrogen are separated as a non-adsorbed
fraction. The carbon dioxide, methane, water vapor, and
hydrogen non-adsorbed fraction is withdrawn from carbon
monoxide pressure swing adsorption system F through feed
conduit 9 to recycle compressor G. Recycle compressor G
compre6ses the non-adsorbed gases to the hydrogen
pre66ure swing adsorption ~eparation pressure. In
general, the recycle compres60r G will compress the non-
adsorbed gases to a pressure in the range from about
150 p~ia to about 600 psia, preferably from about
150 psia to about 400 psia, and more preferably from
about 150 psia to ~bout 310 psia. The compressed non-
adsorbed gases from recycle compressor G are thenrecycled to hydrogen pressure ~wing adsorption 6ystem A
through feed conduits 10 and 2.
2~5 ï~
- 18 -
After the carbon monoxide adsorption step,
carbon monoxide pressure swing adsorption ~ystem F
undergoes a pressure egualization step, a carbon monoxide
product gas purge ~tep, and a carbon monoxide product
production step (a vacuum desorption step). During the
pressure equalization step, a portion of the void gas,
which contains mainly non-adsorbed ga~eou~ impurities, iB
withdrawn from the outlet (top) end of the bed being
depre~surized and pas~ed to the outlet (top) end of the
bed beinq pressurized to enrich the depressurized bed in
the adsorbed carbon monoxide component. During the
carbon monoxide product gas purge ~tep, carbon monoxide
is introduced into the inlet (bottom) end of the
depressurized bed in a cocurrent direction to purge and
force remaining non-adsorbed gaseous i~purities to the
outlet (top) end of the bed and into the purge exhaust
gas recycle tank L (see FIGURE 3) for recycle into the
feed gas. During the carbon monoxide product production
step, carbon monoxide i8 desorbed and withdrawn ~rom the
inlet (bottom) end of carbon monoxide pressure swing
adsorption system F via feed conduits 12, 13 and 14 by
applying vacuum from vacuum pump H via feed conduit 12.
The carbon monoxide product (merchant grade, léss than
about 1500 vpm impurities) is passed through feed
conduits 12, 13, and 14 to carbon monoxide reservoir I.
FIGURE 2 illustrates a first (hydrogen
separation) pressure swing adsorption system for
separating hydrogen from ~ feed ~ixture comprising
hydrogen, carbon monoxide, carbon dioxide, and methane
according to the present invention. A~ set out in
FIGURE 1, the feed mixture from the hydrocarbon eteam
reformer i5 passed through feed conduit~ 1 and 2 to
hydrogen pressure swing adsorption system A.
In FIGURE 2, the hydrogen pressure swing
adsorption system A comprises adsorption beds A, B, C,
and D, carbon monoxide storage vessel C, carbon dioxide-
rich gas buffer vessel J, hydrogen product reservoir B,
-- 19 --
hydrogen product pressure control valve PCV1, carbon
dioxide-rich fuel gas pressure control valve PCV2,
carbon monoxide product pressure control valve PCV3,
repressuri2ation flow control valve FCV1, hydrogen
product purge ga~ flow control valve FCV2, ~top
valves 21 through 49, and non-return valves S0 and S1.
Ad60rption beds A through D are connected in
parallel. Each of the adsorption bed6, A through D, i6
physically divided into two bed parts, a first adcorption
bed and a second ad~orption bed, Al/A2, Bl/B2, Cl~C2, ~nd
D1/D2, respectively, which are connected in 6eries. Each
adsorption bed contains an inlet (feed, bottom) end and
an outlet (discharge, top) end. The two part ad60rption
bed6 facilitate removal of a carbon monoxide stream from
an intermediate position in the bed. The carbon monoxide
stream i8 drawn at an intermediate pressure, for example
at about 25 psia, and passed to carbon monoxide ~torage
vessel C. The adsorbent bed comprises an adsorbent
having a greater a~finity for carbon dioxide, methane,
and carbon monoxide than for hydrogen. For example, the
first adsorption bed (for example, bed A1) comprises an
activated carbon adsorbent material and the 6econd
adsorption bed ~for example, bed A2) comprises a lower
level of an activated carbon adsorbent material and an
upper level of a zeolite molecular 6ieve adsorbent.
The fir6t (hydrogen separation) pressure 6wing
adsorption sy6tem is operated in accordance with the full
cycle 6equence ~hown in Table 1. The ~eguence is
described below in detail using bed~ Al/A2. 8eds 81/82,
Cl/C2, and Dl/D2 are employed in the same ~equence but at
an offset as 6hown in Table 1. All stop valves may be
controlled automatically on a predetermined schedule.
2 ~ 3
-- 20 --
o ~o o
~1 ~I N r O~ 11~ ~ ~ O 'r 1O ~1
:~N r N ~t N ~r N ~ N ~ N ~ ~ q' N
O ~ ~ Y 8'
F~ e t: 0 ~ 0
m .~ ~ O ~ Y
X U U ~ 8 - U V O
O ~ V V ~ --
~ o ~ o ~ . fi O ~0
m ~ O
C
c
C: 0 N 11~ m 0 c c ~ c
O 1 1~ O ~ 10 -- O i~
~.,~ ~ ui 0 ~ ~ m ~ 0 ~ 0 oq ~ o ~ ~0 ~ ~ 0 6~ J 0
~a o o ~ o C~l o o o o o P' ~ ~o ~ c '
' ~ P h _ ~ c ~
a m ~ v P~ .. ,, " .. ~,
O ~ o o~ -
o
~\ ~ fi .,
N ~ 0 ~ c e ~o c ~
_ o e: o 1: ~ " ~Z c '
. . ~ o ~ o tr~ O Q ~ _ Q
0 0 ~ 0 0 0 0 ~
P~ 0 0 0 ~ 8~ U D~ U 0 0 U U U ~ ~ o c ~ o _
o ~ ~o ~ ~o ~ o ~ ~o ~o ~ ~
~ o ~ o ~ - o
t ~
m m 0~ _ O O O 0 0~ _ 3
0 u~ 0 m ~ 2,, 2 2 2
a J~ Y~ 0 u ~ u ~ ~ 0 ~
cru D`O ~ ~ ~ O ~ O u ~ ~ ~ U
o ~ o ~ o o o ~ ~ o ~O
m ~ 8 ~ h ~ S " v ~ ~
. ~ o' ~ ~
N1''~ F 8 ~
2~3~ih
- 21 -
At the start of the pressure swing adsorption
cycle, bed Al/A2 is in the hydrogen production step.
Feed mixture from the hydrocarbon steam reformer is
passed to the inlet end of bed A1 via feed conduits 1 and
2 (see FIGURE 1) and open ~top valve 21 at a pressure
typically in the range from about 150 psia to about
600 psia. ~he feed mixture is adsorbed in adsorption
bed A1/A2 to selectively sieve hydrogen as a non-ad~orbed
product ~nd carbon dioxide, carbon monoxide, and Dethane
as adsorbed products. Non-adsorbed hydrogen product gas
is withdrawn from the outlet end of bed A2 via open stop
valve 37 and passed to hydrogen product reservoir B via
hydrogen product pressure control valve PCVl. The
hydrogen product gas typically contains less than about
10 vpm impurities.
During the hydrogen production step, the
activated carbon adsorbent material in bed hl/A2 adsorbs
carbon dioxide and water vapor more strongly than carbon
monoxide and methane, which in turn are more strongly
adsorbed than hydrogen. As the feed mixture flows
through the adsorbent bed, the non-adsorbed mixture
becomes enriched in hydrogen. The zeolite ~olecular
sieve adsorbent removes all but traces of other gases and
yields a hydrogen product substantially free of
impurities. The flow of feed mixture into the inlet end
of the first adsorbent bed and flow of product gas from
the outlet end of the second adsorbent bed are stopped
just before the breakthrough point of non-hydrogen
components fro~ the outlet end of the second adsorbent
bed. A typical feed and production cycle iB conducted
for a period of about two to about ~ix minute~.
When the non-hydrogen co~ponent~ in the feed
mixture advance close to the outlet end of bsd A2, the
hydrogen production step in bed Al/A2 is stopped. Stop
valves 21 and 37 are closed stopping the production of
hydrogen. Bed Al/A2 i8 then depressurized and bed Cl/C2
i~ repressurized by pressure equalization of the beds.
2 ~
- 22 -
Stop valves 38 and 44 are opened and lean gas i6 passed
from the outlet end of bed ~2 to the outlet end of bed C2
to substantially equalize the pressure of bed Al/A2 and
bed Cl/C2. This pressure egualization step typically is
conducted ~or a period of about twenty to about forty
~econds.
During the presgure equalization ~tep, the
pressure in bed Al/A2 decrease~ causing carbon monoxide
to be desorbed from the adsorbent material in preference
to methane, carbon dioxide, and water vapor. Optionally,
bed A1/A2 may be pressure equalized with an equalization
tank through the outlet end of bed A2. The gas collected
in the equalization tank is subseguently used to
repressurize a bed in the pressure swing adsorption
~ystem.
After the preesure egualization step is
complete, bed A1/A2 begins the carbon monoxide production
step. Bed Al/A2 is depressurized by an intermediate
depressurization step to withdraw and produce a carbon
monoxide-rich fraction. Stop valves 38 and 44 are closed
and ~top valve 33 is opened to withdraw the carbon
monoxide-rich fraction from a position intermediate
between be~ Al and bed A2. The carbon monoxide-rich
fraction i~ drawn at an intermediate pressure, for
example at about 25 psia, and pas6ed to carbon monoxide
storage vessel C. The carbon monoxide-rich fraction from
carbon monoxide storage vessel C i8 then passed to carbon
monoxide pressure swing ad~orption system F via feed
conduits 7 ~nd 8 (see FIGURE 1).
During the intermediate depressurization step,
carbon monoxide, desorbed into the void spaces during the
pressure egualization 6tep, iB withdrawn. Withdrawal of
carbon monoxide during the intermediate depressurization
6tep causes the pressure in the bed to further decrease
resulting in ~dditional carbon monoxide desorption.
Withdrawal of carbon monoxide from a location
2 ~ 5 ~ ~ 2 ~
- 23 -
intermediate between bed A1 and bed A2 minimizes
retention of carbon monoxide (and carbon dioxide) in
bed A2 which could contaminate a subsequent hydrogen
production ~tep. The time for the carbon monoxide
production ~tep is typically about two minutes. The
re~ulting carbon monoxide-rich fraction, which is
produced at a pressure between about 25 psia and abou~
40 psia, generally contains carbon monoxide at leact at
about a volume fraction of about 2.5 times that in the
feed gas, the remainder being ~ainly hydrogen with up to
about 2% of ~ethane and carbon dioxide.
When the intermediate depressurization carbon
monoxide production 6tep is complete, bed Al/A2 undergoes
carbon dioxide-rich fraction production steps ~a
depressurization step and a hydrogen product gas purge
step). In the first carbon dioxide-rich fraction
production step, a carbon dioxide-rich fraction i8
withdrawn by depressurizing bed A1/A2. During the
depressurization ~tep, ~top valve 33 i~ closed and stop
valve 23 i~ opened. The carbon dioxide-rich fraction is
withdrawn from the inlet end of Bed A1. The flow of the
carbon dioxide-rich fraction ls countercurrent to the
flow of the feed mixture during the hydrogen production
step. In general, the carbon dioxide-rich fraction is
produced at a pressure of about 20 psia. Ths reduction
in pressure during the depressurization step and
withdrawal of the carbon dioxide-rich fraction cause~
desorption of carbon dioxide from the adsorbent.
Generally, the withdrawal of the carbon d$oxide-rich
fraction i8 conducted for a period of one to two minutes.
In the second carbon dioxide-rich fraction production
stept ~ carbon dioxide-rich fraction i8 withdrawn by
purging bed Al/A2 w$th ~ydrogen product ga~. Stop
valve 38 ~6 opened. Hydrogen product purge flow control
valve FCV2 is opened and bed Al/A2 is purged with
hydrogen product gas from bed Cl~C2. The flow of
hydrogen product purge gas iB countercurrent to the flow
of the hydrogen gas during the hydrogen production step.
2~C~ 9
- 24 -
Generally, the product gas purge is conducted for a
period of about three minutes. The resultinq carbon
dioxide-ric~ fraction generally contains at least about
50% by volume carbon dioxide and less than 10% by volume
carbon monoxide, traces of methane and water vapor, with
the balance being hydrogen. All water vapor in the feed
ga~ iB separated into the carbon dioxide-rich fraction.
After the carbon dioxide-rich fraction
10 production ~teps are complete, bed Al/A2 iB repressurized
and bed C1/C2 iB depressurized by pressure equalization
of the beds. Stop valve 23 and hydrogen product purge
flow control valve FCV2 are closed and stop valves 44 is
opened. Void gas is passed from the outlet end of bed C2
15 to the outlet end of bed A2 to substantially equalize the
pressure of bed Al/A2 and bed Cl/C2.
After the pressure equalization 6tep
(repressurization step) iB complete, bed Al/A2 i8
20 back~illed with hydrogen product gas. Stop valves 38
and 44 are clo~ed and bed Al/A2 iB repressurized by
backfill with product gas. Product gas from producing
bed Dl/D2 i5 passed through open repressurization flow
control valve FCVl and into the outlet end of bed A2 to
25 backfill bed Al/A2 through open 6top valve 39.
When the backfill step is complete, bed A1/A2
is ready to again begin the hydrogen production ~tep.
Repressurization flow control valve FCVl and ~top
30 valve 39 are closed and 6top valves 21 and 37 are opened
to admit feed mixture from feed conduit 2 to the inlet
end of bed Al. The hydrogen production step in bed Al/A2
is begun and the cycle i8 repeated. Beds Al/A2, Bl/B2,
C1/C2, and D1/D2 operate in the sequence set out in
35 Table 1. In general, the time to complete a cycle (cycle
time) is in the range from about 60 seconds to about
1500 seconds, preferably from about 180 seconds to about
960 seconds, and more preferably from about 240 seconds
to about 720 ~econds.
- 25 - ~a ~2 a
FIGURE 3 illustrates a second (carbon monoxide
separation) pressure swing adsorption system for
separating carbon monoxide from a feed mixture comprising
hydrogen, carbon monoxide, carbon dioxide, and methane
according to the present invent$on. As set out in
FIGURES 1 and 2, the feed mixture from hydrogen pressure
swing adsorption ~ystem A ie passed through carbon
monoxide 6torage vessel C and carbon monoxide
compressor E to carbon monoxide pressure swinq adsorption
system F.
In FIGURE 3, the carbon monoxide pressure
swing adsorption system F comprises adsorption beds A',
B', C', and D', carbon monoxide compressor E, carbon
monoxide storage vessel X, carbon monoxide product
reservoir I, purge gas recycle vessel L, stop valves 101
through 131, and pressure control valves PCV101, PCV102,
PCV103, and flow control valves 101 and 102. Adsorption
beds A' through D' are connected in parallel and each
adsorption bed contain~ an inlet ~feed, bottom) end and
an outlet (discharge, top) end. The adsorbent bed
comprises an adsorbent having a greater affinity for
carbon monoxide than ~or hydrogen, carbon dioxide, and
methane. For example, the adsorption beds may contain an
adsorbent such a~ copper exchanged aluminosilicate
zeolite molecular sieves, copper exchanged alumina, and
copper exchanged activated carbon, and mixtures thereof.
The second (carbon monoxide) pressure swing
adsorption system is operated in accordance with the full
cycle sequence ~hown in Table 2. The sequence is
described below in detail using bed A'. Beds B', C', and
D' are employed in the ~ame eequence but at an offset as
shown in Table 2. All ~top valves may be controlled
automatically on a predetermined schedule.
- 26 ~ J;~
N N N ~ ~1 ~ ~ N ~ N ~`1 t~l
o ~ o In ~D O ~O O ~i~ O ~ ~1 ~i ~o o o~
O ~ O ~ ~1 0 ~ O ~1 ~ O ~ O ~ ~7 0 ~ O ~ _I
~I rl 1~~I r~ r N CO 0~ r ~ ~ ~I N
O ~ O ~10 ~ ~10 ~10 ~ ~ O ~10 ~ r~ O ~10 ~ ~ ~"
o~
-
111 N ~11 0 0 ' ~ ~
e ~ O ~ '
U ~ ~ O O ~ ~ ~ ~ ~ ~, _
0 ~a 0 ~ ~ ~ ~D 0~ ~10 ~~ ~ .--o x O
~ ~ ~ u u o 0 ~~ o ~u ~u 2
a ~ h 0 ~ ~ ~ _
o~ ~ a ~0 ~ ~0 ~ c~o ~ ~ o
o ~ ~ n ~ o S~ _
~ _ .
N 1~1 0 ~0 ID ., " ~ , _
~o 0 O ~
N ~ U~U ~U 10 UtJ~ ~ ~ U 1~ 1 U ~ _ o L ~
o ~ o o . ~ ~ o ~ o ~ o ~ ~ ~ ~o ~ ~ - 3
~ 8 ~ ~,, ., ~ v~
o ~ o
U ~ ~U
p~ N 0 ~ o v C ~ v
~ ~ , ~ y _
8 ~
'O 0 ~0 :1 0 ~11 CO 111 ~ ~d r4 ~ o 9. t- o v
n u~ u ~ u ~ ~ ~ u u uB 2 Y ~ ~ ~
o .. ~
~ 0~ ~ 'a 'aO P~ ~U '~o5 ~ ~o ~ ~ 3 - -
o s~ :~o ~1 0 ~ " ~,, ,
~i3-- X C~- ~ P.~ P. ~--V P. P. C.) C4 U P.- ~ - o _
o to ~
0 ~0CO 0 C
o ~ ~ ~t ~0 ~
m ~ ~u ~u ~ 0 ~ u u u ~ ~ ~ u æ
~a ~, ~ ~ Y P~
O ~ ~U U O ~ ~ . ~ ~:1. u
O ~ 0 ~ ~ ~ O ~ ~O ~ O ~
~ ~ ~ ~
~q z ~ ~ ~ ~r . ~ u ~ ~
- 27 - ~ 3 ~; 2 ~
At the 6tart of the pressure 6wing adsorption
cycle, bed A' is in the carbon monoxide adsorption step.
Carbon monoxide-rich feed mixture from carbon monoxide
compressor E is passed to the inlet end of bed A' via
open stop valve 101 at a pressure typically in the range
from about 25 psia to about 600 psia. The feed ~ixture
i8 adsorbed in adsorption bed A' to ~electively sieve
carbon monoxide as an adsorbed fraction~ Non-adsorbed
carbon dioxide, hydro~en, ~ethane, ~nd any carbon
monoxide are withdrawn from the outlet end of bed A' via
open ~top valve 105 and passed to feed conduit 9 for
recycle to hydrogen pressure ~wing adsorption ~ystem A
(see FIGURES 1 and 2).
During the carbon monoxide adsorption ~tep, the
adsorbent bed of copper alu~inosilicate zeolite molecular
sieves adsorbs carbon monoxide more strongly than carbon
dioxide, hydrogen, and methane. As the feed mixture
flows through the adsorbent bed, the bed becomes enriched
in carbon monoxide. The flow of carbon monoxide-rich
ieed mixture into adsorbent bed A' is stopped just before
the breakthrough point of carbon monoxide from the outlet
end of adsorbent bed A'. A typical feed and carbon
monoxide adsorption cycle i8 conducted for a period of
about two to about six minutes.
When carbon monoxide in the feed mixture
advances close to the outlet end of bed A', the carbon
monoxide ~dsorption step in bed A' is stopped. Stop
valves 101 and 105 are closed stopping the carbon
monoxide ~dsorption step. Bed A' is then depressurized
and bed C' is repressurized by pressure equalization of
the bed6. Stop valves 114, 130, and 125 ~re opened and
lean gas is passed from the outlet end of bed A' to the
outlet end of bed C' to 6ubstantially equalize the
pressure of bed A' and bed C'. ~his pressure
equalization ~tep typically i8 conducted for a period of
about twenty to about forty seconds. Optionally, bed A'
may be pressure equalized with an equalization tank
~ ~3
- 28 -
through the outlet end of the adsorption bed. The gas
collected in the equalization tank i8 subsequently used
to repressurize a bed in the pressure 6wing adsorption
~ystem. If the adsorption pres6ure i8 very low (for
example, under 25 psia or lower), the pressure
equalization step i~ not employed or a partial pressure
egualization step i8 employed.
After the pressura egualization 6tep, a carbon
monoxide product cocurrent purge step i~ employed to
displace the impurities ~carbon ~onoxide, hydrogen, and
methane] in the void gas left in the bed ~fter the
adsorption and pressure equalization ~teps. Bed A' i8
purged from the inlet end with carbon monoxide product
gas from carbon monoxide product gas reservoir I. Stop
valve 130 is closed and stop valves 110, 128, and 139 and
product purge flow control valve FCV102 are opened.
Product purge gas effluent is then passed to purge gas
recycle vessel L. The flow of carbon monoxide product
purge gas i5 cocurrent to the ~low of the carbon monoxide
gas during the carbon monoxide adsorption ~tep. The
product purge gas from purge gas recycle vessel L is then
recycled to the carbon monoxide pressure swing adsorption
6ystem via carbon monoxide compressor E. Generally, the
purge with product gas is conducted for a period of about
three minutes.
After the cocurrent carbon monoxide purge
6tep, bed A' undergoe6 a carbon monoxide production step.
Stop valves 128, 110, 114, and 129 are closed and stop
valve 119 is opened. The adsorbed carbon monoxide is
removed from the inlet end of bed A' by applying a vacuum
u6ing vacuum pump H and open 6top valve 119. The
resulting carbon monoxide-rich fraction generally
contain~ at least about 98% by volume carbon monoxide
with the balance being trace6 of hydrogen, carbon
dioxide, and methane. In general, the desorption
pressure i~ from about 75 torr to about 300 torr,
preferably from about 100 torr to about 150 torr.
. ~3
- 29 -
After the carbon monoxide production ~tep
(vacuum regeneration step) iB complete, bed A' is
repre~surized and bed C' i8 depressurized by pre6sure
squalization of the beds. Stop valves 130 and 123 ~re
opened and void gas iB passed from the outlet end of
bed C' to tbe outlet end of bed A' to Qubstantially
egualize the pre~sure of bed A~ and bed C'.
After the repressurization ~tep, bed A' is
bac~filled with hydrogen-rich product gas. Stop
valves 130 is closed and stop valves 131 is opened.
Product gas from producing bed D' is passed through
repressurization flow control valve FCVlO1 into the
lS outlet end of bed A' to backfill and repressurize bed A~.
When the backfill ~tep is complete, bed A' is
ready to again begin the carbon monoxide adsorption 6tep.
Repre~surization flow control valve FCV101 and stop
valves 131 and 123 are closed and stop valves 101 and 105
are opened to admit carbon monoxide-rich feed mixture
from compressor E to the inlet ~nd of bed A'. The carbon
monoxide adsorptiGn step in bed A' is begun and the cycle
i6 repeated. Beds A', ~', C', and D' operate in the
sequence set out in Table 2. In general, the time to
complete a cycle (cycle time) is in the range from about
60 seconds to about 1500 ~econds, preferably from about
240 ~econds to about 960 seconds, and more preferably
from about 240 seconds to about 720 ~econds.
In a preferred ~mbodiment, the present
invention i~ directed at a method for producing hydrogen
and carbon monoxide from ~ feed mixture comprising
hydrogen, carbon monoxide, carbon dioxide, and methane,
which comprises the steps of:
ta) passing the feed mixture through a first
pressure swing adsorption ~ystem containing an adsorption
bed comprising an adsorbent having a greater ~ffinity for
carbon dioxide, methane, and carbon monoxide than for
2~3
- 30 -
hydrogen to separate hydrogen as a pure non-adsorbed
product and carbon dioxide, methane, and carbon ~onoxide
as an adsorbed fraction;
(b) desorbing carbon monoxide from the
pre~ure ~wing adsorption ~y~tam in ~tep ~a) to form a
carbon monoxide-rich fr~ction;
(c) desorbing carbon dioxide and methane from
the pressure swing adsorption ~ystem in step (a) to form
a carbon dioxide-rich fraction;
(d) passing the carbon monoxide-rich fraction
from step (b) to a second pressure swing adsorption
system containing an adsorption bed comprising an
adsorbent having a greater affinity for carbon monoxide
than for hydrogen, carbon dioxide, and methane to
separate carbon monoxide as an adsorbed fraction and
hydrogen, carbon dioxide, and methane as a non-adsorbed
fraction; and
(e) desorbing carbon monoxide from the
pressure swing adsorption system in step (d) to form a
pure carbon monoxide product.
FIGURE 4 illu6trates a second embodiment of the
present invention for producing hydrogen and carbon
monoxide from a feed mixture comprising hydrogen, carbon
monoxide, carbon dioxide, and methane by using a two
stage (first stage/second stage, top stage/bottom stage)
pressure swing adsorption system. In this embodiment,
hydrogen is the unadsorbed gas, carbon dioxide and
methane are the first desorbed gases, and carbon monoxide
30 iB the strongly ~dsorbed gas. In FIGURE 4, ga~eous feed
mixture is fed through feed conduit 70 to two 3tage
pressure swing adsorption ~ystem M to separate the
mixture. Typically the feed mixture from the hydrocarbon
steam reformer will enter two stage pressure swing
adsorption system M at a pres~ure in the range from about
150 psia to about 600 psia, preferably from about
150 psia to about 400 p6ia, and more preferably from
about 150 psia to about 300 psia. After being cooled,
2 ~3
- 31 -
the feed mixture entering pressure swing adsorption
system M will be at ambient temperature.
During the hydrogen product production ~tep,
feed mixture i8 fed into and hydrogen product i8
withdrawn from two ~tage pressure swing adsorption
system M. Hydrogen product is separated as a pure non-
adsorbed product and carbon monoxide, carbon dioxide,
methane, and water vapor i~ separated a~ an ~dsorbed
fraction. Hydrogen product (merchant grade, less than
about 10 vpm impurities) is withdrawn from two 6tage
pressure swing adsorption system M through feed
conduit 71 and passed to hydrogen reservoir B.
After the hydrogen product production step,
pres~ure swing adsorption system ~ undergoes a pressure
equalization step, an intermediate depressurization step,
and a ~econd 6tage purge/first stage purge step (carbon
dioxide-rich fractlon production 6teps). Durlng the
intermediate depres~urization step, carbon dioxide-rich
gas i8 collected as 6econdary product, compressed, and
recycled to the reformer feed gas. Durinq the second
stage purge/first stage purge step, the second 6tage is
purged with hydrogen from another 6tage and the first
stage is purged with carbon monoxide product gas from the
receiver. The depressurization and purge effluent gases
are passed to carbon dioxide reservoir N and collected as
secondary product via feed conduit 72. The gases are
then passed to compressor 0 via feed conduit 73 and
compressed And recycled to the refGrmer feed gas v~a feed
conduit 74. In general, carbon dioxide compres~or 0 will
compress the carbon dioxide-rich fraction to a pressure
in the range from about 150 psia to about 600 psia,
preferably from about 150 psia to about 450 psia, and
more preferably from about 150 psia to about 350 psia.
After the carbon dioxide-rich fraction
production steps, pres6ure ~wing adsorption 6y6tem M
undergoes a ~econd stago purge/first 5tage evacuation
- 32 - 2~
step (sarbon monoxide production step). In the ~econd
stage purge/first stage evacuation step, the 6econd stage
is purged with hydrogen ga~ and the first 6tage is
evacuated using vacuum pump P to remove carbon monoxide
S product gas. The carbon monoxide product gas i8
withdrawn through feed conduits 77 and 78 and pas6ed to
carbon monoxide reservoir Q.
After the carbon monoxide production step,
pressure swing adsorption system ~ undergoe~ ~ pre~sure
equalization ~tep (repressurizAtion). During the
pressure equalization step, the bed is repressurized by
pressure equalization with another bed. The bed is then
repressurized to adsorption pressure using hydrogen gas
from the pressure swing adsorption 6ystem. The carbon
monoxide product (merchant grade, le88 than about
1500 vpm impurities) is passed through feed conduits 77,
78, and 75 to carbon monoxide reservoir Q.
FIGURE S illustrates a two stage pressure
swing adsorption method for separating carbon monoxide
and hydrogen from a feed mixture comprising hydrogen,
carbon monoxide, carbon dioxide, and methane in accord
with the ~econd embodiment of the present invention. As
set out in FIGURE 4, the feed mixture from the
hydrocarbon 6team reformer is passed through feed
conduit 70 to pressure 6wing adsorption system M.
In FIGURE 5, the two stage pressure swing
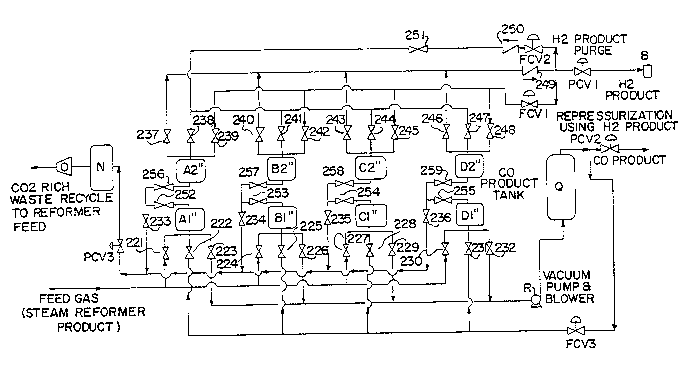
adsorption ~ystem M comprises adsorption beds A'', ~3'',
C'', and D'', carbon monoxide ~torage vessel Q, carbon
dioxide-rich gas buffer ve~sel N, hydrogen product
reser~oir B, hydrogen product pressure control
valve PCVl, carbon monoxide-rich product gas pressure
control valve PCV2, carbon dioxide product pressure
control valve PCV3, repressurization flow control
valve FCVl, hydrogen product purge gas flow control
valve FCV2, 6top valves 221 through 259, and non-return
valves 249 and 250.
- 33 - 2~3~
Adsorption beds A'' through D'' are connected
in parallel. Each of the adsorption beds, A''
through D'', is physically divided into two stages, a
S first (bottom) stage and a ~econd (top) stage, Al''/A2'',
B1''/B2'', C1''/C2'', and D1''/D2'', respectively, which
are connected in 6eries. Each stage contains an inlet
(feed) end and a outlet (di6charge) end. The first stage
and second staqe of each bed are i601ated by two stop
valves for sequential depressurization and carbon
monoxide production steps (i.~., first stage Al'' and
6econd stage A2'' are i601ated by 6top valves 252 and
256). The two part ~tages f~cilitate removal of a carbon
dioxide stream from an intermediate position in the bed.
The carbon dioxide stream is drawn at an intermediate
pressure, for example at about 25 psia, and passed to
carbon dioxide 6torage vessel N. The first adsorption
bed stage (for example, first 6tage Al'') compri6es an
adsorbent having a greater affinity for carbon monoxide
than for hydrogen, carbon dioxide, and methane and may be
~lected fro~ the group consisting of copper exchanged Y-
type aluminosilicate zeolite molecular sieves, copper
exchanged alumina, and copper exchanged activated carbon.
The second adsorption bed stage (for example, 6econd
stage A2'') comprises an adsorbent having a greater
affinity for carbon dioxide, methane, and carbon monoxide
than for hydrogen and may be a molecular sieve or
activated carbon, and preferably i8 a combination of
molecular 6ieves and activated carbon.
The two 6tage pressure swing adsorption system
i8 operated in accordance with the full cycle sequence
shown in Table 3. The sequence i~ described below in
detail using stages Al''/A2''. Stages Bl''/B2'',
Cl''/C2'', and Dl''/D2'' are employed in the 6ame
sequence but at an offset as shown in Table 3. All stop
valves may be controlled automatically on a predetermined
schedule .
2 ~ ;~ 3 7 2
-- 3~ --
~ ~ N~ ~N ~ ~ N~
NN NNN NN NNN NN NNN NN NNN
~ ~ ~ ~N~ ~ ~ u~
NNN NNN NNN NNN NNN NNN NNN NNN
~ N~ ~ ~ ~N~ ~ ~ ~ m _
X C NNN NNN NNN ~NN NNN NNN NNN NNN ~ ~
~ ~ ~ 0~ ~N~ ~ ~ ~N~ o~ c
~ 0 NNN NNN NNN NNN NNN NNN NN~ NNN
~ ~ ~ ~ ~N~ ~ ~ 0~ 0~ ~y~
N~ N~ N~ N~ N~ ~ ~ ~ -~o~
U ~ NNN NNN NNN NNN N N N N N N NNN N~N j -~
0
e ~ ~ 0 ~o Y "
ON ~ ~ N
~ ~ ~ 0 51 .8 u
P~ ~q 0 ~ m ~ ~ ~ a0~ 0 ~0 ~ y ~ ~ d
2 C~ , V a~ N ~ 5 ~o ~ C
~ 00 ~ a L ~
~ ~ 2 ~ u8 u 2 a ~
~ N ~ ~ ~- ~ ~Co~
5j5~ 0 C
o ~ ~ ~
_I ~J ~ ~ Eo~ ~ _ ~ o
~s ~ s æev e~ Y
~ m ~ ~N ~ 8 ~ ,s
~, 0 ~ ,0
0 o 0 ~P
a E~ 8 ~--~;$
~ ~ ~ S
o ~ ~ 0~ 0~ v
N ~ ~ ~ ~ ~ ~ ~,~
2 ~
- 35 ~
At the start of the pressure ~wing adsorption
cycle, first stage Al'' and 6econd fitage A2'' are in the
hydrogen production step. Feed mixture from the
hydrocarbon ~team reformer is passed to the inlet end of
first 6tage Al'' via open stop valve 221 at a pressure
typically in the range from about 150 psia to about
600 psia. The feed mixture pas6es from the outlet end of
the first ~tage A1~' through stop valve~ 252 and 2S6 to
the inlet end of the ~econd 6tage A2''. The feed mixture
0 iB adsorbed in first 6tage A1'' and ~econd ~tage A2'' to
6electively 6ieve hydrogen as an non-adsorbed fraction
and carbon dioxide, carbon monoxide, and methane as
adsorbed products. Non-adsorbed hydrogen product gas is
withdrawn from the outlet end of 6econd ~tage A2'' via
open stop valve 237 and passed to hydrogen product
reservoir B via non-return valve 249 and hydrogen product
pressure control valve PCV1. The hydrogen product gas
typically contains less than about 10 vpm impurities.
During the hydrogen production step, the copper
exchanged 6ubstrate in first stage Al'' preferentially
ad60rbs carbon monoxide. The activated carbon/molecular
6ieve adsorbent material in second 6tage A2''
preferentially adsorb~ carbon dioxide, water vapor, and
methane more strongly than hydrogen. As the feed mixture
flows through the adsorbent stages, carbon monoxide
becomes concentrated in first 6tage Al'', carbon dioxide,
water vapor, and methane become concentrated in second
stage A2'', and the mixture exiting the bed ~tages
becomes enriched in hydrogen. The zeolite molecular
sieve adsorbent removes all but traces of other gase6 and
yields a hydrogen product substantially free of
impuritie6. The flow of feed mixture into the inlet end
of the first 6tage and flow of product ga~ from the
outlet end of the 6econd stage are ~topped ~u~t before
the breakthrough point of non-hydrogen components from
the outlet end of the second 6tage. A typical feed and
production cycle i~ conducted for a period of about two
to about six minutes.
2 ~ 2 i~
- 36 -
When the non-hydrogen components (i.e.,
methane) in the feed mixture advance close to the outlet
end of second stage A2'', the hydrogen production step in
~irst ~tag~ A1''/A2'' ia stopped. Stop valves 221
and 237 are clo~ed ~topping the production o~ hydrogen.
First 6tage Al'' and second ~tage A2'' are then
depressurized (treated as one ~ed with ~top valves 252
and 256 open) and stage Cl''/C2'' i8 repressurized
(treated as one bed with Etop valves 254 and 258 open) by
pres6ure equalization of the beds through the outle~
(top, discharge) ends of the beds. Stop valves 238
and 244 are opened and void gas is passed from the outlet
end (top) of second stage A2'' to the outlet end (top) of
bed C2'' to substantially equalize the prçssures in
beds Al''/A2'' and beds Cl''/C2''. This pressure
equalization 6tep typically is conducted for a period of
about twenty to about forty seconds.
During the pressure equalization 6tep, void gas
containin~ carbon dioxide and methane i8 passed to the
repressurized bed and the pressure in first
stage A1''/A2'' decreases. Optionally, first
stage Al''/A2'' may be pressure egualized with an
equalization tank through the outlet end of second
stage A2''. The gas collected in the equalization tank
is ubsequently u~ed to repressurize a bed in the
pressure ~wing adsorption ~ystem.
After the pressure equalization 6tep is
comple~e, first ~tage A1''/A2'' begins the intermediate
depressurizAtion ~tep (~ ~ir~t carbon dioxide-rich
fraction production ~tep). During the intermediate
depres~urization ~tep, ~ir~t stage Al'' ~nd 6econd
6tage A2'' ~re depressurized from an intermediate
location to withdraw and produce a carbon dioxide-rich
fraction. Stop valves 238 and 244 are closed and stop
valves 233, 252, and 256 are opened to withdraw the
carbon dioxide-rich fraction from a position intermediate
- 37 - 2~3~
between first stage A1'' and second stage A2''. The
carbon dioxide-rich fraction i8 drawn at an intermediate
pressure, for example at about 25 psia, and passed to
carbon dioxide storage vessel N. The carbon dioxide-rich
fract~on ~rom carbon dioxide storage vessel N is then
passed to the reformer feed gas via compressor 0.
During the intermediate depressurization 6tep,
void gas in second stage A2'' which i~ predominantly
carbon dioxide, i~ withdrawn from the inlet (bottom) end
of the bed. Withdrawal of carbon dioxide-rich void gas
from the outlet (top) end of first ~tage A1'' causes the
carbon monoxide, adsorbed near the bottom of the bed, to
be desorbed displacing additional void gas. Withdrawal
of carbon dioxide from a location intermediate between
first stage A1'' and second stage A2'' minimizes
retention of carbon dioxide in the top region of second
stage A2'' which could contaminate a subsequent carbon
monoxide production step. The time for the intermedi~te
depre~surization step i~ typ~cally about two minutes.
When the intermediate depressurization carbon
monoxide production step i~ complete, first
stage A1''/A2'' undergoes a second stage purge/first
stage purge step (a second carbon dioxide-rich fraction
production 6tep). During the second 6tage purge/first
stage purge step, ~econd stage A2''i~ purged with
hydrogen gas and first stage Al'' is purged with carbon
monoxide product gas from carbon monoxide reservoir Q.
During the purge of ~econd stage A2'' with hydrogen gas,
~top valves 251, 138, ~nd 233 are opened. Hydrogen from
hydrogen reservoir 8 is t~en passed through open flow
control valve FCV2, open non-return valve 250, and open
~top valves 251 ~nd 238, through second stage A2'', and
open stop valve~ 256 and 233 to carbon dioxide
reservoir N. During the purge of first stage A1'' with
carbon monoxide ga~, stop valves 222, 252, 230 are
opened. Carbon monoxide from carbon monoxide reservoir Q
is then passed through open flow control valve FCV3, open
- 38 - 2 ~3~h
stop valve 222, through first ~tage Al'~, and open 6top
valves 252 and 233 to carbon dioxide reservoir N. The
flow of hydrogen through second 8tage A2'' i8 in a
direction countercurrent and the flow of carbon monoxide
$s in a direction cocurrent to the flow of the feed
mixture during the hydrogen production step. Generally,
the purge steps are carried ~ut simultaneously and are
conducted for a period of one to two minutes. The
resulting carbon dioxide-rich fraction generally contains
at least about 50% by volume carbon dioxide and less than
10% by volume carbon ~onoxide, and small amount~ of
methane, with the balance being hydrogen.
When the ~econd stage purge/first stage purge
step i6 complete, stage6 A1'' and A2'' undergo a 6econd
stage purge/first 6tage evacuation step ~carbon monoxide
production step). During the second stage purge/first
stage evacuation step, second stage A2''is purged with
hydrogen gas and first stage Al'' i8 evacuated using
vacuum pump R to withdraw carbon monoxide product gas
from the inlet end o~ first ~tage A1'' for passage to
carbon monoxide reservoir Q. During the production of
carbon monoxide, stop valves 252 and 222 are closed.
Carbon monoxide iB then passed from first stage A1''
through open stop valve 223 to carbon monoxide
reservoir Q. Generally, the purge and production steps
are carried out simultaneously and are conducted for a
period of one to two minutes.
~he resulting carbon Donoxide-rich fraction,
which iB produced at a pressure at about 25 psia,
generally contains carbon monoxide having a purity
exceeding 98%.
After the carbon monoxide production step is
complete, first ~tage A1'' and second stage A2'' is
repressurized and bed C1''/C2'' is depressurized by
pressure equalization of the beds. Stop valves 223, 233,
and 251 are closed and stop valve 244 i~ opened. Void
.~ ~ I r
-- 39 --
ga6 i6 passed from the outlet end of ~econd 6tage C2'' to
the outlet end of second stage A2'' to 6ubstantially
equalize the pressure of bed A1''/A2'' and bed C1''/C2''.
After the pressure equalization step
~repressurization step) iB complete, fir~t stage Al'' and
second ~tage A2'' are back~illed with hydrogen product
ga6. Stop valves 238 and 244 are closed and
bed A1''/A2'' is repressurized by backfill with product
gas. Product gas from producing bed D1''/D2'' is passed
through open repressurization flow control valve FCV1 and
into the outlet end of second stage A2'' to backfill
bed Al''/A2'' through open stop valve 239.
When the backfill step is complete, first
6tage Al'' and second 6tage A2'' are ready to again begin
the hydrogen production 6tep. Repressurization flow
control valve FCVl and stop valve 239 are closed and stop
valves 221 and 237 are opened to admit feed ~ixture to
the inlet end of ~irst ~tage Al''. The hydrogen
production step in bed Al''tA2'' ls begun and the cycle
is repeated. Beds Al''/A2'', Bl''/B2'', Cl''/C2'', and
Dl''/D2'' operate in the 6equence 6et out in Table 3. In
general, the time to complete a cycle (cycle time) is in
the range from about 60 ~econds to About 1500 seconds,
preferably from about 180 6econds to about 960 6econds,
and more preferably from about 240 ~econd6 to about
720 second6.
In a preferred embodiment, the present
invention i~ directed at a method for producing hydrogen
and carbon monoxide from ~ feed mixture comprising
hydrogen, carbon monoxide, carbon dioxide, and methane,
which comprises the Rteps of:
(~) providing a pre6sure swing adsorption
system having a fir6t 6tage and a 6econd 6tage, wherein
the first stage contains an adsorption bed comprisinq an
adsorbent having a greater affinity for carbon monoxide
than for hydrogen, carbon dioxide, and methane, the
2 ~ 2 v
- 40 -
second 6tage contains an adsorption bed compri6ing an
adsorbent having a greater affinity for carbon dioxide,
methane, and carbon monoxide than for hydrogen, and the
first and second stages are connected in ~eries and each
~tage contains an inlet end and an outlet end;
(b) pa6~ing th~ Peed mixture through the fir6t
stage of the pre6sure ~wing adsorption system to ~eparate
carbon monoxide a6 an adsorbed fraction and hydrogen,
carbon dioxide, and methane as a non-adsorbed fraction;
(c) passing the non-ad60rbed ~raction from
step (b) through the second ~tage of the pre6~ure 6wing
adsorption 6ystem to separate carbon dioxide and methane
as an adsorbed fraction and hydrogen as a non-adsorbed
pure product;
(d) desorbing carbon dioxide and methane from
the first and ~econd 6tages of the pressure 6wing
adsorption system to form a carbon dioxide-rich fraction;
and
~ e) de~orbing carbon monoxide from the first
6tags of the pressure swing adsorption sy6tem to form a
pure carbon monoxide product.
The selectivity of the adsorbent material in
the bed of the pressure swing adsorption system for a
gaseous component i8 generally governed by the volume of
the pore size and the distribution of that pore size in
the adsorbent. Gaseous molecules with a kinetic diameter
- less than, or equal to, the pore size of the ad60rbent
are ad60rbed and retained in the adsorbent while gaseous
~olecules with a diameter larger than the pore size of
the ad60rbent pass through the adsorbent. The adsorbent
thus sieves the gaseous molecules according to their
molecular ize, The adsorbent may also separate
molecule~ according to their diffsrent r~tes of diffusion
in the pores of the adsorbent.
Zeolite molecular ad60rbents adsorb gaseous
molecules with some dependence upon crystalline 6ize. In
general, adsorption into zeolite is fast and equilibrium
~a3~2~
- 41 -
is reached typically in a few 6econds. The ~ieving
action of zeolite is generally dependent upon the
difference in the equilibrium adsorption of the different
components of the gaseous mixture. When air i8 separated
by a zeolite adsorbent, nitrogen iB preferentially
adsorbed over oxygen and the pre~sure swing adsorption
method may be employed to produce an oxygen enriched
product. When hydrogen, carbon ~onoxide, carbon dioxide,
and methane are separated by a zeolite adsorbent, carbon
dioxide, carbon monoxide, and methane are the adaorbed
components, in the order indicated, and hydrogen is the
unadsorbed component.
During the carbon monoxide pressure swing
adsorption separation, carbon dioxide, hydrogen, and
methane are removed from the feed mixture as vent gas
during the pressure egualization step. A certain amount
of carbon monoxide is lost with the vent gas. ~his loss
of carbon monoxide results from carbon monoxide not
adsorbed in the sieves at the pressure swing adsorption
operation pressure, and carbon monoxide present in the
bed voids and discharged during the pressure egualization
step. This vent gas containing carbon monoxide is
recycled to the pressure swing adsorption system as feed
gas during the carbon monoxide cocurrent purge ~tep.
Although a particular carbon monoxide pressure
swing adsorption cycle seguence was illustrated as a
preferred embodiment (Table 1 and Table 2), other
variations of pressure swing adsorption process cycle
6eguences may be employed. A simple pressure 6wing
adsorption process cycle 6eguence may consist of the
following 6teps: (i) adsorption wherein feed mixture
enters the inlet end of the adsorbent bed and the product
gas exits the outlet end of the adsorbent bed, (ii)
bed pressure egualization through the outlet and inlet
ends of the bed to depressurize the bed, (iii)
countercurrent vent, (iv) vacuum regeneration to remove
components strongly adsorbed in the bed, (v) bed pressure
r~ r~
-- 42 --
equalization to partially repressurize the regenerated
bed, and (vi) repressurization using a product backfill.
The proces~ cycle sequence illustrated in
Table 1 can increase the carbon monoxide yield to
approximately 70S. The carbon monoxide product
withdrawal step incorporated in the proce~s ~eguence
referred to in Table 1 reduce6 carbon ~onoxide 106a by
permitting the withdrawal of substantial amounts of
~0 carbon monoxide containing bed ~oid ga~ and gas weakly
bound to the adsorbent. The selection of nn intermediate
location for wlthdrawing the carbon monoxide-rich
fraction increase6 the amount of carbon monoxide
withdrawn without affecting the hydrogen product purity.
If the carbon monoxide-rich fraction i6 withdrawn from
the outlet end of the adsorption bed, then the quantity
of the product withdrawn, without affecting hydrogen
product purity, will be limited and the net carbon
monoxide recovery that can be achieved will only be
between about 30% and about 50%. When the carbon
monoxide-rich fraction is withdrawn from an intermediate
position in the bed, the beds need not be separate
vessels (first ~tage A1'' and second ~tage A2'', for
example) but may be t~o regions inside a single vessel
between which a side port i6 located for withdrawing the
carbon monoxide product.
The carbon monoxide yield may be improved to
85% or greater by including a cocurrent purge during the
carbon monoxide production step in the carbon monoxide
pressure swing adsorption system. In this variation of
the cycle, a portion of the carbon dioxide-rich fraction
i~ compressed and fed as cocurrent purge gas to the inlet
end of the adsorption bed (inlet end of bed A', for
example) during the carbon monoxide production step. The
cocurrent purge, also referred as 6weep or displacement
gas, displace~ the carbon monoxide near the inlet end of
the adsorption bed (inlet end of bed A', for example)
2 ~
- 43 -
further along and permits remo~al of more carbon
monoxide.
In yet another process cycle variation, the
carbon monoxide production step in the hydrogen pre~sure
~wing adsorption system iB 6plit into two parts. In the
first part, the outlet end ~econd stage A2'', f or
example) i5 opened to the carbon monoxide product line
while cocurrent purge ga6 (carbon diox$de-rich fraction)
0 iB admitted to the lower region (first ~tage Al'', for
example). In the ~econd part, the outlet region is
isolated and the inlet region i8 opened to the carbon
monoxide product line. To conduct this process cycle
variation, two additional 6top valves must be provided to
isolate the two regions from each other and from the
carbon monoxide product line.
In a preferred embodiment, the carbon monoxide
depressurization product from the pressure swing
adsorption system is withdrawn from the outlet end o~ the
bed in the pressure ~wing adsorption ~ystem. In another
preferred embodiment, the carbon monoxide
depressurization product from the pressure swing
adsorption system is withdrawn from an intermediate
location in the bed in the pressure swing ad~orption
~ystem.
The carbon monoxide product withdrawal location
i8 preferably a6 close as possible to the outlet end of
the adsorption bed. The volume of 6econd stage A2'', for
example, must be as s~all a6 pos6ible compared to the
volume of first stage A1''. The volume of second
stage A2'' mu~t, however, be large enough co that the
hydrogen enriched product purity 18 not ~ffected a8 a
result of carbon monoxide product ~roduction. During the
production cycle, concentration fronts are formed for
each of the component~ in the feed. Components that are
strongly adsorbed (e.g. carbon dioxide) exist at feed
concentration in the gas phase near the entrance of the
L~
- 44 -
bed. Over a length egual to the eguilibrium saturation
zone, the gas phase concentration i~ constant. Beyond
this length the concentration of the adsorbed component
decreases sharply. In the present separation (hydrogen,
carbon monoxide, carbon dioxide, and methane from the
feed mixture), the production of hydrogen-rich primary
product must be ~topped when the eguilibrium f ront ~6
well within the pres6ure swing adsorption bed. ~he
outlet end of a pres6ure ~wing ad~orption bed at the
completion of the product~on step thu~ contain6
predominant amounts of hydrogen which mainly account~ for
the hydrogen losses with the vent. It i~ desirable to
collect a carbon monoxide product in a direction
cocurrent to feed by moderate pressure reduction of the
pressure swing adsorption beds before carbon monoxide
product vent is initiated. The carbon monoxide product
contain~ a significant amount of hydrogen.
When a ~mall quantity of carbon monoxide
product, for example 5-10% Or feed, is collected, it is
pref~rable to withdraw the carbon monoxide product stream
from the outlet of the bed. If greater than 10~ of the
feed i~ collected, the carbon monoxide product stream
should be withdrawn from an intermediate location on the
bed. Thi~ method prevents contamination of the high
pressure hydrogen-rich product.
The pressure swing adsorption unit mu6t be
regenerated periodically. Suitable modes of regeneration
include (i) regeneration at or below 25 psia coupled with
product purge or purge from an extern~l ~ource, and ~ii)
vacuum regeneration.
Throughout this application, various
publications have been referenced. The disclosures in
these publications are incorporated herein by reference
in order to more fully describe the state of the art.
2 7~ a ~
- 45 -
The embodiments de6cribed herein are merely
exemplary and a person 6killed in the art may make many
variations and modificat~ons without departing from the
6pirit and scope of the invention. All 6uch
S modifications and ~ariations are intended to be included
within the scope o~ the invention as defined in the
appended claims.