Note: Descriptions are shown in the official language in which they were submitted.
20a61~
HOECHST ARTIENGE5ELLSCHAFT HOE 90JF 353 Dr. JA/AP
Description
Aminodiol derivatives
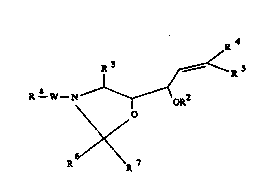
The invention relates to compounds of the formula Ia
or Ib
R 3 (S) ~ R S
R - N ~ C~R
~ (Ia)
6/\
R 4
R 3 ~ R 5
R--N"" (R~--~0 R2
~ O (Ib)
,~
R R7
and their salts, in which R1 is R -W-;
W is -CO-, -O-CO-, -SO2- or -NH-CO-;
R~ is hydrogen, (C1-C10)-alkyl which is optionally mono- or
diunsaturated and which is optionally ~ubstituted by up
; '' '
2~5B~
-- 2 --
to 3 identical or different radicals from the series
comprising hydroxyl, (Cl-C7)-alkoxy, (C1-C7)-alkanoylo y,
carboxyl, (Cl-C7)-alkoxycarbonyl, halogen, amino, (C1-C7)-
alkylamino, di-(Cl-C7)-alkylamino, (C1-C5)-alko ycarbonyl-
amino and (C7-CIs)-aralko ycarbonylamino, (C3-C8)-cyclo-
alkyl, (C3-C8)-cycloalXyl-(Cl-C6)-alkyl, (C6-Cl~)-arylwhich
is optionally substituted by one or two identical or
different radicals from the series comprising F, Cl, Br,
I, hydroxyl, (cl-c7)-alko y, (Cl-C7)-alkyl, (Cl-C7)-alkoxy-
carbonyl, amino, and anilino and trifluoromethyl whichare optionally ~ubstituted by up to two halogens,
(C6-Cl4)-aryl-(Cl-C6)-alkyl, in which the aryl moiety is
optionally substituted by one or two identical or dif-
ferent radicals from the series comprising F, Cl, Br, I,
hydro yl, (C1-C7)-alXoxy, (C1-C7)-alkyl, (C1-C7)-alkoxy-
carbonyl,amino,(C1-C,)-alkylamino,di-(C1-C,)-alkylamino,
carboxyl, carboxymethoxy, amino-(C1-C,)-alkyl, (Cl-C,)-
alkylamino-(cl-c7)-alkyl~ di-(C1-C7)-alkylamino-(Cl-C7)-
alkyl, (C1-C,)-alkoxycarbonylmethoxy, carbamoyl, sulfa-
moyl, (Cl-C,)-alkoxysulfonyl and sulfo, or is the radical
of a 5- or 6-membered monocyclic or 9- or 10-membered
bicyclic heterocycle having at least one carbon atom, 1
to 4 nitroqen atoms and/or 1 sulfur or oxygen atom ac
ring members, and which is optionally mono-, di- or
trisubstituted by one or more radicals from the ~eries
comprising F, Cl, Br, hydroxyl, (C1-C,)-alkoxy, (Cl-C,)-
alkyl, (C1-C8)-alkoxycarbonyl, amino and trifluoromethyl.
R2 is H or a hydroxyl protective group, such as are
de~cribed, for example, in T.W. Greene, ~Protective
Groups in Organic Synthesis"; A. Wiley-Interscience
Publications; New York-Chiche~ter-Bri~bane-Toronto-
Singapore, 1981. Of these, benzyl, p-metho ybenzyl,
methoxymethyl, metho yethyl, benzyloxymethyl, acetyl,
trimethylsilyl, tert.-butyldimethylsilyl and tert.-
butyldiphenylsilyl are particularly suitable.
R3 is (cl-cl2)-alkyl~ mono-, bi- or tricyclic (C3-Cl8)-
cycloalkyl or mono-, bi- or tricyclic (c3-cl8)-cycloalkyl-
:
. ~
:
,
2~G~6~
-- 3 --
(C,-C6)-alkyl, where the cycloalkyl moiety is in each case
optionally substituted by (Cl-C6)-alkyl.
R4 and R5 are identical or different and are hydrogen,
(cl-cl2)-alkyl~ (C3-C,2)-cycloalkyl, (C3-Cl2)-cycloalkyl-
(cl-c6)-alkyl~ (C6-Clj)-aryl-(Cl-C6)-alkyl, where aryl can
in each case be substituted by one, two or three identi-
cal or different radicals from the group comprising
(Cl-C6)-alkyl and halogen, Het or Het-(Cl-C6)-alkyl, where
Het i8 a S-, 6- or 7-membered heterocyclic ring which is
optionally fused to benzene and can be aromatic, partial-
ly hydrogenated or completely hydrogenated and which as
a hetero element contains one or two identical or dif-
ferent radicals from the group comprising N, 0, S, N0, S0
and S02 and which can be substituted by one or two iden-
tical or different radicals from the group comprising(Cl-C6)-alkyl, (cl-c4)-alkoxy and halogen.
R6 and R7 are identical or different and are hydrogen,
(C1-C12)-alkyl~ (C3-C~2)-cycloalkyl, (C3-C,2)-cycloalkyl-
(C,-C6)-alkyl, (C6-C,4)-aryl-(C,-C6)-alkyl, where aryl can
in each case be substituted by one, two or three identi-
cal or different radicals from the group comprising
(C~-C6)-alkyl, (C,-C4)-alkoxy and halogen; or R~ and R7,
together with the carbon atom carrying them, are (C3-Cl2)-
cycloalkyl.
The compounds of the formulae Ia and Ib are enantiomers.
The invention relates both to the optically pure com-
pounds and to their stereoisomer mixtures, such as
racemates.
Alkyl can be straight-chain or branched. The same applies
to radicals derived therefrom such as alkoxy.
Cycloalkyl is understood as meaning, for exa~ple, cyclo-
propyl, cyclobutyl, cyclopentyl, cyclohexyl or
cycloheptyl.
A Het radical preferably has one of the following
- 4 -
meaningss pyridyl, thiazolyl, thienyl, pyranyl, benzo-
furyl, isobenzofuryl, furyl, pyrrolyl, imidazolyl,
pyrazinyl, indazolyl, guinolyl, isoquinolyl, phthal-
azinyl, pyrimidinyl, pyrazinyl, indolizinyl, isoindolyl,
indolyl, quinoxalinyl, quinazolinyl, cinnolinyl, oxazo-
ly', isoxazolyl or isothiazolyl. The radicals can be
aromatic, partially hydrogenated or completely hydrogen-
ated. They can be substituted by one or two identical or
different radicals from the group comprising (Cl-C6)-
alkyl, (C,-C4)-alkoxy and halogen.
(C6-C14)-Aryl i8 understood as meaning, for example,
phenyl, naphthyl or biphenylyl; phenyl is preferred.
Halogen i8 fluorine, chlorine, bromine or iodine.
Suitable salts are in particular salts with mineral
acids, such as HCl, H2S04 or H3P04, into which the com-
pounds of the formula Ia or Ib are converted, for example
for the purposes of storage.
Preferred compounds of the formula Ia or Ib are those in
which
W is as defined above,
R^ is hydrogen, (C,-C10)-alkyl which is optionally mono- or
diunsaturated, (C3-CB)-cycloalkyl, (C3-C8)-cycloalkyl-
(C,-C6)-alkyl, (C6-C,4)-aryl, (C6-C,4)-aryl-(C,-C8)-alkyl, or
the radical of a 5- or 6-membered monocyclic or 9- or 10-
membered bicyclic heterocycle additionally having atleast 1 carbon atom, 1 to 4 nitrogen atoms and/or
1 sulfur or oxygen atom as ring members,
R2 is as defined above,
R3 is (C1-C6)-alkyl, mono-, bi- or tricyclic (C3-Cl~)-
cycloalkyl or mono-, bi- or tricyclic (C3-Cla)-cycloalkyl-
(C1-C3)-alkyl;
.
2 0
-- 5 --
R4 and R5 are aY defined above; and
R6 and R7 are identical or different and are hydrogen,
(C1-C~z)-alkyl, (C3-Cl2)-cycloalkyl or (C3-C12)-cycloalkyl-
(cl-c6)-alkyl; or, together with the carbon atom carrying
them, are (C3-C12)-cycloalkyl;
and their salts.
Particularly preferred compounds of the formula Ia or Ib
are those in which
W is -CO-, -O-CO- and R~ is hydrogen, (cl-clo)-alkyl which
is optionally mono- or diunsaturated, (C3-CB)-cycloalkyl,
(c3-c8)-cycloa~ l-c6)-a~ (C6-Cl4)-aryl, (C6-Cl4)-
aryl-(Cl-C6)-alkyl, or the radical of a 5- or 6-membered
monocyclic or 9- or 10-membered bicyclic heterocycle
having at least l carbon atom, l to 4 nitrogen atoms
and/or 1 sulfur or oxgen atom as ring member~;
R2 is as defined above; and
R3 is methyl, ethyl, n-propyl, n-butyl, 2-methylpropyl,
2-ethylbutyl, cyclopentylmethyl, cyclohexylmethyl or
cycloheptylmethyl;
R4 and R5 are as defined above; and
RB and R7 are identiGal and are hydrogen or (C1-C4)-alkyl,
or, together with the carbon atom carrying them, are
(Cs~c7)~cYcloalkyl;
and their ~alts.
Very particularly preferred compounds of the formula Ia
or Ib are those in which
R1 and R2 are as defined above,
2 ~
- 6 -
R9 is methyl, ethyl, n-propyl, n-butyl, 2-methylpropyl,
2-ethylbutyl, cyclopentylmethyl, cyclohexylmethyl or
cycloheptylmethyl;
R~ and R5 are identical or different and are hydrogen,
(Cl-C~)-alkyl, (C5-C,)-cycloalkyl, (C5-C,)-cycloalkyl-
(Cl-C3)-alkyl, phenyl, benzyl, 2-pyridyl, 3-pyridyl, 4-
pyridyl, 2-thiazolyl, 4-thiazolyl, 5-thiazolyl, 2-thi-
enyl, 3-thienyl, 1-methylimidazol-2-yl, l-methylimidazol-
4-yl or 1-methylimidazol-5-yl; and
R6 and R7 are identical and are hydrogen, (C1-C4)-alkyl or,
together with the carbon atom carrying them, are a
(C5-C~)-cycloalkyl,
and their salts.
~ he invention furthermore relates to a process for the
preparation of compounds of the formula Ia or Ib, which
comprises reacting a compound of the formula IIa or IIb
,, , -
..
h O ~
- 7 -
R 3 CHO
R - N ~ ~R
~ (IIa)
-~\
R R 7
R CHO
R--N ~ OR
~ (IIb
~/\ ' . :
R R 7
in which the radicals R1, R2, R3, R6 and R7 are as defined
above, with a phosphorane of the formula III
R ~ R 4
p ~ (III)
R l10 R5
in which R4 and R5 are a8 defined above and R8, R~ and Rl
are identical or different aryl radical~, preferably
phenyl.
To prepare a compound of the formula ITa or IIb, sultably
protected amino acid dQrivativQs can be used as stsrting
materials. ThesQ yield IIa or IIb as a result of double
reaction with the nucleophilic formyl equivalent 2-
trimethylsilylthiazole TST (Scheme 1) (A. Dondoni et al.,
J. Org. Chem. 1990, 55, 1439) in a solvent which is inert
.
- . . .. ....
. , .. . ~. :
.
,:
~a69~
-- 8 --
to this nucleophile such as diethyl ether, di-n-butyl
ether, MTB, dichloromethane, DIP, THF, tetrahydropyran or
DME at a temperature between -40C and the boiling point
of the solvent, preferably between -40-C and +25-C. The
first addition of TST take~ place with high syn selec-
tion, the second with high anti selection. The resultant
hydroxyl function is protected in the next reaction step
using methods known from the literature. The liberation
of the formyl group can be carried out by the following
reaction sequence:
1) N-Alkylation with a suitable alkylating reagent such
as, for example, dimethyl sulfate or methyl iodide
without solvent or in a suitable inert solvent such
as, for example, acetonitrile, THF, MTB, DIP or EA
at temperatures between 25C and the boiling point
of the appropriate solvent.
2) Reduction using a hydride transfer reagent ~uch as,
for example, NaBH4 in a solvent suitable for these
types of reaction such as, for example, methanol or
ethanol at temperatures between -30C and 25C,
preferably between -20C and 0C.
3) Liberation of the aldehyde group by action of
reagents for the cleavage of thioacetals (see Tamura
et al., Synthesis 312 (1973), Seebach et al.,
ibid. 357 (1977) and literature cited therein),
preferably by means of N-bromosuccinimide in a
mixture of acetone and water, preferably at O-C to
30-C.
. .. .
' ~ ' ` ~ , . ;
,. :. !
:- ' ~ .
_ 9 _ 2 3~ t~
Scheme 1
Rl N~H > R--N~S
H OH
protection 1 ,~<N 3 "Me+"
>R--N S >
\~o
R 6~\R 7 3
Me
Rl N~s3
6 4
R R
2~6~6t,
10 --
CH3
R3 HN_
R--N~S
e.a. NBS
\~0
6A S
R \ 7
R
R3 0
Rl N ~ H 3 'Me-l'' > ~b
~ 4."H ~"
R 6-\ 7 6 ~. e . g NBS
R
The Wittig reaction with a suitable phosphorane in an
inert solvent such as diethyl ether, di-n-butyl ether,
MTB, DIP, THF, DNE or dioxane at -30-C up to the boiling
point of the solvent, preferably between 0C and 30C,
yields the title compounds of the formula Ia or Ib. The
aldehyde of the formula IIa or IIb can also be reacted
with the anion of a suitable phosphine oxide with the
formation of the title compound (Horner; see Krauch,
Kunz, Reaktionen der Organischen Chemie (Reactions of
Organic Chemistry), H~thig Verlag Heidelberg 1976,
page 241).
The compoundc of the formulae Ia and Ib according to the
invention are valuable intermediates for the preparation
of pharmaceuticals, in particular of inhib~tors of renin
and of HIV protease. Inhibitors in whose synthesis these
compounds can advantageously be used are described, for
example, in FEBS Lett. Vol. 230, 38 (1988), J. Med. Chem.
Vol. 31, 2264 (1988), ibid. 2277, Biochem. Biophys. Res.
.. ; ~ . , .
20a6~63
-- 11 --
Commun. Vol. 146, 959 (1987) and EP-A-370,454.
List of the abbreviations used:
TLC thin-layer chromatography
DCI desorption chemical ionization
DIP diisopropyl ether
DNE 1,2-dimethoxyethane
EA ethyl acetate -
FAB fa~t atom bombardment
Hep n-heptane
~ molecular peak
MeOH methanol
NS mass spectrum
MTB methyl tert.-butyl ether
Et20 diethyl ether
R.T. room temperature
m.p. melting point
THF tetrahydrofuran
NBS N-bromosuccinimide
TST trimethylsilylthiazole
20 Red-Al sodium bis(2-methoxyethoxy)dihydroaluminate
CH2Cl2 dichloromethane
The examples below are used to illustrate the present
invention, without restricting it theretos
Example 1
(2S,lRS)-2-t-Butyloxycarbonylamino-3-cyclohexyl-1-(2-
thiazolyl)propanol
3.14 g (10 mmol) of N-Boc-L-cyclohexylalanine-N,O-di-
methylamide are dissolved in 30 ml of dry THF and cooled
to 0C. 5.7 ml (20 mmol) of Red-Al (70 % in toluene) are
added dropwise. After ~tirring at ODC for 2 hours, the
mixture i8 poured into 200 ml of ice-water and extracted
3 x with ethyl acetate. The combined organic pha~es are
washed with saturated NaCl solution, dried with Na2SO~
~, ~ ~ ' ' ; ,. --, - . . .
- 12 -
and concentrated. 2.6 g of N-Boc-L-cyclohexylalaninal are
obtained, which is dissolved as the crude product in
30 ml of dry CH2C12 and treated dropwise at -30C with
3.13 g (20 mmol) of 2-trimethylsilylthiazole. The mixture
is kept in a deep-freeze at -25-C for 14 hours. After
concentrating, the re~idue i8 dissolved in 30 ml of
THF/5 ml of H20 and treated with 3 g (20 mmol) of cesium
fluoride. After refluxing for 7 hours, the mixture iB
diluted with ethyl acetate and washed twice with satd.
NaCl solution. It i8 concentrated after drying over
Na2SO4 .
The product is separated from small amounts of unreacted
aldehyde on silica gel (eluent EA/Hep 1 : 2). 1.83 g
(54 %) of the title compound are obtained. According to
NMR, it is a 5 : 1 mixture (R- or S-configuration on
carbon C-1).
R (EA/Hep 1:1) = 0.21; MS (DCI) = 341 (M + 1)
Example la
(4S,5R)-3-t-Butyloxycarbonyl-4-cyclohexylmethyl-2,2-
dimethyl-5-(2-thiazolyl)oxa~olidine
23 g of the title compound 1 (67.6 mmol) in 250 ml of
toluene are heated to reflux for 48 hours with 40 ml of
2,2-dimethoxypropane and 2.5 g of p-toluenesulfonic acid.
After cooling, the mixture is washed with 1 N NaHC03
solution, dried with MgS04 and concentrated. The crude
product is purified on SiOz using EA/cyclohexane (1 s 4).
12.5 g of the title compound are obtained.
Rr (EA/Hep 1:1) = 0.55; MS (DCI) = 381 (M + l); m.p.
= 89 - 91C, [~]DZ = -67.9 (c = 1, CH30H).
Example lb
(4S,5R,lR)-3-t.-Butyloxycarbonyl-4-cyclohexylmethyl-2,2-
dimethyl-5-tl'-hydroxy-1'-(2-thiazolyl)methyl~oxazolidine
,
~ .
- 13 _ 2~36~
12.S g (32.8 mmol) of the title compound la are dissolved
in 70 ml of acetonitrile. 3.9 ml (1.25 equivalents) of
dimethyl sulfate are added and the mixture is heated to
boiling for 7 hours. After standing overnight at RT, it
is concentrated to dryness. The solid re~idue iB dis-
solved in 150 ml of methanol. 4.3 g (0.013 mol) of ~odium
borohydride are added in portions with efficient cooling,
during which the temperature should not rise above -lO-C.
After subsequently stirring at 0C for 30 minutes, 40 ml
of acetone are added. After concentrating, the residue is
taken up in 500 ml of EA and washed with 200 ml of satd.
NaCl solution. After drying with Na2S04, the solution is
concentrated and the crude product is sub~ected to coarse
purificstion by filtration through a little silica gel,
14 g being obtained (Compound 5, Scheme 1). This product
is dissolved in 250 ml of acetone/H20 (95 s 5) and 16.5 g
of NBS are added with cooling (s 25C) in the course of
10 min. After the end of the addition, the mixture i8
strongly cooled and treated in small portions with 500 ml
of satd. Na2S03 solution (s 25-C, strongly exothermic~).
After diluting with 500 ml of H20, the mixture is ex-
tracted 3 x with Et20. The combined extracts are washed
twice with H20 and once with saturated NaCl solution,
dried with Na2S04 and concentrated. Water residues are
removed by azeotropic di~tillation with toluene on a
^Rotavapor. 5.9 g of aldehyde (Compound 6, Scheme 1) are
obtained, which is immediately dissolved in 80 ml of
CH2Cl2 and cooled to -40C. 5.88 g (37.4 mmol) of tri-
methylsilylthiazole are in~ected. The mixture is ~lowly
warmed to RT overnight. After concentrating, it i~ t~ken
up in 80 ml of THF nnd treated with 11.8 g (37.4 mmol) of
tetrabutylammonium fluoride trihydrate. After 2 hour~,
the mixture is diluted with EA, wa~hed twice with H20 and
once with satd. NaCl ~olution, dried with Na2S0~ and
concentrated. The crystalline crude product is recry~tal-
lized from acetonitrile, 4.7 g of the title compound
being obtained.
R~ (DIP) = 0.4; NS (DCI) = 411 (M + l); m.p. = 176 -178C;
1~]2 = +35.7o (c = 1, CH30H)-
2 ~ 3
- 14 -
Example lc
(4S,5R,1'R)-3-t.-Butyloxycarbonyl-4-cyclohexylmethyl-2,2-
dimethyl-5-[1'-benzyloxy-1'-(2-thiazolyl)methyl]-
oxazolidine
5.4 g (13.2 mmol) of the title compound lb are dissolved
in 100 ml of THF and added dropwise to 0.7 g of sodium
hydride (50 % in oil, washed twice with Hep) in 20 ml of
THF. After 20 min at reflux, the mixture is cooled to
50C and 0.5 g of tetrabutylammonium iodide and 1.76 ml
of benzyl bromide are added. After stirring at RT for
3 hours, the mixture is taken up in about 500 ml of satd.
NaCl solution. After extracting twice with ethyl acetate,
the extract is dried with Na2S0~ and concentrated, 5 g of
the title compound being obtained, which can be further
reacted without further purification.
Rf (DIP) = 0.45; NS (DCI) = 500 (M + l); m.p. = 96C;
t~]DO = +46.3 (c = 1, CH30H).
Example ld
(4S,5R,l'R)-3-t.Butyloxycarbonyl-4-cyclohexylmethyl-2,2-
dimethyl-5-(1'-benzyloxy-l'-formyl)oxazolidine
2.1 g (4.2 mmol) of the title compound lc are dissolved
in 10 ml of acetonitrile and 0.55 ml of dimethyl sulfate
are added. After refluxing for 8 hours and standing
overnight at RT, the mixture i8 concentrated to drynes#.
The crude product is dissolved in 15 ml of CH30H, and
0.5 g of NaBH4 is added in small portlon~ at -10C and
the solution is concentrated. The residue is taken up in
EA, and the solution is washed with satd. NaCl solution
and dried with Na2S04. After concentrating, the crude
product is di~solved in 23 ml of acetone/HzO (95 s 5) and
added dropwi6e (15 min, -10C) to 2.25 q of NBS in 22 ml
of acetone~H20 (95 s 5). 80 ml of satd. Na2S04 solution
are added in small portions such that the temperature
does not rise above +20C. After diluting with 100 ml of
. .
- 1S ~3~3
H20, the mixture i8 extracted 3 x with Et20. The combined
extracts are washed twice with H20, dried with MgSO~ and
concentrated, 1.75 g (95 ~) of the title compound being
obtained, which are reused a8 a crude product.
Rs (DIP) = 0.4; NS (DCI) = 446 (M + 1).
Example le
(4S,SR,l'S)-3-t.-Butyloxycarbonyl-4-cyclohexylmethyl-2,2-
dimethyl-5-~1~-benzyloxy-3~-(2-pyridyl)-prop-2'-enyl]-
oxazolidine
1.72 g (4 mmol) of 2-picolyltriphenylphosphonium chloride
are suspended in 30 ml of dry THF. 0.43 g of potassium
t.-butylate are added under argon. After 2 hours at RT,
0.85 g of the title compound ld in 5 ml of THF is added
dropwise (-15C) to the lemon-yellow solution. After
standing at RT overnight, the mixture is diluted with
H20, extracted (3 x) with EA, washed with satd. NaCl
solution, dried (Na2SO4) and concentrated. Chromatography
on SiO2 yields 0.11 g of cis-isomer and 0.4 g of trans-
isomer of the title compound as an oil.
R~ (DIP) = 0.45 (ci8)
0.3 (trans);
NS (DCI) = 521 (N + l);
1~]D = +26.8 (c = 1, NeOH, trans)