Note: Descriptions are shown in the official language in which they were submitted.
~~~6~~~
- 1 -
INTRAOCULAR AND INTRAORBITAL IMPLANTABLE
DEVICES FOR THE CONTROLLED RELEASE
OF PHARMACOLOGICAL AGENTS
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to a device.
and method for intraocular or intraorbital
controlled release of pharmacological agents.
2. Description of the Related Art
A number of devices have been proposed
which permit controlled release of pharmacological
agents to various parts of the body and have
facilitated dramatic advances in the medical field.
Indeed, slow release of insulin from such devices
can eliminate the need for repeated insulin
injections in the case of a diabetic and the slow
release of bactericides can minimize the likelihood
of post-operative infection in other cases.
The controlled release of agents for
intraoc~.~lar or intraorbital use would allow
optimized therapeutics for AIDS (CMV retinitis), for
endophthalmitis (prevention ofJcure of bacterial,
viral or fungal infections), in proliferative
diseases (such as proliferative vitreoretinopathy
[PVR), lens epithelium proliferation (secondary
cataract), for malignant intraocular tumors and
other diseases. In addition, intraorbitally, such a
technique would allow control of wound healing
(e.g., reduction of scarred tissue volume in
glaucoma filtration surgery, retinal detachment
surgery (scleral buckling procedure), and strabismus
- ~~~~i~~~
2
surgery). However to date, devices for the
controlled release of appropriate agents for
intraocular or Intraorbital use have not been
developed.
It would therefore be desirable to provide
a device for intraocular or intraorbital use which
permits the controlled release or slow release of
pharmacological agents.
SUMMARY OF THE INVENTION
The present invention provides a series of
devices or implants for intraocular or intraorbital
use which permit the controlled release of
pharmacologlcal agents. The devices provided in
accordance with the present invention are
substantially "C"-shaped rings and are flexible for
insertion through small incisions made in the eye
wall or are sutured around the globe. The device
provided in accordance with the present invention
are designed to conform with existing surgical
techniques (vitrectomy, cataract surgery,
penetrating keratoplasty, scleral buckling
procedures and glaucoma and strabismus surgery).
The implant releases the selected pharmacological
agents via biodegradation or by osmosis. The
reieased drug then reaches targeted tissue by
diffusion.
~0~~~~~
3
BRIEF DESCRIPTICLN OF THE DRAWINGS
FIGURE 1 is a schematic front elevational view showing an
implant provided in accordance with the present invention within
the vitreous cavity.
FIGURE 2 is a view taken along line 2-2 of FIGURE 1;
FIGURE 3 is a front elevational view showing an alternate
size and location for an implant located in the eye's anterior
chamber provided in accordance with the present invention;
FIGURE 4 is a view taken along line 4-4 of FIGURE 3;
FIGURE 5 is a front elevational view showing the implant of
the invention disposed at the equator of the lens capsular bag;
FIGURE 6 is a view taken along line 6-6 of FIGURE 5;
FIGURE 7 is a front elevational view showing an alternate
location and configuration of the implant provided in accordance
with the present invention;
FIGURE 8 is a view taken along line 8-8 of FIGURE 7;
FIGURE 8A is a view similar to FIGURE 8 showing the eye wall
deformation when the buckle compresses the eye's equator;
FIGURE 8B is a front elevational view showing an alternate
location and configuration of the implant provided in accordance
with the present invention;
FIGURE 9 is a front elevational view snowing yet another
implant location for the device of the invention;
FIGURE 10 is a view taken along line 10-10 of FIGURE 9;
CA 02056138 1999-08-31
4
FIGURE 11 is a cross-sectional view of a
device in accordance with one embodiment of the
present invention; and
FIGURE 12 is a cross-sectional view
showing an implant inserter provided in accordance
with the present design.
DETAILED DESCRIPTION OF THE PRESENTLY
PREFERRED EXEMPLARY EMBODIMENT
Referring to the drawings, the implant of
the invention is shown disposed at various locations
in and about a patient's eye. Each disposition of
the implant is selected to facilitate optimal drug
delivery for the particular treatment being effected
and the contemporaneous surgical procedure. For
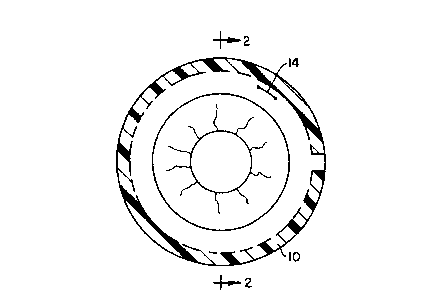
example, referring to FIGURES 1 and 2,
intravitreally positioning the "C" ring implant 10
at the equator of the globe 12 is ideal for drug
release in the vitreous cavity following
vitreoretinal surgery. The implant 10 can be
inserted through an incision as at 14 and is
preferably preformed so as to have a diameter
slightly larger than the diameter of the vitreous
cavity 12. Once inserted, then, the implant 10 will
not encroach upon the visual axis and will be
maintained in position initially by is resiliency
and, within two to four weeks, probably by
encapsulation, This device could also be utilized in
the treatment of ocular malignancy when loaded with
appropriate antimitotic agents such as but not
limited to 5-fluorouracil, daunomycin, and others,
5
and for the treatment of CMV retinitis in AIDS
patients when loaded with ganciclovir.
Referring to FIGURES 3 and 4, a smaller
"C" ring 16 can be made to fit into the,angle 18 of
the anterior segment 20 following penetrating
keratoplasty (corneal transplantation), intraocular
cataract surgery, vitrectomy, glaucoma filtering
surgery, etc. Again, the implant can be inserted
through the surgical incision 22 and could be
provided for releasing antibiotics, antimicotics,
antimitotics, and/or growth factors.
Referring to FIGURES 5 and 6, another
location for a "C" ring 24 provided in accordance
with the present invention is at the equator of the
lens capsular bag 26. Such a "C" ring 24 can be
introduced during extracapsular cataract procedures,
after removal of nucleus and cortex, to release
drugs preventing lens epithelium proliferation, the
cause of posterior capsule opacification (secondary
cataract). In the alternative, the "C" ring of the
invention could be used as an element for
lntraocular lens (IOL) construction or as an IOL
coatlng.
By modifylng the ring design slightly as,
shown in FIGURES 7 and 8, the "C" ring 28 can be
used in the orbit external to the globe 30 (as a
buckle) and provide therapeutic drug release to the
orbital tissue, ocular muscles, the sclera, and, by
diffusion, into intraocular tissues. The ends 32,
34 of ring 28 could be made to overlap (FIGURE 8B)
or butt and serve as a temporary or permanent buckle
(elastic band used in retinal detachment surgery). In
the alternative, as shown in FIGURE 8A, the ring 28
2,Q~~~.
6
could be molded to have a diameter slightly less
than the diameter of the globe 30 so that the
resiliency of the ring tends to clamp it about the
globe and deforming it as for conventional scleral
buckling procedure performed with silicone elastic
bands wherein the buckle compasses the eye°s eguator
bringing the eye tissue outer coat (sclera) into
contact with the detached retina (inner coat). The
ring can also be sutured about the globe to ensure
retention of the ting in an appropriate position.
Even further, as shown in FIGURES 9 and
10, positioned under the conjunctiva 36 the "C" ring
38 can release antimitotic agents in order to
modulate Would healing and prevent early closure of
the filtering bleb in glaucoma surgery and minimize
scarring during strabismus and retinal reattachment
procedures.
Intraocular insertion of the flexible "C"
rings described above can be accomplished by
injection with a simple surgical instrument during
the contemporaneous procedure (e. g.; vitrectomy,
cataract surgery, penetrating keratoplasty, glaucoma
filtering surgery) through a small incision made in
the eye wall through the conjunctiva as shown, for
example, at 14, 22, and 40.
Referring to FIGURE 12, one embodiment of
an instrument 42 for implanting the device 44 which
includes a cannula~46 for receiving the implant 44
in a straightened configuration. The cannula 46
preferably has a beveled forward end 48 for
facilitating insertion of the same into an incision
in the eye wall. The cannula 46 further preferably
includes a side arm structure 50 for enabling
CA 02056138 1999-08-31
7
infusion and/or irrigation and aspiration of
materials. A modified syringe structure 52 having a
piston 54 extending forwardly from the plunger 56
can be employed to eject the implant 44 from the
cannula.
In one form of the present invention, the
device substrate is made from synthetic
biodegradable polymers. The drug is mixed with the
polymer (both in fine powder form or with the drug
in fine powder form and the polymer in the liquid
state) and processed into a "C" ring-shaped implant
by solvent casting or by thermoforming through
compression or injection molding techniques.
Biodegradation of the polymer releases the drug.
For a given application site (e. g. intraocular,
intraorbital, etc.) Release time is a function of
the polymer and implant shape selected and can be
made to vary from about one week to about one year.
In another form, the drug is covalently
bonded to the polymer backbone via water soluble or
hydolyzable organic links (e. g.. peptides). Drug
release occurs by biodegradation of these links.
In yet another form of the invention,
covalent bonds are formed between the polymer and
drug molecules to create a polymeric drug system.
Polymeric degradation releases the drug.
In a fourth form, the drug is contained in
a hollowed flexible polymeric cocoon 58 shaped as a
"C" ring (FIGURE 11). The drug 60 diffuses through
the wall of the ring into tissue via osmosis.
Biodegradable polymers have been tested
subconjunctivally, intraorbitally and intraocularly
~~)~~~. ~8
8
both in the anterior and the posterior (vitreous
cavity) chambers.
Twenty-six of 140 biopolymers tested were
found to have adequate physical properties for the
fabrication of ocular implants. Three were found
intraocularly biocompatible and two of these were
selected for the fabrication of implants. Using a
10/90 lactide-glycolide copolymer, biodegradable
retinal fixation tacks (biopins) were fabricated and
implanted in a series of 30 rabbits. In
vitrectomized and non-vitrectomized eyes, the
biopins fully biodegraded at 8 and 12 months
respectively. As with their metallic counterparts,
encapsulation of the biopins develops at
approximately 1 month. No foreign body or toxic
reaction was observed histologically.
A biodegradable polymer controlled drug
release matrix containing 10% 5-FU has been
fabricated and tested in vitro. More particularly,
using a 50/50 lactide-glycolide copolymer, a 10%
5-FU controlled drug released matrix designed for
rapid release was developed. An in vitro technique
was developed to assess the pharmacokinetics of the
matrix.
Usirig a 10/90 lactide-glycolide compound,
each blopin was designed to release long of drug per
hour over a 4 month period. An increased drug rate
of l0ug/hr can be achieved with a "C zing" implant.
Injectable through a 20ga gars plana incision, this
larger implant can be designed to fit the retinal
equator thereby avoiding retinal perforation.
~o~~~~~
9
While the invention has been described in
connection with what is presently considered to be
the most practical and preferred embodiment, it is
to be understood that the invention is not to be
limited to the disclosed embodiment, but on the
contrary is intended to cover various modifications
and equivalent arrangements included within the
spirit and scope of the appended claims.