Note: Descriptions are shown in the official language in which they were submitted.
20~6~81
--1--
D~SCRIPTION
Microbial Inhibition Test Yit and Method
Reference to Prior Applications
This application is a continuation-in-part of U.S. S.N.
07/614,729, filed November 16, 1990 which is a continuation
application of U.S. S.N. 07/190,041, filed May 4, 1988, now
abandoned.
Background of the Inve~tion t
Antimicrobial drug~, particularly those used in animal
feed or to treat animals, have been found in mllk and food
products, such as for example, beta lactams like penicillin
and/or tetracyclines, and more recently sulfa drugs like
sulfonamides, such as sulfamethazine, have been detected in
food products. The detection of these drugs at low levels are
most important, particularly sulfa drugs which are allergenic
and possibly carcinogenic in nature, even at low levels. Milk
is routinely tested for the presence of drugs, like beta lactam,
by the use of an inhibition disc assay method. However, such
assay method does not detect sulfa drugs or certain other
antimicrobial drugs at very low levels.
One technique for detecting sulfa drugs in milk at low
level~ is by the employment of high pressure liquid chromatography
~HPLC) or by receptor assay test method (see U.S. Patents
4,239,745 and 4,239,852, both issued December 16, 1980). Both
of such test methods are cumbersome and require costly equipment
and highly trained technicians.
A microbiological growth inhibition test method to detect
sulfonamides in milk for example has been reported and described
in European patent specification 79200277.6 by Beuker3 et al,
30 published December 12, 1979. This te3t method has been described
as an improvement on the test described in ~ritish patent
specification 1 q67 ~39, published March 16, 1977 to Lameris
-2- 20~6~8~
et al. The Beukers et al test method places spores of 8acillus
stearothermophilus (BST) into a buffered agar solution, and
the agar is solidified to form a support medium. The BST in
the agar solution is prevented from germinating by lack of
nutrients in the agar and/or by low temperature. The nutrients
for the growth of the BST are prepared separately and are either
placed in a tablet or on a disc, with the tablet or disc placed
on the solid agar medium containing the BST before carrying
out a test. A test sample is then added and the sensitivity
for sulfa drugs i9 increased by the addition of trimethoprin
while an indicator is added either to the agar solution prior
to solidification or to the nutrients 90 as to provide for
increased sensitivity for sulfa drugs. However, sensitivlty
is not less than about 100-500 ppb.
It is desirable to provide for a microbial inhibition test
kit, method and apparatus which is simple and effective to employ
and is sufficiently sensitive to detect very low levels of drugs,
particularly sulfamethazine in milk and other products.
SummarY of the Inve~tio~
The present invention relates to a test kit and method
for the detection of drugs, and more particularly to a test
kit, including a controlled heating and cooling apparatus, and
method which is sensitive to sulfa and other drugs particularly
at a level of less than about 50 ppb.
The present invention concerns a microbial inhibition test
kit suitable for the screening of drugs at low concentration
levels, particularly sulfa drugs, like sulfonamides and more
particularly, sulfamethazine, for example, in various products,
such as food products, and particularly milk and dairy products.
The test kit comprises a first BST tablet containing a mixture
which includes a thermophilic, spore-forming bncteria sensitive
to the drugs to be tested, And more pArticulArly UAcillus
stearothermophllus (BST), the BST in n low, but effective amount
and which BSq~ is inhibited in growth by the presence Oe a drug
in the sample. Typically, the first tablet is a low
2~565~
--3--
moisture-containing, compressed, BST-containing tablet which
also contains a stabilizer for the BST spores in an inert filler-
type material. The tablet contains a low moisture lev~l, for
example, less than 2 percent, and more particularly, less than
1 percent moisture and is compressed under such conditions so
as to reduce any damage to the BST spores with generally
compression being accomplished with a pressure for example of
5 to 10 newtons, for example, 3 to q newtons. The BST tablet
generally contains a stabili~er for the BST spores, such as
for example a carbohydrate, such as lactose, or an amino acid
source, such as peptone, or more particularly, a comblnation
thereof. The BST tablet usually contains a powdered, inert,
water insoluble filler material to provide bulk for compression.
Such a material may vary widely, but usually comprises for example
a cellulose-type material such as an ethyl or propylcellulose
or micro crystalline cellulose or celluloid powder. Compression
of the BST tablet should take place under such time, temperature,
pressure and friction conditions so as to prevent any substantial
destruction of the BST spores in the tablet. The first 8ST
tablet may also contain other ingredients, including a small
amount of agar or other medium.
The test kit also includes a medium tablet which contains
nutrients for the BST spores ln the first BST tablet, the amount,
type and concentration of nutrients present in the medium tablet
are present sufficient to permit the detectable growth of the
BST spores, but sufficiently low to provide low resistivity
to the drug to be tested, 90 as to stress the BST during the
test procedure and to provide for the detectlon of an
antlmicrobial drug, particularly such as a sulfanomide, to a
detection concentration of less than about 50 ppb and as low
as 0.5 to 20 ppb. The medium tablet containin~ the nutrients
may comprlse for example ~ E~ortlon of n nutrient broth at a
level of for example O.q grams per liter or less, and more
particularly carbohydrates as nutrients at a total amount of
about 1.0 grams per liter or less. Suitable carbohydrates would
2~6~81
--4--
include, but not be limited to: glucose, fructose: sucrose:
and dextrose. Generally, amino acids may include, but not be
limited to: tryptone, peptone and beef extract and similar
products for example at a level of about 0.15 grams per liter
or more particularly, 0.08 to 0.12 grams per liter.
Generally, the tablets include a buffer agent, such as
alanine, and for example, a potassium or sodium phosphate buffer
may be added to provide a pH of about 7.5. The tablets may
include for example a salt, such as sodium chloride, as well
as a non-ionic-type detergent, to enhance the 3ensitivity of
the test. Further additives may include for example an
antibiotic-free, non-fat dry milk where a test sample is derived
from milk or a dairy product to reduce the variation in the
test between test samples. The tablets also may include a
thickening agent, such as a colloidal silica: a lubricant, such
as a glycol, like polyethylene glycol: vitamin and phosphate
sources. Other additlves may include for example a
calcium-chelating agent present in an amount to complex or chelate
calcium in the test sample, such as ethylenediaminetetraacetic
acld ~EDTA) or ethylene glycol tetraacetic acid (EGTA~ which
additives are particularly effective in provlding a sensitive
test to tetracycline and tetracycline-type antibiotics, providing
a sensitivity for example of 25 to 50 ppb or less. Other
additlves include antifoliate agents, such as trimethoprin (TMP)
or methotrexate (MTX) or primethamine (PMA) to enhance the
sensitivity of the test. The test results may be interpreted
by the employment of a pH color indicator which can be based
on an acid-base or redox or glucose monitoring and particularly
where a pH color indicator is used, may comprise bromocre301
purple or phenol red or a redox color lndicator. UsUally, the
pl~ color indicator is added to the medium tablet while the calcium
chelating or anti~oli~te agents and buffers are u9ually Added
to the medium tablet.
The test kit also includes a test container, such as for
example a multiwell plate, a test tube in which the 8ST tablet,
2~6~8~
--5--
the medium tablet and test sample may be introduced, heated,
cooled and incubated so as to permit the rapid and ef~icient
assay screening of druqs by the absence of change or change
in color of the pH indicator included or by monitoring the
glucose.
The test method iB dlrected to a test or the determlnation
of drugs, such as sulfa drugs and other antimicrobial drugs,
in a test sample at a defined, low concentration level,
particularly at concentration levels where sulfamethazine and
sulfa drugs are below about 50 ppb. The test method comprises
placing a defined amount of a test sample into a test container
and heating the sample to a temperature sufficiently high to
destroy the natural inhibitors in the sample, and thereby
enhancing the further sensitivity of the test, such as for
example, heating a test sample derived from raw or pasteurized
mllk to a temperature of about 100C for about one to five
minutes. The test method includes cooling the heated sample
to a defined lower temperature, for example, to a temperature
of less than about 85C and more particularly, about 80C to
85C. The test method then includes adding the BST-containing
tablet to the cooled test sample, the tablet comprising a low
moisture-containing, compressed tablet of the BST spores, a
stabilizer and an inert bulking and filler material. Thereafter,
the test container with the BST spores and the sample are rapidly
heated to a defined temperature to heat shock the BST ~pores
so as to affect generally synchronous germination of the BST
spores. Generally, the rapid heating of the BST spores in the
test sample is done to a temperature of about 100C or more
for about 0.1 to 2 minutes. Antifoliate agents such as TMP
tend to be destroyed on heating therefore, TMP is typically
added to both the ~ST tablet and the medium ~ablet~ to insure
the presence of TMP in the te~t to enhanas ~ensitivity
particularly for sulfa drugs.
The test method includes adding a medium tablet containing
the nutrients to the heat-shocked BST ~pores and test sample
-6- ~5~8~
in the container. A medium tablet comprising nutrients merely
sufficient for the growth of the 8ST spores, but sufficiently
low to provide for low resistivity to the drug to be tested
so that the test i9 particularly ~ensitive. The test container
s containing the test sample, BST and medium with the nutrients
are then incubated, for example, at an incubation temperature
of 65C~1C and incubation time for about 2 hours 45 minutes
to 3 hours 15 minutes. The test method includes terminating
the incubation at the defined end of the incubation period,
either by removing the test sample from the incubator or by
rapidly increasing the incubating temperature to over about
75C for a time period to terminate further growth of the BST.
Thereafter, the presence or absence of the drug in the sample
within a defined concentration level is detected, particularly
simply by observing the absence or change of color of the pH
color indicator included or by glucose monitoring or a redox
indicator. A sulfa drug, particularly a sulfamethazine, present
in an amount of less than 20 ppb will test positive. The
sensitivity may be as low to sulfa drugs like sulfamethazine,
as low as 10 ppb or lower, thereby providing for an extremely
3ensitive test kit and method for hitherto available to possible.
The test kit and method permits the sensitive determination
of antimicrobial drugs in a wide variety of materials,
particularly in food products and body fluids, for example,
but not limited to: raw and pasteurized milk: urine; and other
body fluids: and meat. Generally, test samples taken from the
material to be tested are placed in liquid form and a deflned
volume of liquid may be optionally added to the test container,
while the tested material placed in liquid form and liquid
aliquots taken. The test kit and method are sensitive for example
to 0.5 to 20 ppb levels o~ sulfonamides depending on the
individual sulfa drugs tested. 'rlle test kit and method also
have excellent sensitivity to aminoglycocides, especially to
gentamicin to a level of about 30 to 80 ppb and neomycin to
a level of about 100 ppb or less. The assay may be psrformed
2~5~
--7--
in any test container, such as test tube or a plate and i!lcubated
in a heat block or water shaker bath.
The test method may be carried out in an automatic system
and apparatus wherein preheating, cooling, heat activation and
incubation are controlled in a single, automated system and
apparatus. The test kit may include a control apparatus which
comprises a metal heating block, such as for example, made oE
aluminum, having at least one and typically a plurality of
openings therein to form and arrange for the insertion of a
test container, more generally a test tube, to be heated with
electrical means, e.g. coils in the block, to heat the metal
block so that the test tube and the test sample will be rapidly
heated to defined temperatures as required in the test method.
The test apparatus also includes a separate metal cooling block,
typically of aluminum, and generally of defined and greater
mass than the heating block to provide for the rapid cooling
of the heating block when placed in contact therewith to the
defined cooled temperature as required in the test method.
Generally, the heating and cooling biocks are placed in a spaced
apart heating position when the heating of the sample containers
are required and are placed in an ad~acent, contacting, heat
exchange cooling position when cooling of the sample containers
are required. The heating and cooling blocks are moved between
positions by tilting the control apparatus to provide for slidable
movement of the blocks. The incubation i5 done in the tilted
position to enhance the oxygen transfer rate. The test apparatus
also includes electrical circuitry and control programming means
based in the test method to provide for the sequential timed
heating of the heating block and to signal the time period for
the movement of the heating and cooling b30aks between heatlng
and cooling positions and to provide signal moans, e.g. light~
or audible means, for the termination of the various test steps
. . and the termination of the incubation and termination of the
test sequence. While the control apparatu3 is useful in the
test method, the control apparatus may be usefully employed
2 ~
--8--
where rapld heating and cooling of test samples at defined test
times are of importance.
The test kit and method have significant advantages Gver
prior art inhibition, disc-type tests in that the test kit and
S method do not require the employment of agar or an agar-type
support. The use of a separate ~ST tablet and a medium tablet
provides for a long shelf life, typically of at least a year,
and where storage may be accomplished at amblent temperatures,
such as 60F to 70F. In contrast, the prior art test method
requires a solid agar medium with the BST which has a limited
shelf life of not more than about three to 8iX months and which
medium must be stored at refrigerated temperatures of 40F.
The test kit and method are also more sensitive than the prior
art test. The test kit and method do not require the employment
of a paper disc or the reading of the test sample by visual
examinatlon of a zone of inhibition around the periphery of
the disc. Therefore, the test kit and method represent a
significant advance, both in simplicity, convenience, shelf
life and sensitivity in the determination of antimlcrobial drugs.
The test kit and method have been described as employing
separate SST and medium tablets; however, it is recognized that
a single tablet may be prepared and used rather than separate
tablets provided that the single tablet or composition preparation
prevents the growth of the BST in the tablet in the presence
of the nutrients, such as for example by segregating, coating
or otherwise treating the BST spore~ or the nutrients to prevent
interaction and provide for long shelf life.
The test kit, including the control apparatus, and method
provide a simple, antimicrobial drug screening test. The test
is sensitive to beta lactams, tetracycline, sulfa drugs, amino
glycocides and macrolides concentration levels as follows, with
the following concentration range ln ppb per mlnimum positive
color:
2~5~
--9--
Penicillin 2.4 to 3.6
Sulfamethazine 15-20
Sulfadimethoxine 8-10
Gentamicin 30-80
Oxytetracycline 80-100
~C l1171~
Tylo~in 40-60
The test kit and method are a qualitative test kit and method
intended for determining antimicrobial drugs, particularly for
raw and pasteurized milk testing. The tablets in the test kit
may still have a shelf life at room temperature at 20C to 25C
for up to one year. The test kit and method develop ln
approximately 2 hours and 55 minutes. The test kit may also
contain for example microbial tabletæ in a blister pack for
example of 20, medium tablets in blister packs for example of
20, negative control tablets in blister packs of 2, a pipet,
water, pipet tips and test tubes. The equipment employed lnclude
an automatic test block to carry out the test sequence, test
tube caps which are reusable, a 0.2 ml pipet and a marker pen.
The ingredients and additives as described for use in the
8ST and medium tablets may vary as desired and may be ad~usted
in order to provide for particularly sensitive detection of
particular drugs. Typically, the 8ST spores should not be used
in excess, but sufficient to permit growth and to provide for
detection of the antimicrobial drug, and generally are employed
for example in the amount of 106 to 107 spores per tablet.
The test kit, method and apparatus of the invention will
be described for the purposes of illustration only in connection
with certain preferred, illustrated embodiments; however, it
is recognized that various changes, modifications, additions
and improvements may be made to the test kit, method and apparatus
by those persons skllled ln the art without departing ~rom the
spirit and scope o~ the invention.
Brief Description of the Drawinqs
Pig. l is a perspective illustrative view from above of
a test apparatus for carrying out the test method of the invention
showing the test apparatus in a heating position.
2~56~
--10--
Fig. 2 is a perspective, illustrative view from above of
the test apparatus of Fig. 1 showing the test apparatus in a
tilted position for cooling and incubation.
Pig. 3 is an illustration of the front control panel of
the test apparatus of Fig. 1 .
Fig. ~ is an illustratlve, partially sectional sido plan
view of the apparatus of Fig. 1
Pig. 5 is an illustrative, partially sectional side plan
view of the test apparatus of Fig. 2.
Fig. 6 is an illustrated electrical circuitry and programming
diagram of the test apparatus.
Pig. 7 is an illustrated electrical circuitry diagram of
the electrical power supply used in Fig. 6.
Fig. 8 is a graphical illustration of a test method employing
the test appara~us showing the temperature in degrees Centigrade
(Temperature Deg C) versus the time in mlnutes ~Tlme Mlns) of
a test sample.
Pig. 9 is an enlarged graphical illustration of a portion
of the test method shown in Fig. 8.
De~cription of the ~bodiment~
Compressed, low moisture ~ST and medium tablets were prepared
with the following composition:
8ST Medium
IngredlentsTablet Tablet
1. Mlcrocrystalline cellulose 22.6 mg 25.5 mg
(tablet bulking agent and binder)
2. Sodlum chloride (isotonlc agent)1.6 mg 1.6 mg
3. Polyethylene glycol (PEG) 600.0 ug 600.0 ug
(lubrlcant)
4. a)Powdered milk - skimmed, dried,953.0 ug 953.0 ug
antibiotic-free ~general supplement
and nutrient)
a) The powdered milk may be substltuted by other materials
where the test i3 designed to test other samples, such as
urine or blood. The presence of the antiblotic-free test
sample materials aids in elimlnatlng dlfferences between
test samples.
2 ~ 8 1
5. Tryptone (amino acid supplement) 100.0 ug 100.0 ug
6. Alamine (amino acid supplement 100.0 ug 100.0 ug
and buffer)
7. Potassium phosphate (buffering 76.0 ug 76.0 ug
agent and phosphate source~
8. b)Peptone (amino acid source) 12.6 ug 12.6 ug
9. Bromocresol purple (pH indicator) 15.0 ug 15.0 ug
10. Colloidal silica (stabilizer and 8.3 ug 8.3 ug
thickener)
11. Beef extract ~vitamin source) 0.74 ug 0.74 ug
12. d)Trimethoprin (optional folic 29.2 ~g 29.2 ug
acid analog-sensitizer)
13. Non-ionic surfactant (to increase 0.15 ug 0.15 ug
penetration of BST spores)
14. EGTA-chelating agent Tween-80 142.7 ug 142.7 ug
15. C)BST spores 2.90 ug -0-
16. Glucose 500 ~g 500 ~g
The above BST and medlum tablets used in a test tube as
the test container with a liquid sample, such as milk, provide
enhanced antimicrobial drug sensitivity in the described test
method, as follows:
b) In the above example, the amino acid source is split between
the tablets; however, 5% to 20~ of less of the amlno Acid
source may be placed in the UST tnblet and the r~st in the
medium tablot. The tota~ amino acid ~ouro~ is eixed to
stress the BST to increase sensitivity.
c) The BST spores may vary in concentration, but never exceed
107/ml.
d) To increase gensitivity for sulfa drugs. The above tablets
have a long shelf life and need not be refrigerated, but
stored at room temperature, 20C to 25C, for up to one
year.
MINIMAL DETECTION LEVELS
Prior
Antibiotic ¦ ~A~t ¦Invention
BETA-LACTAMSl l
5 Cephalexin ¦50 ¦ 50
Oxacillin I__ ¦ 8
Hetacillin ¦8 ¦ 10
Penicillin ¦2 ¦ 3
(various)
10 Cephapirin ¦8 ¦ 10
Cloxacillin ¦20 1 30
Ceftiofur 150 ¦ 40
Ampicillin ¦4 ¦ 4
Cefalonium
15 Cefadroxil
Amoxicillin
-13- 2~6~
I Prior
Antibiotic I Art IInvention
SULFONAMIDES l l
Sulfamethoxazole I ~ 5
Sulfaquinoxaline I___ ¦ 8
Sulfamethizole ¦100 ¦ 10
Sulfisoxazole ¦100 ¦ 8
Sulfadimethoxine ¦100 ¦ ~
Sulfapyridine ¦250 ¦ 20
Sulfamethazine ¦500 ¦ 15
Sulfadiazine ¦250 ¦ 20
Sulfachloropyridazine ¦ --- ¦ 5
Sulfamerazine I___ ¦ 20
Sulfathiazole ¦100 ¦ 10
Dapsone I___ ¦ 0.5
Sulfacetamide I___ ¦ 50
Sulfanilamide ¦1000 ¦ 50
Sulfadoxine I ~ 5
AMINOGLYCOSIDES¦
Streptomycin ¦ 4000 ¦ 600
Kanamicin ¦ 10000 ¦1500
Gentamicin ¦ 250 ¦ 50
Neomycin ¦1000 ¦ 75
--lq--
Prior
Antibiotic ¦ Art ¦ In~ention
MACROLIDES¦ l
Erythromycin ¦ 400 ¦ 200
Tylosin ¦ 100 ¦ 40
TET~ACYCLINES¦
Tetracycline ¦200 1 5,0
Chlorotetracycline ¦ 500 1 150
Oxytetracycline ¦200 1 80
OTHERS
Novobiocin ¦ 500 ¦ 600
I
2~6~ ~
-15-
The test kit and method provides for nutrients and bacteria
or BST spores stabilized in dry, compressed tablets (separately
or together1 which are introduced into a liquid sample. The
test method in the liquid phase does not require any agar or
diffusion barrier, thus better contact of inhibitors and nsT
spores or bacteria is achieved. The test method may use test
tubes and multiwell plates and be incubated in the standard
form heat block/water bath or employing the control test
apparatus. The composition of the tablets and test method,
e.y. heating times, may vary to provide for select sensitivity
to particular antimicroblal drugs, such as the variation in
BST spore concentration. As illu~trated, a pH color change
indicator usually shows the test results with a yellow color
indicating a negative test,and a blue color indicating a positive
test e.g. sulfa drug present at a concentration level of greater
than 10 ppb.
The microbial inhibition test method may be carried out
without the use of the control special heating incubator-cooling
test apparatus. The test method provides a single screening
test based on color change with the test results for all members
of an antimicrobial drug family. For example, in the described
tablets, the concentration range ~ppb) for a minimum positive
(blue) color would be: penicillin, 2.4-3.6s sulfamethazine,
15-20 sulfadimethoxine, 8-10: gentamicin, 30-80: and
tetracycline, 30-80.
The test kit may comprise a plurality of separate BST
and medium tablets generally in a blister package form and one
or more negative control tablets and requires distilled water.
The equipment required includes a fixed pipet, e.g. 0.2, and
disposable pipet tips, test tubes or multiwell plates, dropp~r
or drop dispenser, 66C shaker water bath with test tube rack
and hot plate to boil water to timer. Then the te~t kit includes
the control apparatus, the water bath, test tube rack and hot
plate are not required.
2~5~
-16-
For esample, for testing a large number of samples, the
test is conducted as follows:
1. Label test tubes (13 x 100 mm) and place in
rack.
2. Add 0.6 ml distilled water into each test tube.
3. Add 0.2 ml of milk sample into teYt tube and
mix well. Use new tip for each sample.
4. Heat test tubes in boiling water for 6 mlnutes.
Remove test tubes to table top to cool.
5. Add one microbial tablet to each test tube by
pushlng the tablet through the blister into the
test tube with the blunt end of a pencil.
6. Place test tube back in boiling water for 2
minuteY. Remove immediately after 2 minutes.
7. Let cool to the touch (tap water may be used
to hasten cooling) and add one medium tablet to
each test tube.
8. Cap test tubes with the plastic caps and place
ln 66C ~ 1C shaker water bath. Set shaker at
100 rpm.
9. Start timer for incubation time specified on
each kit, e.g. 3 hours.
10. When time i9 Up, remove test tubes from incubator.
11. Observe color change. Blue indlcates positive;
yellow-green is neg~tive.
Color can be stabilized for up to 24 hours by placing
test tubes in boiling water for 2 minutes.
The time-temperature profiles will be the same or similar
to those graphical profiles of a test shown in Pigs. 8 and 9
of the drawings.
The test method may be carrled out particularly for a
continually and largc number of te~t samples employing n control
test apparatus as illustrated particularly in Plgs. 1-5 of the
drawings. The test apparatus is a combined automatic hcating
incubator and coolex with circuitry and programming to provide
-17-
for the signal and timed sequence of the test steps for ease
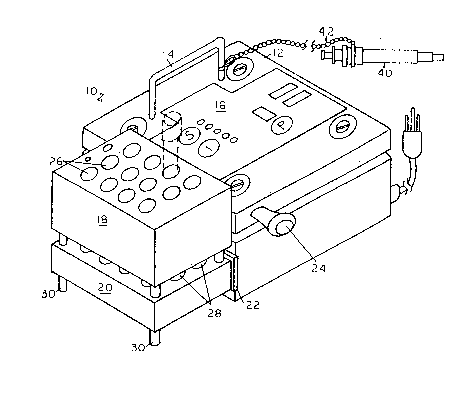
of use of the test user. The apparatus 10 comprises a control
housing 12 including a power supply (see Pig. 7~ and electrical
circuitry (see Pig. 6) with a raised handle 14 and a control
panel 16 on the top surface and a short support leg 24 on one
side of the housing 12, the handle 14 permitting the user to
tilt the apparatus 10 to a tilt position, for example, about
75 from hori~ontal and to rest the housing on support leg 24
in the tilt position. The apparatus includes a solid, slidable
aluminum cooling block 18 with a plurality of uniformly disposed
holes 26 passing therethrough for the insertion of test tubes
36 containing the test samples to be incubated. The apparatus
10 includes a golid dimension heating block 20 secured to the
one side of the apparatus 10 and heat Lnsulated therefrom by
a layer of rubber-foam insulating material 22. The heating
block 20 also has a plurality of uniformly distributed holes
26 therein aligned with holes 28 of the cooling block 18 to
receive and retain the bottom portion of the test tubes 36.
The cooling block 18 is designed of sufficient mass to provide
for the desired rapid cooling of the heating block 20 and retained
test tubes 36, while the heating block 20 has electrical heating
coils (see Fig. 6) to provide for the heating-incubation of
the test tubes 36 with the test samples. The test tubes 36
are illu~trated in dotted lines in Pig. 1 and 2 and shown with
sealed caps in Pig. 3 and 4. The heating block 20 includes
legs 30 to support the one side of the block 20. The cooling
block 18 includes a central, fixed length rod 32 secured to
the cooling block 18 and extending through a hole (not shown)
in heating block 20 and designed to support the cooling block
18 in a spaced apart, non-cooling position above the heating
block 20 when apparatus 10 i3 in a generally horizontal position
~see Figs. 1 and 4), the bottom portion of the rod 32 resting
on the table support-like leg8 30. When the apparatus 10 is
placed in a cooling position, the apparatus 10 ls tilted to
rest on support 24 (see Fig. 2 and 4) through the use of handle
2 ~
14, so that the tilt permits the lower surface of the cooling
block 18 now unsupported by rod 32 to slide into direct heat
exchange contact with the top surface of heating block 20 (the
heating or incubating then finished) and to permit rapid cooing
of the block 20 and the retained test tubes 36 with the liquid
samples 38. The tilt position is also designed to expose the
maximum top surface of the liquid test sample 36 in the tilt
position to expose the maximum surface of the test sample 38
to the air (see Pig. 4). The test apparatus optionally may
include an Eppendorf fixed pipet 40 secured by chain or cable
52 to a screw on the housing (see Fig. l; not shown in Pigs.
2-4). The control apparatus provides for automatic, preset,
preheating, cooling, incubation and fixation of test re~ults
(tilt position).
The control panel 16 of apparatus 10 is shown more
particularly in Pig. 3 and includes a start button S to start
the automatic test cycles when the test tube 36 with sample
38 is inserted in holes 26 and 28. The panel includes aligned
signal lights 44 with printed in3tructions opposite each light,
the lights operating to signal action in the test method by
the user. The panel 16 includes comparative negative and positive
color indicators 46 so the test results can be compared with
standard indicator colors. The test program can be reset by
pressing buttons I and S together. Button I controls the start
of the selected incubation period. The panel includes program
button P with a digital LED readout 48. The user sets the proper
program (time of incubation, etc.) by pushing button P to display
a number according to the number on the microbial tablet, for
example, numbers 1 to 9, with the tablet varied in concentration
and ingredients for the detection of particular nntimicrobial
drugs to a selected sensitlvity, and the proqram salected d~signed
for particular matrices, such a8 milk, serum, urine, meat and
eggs. For example, the number 5 as illustrated in display 48
is for fixed incubation time of 2:55 minutes with lower program
numbers of lesser incubation time and higher program numbers
-19- ~~t~
of greater incubation time for a variation of ~25-30 minutes
in incubation time permitted to the user by the program P button.
Fig. 6 shows the program circuitry of the test apparatus
10, while Fig. 7 shows the power supply circuitry.
The test method for the test apparatus in the determination
of 10 ppb or less of sulfa drugs in milk samples is as follows:
1. Press S and I buttons together to reset system.
Set proper program number on apparatus 10 by button
P on panel 16 according to microbial tablet label,
e.g. 5 as illustrated, incubation time 2:55 minutes.
2. Label test tube 36 (13 x 100 mm) to ldentify
test samples using marker pen.
3. Draw 0.6 ml supplied dlstilled water and di3pense
into test tube.
4. Mix milk test sample vigorously to resuspend
fat. Add 0.2 ml milk to each tube, using pipet
40 and a new pipet tip for each sample.
FIRST TIM~ US~RS: Run a negative control along with
samples until famlliar with colors. Add negative
control tablet to 0.6 ml supplied distilled water.
Using pipet and a new pipet tip, add 0.2 ml additional
supplied water.
5. Place test tube 36 in holes 26 and 28 of blocks
18 and 20 and press S button to start selected
program.
6. When first green light turns on (approx. 6 min.,
30 sec. preheat period), tilt apparatus 10 to rest
on support 24 and to place block 18 and 20 in contact
tsee Figs. 2 and 4) to cool test tube. When second
green light turns on (approx. 1 min., 30 sec.),
tilt block upright and immediately add one microbial
tablet to each test tube by pushing through blister
pack into test tube with the blunt end of A pen
or pencil. Press I button.
7. When third green light turns on (approx. 1 min,
45 sec.), tllt block down.
8. When fourth green light turns on (approx. 6
mln.), tllt block upright and immedlately add one
medlum tablet to each test tube by pushing through
bli~ter pack into test tube. Shake test tube from
side to side to disperse tablet (5 sec.). Cap test
tube and immediately tilt block down.
2~56~81
~20-
9. When red light turns on (approx. 2 hrs., 55
min. incubatlon time), test is complete. (Caution:
heating block and test tube may be hot.)
10. Observe color of sample in fluorescent light
or daylight and compare to color references to
determine positive or negative.
The temperature C versus time in minutes graphical profile
of the test sample above is illustrated in Fig. 8 (with the
additional step of heating the test sample after incubation
and observation of the test results to preserve the assay results
heat to 90C for 2 minutes). Pig. 9 shows the enlarged
temperature C versus time in minutes graphical profile of a
representative test with the time in minutes at each point shown
on the graph. This graphical profile is an enlarged portion
lS of the first part of Fig. 8 shown in dotted lines in Fig. 7
for a specific sulfa drug test.
The invention provides a unique sensitive test method
for the detection of low concentrations of antimicrobial drugs
and other antimicrobial compounds, either manually or by use
of the control apparatus iliustrated, which test method, test
compositions and test kit and control apparatus have many
advantages over the present prior art test methods and kits.