Note: Descriptions are shown in the official language in which they were submitted.
205665~
BACKGROUND OF THE INVENTION
The present invention is drawn to a method and
apparatus for treating a gas sample containing sulfur
and, more particularly, a method and apparatus for
treating a gas sample containing sulfur so as to
prohibit the degradation of the sulfur content of the
gas sample over time when stored.
One of the major concerns facing industry today
is the control of environmental pollution. One of the
most harmful pollutants present in process gases used
and/or produced by industry are sulfur compounds. The
undesirability of sulfur compounds are exhibited not
only in environmental pollution caused by burning of
gas containing sulfur but also corrosion of plant and
laboratory equipment which results from contact with
sulfur compounds.
In light of the foregoing, it is highly desirable
to be able to accurately analyze process gases and the
like and their sulfur content in order to insure
proper treatment of said gases so as to avoid the
deleterious effects attributed to the sulfur in such
process gases. To date, the sampling and storing of
sulfur gases has remained an unsolved problem. The
main reason for this is that the sulfur content of a
gas sample which is being analyzed tends to degrade
over time and, therefore, the value measured tends to
be lower than the true value of the sulfur content of
the gas actually being employed in various commercial
situations. This decomposition of sulfur has been
observed even at sulfur concentrations below 5% and
occur in all types of gases whether they be natural
gases, process gases or air.
~ .
~, .
. '~
.
2056659
The prior art has attempted to address the
foregoing problem. "Methods of Sampling and Storage
of Air Containing Vapors and Gases", Int. J. Air.
Poll., Vol. 2, pp. 142-158, 1959, quantifies several
of the difficulties encountered in the storage of
sulfur compounds including the difficulty of measuring
concentrations of sulfur compounds as compared to
various other compounds, and the fast decomposition
time of the sulfur compounds over time. While it was
found that the decomposition time of sulfur dioxide
could be extended by proper choice of storage
container, decomposition nevertheless occurs over a
rather short period of time. Thus even with a proper
choice of materials, effective long-term storage is
not possible.
In addition to the difficulties of storing sulfur
compounds, it is likewise difficult to accurately
measure the concentration of sulfur compounds in
gases. One of the most reliable alternatives
available to date for the quantitative determination
of sulfur content is the Drager method. Although the
Drager method allows measurements to be made in the
field, the method has several limitations: reduced
accuracy, the method is sensitive to only some sulfur
species, it requires a great deal of expertise by the
operator, and optical interference appears whenever
more than one sulfur compound is present. Further,
the Drager method provides no means for storage of the
gas samples.
Other techniques have also been developed for the
analysis of sulfur compound.
2056659
Kimbeli, U.S. Patent No. 3,756,781 teaches a
method of analyzing sulfur content in hydrocarbons by
first breaking the hydrocarbons down into simpler
molecular structures.
Sisti, U.S. Patent No. 4,293,308 discloses a
method and apparatus for determining very small
percentages of sulfur in gas samples.
Overall, sulfur compounds in gases are very
dif~icult to measure with time due to the breakdown of
the compounds while in storage. Yet, as the harmful
effects of these compounds appear from even very low
concentrations, it is desirable to detect
concentrations of sulfur in the parts per billion
range. There exists a need for a simple method and
apparatus to sample and store gases containing sulfur
compounds with little or no breakdown of the compounds
while in storage, so that sulfur concentrations can be
accurately measured at later times. In addition, to
be used more effectively, such an apparatus should be
portable so that it may be moved to the supply of gas.
There are many applications which require an accurate
sulfur reading at later times, and none of the prior
art suggests such a solution for this problem.
Accordingly, as aforementioned, it would be
highly desirable to provide an efficient method and
apparatus for treating sulfur containing gases so as
to prohibit the degradation of the sulfur contained in
the gases over time thereby allowing for an accurate
measurement of the actual sulfur content of the gases.
Accordingly, the present invention seeks to
provide a method and apparatus for storing sulfur
containing gases so as to prohibit the degradation of
the sulfur content of the gas over time.
3 --
2056659
In particular the present invention seeks to
provide a method as aforesaid wherein the sulfur
containing gas is pretreated prior to storage so as to
prohibit degradation of the sulfur content of same
over time.
Still further the present invention seeks to
provide a storage container which is non-reactive to
sulfur contained in a gas sample.
Still further the present invention seeks to
treat a sulfur containing gas so as to prohibit the
degradation of same over time by dehydrating the gas
and storing the gas in non-reactive containers.
Further advantages of the present invention will
appear hereinbefore.
SUMMARY OF THE INVENTION
In accordance with the present invention, the
foregoing intentions and advantages are readily
obtained.
In accordance with one aspect of the invention
there is provided a method for treating a sulfur gas
containing gas ample to be later analyzed so as to
prohibit the degradation of the sulfur gas content of
the gas sample over time comprising the steps of: a)
providing a sulfur gas containing gas sample; b)
dehydrating said gas sample so as to obtain a water
content in said gas sample of less than 100 ppm to
produce a dried gas samplei c) storing said dried gas
sample in a container which is non-reactive with the
sulfur gas in the dried gas sample; and d) thereafter
removing and analyzing said dried gas sample for
sulfur gas content.
r:
r
2056659
In accordance with another aspect of the
invention there is provided an apparatus for treating
a sulfur gas containing gas sample so as to prevent a
change in sulfur gas content over time when stored
when apparatus comprises: means for obtaining a gas
sample containing sulfur gas; conduit means for
transporting said gas sample to a dehydration zone;
means for dehydrating said gas sample in said
dehydration zone thereby producing a dried gas sample
with a water content up to 100 ppm; conduit means for
transporting said dried gas sample to a storage
containeri means for storing said dried gas sample in
said storage container wherein said container is non-
reactive with the sulfur gas in the dried gas samplei
and means for removing and analyzing said dried gas
sample for sulfur gas content.
In accordance with yet another aspect of the
invention there is provided a method for treating
sulfur containing gases so as to prohibit the
degradation of the sulfur content in these gases over
time comprising the steps of: a) providing a sulfur
containing gas sample having a known water content; b)
contacting said gas sample with a dehydrating agent in
an amount of greater than or equal to 1.50 kg of said
dehydrating agent per liter of water to be removed
from said gas sample so as to obtain a dried gas
sample; and c) storing said dried gas sample in a
container which is non-reactive to sulfur in the dried
gas sample.
2056659
The present invention is drawn to a method and
apparatus for treating and storing gas samples
containing sulfur and, more particularly, a method and
apparatus for treating a gas sample containing sulfur
wherein the degradation of the sulfur content of the
gas sample over time is prohibited.
In particular, in the method of the invention, a
gas sample containing sulfur is subjected to
dehydration so as to obtain a dried gas sample having
a water content of less than 100 ppm. In accordance
with the present invention, it has been found
especially appropriate to treat the gas sample with a
dehydrating agent in an amount of greater than or
equal to 1.5 kg of dehydrating agent per liter of
water to be removed from the gas sample. The
dehydrated dried gas sample is thereafter stored in a
container which is non-reactive to the sulfur
remaining in the gas and which is also, suitably,
water impermeable thereby prohibiting the
reintroduction of water into the stored gas sample.
~ .
... . .
2056659
Suitably, the dehydrating agent employed in the
process is selected from the group consisting of
carbon, magnesium perchlorate, glycol, silica gel,
alumina and mixtures thereof with magnesium
perchlorate being pre~erred. In accordance with a
preferred feature of the process of the present
invention, the temperature, pressure and flow rate of
the gas sample to the dehydration zone is maintained
under controlled conditions so as to maximize the
dehydrating effect of the dehydration agent on the gas
sample.
The apparatus in accordance with the present
invention suitably comprises a dehydrating zone
consisting of at least one trap containing a
dehydrating agent through which the gas sample passes.
In accordance with the preferred embodiment of the
present invention, the dehydration zone comprises a
plurality of traps which are selectively fed with the
gas sample upon sensing of saturation of the dehydrant
in any one of the traps. The apparatus of the present
invention further suitably includes the employing of
storage containers which are non-reactive to sulfur
contained in the gas sample and impermeable to water.
The most preferred storage
~ . .
205665~ 90-311
containers could be selected between aluminized plastic
bags of the type sold under the Trademark Calibrated
Systems, and Aluminium Cylinders with Spectra-Seal
treatment sold under the Trademark Airco.
The method and apparatus of the present invention
makes it possible to obtain accurate readings of the
sul~ur content o~ gas samples many days after the gas
samples are withdrawn from their source. The improved
accuracy allowed by the apparatus of the present
invention has numerous benefits including (1) quality
control in the production of natural gas, (2) accurate
guarantees of qualities and composition of the natural
gas to suppliers and purchasers, (3) reduced corrosion
in transportation pipes and facilities employing the
gases, and (4) reduction in harmful environmental
consequences resulting from the use of said sulfur
containing gases.
~3RIEF DESCRIPTION OF THE DRAWINGS
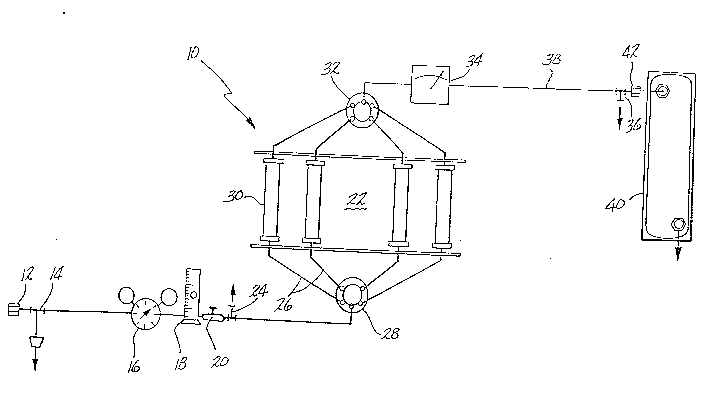
Figure 1 is a schematic illustration of the
apparatus and process of the present invention.
Figure 2 is a graph illustrating the degradation of
sulfur over time in wet and dehydrated gas samples.
Figure 3 is a graph showing the efect of various
storage containers on the degradation of sul~ur in
dehydrated gas samples.
9~-3~ 9
Figure 4 is a graph further illustrating the effect
of storage containers on the degradation of dehydrated
gas samples over time.
DETAILED DESCRIPTION
Figure 1 is a schematic illustration of the system
of the present inven~ion ~or carrying out the method for
dehydrating and storing sulfur containing gas samples
without sulfur degradation over time.
The system 10 is provided with a connection 12 for
tapping into a gas line and removing a gas sample
therefrom. The gas sample might be generated from any
source such as oil wells, environment, etc. Usually,
depending on the source, the moisture content of the
sample may be as high as 20,000 ppm water and the sulfur
content could be as low as 10 ppm. The gas is permitted
to flow through the pipe 14, a steel Teflon type pipe
preferably, to which are connected successively a
nanometer 16 for regulating the pressure, a thermometer
18 for measuring the temperature and a rotameter 20 to
control the flow rate of the gas to a dehydrating zone
22. The gas samples are preferably at a pressure
between 10 and 100 psi, temperatures no higher than 60C
and a flow rate from 0.2 to 2.0 l/min. The reason why
these parameters have to be maintained at the aforesaid
values are the following~ at pressures higher than
90-311 ~5~6~ 9
40 psi the dehydrating apparatus would need to be
reinforced; (2) at temperatures higher than 60C, the
grain shape of the dehydration agent used in the method
would deteriorate and consequently would not be
effective for dehydration purposes; and (3) at higher
flow rates, the gas sample would not have enough
residence time with the dehydration agent to effect the
required dehydration. The dehydration agent may be
selected from a group of well known substances such as
silica gel, alumina, magnesium perchlorate, carbon,
glycol, and mixtures thereof with magnesium perchlorate
being most preferred.
Pipe 14 is also provided upstream of elements 16,
18 and 20 with a drainage valve 24 which is used for
removing from the system all liquids which may be
present in the gas sample. Most natural gases contain a
liquid phase which consists basically of C6 - C14
fractions which are carried with the gas. The liquid
phase arises due to condensation which occurs by
expansion effects due to the pressure difference between
the main gas line and the system.
The valve 24 has two positions for (a~ connecting
the flow line 14 with the dehydration zone 22 or for (b)
connecting the flow line 14 with atmosphere so that the
three parameters (temperature, pressure and flow rate)
can be controlled and excess gas pressure can be
--10--
205665~
released out of the system. It must be appreciated that
the system of the invention is a dynamic system which
~eans ~he gas flows constantly through the whole
system. With the valve 24 in position (b), it is
~ossible to measure the sulfur content of the gas sample
to obtaitl a reference valve of the sulfur conten~ so
that the 6ulfur content measured after the dehydration
~nd every several days storage can be compared and the
performance of the system can be evaluated. The sulfur
content is measured in known manner by connecting to
valve 24 a DRAGER tube. With the valve 24 in position
(a~, the gas sample is allowed to reach the distribution
valve 28. Valve 28 is a single-inlet, multiple-outlet
type valve and preferably a five way type valve. The
distribution valve 28 allows the gas samples to be
selectively directed to any of a plurality of plexiglass
traps 30 which are filled with the dehydration agent to
be used in the invention for removing or reducing the
moisture content of the gas sample. Each outlet of said
distribution valve 28 is connected to one plexiglass
trap 30 by lines 26 so that only one trap 30 is in use
at any one time. Thus, the number of plexiglass traps
30 correspond to the number of outlets on distribution
valve 28.- In a preferred embodiment of this invention,
just one of the traps 30 is used for dehydrating a gas
sample flowing through it; however, if necessary, it is
* Trade Mark
~;~ -11-
"~,,.,~ .
gO-311 ~'0 ~
possible to use a plurality of plexiglass traps 30. The
feature which is critical is that the gas samples
leaving the traps 30 must have a moisture content below
100 ppm as it will be demonstrated hereinafter.
Distribu~ion valve 32 which is a multiple
inlet-single outlet type valve connects each trap 30
~ith a moisture meter 34 where ~he moisture content of
the gas sample is removed.
The saturation point of the dehydrating agent in
any one of the traps 30 can be observed at said moisture
meter 34 by measuring the moisture content of the gas
leaving the trap. When the saturation point of the
agent in a trap is approached, one turns the
distribution valve 28 in order to direct the gas flow
from trap 30 filled with the saturated dehydrating agent
to another trap 30 filled with unused dehydrating
agent. The ratio of dehydration agent to water content
in the gas sample must be maintained higher than 1.50
~y/lt in order to obtain effective dehydration within
the designed system. The valve 36 is provided
downstream of the moisture meter 34 for (a) connecting
the flow line 38 coming from the moisture meter 34 with
storage tank 40 or for (b) connecting the system with
atmosphere in order to reduce pressure in the system.
Valve 36 in position (a) controls the volume of gas
lowing into the container 40. The container 40 to be
-12-
90-311 2~6~59
used in the invention is connected to the rest of the
system by means of the air-tight connecting device 42.
The containers used in the apparatus and method of the
present invention are selected based on materials which
have no capacity for reacting with sulfur and which are
no~ water permeable so as to raise the water content of
the sulfur ~uring storage. The containers preferably
used in this invention are plastic bags with aluminized
outside and aluminum cylinders coated with plastic such
as epoxy or the like.
EXAMPLE I
In order to demonstrate the effect of water content
on the degradation of sulfur in a gas sample when
stored, a natural gas sample having a water content of
100 ppm was treated in accordance with the method of the
present invention. The natural ~as having a water
content of 100 ppm is considered to be a very dry gas
sample compared to typical natural gas samples. The gas
sample was fed at a pressure of 30 psi, a temperature of
55C and a flow rate of 1.5 liters per minute to a
dehydration zone containing magnesium perchlorate. The
magnesium perchlorate in the reaction zone was present -
in an amount sufficient to reduce the water content of
the gas sample by approximately 90~, that is, in an
amount of 1.50 kg per liter of water be removed. The
-13-
~ 90-311 ~Q~6659
sulfur content of the dehydrated dry gas sample was
measured and found to be 12 ppm.
The dry gas sample was thereafter fed to an
aluminized storage container having the interior surface
thereo~ electrolytically coated. The cylinder is a
commercially available cylinder sold by Airco. An
untreated gas sample having the water content of 100 ppm
was likewise stored in an identical storage container.
After three (3) days of storage, the sulfur content of
the gas samples was again measured and the sulfur
content of the treated dehydrated gas sample was
measured to be 11.9 ppm which is virtually identical to
that of the original dehydrated gas sample whereas the
sulfur content of the non-treated wet gas sample having
an original water concentration of 100 ppm was found to
be 1.~ ppm. This example clearly illustrates the
benefit of treating by dehydration prior to storing on
the degradation of sulfur in gas samples. The results
are graphically illustrated in Figure 2.
EXAMPLE II
Example II was carried out to demonstrate the
effect of storage containers on the degradation of
sulfur over time when stored. In this example, the gas
sample of Example I which was dehydrated was stored in
three (3) different containers. The first container is
-14-
90-311 20566~9
an aluminized plastic bag sold by Calibrated Systems.
The second sample was stored in a Teflon coated steel
cylinder. The third sample was stored in a plexiglass
syringe sold by Hamilton. As can be seen in Figure 3,
the sample stored in the Calibrated System's aluminized
bag showed less deterioration than the sample stored in
~he aluminized cylinder of Example I. The sample stored
in the Teflon coated steel cylinder was slightly
inferior to both the aluminized bag and the aluminium
cylinder. The sample stored in the plexiglass syringe
sold by Hamilton showed nearly total sulfur degradation
after a time of three (3) days. This example
illustrates that the preferred storage container for
prohibiting the degradation of sulfur in a gas sample is
the aluminized bag container sold by Calibrated Systems.
EXAMPLE III
Again, in order to demonstrate the advantages of
the process of the present invention, and particularly
the effect of dehydration on the degradation of sulfur
over time, a further test was conducted wherein a gas
sample having a sulfur content of 25 ppm was stored in
the preferred aluminized bag discussed above with
reference to E~ample II. A wet gas sample having a
water content of 15,000 ppm was likewise stored in
another aluminized bag. The sulfur content of both
~ 90-311 20~66~9
samples were measured after eight (8) days and again
after sixteen (16) days. As can be seen in Figure 4,
the sample content of the wet gas sample totally
degraded in terms of sulfur content after sixteen (16)
days as compared to the gas sample treated in accordance
with the process of the present invention which had a
reduced sulfur concentration of less than 20%.
The foregoing examples clearly demonstrate the
positive effect of the process of the present invention
on prohibiting the degradation of sulfur in a gas sample
over time when stored in optimum storage containers.
This invention may be embodied in other forms or
carried out in other ways without departing from the
spirit or essential charac~eristics thereof. The
present embodiment is therefore to be considered as in
all respects illustrative and not restrictive, the scope
of the invention being indicated by the appended claims,
and all changes which come within the meaning and range
of equivalency are intended to be embraced therein.
-16-