Note: Descriptions are shown in the official language in which they were submitted.
205697~
Improved process for the preparation of
ketone compounds
Field of the invention
,! The invention relates to an improved, large scale
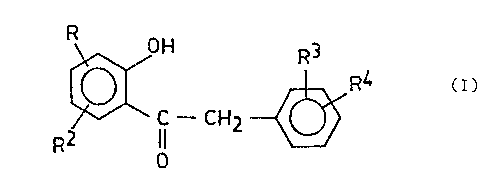
process for the preparation of ketones of formula (I)
lo ~F - C~2 ~ ( )
wherein
R is halogen atom or hydroxyl,
R is hydrogen atom or hydroxyl,
R3 and R4 are hydrogen atom or alkoxy having 1-6 carbon atoms,
and
Background of the invention
It is known that the ketones of formula (I) can be
used as intermediates for the preparation of isoflavone
69514-77 OE/Hoj
20~97~
--2--
derivatives (see e.g. HU PS No. 163,515) as well as
anabolics since they effect the metabolism.
Out of industrial point of view those processes are
- the most advantageous wherein resorcinol is used as
starting material, e.g. the desired product may be
obtained according to the Houben-Hoesch reaction, wherein
the resorcinol is reacted in anhydrous medium with benzyl
cyanide in the presence of dry hydrogen chloride gas and
anhydrous tin chloride (see e.g. J. Chem. Soc. /1923/,
404 and J. Am. Chem. Soc. 48, /1926/, 791). The yield in
this reaction is 50% and the drawback of this process is
that the hydrolysis of the "ketimine" derivate intermed-
iate is a very corrosive procedure.
Alternatively 2-hydroxy-4-n-butoxy-phenyl-benzyl
ketone or 4-hydroxy-2-n-butoxy-phenyl-benzyl ketone may
be obtained when reacting the mono-n-butyl ether of
resorcinol with phenyl-acetyl- chloride in the presence
of pyridine, then removing pyridine by distillation,
dissolving the residue in ether, extracting the solution
with hydrogen chloride several times, removing the ether
by distillation, thereafter treating the l-phenyl-acetyl-
oxy-4-n-butyloxy-phenol thus obtained in nitrobenzene
with aluminium chloride and steam distilling the mixture
thus obtained (see Example 7 of HU PS No. 168,744). The
starting material of the first step, i.e. the mono-n-
-butyl ether of resorcinol, can be obtained e.g. when
reacting resorcinol with n-butyl bromide in the presence
of dimethyl formamide. Regarding that from resorcinol
-3- 23~97~
diether derivatives may also be formed, in order to
obtain an end product of good quality the monoethers have
to be purified before the second reaction step.
- The analogous phenol compound can be prepared by the
known, so called Bouveault reaction too, wherein 2 moles
of anhyrous aluminium chloride are reacted with phenol.
In the first step of this reaction phenoxy-aluminium di-
chloride forms while hydrogen chloride gas is released.
In the second reaction step said phenoxy-aluminium di-
chloride is then reacted with the acid chloridederivative in the presence of a further mole of aluminium
chloride (Olah, Gy:Friedel Crafts and related reactions,
Vol. I, page 97, 1963).
The drawbacks of these known processes are as
follows:
- the reaction procedure itself and the technology
too, are rather difficult,
- large amount of aluminium chloride (2 moles) is
required,
- the released hydrogen chloride is corrosive.
Summary of the invention
The present invention relates to a process for the
preparation of ketones of formula (I) and salts thereof
205697~
,~f--CH2 ~S
wherein R, R , R , R , R are the same as mentioned above
wherein phenols of formula (II)
R~OH
~ (II)
are reacted with acid chlorldes of the formula (IV)
R3
~ ~ .CH2COCt (IV)
such as phenyl acetyl chloride, in the presence of inert,
anhydrous organic solvent and anhydrous aluminium chloride by
method known per se, the mixture thus obtained is decomposed
with an aqueous acid solution and the phases obtained are
separated, characterized by reacting said phenol derivative
with 1 mole aluminium chloride - calculated for the phenol
derivative - at a temperature between 0~C and 45~C, preferably
;
~ 23305-1198
2056979
,.
between 0~C and 40~C in the presence of halogenated
hydrocarbon, preferably dichloro ethane, thereafter reacting
the complex of the formula (III) thus obtained
R
~ ~ICI~ (III)
_
with an acid chloride of the formula (IV)
~3
R~ ~ CH2COCI (IV)
preferably in the presence of the solvent used prevlously at a
temperature ranging from 10~C to 60~C, preferably 20~C to 50~C
thereafter adding an aqueous acid solution to the mixture thus
obtained, separating the phases and recovering the desired
ketone compound from the organic layer.
Detailed descriPtion of the invention
We have surprisingly recognized that when reacting
e.g. resorcinol with 1 mole of anhydrous aluminium chloride,
-~~ 23305-1198
2(j~979
--6--
without hydrogen chloride formation a hydrogen-aluminium-
-trichloro-3-hydroxy phenolate (further "complex") is
obtained, which dissolves in the used reaction medium.
This complex is very active and it is able to react with
the acide chloride without adding additional aluminium
chloride.
The presently claimed process is based on the above
recognition and phenol derivatives of formula (II) are
used as starting material.
In the process according to the invention halogenat-
ed hydrocarbons, preferably dichloro ethane, are used as
solvent in 3-10-fold excess. The reaction temperature
depends on the used starting material, in case of
resorcinol and dichloro ethane the reaction is preferably
carried out at a temperature of 10~ to 25~C.
The reaction of the complex with the acid chloride
can be carried out by adding the aromatic acid chloride
or the solution thereof to the solution or to the
suspension of the complex or alternatively the solution
or suspension of the complex is added to the acid
chloride or to the solution of same.
In a preferred embodiment of the process according
to the invention the preparation of the complex and the
subsequent reaction steps are carried out in the same
aprotic solvent, preferably in halogenated hydrocarbons.
Another important recognition of the present inven-
tion enables the pure isolation of the desired product.
We have found that the ketones of the formula (I),
_7_ 2056q7q
which are obtained from the resorcinol derivatives of the
formula (V)
R6
~ (V)
OH
- wherein R6 is hydrogen atom or hydroxyl -
Gan react with potassium carbonate to form the double
salts of formula (VI)
KO
\~ OH R
R~ C--CH2~ ~ KHC03
- ~herein R3, R4 and R6 are the same as mentioned above -
which are not soluble in aprotic solvents. So, accord-
ing to a preferred embodiment of the process according to
the present invention, the product is isolated (e.g.
separated by filtration) in the form of this salt and so
can selectively be separated from the side products or
other accompanying materials being present or are formed
in the reaction mixture. The ketone of formula (I) can be
obtained from the double salt of formula (VI) after
dissolving it in water or aqueous alcohol and acidlfying the solution thus
23305-1198
8 20~9~
obtained to pH=3.5-4.5.
The above mentioned step is especially preferred if
the starting resorcinol or acide chloride are not
sufficiently pure. When pure starting material is used,
ketones of appropriate quality can be obtained by the
optional removal of the solvent of the organic layer and
by recrystallization, preferably from toluene, of the
residue.
The advantages of the present invention are e.g. as
follows:
- the synthesis can be carried out without the separat-
ion of the intermediates, especially without the
preparation of the mono-n-butyl ether of resorcinol
and without the use of nitromethane, nitrobenzene or
ether solvents,
- the amount of the aluminium chloride is decreased to
the half of the amount used in the known processes,
- the considerable corrosion of the Houben-Hoesch
method can be eliminated,
- the yield amounts to 82-85%, which is substantially
higher than that of any known method,
- the quality of the product is very good.
The process according to the invention is illustrat-
ed in detail by the following Examples.
Example 1
55 9 (0.5 mole) of resorcinol were suspended in 250
ml of dichloro ethane and at 20~C 67 9 (0.502 mole) of
_9_ 2~97~
anhydrous aluminium chloride were added. To the
obtained homogeneous dark solution, containing the hydro-
gen-aluminium-trichloro-3-hydroxy-phenolate, 77.2 9 (0.5
mole) of phenyl-acetyl chloride in 100 ml of dichloro
ethane were added over one hour while the temperature
raised to 35-40~C. The reaction mixture was stirred for
one hour, the solution thus obtained was added to an
aqueous hydrochloric acid solution, the two layers were
separated, the organic layer was washed with water to
neutral, the solvent was distilled off and the residue
was optionally crystallized from toluene. 96.9 9 of 2,4-
dihydroxy-phenyl-benzyl--ketone were obtained, m.p.: 112-
114~C, yield: 85%. After an optional recrystallization
from toluene the melting point was 113-114~C. Elemental
analysis f~rC14H12~3 ~w: 228):
Calculated: C%: 73.68, H%: 5.26
Found: C%: 73.6, H%: 5.3
NMR spectrum (Bruker WP 80 spectrometer, in DMS0-D6
solvent, TMS internal standard):
lH
6 C-H 7.90 ppm /d/ 3J-9Hz
5 C-H 6.37 ppm /dd/
3 C-H 6.25 ppm /d/ 4J-2Hz
13c
4-C 165.1 ppm
20~97~3
-10 -
Example 2
The procedure described in Example 1 was followed.
~ After separating the two phase mixture, the organic layer
was washed to neutral with water, the dichloro ethane
layer was separated and 69 9 (0.5 mole) of anhydrous
potassium carbonate were added. From the reaction
mixture the precipitated 2,4-dihydroxy-phenyl-benzyl-
-ketone-potassium-potassium hydrogencarbonate double salt
(C14H1103K.KHC03) was separated by filtration (166 g))was
dissolved in methanol:water=1:3 and the solution thus
obtained was acidified (pH=4) with 33% acetic acid. The
precipitated product was filtered and after drying 96.2 9
of 2,4-dihydroxy-phenyl-benzyl-ketone were obtained, m.p.
113-114C.The quality of the product thus obtained was
identical with the product of Example 1 obtained after
recrystallization. The melting point of a mixture (1:1)
did not show depression.
Ele ental analysis for C14H1103K.KHC03 ~ 366):
Calculated: C%: 49.18, H%: 3.27,
Found: C%: 49.6 H%: 3.32
NMR spectrum:
lH
6 C-H 7.63 ppm /d/ 3J=9Hz
5 C-H 6.00 ppm /dd/
3 C-H 5.78 ppm /d/ 4J=2Hz
-1 1 - 2 ~
-
4-C 174.2 ppm
(The potassium salt in the double salt of 2,4-
- -dihydroxy-phenyl-benzyl ketone appears in the 4-
-position).
Example 3
64.25 9 (0.5 mole) of 2-chlorophenol were dissolved
in 200 ml of dichloro ethane and 67 9 (0.5 mole) of
anhydrous aluminium chloride were added to the solution.
Thereafter 77.2 9 (0.5 mole) of phenyl-acetyl chloride in
100 ml of dichloro ethane were added over 1 hour under
stirring while the reaction temperature raised from 15-
-20~C to 35-40~C. After a one-hour stirring the reaction
mixture was admixed with aqueous hydrogen chloride, the
two-phase mixture was separated, the organic layer was
washed with water to neutral and the solvent was removed.
106.1 9 of 2-hydroxy-3-chloro-phenyl-benzyl-ketone were
obtained, m.p.: 62-64~C. After a recrytallization from
aqueous isopropanol (1:2), m.p. 63-67~C.
Elemental analysis for C14H11C102 ~w:246.5):
Calculated: C%: 68.15, H%: 4.46, C1%: 14.40
Found: C%: 68.55, H%: 4.70, C1%: 14.00
Example 4
22 9 (0.2 mole) of hydroquinone were dissolved in 60
ml of dichloro ethane and 26.8 9 (0.2 mole) of anhydrous
aluminium chloride were added to the solution. To the
obtained complex 30.8 9 (0.2 mole) of phenyl-acetyl
~ -12- 20~7~
chloride in 30 ml of dichloro ethane were added. Further
the procedure of Example 1 was followed. lO.1 9 of 2,5-
~ -dihydroxy-phenyl-benzyl-ketone were obtained, m.p.: 118-
-120~C.
Elemental analysis for C14H12C3 ~w: 228):
Calculated: C%: 73.6B, H%: 5.26
Found: C%: 73.62, H%: 5.58
Exapmle 5
24.7 9 (0.196 mole) of floroglucinol were dissolved
in 70 ml of dichloro ethane and 26.6 9 (0.2 mole) of
anhydrous aluminium chloride were added to the solution.
To the obtained complex 30.1 9 (0.196 mole) phenyl-
-acetyl-chloride in 30 ml of dichloro ethane were added.
Further the procedure of Example 1 was followed.
15 9 of 2,4,6-trihydroxy-phenyl-benzyl-ketone were
obtained, m.p.: 117-120~C.
Elemental analysis for Cl4H1204 (Mw: 244):
Calculated: C%: 6a.85, H%:4.92
Found: C%: 69.05, H%: 4.67
Example 6
120 kg (1.09 kmole) of resorcinol were suspended in 660
1 of dichloro ethane and 150 kg (1.12 kmole) of anhydrous
aluminium chloride were added to the suspension while the
temperature raised from 15~C to 25~C. The complex
obtained dissolved in the reaction medium. 171 kg (1.10
kmole) of phenyl-acetyl chloride were added over a period of
-13- ~6979
one hour while the temperature raised to 35-40~C. The
mixture was stirred for one hour thereafter it was
- admixed with diluted hydrogen chloride (the mixture of
300 1 of hydrogen chloride and 600 1 of water), and it
was treated as described in the preceding Examples. The
solvent was removed by distillation, the residue was re-
crytallized from toluene, the product obtained was cent-
rifuged and dried at 45-50~C. 205-210 kg of 2,4-
-dihydroxy-phenyl-benzyl-ketone were obtained, yield 82-
-84.5%. Calculated amount: 248.5 kg. The physical data
are identical with the data given in Example 1.
Example 7
27.5 9 (0.25 mole) of resorcinol were suspended in
150 ml of dichloro ethane and 33.5 9 (0.25 mole) of
anhydrous aluminium chloride were added. To the
solution containing the formed complex 42.9 9 (0.2 mole)
crude 3,4-dimethyl-phenyl-acetyl-chloride in 50 ml of
dichloro ethane were added and was stirred for 4 hours.
Thereafter the complex was decomposed by adding 1:1
aqueous hydrogen chloride, the dichloro ethane solution
containing the desired product was washed with water, the
solvent was removed and the residue was recrystallized
from toluene. 45.9 9 product were obtained, m.p.: 171-
-173~C, yield 79.8%. Calculated amount: 57.6 9.
Elemental analysis for C16H1605 ~w: 288):
Calculated: C%: 66.66, H%: 4.17
Fcund: C%: 66.45, H%: 4.10
-14- 2Q~7~
The NMR spectrum proved the desired compound.
TLC:
Developing system: toluene/n-butyl acetate/acetic
acid=8/2/1
Adsorbent: Kieselgel 60 F254 (Merck)
Application: 0.2 9/10 ml dimethyl formamide-lOO~ug
Front: 16 cm
Development in UV-light, 254 nm
Rf~J 0.6
Example 8
27.5 9 (0.25 mole) resorcinol were suspended in 150
ml of dichloro ethane and 33.5 9 (0.25 mole) of anhydrous
aluminium chloride were added to it. To the solution
containing the obtained "complex" 48.5 9 (0.2 mole) of
3,4-diethoxy-phenyl-benzyl-acetyl chloride in 5û ml of
dichloro ethane were added. Therafter the procedure
described in Example 7 was followed. 53.7 9 of 2,4-di-
hydroxy-3',4'-diethoxy-phenyl-benzyl-ketone were obtained
after a recrystallization from toluene, m.p.: 141-143~C.
Theoretical amount: 63.2 9. Yield 85%.
Elemental analysis for C18H2005:
Calculated: C%: 68.55, H%: 6.23
Found: C%: 68.35, H%: 6.29
The NMR data corresponds to the desired product.
TLC: (carried out as described in Example 7): Rf~ 0.7