Note: Descriptions are shown in the official language in which they were submitted.
2~ f372
W-22
EO BIOLOGICAL T~ST PACR
BAC~GRO~ND_OF T~E INVENTION
FI~D O~ T~E INV~NTION
The present inventlon relate to a biological test
pack of the type used in hospitAl sterilization procedures.
In particular, the Lnvention relates to a biological test pack
o~ the type used in ethylen~ oxide (EO) sterilization
procedures.
~ ESCRIPTION OF THE P~EQR A~
Ethylene oxide gas is commonly us~d in sterilizing
_tems for use in health care facilities. A common method of
testing for the ef~icacy o~ the EO sterilization proces3 is
to includa a biological indica~or in the load being
steri~iz2d. A biologicz~l indicator i~ a suspQnsion of a large
number of bacterial spore~ that has been dried on a carrier,
e.g., paper, and then inserted into a package, such a~ a
glassine envelope or a plastic vial. The spore suspension is
typically comprised of a bacterial species that is very
resistant to EO. For example, Bacillu~ subtilis may be used,
20~7372
and it may be present on the carrier in large numbers.
Accord mgly, the spore suspension acts as an indicator for the
effectiveness of the EO sterilization process. If large
numbers of a very EO resistant organism are placed in a load,
and i~ the sterilizatlon process kills those resistant
spores, then it is reasonable to conclude that the EO
sterilization process was ef~ective.
Since most items that are beLng EO sterilized are
held in some sort of packaging whlch is intended to maintain
the sterility of the contents of the pack until the time of
their use, it is prudent to enclose the biological Lndicator
inside similar packaging in order to egualize the challenge of
k ~ ing the spores on the biological indicator.
In order to standardize the packaging challenge,
the Association ~or the Advancement o~ Medical Instrumentation
(AAMI) ha~ issued an AA~I ~ecommended Practice entitled "Good
Hospital Practice: Per~ormance Evaluation oS Ethylene Oxide
Sterilizer3 -- Ethylena Oxlde TQst Packs". That document
recommends the use of a standardized routlne test pack for
general purpose EO sterllizers in which the biological
indicator as well as a chemical indlcator can be positioned,
and also the use o~ a more resistant challenge pack for newly
in~talled EO sterilizer~. The recommended standard challenge
test packs consist o~ a plastic syringe enclosing a biological
indicator, the syringe being wrapped in a properly-sized
2Q~7~2
cotton towel, and this entire assembly enclosed inside a
wrapping or pouch.
The makmg of an EO test pack is often a tedious
process, as the test packs are laborious to construct, and not
all of the necessary materials and components may be present
at the in~tltution. In addition, there is no standardized
method of manuf acturing or selecting the components.
Compounding this problem is the fact that the surgical
(huckaback) towel component of the test pack, which acts as a
heat sink and moisture absorber in the te~t pack, ls
inherently susc~ptible to variation, because surgical towels
are subject to large changes in their characteristics. For
example, after each succQ~sive laundering, a surgical towel
108e8 some of its flber content and thus its capacity to
provide a heat and moisture challenge. I~ it is laundQred and
then ironed, the towel may be so dry that it proYides too much
o~ a challenge to moistuxe absorption.
In order t:o minimiz~ the above prGblems, an
improved E0 mdicator tQst pack haa b~n developed and is
dascribed in U.S. Patent 4,828,797 (Zwarun et al.), as~igned
to the assignea hereof. This patQnt is incorporatad by
re~erenca herQin and disclos~ a test pack which yields the
same rate of survlval or death of a biological indlcator as
doQs the AAMI-described pack, but which is fully disposable.
This test pack is of standardized construction and eliminates
variation~ from pack to pack by being preloaded with an
appropriate biological indicator to eliminate labor required
for a~sembly. The pack behaves in a manner which is
equivalent to the biological indicator tes~ pack recommended
by AAMI for routme use in general purpose E0 sterilizers.
While the aforementioned te~t pack constitutes a
signiflcant advance over the prior art, some improvements have
been discovered. For example, the volume of materials used in
this test pack required that the pack be fairly large to
accommodate the quantity of blotter paper necessary to absorb
the humidity and ethylene oxide to a sufficient degree to
create a challenge equal to a huckaback towel. It has been
found that by decreasing the size o~ the tray holding the test
pack components wh~le substltuting an alternative absorptive
material for the blotter paper an equivalent amount of
absorption of humidity and ethylene oxide could be achieved
al~o while providing a tortuou~ path. D~pendiny upon the
ef~iciency o~ the alternative absorptive material, the entire
test pack could be made in a range of selected smaller size~
because tha relative ratio o~ tho volume of blotter paper to
the total volume Or th~ original tr~y could be made equal to
the ratio of the volu~le of the altorn~tlv~ absorptive materlal
to the volu~o of the enclo ur~ containing this material.
In the previous test pack, the syringe was situated
within a tray which also contained tha blotter paper and wa~
covered by a permeable membrane. The relationship between the
tray volum2 and the syringe and the mass and volume of the
2~7~7~
blotter paper in the tray created the appropriate challenge
that was equivalent to the AAMI described test pack. In this
prior art device the volume of the syringe itself was not
critical in relation to the volume of the tray. In the
present invention, the volume of the tray is st ~ irrelevant
so long aR there i5 no impediment to the transfer of ethylene
oxide through the tray cover. However, it has been found that
an absorptive material, when inserted into the chamber of the
standard syringe maintains the same relationship to the
relevant volume in~ide the syringe as did the blotter paper to
the relevant volume Ln~ide the prior art tray. That is, the
absorptivQ material used in the present Lnvention i5 about 97%
smaller in volume than the previous blotter paper and is
placed inside the syringe, the volum~ of which i~ about 96%
smaller than the volume of th~ tray. The absorption of
humidity achieved by this amount o~ absorptive material i5
esssntlally the same a~ th~ ab~orption achieved by the blotter
paper u~ed in the previous ver~ion (i.Q. about ~-10% by weight
of the absorptivR material). In ef~ect, the invention enables
a much smaller volume to ~e used to produce the equivalent
challenge.
It is accordingly an ob~ect of this invention to
produce a standard ethylene oxlde te~t pack in smaller sizea
than tho e previously available.
- 5 -
2 ~ 7 2
It is yet another object of this invention to
produce a standard ethylene oxide test pack able to achieve
the results of previously known te~t packs while being
constructed m a more simplified manner.
SIJMXARY OF T~l: INVENTION
These and other objects of the invention are
achieved by the preferred embodiment thereof which is a
standard, disposable ethylene oxide biological test pack
comprising: (a) a clear, colorles~ plastic tray including
syringe holding means molded therein for holding a syringe
containing a biological indicator: (b) indicator card holding
mean associated with said tray for holding an indicator card
ad~acen~ to tha bottom of said tray in a po6ition in which
said Lndicator card is raised away from po~ible condensate
and is vi6ible fro~ outside o~ said tray; (c) a syringe
containing a biological indicator of the type Lncluding a
glassine enclosed or a sel~-contained biological indicator,
~aid syrinqe b~ing held in place wlthin said tray by said
syringe holding m~ans; (d) an indicator card having a
sterilization s~nsitiv~ mk i~printed thereon, said indicator
card bemg held m sald tray by said indicator card holding
mQans with said ink imprint ~acing out of said tray; and (e)
an absorb0nt member situated within said syringe ad~acent the
opening thereof, whereby tha combination creates a
2~372
rate-limiting tortuous opening; and (f) means for sealing the
top of said tray until it is ready for use.
BRIEF DESCRIPTION_OF T~g DRA~INGS
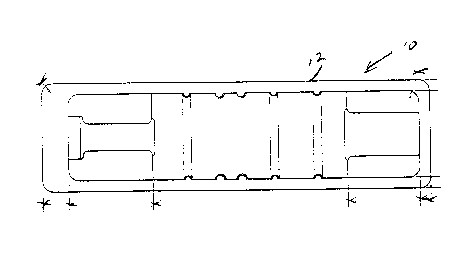
Figure 1 is a top view of the tray of the test pack
of the present invention;
Flgure 2 is a side view of the syringe included in
the test pack of the present invention: and
Flgure 3 is a sida ViQW of the test pack of Figure
1 including the syrmge of Flgure 2.
~SC~Ip~E~ON OF T~E P~F~ED ~O~I~ENT
R~ferrmg generally to Figures 1-3, the test pack
10 of the present invention is constructed of a clear plastic
blister tray 12 which i~ s~aled with a peel off lid 14,
compri~ed of Tyvek in the preferred embodlment of the
invention. Alternati.vely, a paper lld can be used. The
structure o~ the invention i3 sim~lar in many respects to the
test pack of aforementioned U.S. Patent 4,828,797. This
patent i5 incorporat:ed by re~erence to disclose various
structural features not specifically mentioned herein~
In~ide the blister tray 12 is a indicator caxd 16
that is placed on raised bumps 18 on the bottom o~ the blister
tray 12 in order to prevent any potential condensate from
- 7 -
3 7 2
damaging the indicator card 162 The indicator card 16 is
printed with an E0-sensitive Lnk so it serves as both an
internal indicator of the sterilization process and as a
record keeping card on which the results of the biological
indicator test can be recorded. The m dicatsr card 16 can
then be stored with a record keeping system a~ter it has been
exposed.
Above the indicator card 16 and resting in a
premolded seat in tray 12 i9 the plastic syringe 22 that
contains the biological indicator 20, which may be an
envelope-enclosed spore strip or a self-contained biological
lndicator of the type known in thh art or of the type refsrred
to in a~orementioned U.S. Patent No. 4,R28,797.
Raplacing the absorbQnt paper of the test pac~
shown in aforementloned U.S. Patent No. 4,828,797 is a~sorbent
plug 30 which is positloned within syringe 22 adjacent the
s~all opening 32 in the end of th~ syringe. It w ~ be
understood that the ratio of the volume of absorbent plug 30
to the volume of the interior o~ syringe 22 is comparable to
the ratlo o~ the volumQ o~ the absorbQnt paper of the test
pack of U.S. Patent: No. 4,828,797 to the volume of the
interior of the tray o~ that te~t pack. The partlcular type
of absorptive material cho~en for use in the invention must
have characteristic~ such that it i5 able to function withm
the lesser volume of tray 12 to perform a function similar to
that of the prior art test pack. Any suitable absorptiv~
- 8 -
~737 ~
materi~l may be used provided its efficiency per unit w2ight
is, in the new, smaller tray, equivalent to that of the
blotter paper used in the previous pack. For example, the
amount of blotter paper necessary to achieve th~ same function
if placed within the volume of the syringe would not leave
enough room in ~he syringe for the biological test pack
indicator. The particular absorptive material used in the
preferred embodiment is a closed cell reticulated (sponge)
foam known as Prestofoam #725 available from the Presto
Manufacturing Co., 2 FranklLn Avenue, Brooklyn, New York.
The new te~t pack is smaller t~an previous devices
and consequently, more inexpensive, more e~icient because it
reduces wa~te (due to th~ nall~r volume of material to be
discarded) and requires less storage space (hecause its volum~
18 slgni~icantly s~allar than the pr~vious pack).
The AAMI standard~ requlre that a biological
indicator bQ placed inside a syri~g~. However, depending upon
the blologlcal indlcator di~erent size~ of syringes may be
used. In tha preferred embodiment shown in the drawings, the
type o~ biological indicator used raquires tha use o~ a 20cc
(internal volume) syringe.
Wh1le a syr~nge is not abcolutely n~cessary to the
Lnvention, it is an acceptable easily recognized structure in
the marketplace. It is certainly possible to create a
dlfferently shaped structure to replacQ the standard syringe
and yet maintain the AA~I challenge.
~737 ~
It w;ll be undorstood by thos~ sk ~ ed in the art
that numerous other modifications and improvements may be made
to the preferred embodiment of ~he invention disclosed herein
without departing from the spirit and scope thereof.
-- 10 -