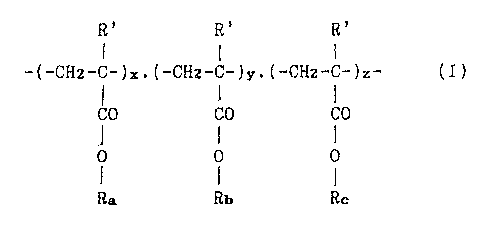Note: Descriptions are shown in the official language in which they were submitted.
2~~~~1
- 1 -
MULTIFUNCTIONAL ADDITIVE FOR LUBRICATING OILS
This invention relates to a lubricating oil viscosity index
improver (V.I.I) with dispersant and antioxidant properties.
It is known in the art to add to lubricating oils an oil-soluble
polymer (V.I.I) able to improve their rheological behaviour on
temperature variation, such as a polymer or copolymer of an
acrylic or methacrylic alkyl ester containing a number of carbon
atoms in the alkyl group such as to make it oil-soluble.
It is also known beneficially in the art to introduce into said
oil-soluble polymer a copolymerizable monomer containing nitrogen
to give the resultant product dispersion characteristics in
addition to viscosity index improvement. Said copolymerizable
monomer containing nitrogen, also known as a dispersant monomer,
is generally chosen from vinylimidazoles, vinyl pyrrolidones,
vinylpyridines and N,N-dialkyl-aminoethyl-methacrylates.
This practice is described for example in British patents
1,272,161 and 1,333,733, USA patent 3,732,334 and Belgian patent
874,068.
V.I.I copolymers with simultaneous dispersant and antioxidant
characteristics are also known, for use in internal combustion
engines to reduce sludge formation and reduce lubricating oil
- 2 -
oxidation during engine operation.
For example, U5A patent 4,699,723 describes ethylene-propylene
copolymers grafted with monomers containing a nitrogen atom and a
sulphur atom, such as 4-methyl-5-vinylthiazole, possessing
dispersant and antioxidant characteristics in addition to
viscosity index improvement.
The use of these polymers, normally defined as multifunctional,
also improves the performance of specific additives contained in
the lubricating oil (for example antiwear additives such as zinc
dithiophosphates, dispersants such as polyisobut enyl succinimides,
detergents such as calcium sulphonates, antioxidants such as
sterically hindered phenols, etc.) and possibly reduces their
required quantity.
The present invention provides sulphur-Free polymeric additives
for lubricating oils which effectively improve their viscosity
index while possessing dispersant and antioxidant properties.
A simple and convenient method has also been found for preparing
said additives.
In accordance therewith, the present invention firstly provides a
polymeric viscosity index improvement additive with dispersant and
antioxidant properties of general formula:
R' R' R'
I I I
-(-CHz-C-)s.(-CH2-C-)y.(-CHz-C-)z- (T)
I
CO CO CO
f i I
0 0 0
Ra Rb R
derived from copolymerization of unsaturated esters, said general
- 3 -
formula (I) representing the type and quantity of reactive
monomers but not their arrangement within the final polymer chain,
where
- R', which can be identical or different, are hydrogen atoms or
alkyl radicals;
- Ra is an alkyl radical or a mixture of linear or branched
alkyl radicals with between 6 and 25 carbon atoms;
- Rb is an alkyl radical with between 1 and 4 carbon atoms or is
identical to Ra;
- Rc consists of one or more linear, branched or cyclic alkyl
radicals with 1 or 2 nitrogen atoms and between 4 and 20 carbon
atoms;
- x, y and z represent the weight percentages of the various
polymerizable monomer units, x being between 80 and 95%, y being
between 0 and 12% and 2 being between 2 and 8%.
According to a preferred embodiment of the present invention:
- x is between 85 and 90%, y is between 3 and 7% and z is
between 4 and 6% by weight;
- R' is a methyl radical, all the polyroerizable esters
represented by formula (I) therefore being methacrylates;
- Ra represents alkyl groups derived from mixtures of natural or
synthetic linear or branched primary alcohols with between 10 and
20 carbon atoms;
- Rb is a methyl radical;
- Ro is the radical of 2,2,6,6-tetramethyl-piperidin-~-ol, the
polymerizable monomer CHz=CR'-COORS therefore being 2,2,6,6-
tetramethyl-piperidin-4-of methac.rylate.
In a further preferred embodiment of the present invention the Ro
methacrylate is a mixture of:
- 50-85 wt% of 2,2,6,6-tetramethyl-piperidin-4-of methacrylate,
- 15-50 wt% of N,N-dimethyl-aminoethanol or N(3-hydroxypropyl)
N'-methyl-piperazine methacrylate or a mixture thereof.
The present invention further provides a process for preparing the
multifunctional additive of general formula (I). The process
consists of copolymerizing a mixture of Ra, Rm and Rc
(meth)acrylates, the Ra (meth)acrylate being present to the extent
of 80-95%, the Rb (meth)acrylate to the extent of 0-12% and the Ro
(meth)acrylate to the extent of 2-8% by weight.
In the preferred embodiment of the present invention the
copolymerized mixtures consist of:
- 85-90 wt% of Rn (meth)acrylates, ie (meth)acrylic esters of
mixtures of natural or synthetic linear or branched primary
alcohols with between 10 and 20 carbon atoms,
- 3-7 wt% of Rb (meth)acrylates, ie (meth)acrylic esters of
methanol,
- R~ (meth)acrylates, ie (meth)acrylic esters of 2,2,6,6 -tetramethyl-
-piperidin--4-ol.or 50-85 ~xt% of 2,2,6,6-tetramethyl-piperidin-4-of
methacrylate, the remaining 15-50 wt~ consisting of N,N-dimethyl-
aminoethanol (meth)acrylate or N-(3-hydroxypropyl)-N'-
methylpiperazine, or a mixture thereof.
The total of Rc (meth)acrylates is 4-6 wt% of the overall
(meth)acrylate mixture.
The present invention also provides a new acrylate or methyl
acrylate of general formula:
- 5 -
CFIz-CHz
CHz=CR'-C00-CHz-CHz-CH2-N N-CHI {II)
\ /
CHz-CHz
where R' is hydrogen or methyl, which pertains to the R~
(meth)aerylate class and is hence useful as a dispersant action
monomer when copolymerized with other acrylates or alkyl
acrylates, it being prepared by usual organic chemistry reactions.
To effect polymerization, these monomers are degassed, either
separately or together, then mixed and diluted with an inert
organic solvent, preferably mineral oil (such as Solvent Neutral
5.4 cSt at 100°C). The reaction mixture is then heated in the
absence of oxygen to a temperature of 70-130°C, in the presence of
a radical initiator' (added either before or after heating), until
60-100 of the (meth)acrylic esters have been transformed into the
relative copolymer. Radical catalysts suitable for this purpose
are generally chosen from tert-butyl-peroctoate, tert-butyl-per{2-
ethyl) hexanoate, tert-butyl-per-isononanoate, tert-butyl-
perbenzoate, azo-bis-isobutyronitrile, dibenzoylperoxide, di-
lauroylperoxide and bis(4-teat-butylcyclohexyl) peroxydicarbonate,
ZO and are used in a quantity of between 0.2 and 3 parts by weight
per 100 parts of methacrylic esters.
Sulphurated substances such as aliphatic mercaptans, thioglycols
and thiophenols (such as tert-dodecylmercaptan and ethanedithiol)
may be present in the reaction mixture, their purpose being to
regulate the molecular weight of the copolymer. These sulphurated
substances generally exhibit their activity when present in a
quantity of between 0.01 and 0.5 parts by weight per 100 parts by
- 6 -
weight of the (meth)acrylic esters. The progress of the reaction
can be monitored by infrared analysis. The monomer conversion
generally reaches the stated value within a time of between 0.5
and 4 hours for the aforestated temperatures and other conditions.
In this manner a solution of the additive of general formula (I)
in an inert solvent is obtained. The copolymer may be isolated as
such by removing the solvent by known methods (such as under
reduced pressure).
The additive can be added as such to the lubricating oil, but its
addition is preferably facilitated by using a concentrate
containing 25-95% by weight, and preferably 40-70%, of the
additive dissolved in a solvent-diluent, which i.n a preferred
embodiment of the present invention can be t he same mineral oil as
that used as the inert solvent for preparing the additive of
formula (I).
The present invention also provides a lubricating oil composition
containing mainly lubricating oil plus a quantity of the described
additive effective as a V.I.I., dispersant and antioxidant.
This effective quantity is generally between 0.5 and 10%, and
preferably between 1.2 and 6% by weight, with respect to the
polymer as such. The additive of -the present invention can be
used in finished lubricants (for example for automotive use) in
combination with other usual additives such as dispersants,
detergents, antiwear agents, antioxidants etc. The following
examples are given to illustrate the present invention.
EXAMPLE 1
148 g of SN 150 mineral oil, 130.31 g of Ciz-Cm linear
_ 7 _
methacrylic alcohol monomers and 1.7 g of 2,2,6,6-tetramethyl-
piperidin-4-of methacrylate are fed into a reactor with a
diathermic oil heating jacket and fitted with an anchor stirrer, a
thermocouple for temperature measurement and a nitrogen injector,
and the system is left stirring for one hour while injecting
nitrogen. While the reaction is proceeding, 5 g of N-(3-
hydroxypropyl)-N'-methyl-piperazine methacrylate, 15 g of methyl
methacrylate and 0.9 ~ of tert-butylperoctoate (TBPO) as
polymerization initiator are degassed separately. The degassed
monomers are then added to the reactor, its temperature raised to
I00°C and the initiator added. Polymerization commences
immediately and .is strongly exothermic, the temperature control
system therefore being set to maintain this temperature constant
until the reaction is complete (2-3 hours). The progress of the
reaction is followed by I.R. analysis, the progressive
disappearance of the bands relative to the double methacrylic
monomer bond at 1320-1340 cm-1 being noted.
The final solution of the polymer in SN 150 has a kinematic
viscosity of 783.83 cSt at 10fl°C.
Evaluation of the additive as a viscosity index imnrover
Kinematic viscosity of a 10% solution in SN 150 at 100°C: 14.77
cSt.
Kinematic viscosity of a 10~ solution in SN 150 at 40°C: 84.74
cSt.
Kinematic viscosity of a I0~ solution in SN 150 at -20°C: 2900
cP.
Viscosity index: 184.
_g_
Evaluation of disoersant Qroperties
The dispersant properties of the additive are evaluated by the so-
called asphaltene test.
Asphaltenes are produced by oxidation of naphthenic oils in the
presence of cupric naphthenate as catalyst. The test method is as
follows: 50 mg of the copolymer of which the dispersant properties
are to be measured are made up to 20 g with SN 150 under slight
heating and stirring. A solution consisting of 30 mg of
asphaltenes dissolved in 10 ml of methylene chloride is prepared
separately and is added to the dissolved polymer. The solution is
placed in an oven at 150'C for one hour to remove volatile
substances and is then allowed to cool. It is transferred into a
turbidimeter cuvette and the turbidity value read from the
instrument, this value increasing with decreasing dispersant
capacity of the polymer. After the first reading the solution is
centrifuged at 7500 rpm for 10 minutes, and then a second
turbidimeter reading is taken. The dispersion index is given by
the equation:
D.I. = (turbidity after centrif./turbidity before centrif.) x 100.
The absolute turbidity values also constitute a. factor of merit so
that for equal D.I. values an additive which gives lower absolute
turbidity is preferred.
The dispersion index of 'the copolymer prepared in this manner was
found to be 100, the absolute turbidity values being 25/25. A
commercial comparison additive gave a dispersion index of 100 and
an absolute turbidity of 73/73.
Evaluation of antioxidant~rouerties
- 9 -
20% solutions of the additive in SIV 450 containing 0.38% of ferric
naphthenate as oxidation catalyst were used. The solution
obtained is temperature controlled at 165'C and kept in air
flowing at a rate of 16.5 litres/h. Samples are taken at hourly
intervals and are tested for increase in IR oxidation band
absorbance at 1700 cm-1. The results are compared with those for
oil samples without additive. The results were as .follows:
Sample without additive:
- IR absorbance after 2 hours = 11.59; after 20 hours = 83.93.
Sample with additive:
- IR absorbance after 2 hours = 13.26; after 20 hours = 65.99.
Again to evaluate the antioxidant properties of the prepared
additive, differential thermal analysis was used to determine the
onset temperature of the exothermal peak corresponding to
25 substrate oxidation. The analyses were carried out on 2U%
solutions of polymer in SN 450 containing 0.38% of ferric
naphthenate operating with oxygen at 10 bar and with a heating
rate of 5°C/min over a 50-350°C range. The oil without additive
had an onset temperature of 174.7°C and the oil with additive an
onset temperature of 180.2°C.
Engine tests
To evaluate the engine properties of the polymer A obtained in
Examgle 1, a SAE 15W50 grade lubricant was used containing 6.5 wt%
of the polymer under examination and 10.5 wt% of conventional
additives consisting of a zinc dithiophosphonate, a superbasic
calcium sulphonate, a polyisobutenyl succinimide and a sterically
hindered phenol. 6.5% of a conventional viscosity index improver
2~~~~~~
- 10 -
based on ethylene-propylene copolymers was also used.
The engine tests used for evaluating the lubricant performance
were: VE sequence (ASTM STP 315H PTIII procedure), IIIE sequence
(ASTM STP 315H PTII procedure), Mercedes M102E black sludge test
(CEC L-41-T-88 procedure) and Petter W1 (CEC L-02-A-78
procedure). It is well known that these tests, incorporated into
official CCMC specifications, evaluate the dispersant and
antioxidant performance of the lubricant and are considered to
have been satisfied if 'the results of the evaluation of the
various engine components at the end of the test fall within the
limits stated in the specification.
The results of the tests on the described lubricant and the
respective limiting values oP the CCMC specification for class G~
lubricants are given in the follo~aing tables.
TABLE 1 -- VE SEQUENCE
Results with Specification
additived limit
oil
Average engine sludge 9.14 9 min.
Rocker arm cover sludge 8.10 7 min.
Average piston skirt 6.58 6.5 m.in.
varnish
Average engine varnish 6.01 5 min.
Oil ring clogging, % 0 15 max.
Oil screen clogging, % 2 20 max.
Compression ring struck, 0 0
No.
Cam wear average, microns130 130 max.
Cam wear max. microns 335.3 380 max.
TABLE 2 - IIIE SEQUENCE
- 11 -
Results with Specification
additived oil limit
Viscosity increase 40C, % 144 300 max.
at
Piston skirt varnish8.9 8.9 max.
Ring land varnish 3.67 3.5 min.
Sludge 9.51 9.2 min.
Ring sticking 0 0
Lifter sticking 0 0
Cam and lifter wear 30 max.
avg, microns 9.3
Cam and lifter 60 max.
wear max., microns
49
TABLE 3 - MERC. M102E(BLACK SLUDGE)
Results with Specification
additived oil limit
Engine sludge merit 9.3 9 min.
TABLE 4 - PETTER
Wl
Results with Specification
additived oil limit
Bearing weight loss, mg 19.8 25 max.
Viscosity increase at 40°C, % 87 not specified
EXAMPLE 2
148 g of SN 150 mineral oil, 125.09 g of Ciz-Cis linear
methacrylic alcohol monomers and 11.8 g of 2,2,6,6-tetramethyl-
piperidin-4-of methacrylate are fed into a reactor with a
diathermic oil heating jacket and fitted with an anchor stirrer, a
thermocouple for temperature measurement and a nitrogen injector,
and the system is left stirring for one hour while injecting
nitrogen. 15 g of methyl methacrylate and 0.9 g of tert~-
- 12 -
butylperoctoate as polymerization initiator are degassed
separately. The methyl methacrylate is then added and the
reaction mixture temperature raised to 100'C. On reaching this
temperature the catalyst is added. Polymerization commences
immediately and is strongly exothermic, the temperature control
system therefore being set to maintain this temperature constant
until the reaction is complete (2-3 hours). The progress of the
reaction is followed by I.R. analysis, the progressive
disappearance of the bands relative to the double methacry.lic
monomer bond at 1320-1340 cm-1 being noted.
The characteristics of the product obtained are determined as
described in Example 1. The results are as follows:
Viscosity undiluted at 100°C: 1100 cst
Viscosity 10% solution in SN 150 at 100°C: 13.93 cst
Viscosity 10% solution in SN 150 at 40°C: 78.92 cst
Viscosity index: 183
Viscosity 10% solution in SN 150 at -20'C: 3000 cP
Dispersion index: 100%
Absolute turbidity values: 118/118
Oxidation stability:
- non-additived oil sample: IR absorbance after 2 hours 14.59
after 20 hours 83.93
- oil sample containing 20 wt% polymer:
IR absorbance after 2 hours 7.20; after 20 hours 71.40
Differential thermal analysis: onset temperature = 189.2°C.
EXAMPLE 3 - Preparation of N-(3-hydroxypropyl)-N'-methylpiperazine
methacrylate
- 13 -
N-(3-hydroxypropyl)-N'-methylpiperazine and methylmethacryiate are
introduced in a 1:2 molar ratio into a cylindrical glass reactor
with a diathermic oil heating jacket and fitted with an anchor
stirrer, a thermocouple for temperature measurement and a
distillation column with a reflux head. 0.05% of phenothiazine by
weight with respect to the reaction mass is added as
polymerization inhibitor together with a basic catalyst such as
dibutyltin dilaurate in a molar ratio to the initial alcohol of
1:135. The residual pressure is reduced to 560 mmHg by a vacuum
pump connected to the column overhead condenser, and the system
temperature is gradually increased to 95°C. The reaction mass
boils at this temperature, the methanol-methylmethacrylate
azeotrope condensing at the top of 'the column with a weight
composition of 85:15. When the temperature at 'the top of the
column has stabilized at about 54-55°C, ie the azeotrope boiling
point, this is withdrawn through a reflux divider, the reaction
being progressively urged to completion, its progress being
followed by gas chromatography analysis. After about 9 hours,
when the converted alcohol exceeds 98%, the excess methyl
methacrylate, the unreacted alcohol and any methanol still present
are removed by high vacuum distillation, and the methacrylate
obtained in this manner is distilled.
- boiling point: 116°C/2 mmHg
- yield after distillation; 95%
- analysis by elements (theoretical values in parentheses): C =
63.4 (63.6); H = 10.1 (9.8); N = 12.1 (12.3)
- I.R. (liquid film): characteristic absorption at 1720 cm-1
(carbonyl group) and at 1640 cm-g (C=C double bond).