Note: Descriptions are shown in the official language in which they were submitted.
METHOD OF SEPARATING HYDROGEN ISOTOPE
[FIELD OF THE INVENTION]
The present invention relates to a method of separating
a hydrogen isotope. More particularly, the present invention
relates to a method of separating a hydrogen isotope, which is
useful for separation, enrichment and removal of tritium in a
nuclear fusion reactor, upgrading of heavy water and enrichment
and removal of tritium in a heavy water reactor, separation and
removal of tritium in nuclear fuel reprocessing, and separation,
recovery and removal of tritium used in a usual test or
research, and further, separation of the other hydrogen isotope
except for tritium in a manufacture of heavy water.
[DESCRIPTION OF THE PRIOR ARTJ
The method of separating a hydrogen isotope based on
isotope exchange reaction between water and hydrogen is
advantageous in that the separation coefficient is high in the
state of equilibrium, permitting an achievement of a very high
separatiing performance using a single apparatus because of the
possibility of constituting a counter-current-type reaction
tower, and because chemically easy-to-handle substances such as
water and hydrogen are only used. While this is a hopeful
method for the manufacture of heavy water, removal and recovery
of tritium from water containing tritium oxides (hereinafter
tritium water), it requires a step of repeating to reduce water
to be enriched with a heavy isotope (for example, heavy hydrogen
- 1 -
~QS~Q~ 9~
~r tritium relative to light hydrogen) to hydrogen, and the
electric power consumption in electrolysis used fox this purpose
is one of the serious uroblems.
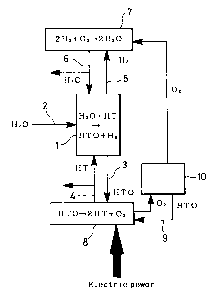
rr~ia.1 illustrates one of the embodiments of the
conventional method of separating tritium water based or_ the
combination of a counter-current-type isotope exchange reaction
tower and an electrolytic cell. As shown in rig. l, tritium
Water as a raw material is supplied through a raw material inlet
port (2) into an exchange reaction tower (1), enriched in the
reaction tower (1), and sent through an enriched water outlet
port (3) to an electrolytic cell (8). In the electrolytic cell
(8), tritium water is decomposed into hydrogen and oxygen
containing tritium at a high concentration, a part of hydrogen
is taken out as a product, and the remaining part is fed through
a hydrogen inlet port (4) into the exchange reaction tower (1).
On the other hand, oxygen, which contains tritium water vapor in
the original state, is sub,~edted to remove tritium water through
a water separator (10), and then sent into a hydrogen
recombinator ('1) by way of an oxygen feed line. After hydrogen
has tritium transfer into water in the exchange reaction tower
(1), hydrogen is sent through a hydrogen outlet port (5) into
the hydrogen recombinator ('I) where hydrogen is oxidized. A
part of thus-produced water is dumpted as depleted water or
cleaned water, and the remaining part is fed again through a
depleted water inlet port (6) into the exchange reaction tower
(1).
In such a conventional method, for example, oxidation
~0~~~3'.:~
of hydrogen and decomposition of water shoud be continuously
carried out to keep the flows of hydrogen and water to be
necessitated for isotope separation. The method is particularly
defective in that it reauires a large amount of electric power
for the decomposition of water, and this seriously results in
preventing the practical application.
The present invention has an object to provide a new
method which permits saving electric power consumption in the
step of repeating to reduce water to be enriched with a heavy
isotope to hydrogen, and does not even reauire electric power
for the decomposition of water.
Other objects, features and advantages of the present
invention will be apparent from the following description taken
in connection with the accompanying drawings.
[BRLEF DESCRIPTION OF THE DRAWINGS
Fig.l illustrates one of the embodiments of the
conventional method of separating tritium water based on the
combination of a counter-current-type isotope exchange reaction
tower and an electrolytic cell;
Fig.2 illustrates one of the embodiments of the present
invention in which an apparatus for transferring oxygen from
water to hydrogen is used;
Fig.3 depicts another embodiment of the present
invention in which a fuel cell as a recombinator is used; and
Fig.4 depicts further another embodiment of the present
invention in which an oxidizing/reducing agent is used as a
- 3 -
parameter for the reduction of water and oxidation of hydrogen. '
(DETAILED DESCRIPTION OF ThB EMBODIMENTS]
According to the present invention, it is possible to
separate a hydrogen isotope With almost no consumption of
electric power for the water decomposition step, or in some
cases, even with a gain of power.
The present invention is described below further in
detail with a separation of tritium from tritium water as an
example.
Fig.2 illustrates one of the embodiments in which an
apparatus simultaneously carrying out to convert water to
hydrogen and from hyarogen to water through transferrig oxygen
from watex to be enriched with a heavy isotope to hydrogen to be
enriched with a light isotope is combined with a Water-hydrogen
isotope exchange tower. More specifically, Fig.2 indicates a
concrete case where tritium water is enriched using an oxygen
ion conductive solid electrolytic cell as a water-hydrogen
converter. Tritium water as a raw material is supplied through
a raw material Water inlet port (12) to a water-hydrogen isotope
exchange reaction tower (11), enriched in this reaction tower
(11), and sent through an enriched water outlet port (13) to a
water-hydrogen exchanger (1'1). A part of the produced hydrogen
is taken out as a product, and the remaining part is sent back
through a hydrogen inlet port (14) to the exchange reaction
tower (11). Hydrogen has tritium transfer to water in the
exchange reaction tower (11), and is sent through a hydrogen
- a -
2~a~~~~
cutlet port (15) to the water-hydrogen converter (1Z), converted
into deleted water. The resultant depleted water is rejected
or supplied again through a depleted water inlet port (16) to
the exchange reaction tower (11). The hydrogen isotope in
tritium water is continuously separated during the process as
described above. The water-hydrogen converter (1?) supplies
water enriched with a heavy isotope on one side of an oxygen ion
conductive solid electrolytic barrier membrane (18), and
hydrogen enriched with a light isotope on the other side of
that. When a barrier membrane having a nature of permitting
permeation or' oxygen comes in contact with water on one side
thereof, and with hydrogen on the other side, the difference in
oxygen potential causes oxygen transfer from water to hydrogen.
This results in simultaneous conversions of oxygen from water to
hydrogen and from hydrogen to water. These conversions are
never mixed up each other because hydrogen isotopes are
seperated through the barrier membrance. Since these
conversions take place spontaneously to some extent, it is
usually unecessary to supply electric power, but oxygen may
forcedly be transferred to the hydrogen side by applying voltage
onto the both sides of the barrier membrance.
The electric power reauired for this transfer of oxygen
is far smaller than that reauired for conventional electrolysis.
It is thus possible to separate a hydrogen isotope and to
consume almost no electric power by the combination of the
isotope exchange reaction and a solid electrolytic cell.
The above-mentioned embodiment is involved in a method
- 5 -
In which electrolysis and recombination reaction are carried out
in a single apparatus. It is also possible to achieve those
with separate apparatuses.
Fig.3 shows a process of electrolyzing water with the
use of electric power produced when the recombination reaction
is utilized in generation or a fuel cell. In Fig.3, while an
isotope exchange reaction tower (11) is the same as shown in
Fig.2, hydrogen enriched with a light isotope is sent through
the hydrogen outlet port (15) to a hydrogen oxygen fuel cell
(19), and water enriched with a heavy isotope is sent through an
enriched water outlet port (13) to an electrolytic cell (20),
respectively. In the case where an oxygen ion conductive solid
electrolytic cell as shown in Fig.2 is used as that electrolytic
cell (20), the generating oxygen is so highly pure that water
and hydrogen are not contaminated by tritium even when supplying
the oxygen to the fuel cell (19). Oxidation of hydrogen in the
fuel cell (19) spontaneously ocurrs and proceeds, enabling to
take out electric power. The electric cell (20) is operated
with this electric power. Because the amount of water supplied
to the electrolytic cell (20) is almost eaual to that of
hydrogen supplied to the fuel cell (19), the amount of electric
generation of the fuel cell (19) serves the electric power
necessary for electrolysis, if electric loss is disregarded.
Furthermore, while decomposition of water in the electrolytic
cell (20) operated at a high temperature of about 900°C reauires
a voltage of about 0.9 V, the electro motive force or' the
hydrogen oxygen fuel cell (19) operated at the room temperature
- 6 -
~fl5~~~~
is about 1.2 V. It is therefore theoretically possible to
achieve a gain of electric power by appropriately setting these
operating temperatures.
Fig.4 depicts one of the embodiments in which energy is
tran3ferred through an aapropriate oxidation/reduction agent
from an oxidation step of rydrogen to a reduction step of water.
For example, a water gas equilibrium (H20 + CO = H2 + C02) is
now explained as follows: Hydrogen enriched with a light
isotope from the isotope exchange reaction tower (11) is sent
through the hydrogen outlet port (15) to a hydrogen oxidation
reactor (21), and water enriched with a heavy isotope is sent
through the enriched water outlet port (13) to a water reduction
reactor (22), respectively. As a water gas eauilibrium(H20 + CO
- H2 + C02) reaction is a reversible equilibrium reaction,
hydrogen and carbon dioxide are produced until the ea_uilibrium
is reached when water and carbon monoxide are supplied to the
water reduction reactor (22). On the other hand, water and
carbon monoxide are produced when hydrogen and carbon dioxide
are supplied to the hydrogen oxidation reactor (21).
By separating carbon dioxide anti carbon monoxide from those
reactors and circulating the same, it is possible to supply
water and hydrogen to the water-hydrogen isotope exchange
reaction tower (11) through oxidation of hydrogen and reduction
of water almost without energy consumption. In the practical
application of this embodiment, the steps of separating carbon
monoxide and carbon dioxide from water and hydrogen may be
omitted by using an oxygen ion conductive solid electrolytic
- 7 -
.:ell as shown in Fig.2 for the reactors (21) and (22).
It is needless to mention that various embodiments of
the present invention are possible in details of the
constitution thereof.
_ g _